Dead Cas Systems: Types, Principles, and Applications
Abstract
:1. Introduction
2. Epigenomic Remodeling Using dCas-X
2.1. Modifying the Methylation State of DNA
2.2. Principles of gRNA Design for Genome Methylation or Demethylation
- Initial methylation of target DNA. Effective suppression of gene transcription by dCas9-DNMTs can be achieved if gRNAs target initially unmethylated or weakly methylated regions [8]. In contrast, dCas9-based systems of DNA demethylation are effective only if gRNAs target heavily methylated DNA regions. DNA methylation levels in different cell lines and tissues can be assessed using several databases, including ENCODE [14] and MethBase [15].
- Methylation sites. Using dCas9-DNMT3A results in methylation of two regions. The first one lies within 27 nt in the 3ʹ-direction from the PAM sequence, and the second is within 27 nt from 5ʹ-end of the gRNA. The site of dCas9 binding (approximately 30 nt) is not methylated [6,9]. Methylation at the two sites occurs due to a flexible peptide linker between the dCas9 protein and the DNMT3A enzyme/catalytic subunit, providing mobility to the methyltransferase enzyme [6]. Peaks of DNA methylation vary upon introducing additional factors to the system, such as binding DNMT3A to DNMT3L [9].
- Methylation window. Methylation of extensive DNA regions is mandatory for stable suppression of gene function. dCas9-DNMT3A methylates regions 25–35 nt in length [6], and thus can only be used for pinpoint methylation [8]. Methylating extensive DNA regions is possible when using multiple gRNAs annealing to proximal DNA regions with the dCas9-DNMT3A system, [6] or a single gRNA combined with dCas9-DNMT3A-3L systems (which can methylate up to 1000 nt) [9] or dCas9-SunTag-DNMT3A (which methylates up to 4500 nt) [10]. DNA demethylation can be induced by dCas9-SunTag-TET1 within 200 nt of dCas9 binding (±100 nt from the dCas9 binding site) [12].
2.3. Regulating DNA Methylation State by dCas-Based Tools: Practical Applications
3. Rewriting Histone Epigenetic Marks
3.1. CRISPRi
3.2. CRISPRa
3.3. Principles of gRNA design for CRISPRa and CRISPRi
3.3.1. CRISPRi
- Target region. CRISPRi approaches should primarily target proximal promoters or enhancers. gRNAs targeting promoters should be designed to anneal at –50 to +300 nt from transcription start site. Highest efficacy has been demonstrated for gRNAs targeting +50 to +100 nt [64]. Transcription start sites can be visualized using FANTOM5 [65] or GeneHancer databases [43].
- Epigenetic state. The most effective binding of dCas proteins occurs in areas of open chromatin determined by peaks of DNase I sensitivity [66]. Moreover, effective interference is observed when using gRNAs targeting sites enriched with marks of active chromatin (H3K27Ac, H3K9Ac, H3K4Me3, H3K4Me2, H3K79Me2) [67]. Epigenetic marks and sites of DNase I hypersensitivity can be monitored using ENCODE database [14].
3.3.2. CRISPRa
- Target region. CRISPRa should target proximal promoters, or, for some systems (e.g., dCas9-p300), distal enhancers. CRISPRa gRNAs to promoters should be designed to interact within −400 to −50 nt from the transcription start site [49].
- Epigenetic state. The most effective activation of genes occurs when CRISPRa are recruited to the sites of DNase I hypersensitivity [66].
3.4. Applications of CRISPRa/i
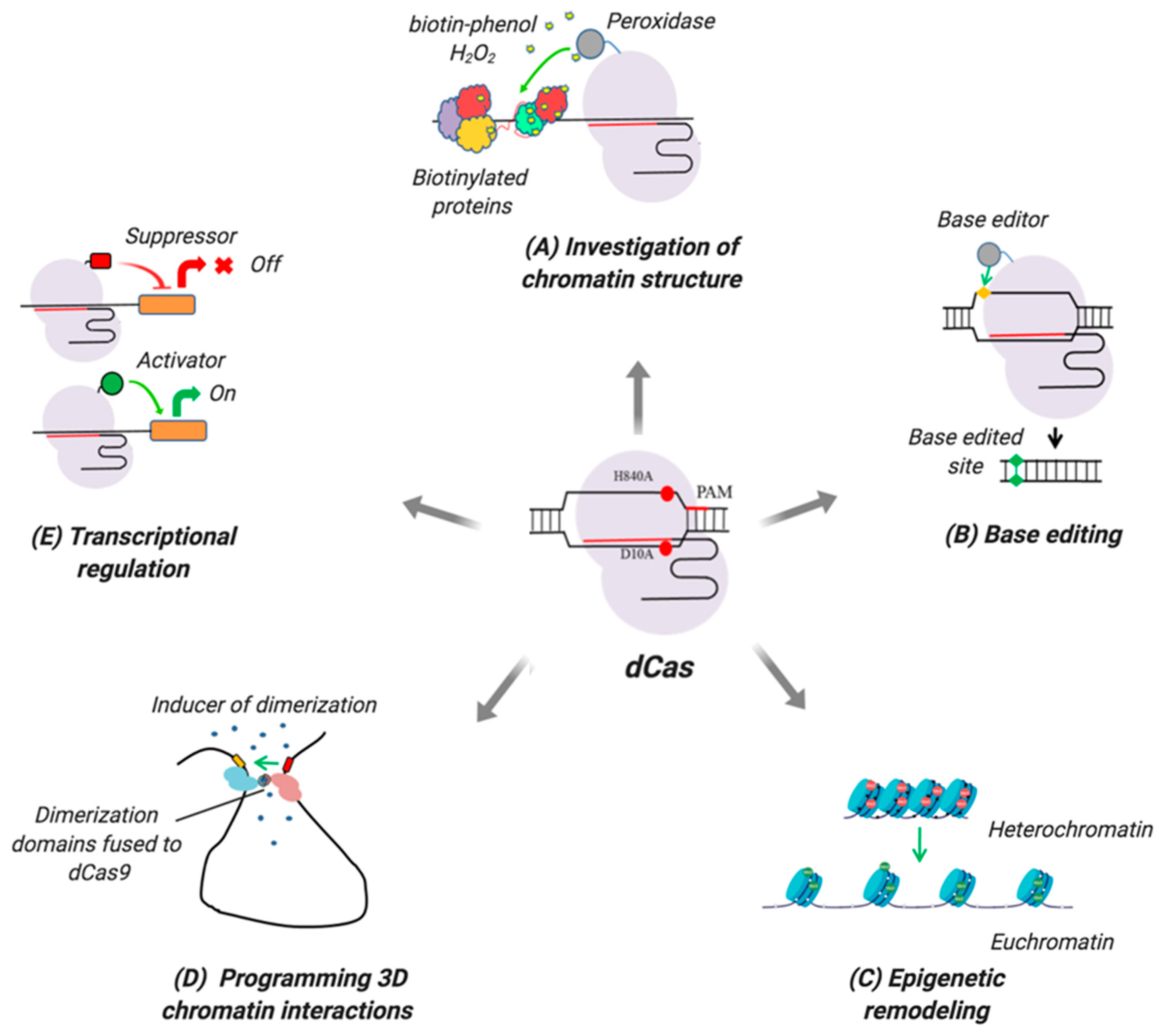
4. Analyzing Factors Involved in Chromatin Remodeling
4.1. dCas Technology for Analyzing Chromatin-Remodeling Factors
4.2. Principles of gRNA Design for CAPTURE, CasID, and CASPEX Methods
- Target site. gRNAs should anneal at the most proximal area of the target region, but should not lie at sites bound by transcription factors to avoid impeding interactions between regulatory DNA elements and proteins [117].
- Off-target interactions. For better consistency, proteome analysis of chromatin architecture should be performed with validated negative controls (cells without dCas9, and cells with dCas9 but without gRNA) and should consider endogenous and non-specific biotinylation [117,118,119,120]. Generating several gRNAs for each site and further comparing the data are strongly recommended to discern factors stably bound to the target region and those with rare and transient interactions [117,118].
5. dCas Systems for Shaping Three-dimensional Chromatin Architecture
6. Editing Nucleic Acids
6.1. DNA Editing Using dCas Tools
6.2. Editing RNA with dCas Tools
6.3. Applications of dCas Base Editors
6.4. Principles of gRNAs Design for Base Editing Applications
7. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.S.; Larson, M.H.; Gilbert, L.A.; Doudna, J.A.; Weissman, J.S.; Arkin, A.P.; Lim, W.A. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 2013, 152, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Chedin, F.; Lieber, M.R.; Hsieh, C.L. The DNA methyltransferase-like protein DNMT3L stimulates de novo methylation by Dnmt3a. Proc. Natl. Acad. Sci. USA 2002, 99, 16916–16921. [Google Scholar] [CrossRef]
- Guo, J.U.; Su, Y.; Zhong, C.; Ming, G.; Song, H. Hydroxylation of 5-Methylcytosine by TET1 Promotes Active DNA Demethylation in the Adult Brain. Cell 2011, 145, 423–434. [Google Scholar] [CrossRef]
- Vojta, A.; Dobrinic, P.; Tadic, V.; Bockor, L.; Korac, P.; Julg, B.; Klasic, M.; Zoldos, V. Repurposing the CRISPR-Cas9 system for targeted DNA methylation. Nucleic Acids Res. 2016, 44, 5615–5628. [Google Scholar] [CrossRef]
- McDonald, J.I.; Celik, H.; Rois, L.E.; Fishberger, G.; Fowler, T.; Rees, R.; Kramer, A.; Martens, A.; Edwards, J.R.; Challen, G.A. Reprogrammable CRISPR/Cas9-based system for inducing site-specific DNA methylation. Biol. Open 2016, 5, 866–874. [Google Scholar] [CrossRef]
- Liu, X.S.; Wu, H.; Ji, X.; Stelzer, Y.; Wu, X.; Czauderna, S.; Shu, J.; Dadon, D.; Young, R.A.; Jaenisch, R. Editing DNA methylation in the mammalian genome. Cell 2016, 167, 233–247. [Google Scholar] [CrossRef]
- Stepper, P.; Kungulovski, G.; Jurkowska, R.Z.; Chandra, T.; Krueger, F.; Reinhardt, R.; Reik, W.; Jeltsch, A.; Jurkowski, T.P. Efficient targeted DNA methylation with chimeric dCas9–Dnmt3a–Dnmt3L methyltransferase. Nucleic Acids Res. 2016, 45, 1703–1713. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Su, J.; Lei, Y.; Brunetti, L.; Gundry, M.C.; Zhang, X.; Jeong, M.; Li, W.; Goodell, M.A. DNA epigenome editing using CRISPR-Cas SunTag-directed DNMT3A. Genome Biol. 2017, 18, 176. [Google Scholar] [CrossRef]
- Choudhury, S.R.; Cui, Y.; Lubecka, K.; Stefanska, B.; Irudayaraj, J. CRISPR-dCas9 mediated TET1 targeting for selective DNA demethylation at BRCA1 promoter. Oncotarget 2016, 7, 46545. [Google Scholar] [CrossRef] [PubMed]
- Morita, S.; Noguchi, H.; Horii, T.; Nakabayashi, K.; Kimura, M.; Okamura, K.; Sakai, A.; Nakashitna, H.; Hata, K.; Nakashima, K.; et al. Targeted DNA demethylation in vivo using dCas9-peptide repeat and scFv-TET1 catalytic domain fusions. Nat. Biotechnol. 2016, 34, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Tao, Y.; Gao, X.; Zhang, L.; Li, X.; Zou, W.; Ruan, K.; Wang, F.; Xu, G.; Hu, R. A CRISPR-based approach for targeted DNA demethylation. Cell Discov. 2016, 2, 16009. [Google Scholar] [CrossRef] [PubMed]
- Dunham, I.; Kundaje, A.; Aldred, S.F.; Collins, P.J.; Davis, C.; Doyle, F.; Epstein, C.B.; Frietze, S.; Harrow, J.; Kaul, R.; et al. An integrated encyclopedia of DNA elements in the human genome. Nature 2012, 489, 57–74. [Google Scholar]
- Song, Q.; Decato, B.; Hong, E.E.; Zhou, M.; Fang, F.; Qu, J.; Garvin, T.; Kessler, M.; Zhou, J.; Smith, A.D. A Reference Methylome Database and Analysis Pipeline to Facilitate Integrative and Comparative Epigenomics. PLoS ONE 2013, 8, e81148. [Google Scholar] [CrossRef]
- Qu, J.; Zhu, L.; Zhou, Z.; Chen, P.; Liu, S.; Locy, M.L.; Thannickal, V.J.; Zhou, Y. Reversing Mechanoinductive DSP Expression by CRISPR/dCas9-mediated Epigenome Editing. Am. J. Respir. Crit. Care Med. 2018, 198, 599–609. [Google Scholar] [CrossRef]
- Mkannez, G.; Gagne-Ouellet, V.; Nsaibia, M.J.; Boulanger, M.-C.; Rosa, M.; Argaud, D.; Hadji, F.; Gaudreault, N.; Rheaume, G.; Bouchard, L.; et al. DNA methylation of a PLPP3 MIR transposon-based enhancer promotes an osteogenic programme in calcific aortic valve disease. Cardiovasc. Res. 2018, 114, 1525–1535. [Google Scholar] [CrossRef]
- Park, J.; Guan, Y.; Sheng, X.; Gluck, C.; Seasock, M.J.; Hakimi, A.A.; Qiu, C.; Pullman, J.; Verma, A.; Li, H.; et al. Functional methylome analysis of human diabetic kidney disease. JCI Insight 2019. [Google Scholar] [CrossRef]
- Saunderson, E.A.; Stepper, P.; Gomm, J.J.; Hoa, L.; Morgan, A.; Allen, M.D.; Jones, J.L.; Gribben, J.G.; Jurkowski, T.P.; Ficz, G. Hit-and-run epigenetic editing prevents senescence entry in primary breast cells from healthy donors. Nat. Commun. 2017, 8, 1450. [Google Scholar] [CrossRef]
- Wu, J.; He, K.; Zhang, Y.; Song, J.; Shi, Z.; Chen, W.; Shao, Y. Inactivation of SMARCA2 by promoter hypermethylation drives lung cancer development. Gene 2019, 687, 193–199. [Google Scholar] [CrossRef]
- Tong, Y.; Sun, J.; Wong, C.F.; Kang, Q.; Ru, B.; Wong, C.N.; Chan, A.S.; Leung, S.Y.; Zhang, J. MICMIC: Identification of DNA methylation of distal regulatory regions with causal effects on tumorigenesis. Genome Biol. 2018, 19, 73. [Google Scholar] [CrossRef] [PubMed]
- Schuijers, J.; Manteiga, J.C.; Weintraub, A.S.; Day, D.S.; Zamudio, A.V.; Hnisz, D.; Lee, T.I.; Young, R.A. Transcriptional Dysregulation of MYC Reveals Common Enhancer-Docking Mechanism. Cell Rep. 2018, 23, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Dai, L.; Wang, Y.; Deng, J.; Lin, Y.; Wang, Q.; Fang, C.; Ma, Z.; Wang, H.; Shi, G.; et al. Targeted demethylation of the SARI promotor impairs colon tumour growth. Cancer Lett. 2019, 448, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.S.; Wu, H.; Krzisch, M.; Wu, X.; Graef, J.; Muffat, J.; Hnisz, D.; Li, C.H.; Yuan, B.; Xu, C.; et al. Rescue of Fragile X Syndrome Neurons by DNA Methylation Editing of the FMR1 Gene. Cell 2018, 172, 979. [Google Scholar] [CrossRef] [PubMed]
- Kantor, B.; Tagliafierro, L.; Gu, J.; Zamora, M.E.; Ilich, E.; Grenier, C.; Huang, Z.Y.; Murphy, S.; Chiba-Falek, O. Downregulation of SNCA Expression by Targeted Editing of DNA Methylation: A Potential Strategy for Precision Therapy in PD. Mol. Ther. 2018, 26, 2638–2649. [Google Scholar] [CrossRef]
- Baumann, V.; Wiesbeck, M.; Breunig, C.T.; Braun, J.M.; Koeferle, A.; Ninkovic, J.; Goetz, M.; Stricker, S.H. Targeted removal of epigenetic barriers during transcriptional reprogramming. Nat. Commun. 2019, 10, 2119. [Google Scholar] [CrossRef] [PubMed]
- Barski, A.; Cuddapah, S.; Cui, K.; Roh, T.-Y.; Schones, D.E.; Wang, Z.; Wei, G.; Chepelev, I.; Zhao, K. High-resolution profiling of histone methylations in the human genome. Cell 2007, 129, 823–837. [Google Scholar] [CrossRef]
- Shinkai, Y.; Tachibana, M. H3K9 methyltransferase G9a and the related molecule GLP. Genes Dev. 2011, 25, 781–788. [Google Scholar] [CrossRef]
- Rea, S.; Eisenhaber, F.; O’Carroll, N.; Strahl, B.D.; Sun, Z.W.; Schmid, M.; Opravil, S.; Mechtler, K.; Ponting, C.P.; Allis, C.D.; et al. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 2000, 406, 593–599. [Google Scholar] [CrossRef]
- Cao, R.; Zhang, Y. SUZ12 is required for both the histone methyltransferase activity and the silencing function of the EED-EZH2 complex. Mol. Cell 2004, 15, 57–67. [Google Scholar] [CrossRef]
- Vire, E.; Brenner, C.; Deplus, R.; Blanchon, L.; Fraga, M.; Didelot, C.; Morey, L.; Van Eynde, A.; Bernard, D.; Vanderwinden, J.M.; et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature 2006, 439, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Fuks, F.; Hurd, P.J.; Deplus, R.; Kouzarides, T. The DNA methyltransferases associate with HP1 and the SUV39H1 histone methyltransferase. Nucleic Acids Res. 2003, 31, 2305–2312. [Google Scholar] [CrossRef] [PubMed]
- Lachner, M.; O’Carroll, N.; Rea, S.; Mechtler, K.; Jenuwein, T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 2001, 410, 116–120. [Google Scholar] [CrossRef] [PubMed]
- O’Geen, H.; Bates, S.L.; Carter, S.S.; Nisson, K.A.; Halmai, J.; Fink, K.D.; Rhie, S.K.; Farnham, P.J.; Segal, D.J. Ezh2-dCas9 and KRAB-dCas9 enable engineering of epigenetic memory in a context-dependent manner. Epigenet. Chromatin 2019, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.; Bannister, A.J.; Myers, F.A.; Thorne, A.W.; Crane-Robinson, C.; Kouzarides, T. Histone H3 lysine 4 methylation patterns in higher eukaryotic genes. Nat. Cell Biol. 2004, 6, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Creyghton, M.P.; Cheng, A.W.; Welstead, G.G.; Kooistra, T.; Carey, B.W.; Steine, E.J.; Hanna, J.; Lodato, M.A.; Frampton, G.M.; Sharp, P.A.; et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl. Acad. Sci. USA 2010, 107, 21931–21936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steger, D.J.; Lefterova, M.I.; Ying, L.; Stonestrom, A.J.; Schupp, M.; Zhuo, D.; Vakoc, A.L.; Kim, J.-E.; Chen, J.; Lazar, M.A.; et al. DOT1L/KMT4 recruitment and H3K79 methylation are ubiquitously coupled with gene transcription in mammalian cells. Mol. Cell Biol. 2008, 28, 2825–2839. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.J.; Lan, F.; Matson, C.; Mulligan, P.; Whetstine, J.R.; Cole, P.A.; Casero, R.A.; Shi, Y. Histone demethylation mediated by the nuclear arnine oxidase homolog LSD1. Cell 2004, 119, 941–953. [Google Scholar] [CrossRef] [Green Version]
- Pasini, D.; Malatesta, M.; Jung, H.R.; Walfridsson, J.; Willer, A.; Olsson, L.; Skotte, J.; Wutz, A.; Porse, B.; Jensen, O.N.; et al. Characterization of an antagonistic switch between histone H3 lysine 27 methylation and acetylation in the transcriptional regulation of Polycomb group target genes. Nucleic Acids Res. 2010, 38, 4958–4969. [Google Scholar] [CrossRef]
- Jackson, A.L.; Bartz, S.R.; Schelter, J.; Kobayashi, S.V.; Burchard, J.; Mao, M.; Li, B.; Cavet, G.; Linsley, P.S. Expression profiling reveals off-target gene regulation by RNAi. Nat. Biotechnol. 2003, 21, 635–637. [Google Scholar] [CrossRef]
- Kampmann, M. CRISPRi and CRISPRa Screens in Mammalian Cells for Precision Biology and Medicine. ACS Chem. Biol. 2018, 13, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.R.; Fredericks, W.J.; Jensen, D.E.; Speicher, D.W.; Huang, X.P.; Neilson, E.G.; Rauscher, F.J. KAP-1, a novel corepressor for the highly conserved KRAB repression domain. Genes Dev. 1996, 10, 2067–2078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakore, P.I.; D’ippolito, A.M.; Song, L.; Safi, A.; Shivakumar, N.K.; Kabadi, A.M.; Reddy, T.E.; Crawford, G.E.; Gersbach, C.A. Highly specific epigenome editing by CRISPR-Cas9 repressors for silencing of distal regulatory elements. Nat. Methods 2015, 12, 1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultz, D.C.; Friedman, J.R.; Rauscher, F.J. Targeting histone deacetylase complexes via KRAB-zinc finger proteins: The PHD and bromodomains of KAP-1 form a cooperative unit that recruits a novel isoform of the Mi-2 alpha subunit of NuRD. Genes Dev. 2001, 15, 428–443. [Google Scholar] [CrossRef] [Green Version]
- O’Geen, H.; Ren, C.; Nicolet, C.M.; Perez, A.A.; Halmai, J.; Le, V.M.; Mackay, J.P.; Farnham, P.J.; Segal, D.J. dCas9-based epigenome editing suggests acquisition of histone methylation is not sufficient for target gene repression. Nucleic Acids Res. 2017, 45, 9901–9916. [Google Scholar] [CrossRef] [Green Version]
- Amabile, A.; Migliara, A.; Capasso, P.; Biffi, M.; Cittaro, D.; Naldini, L.; Lombardo, A. Inheritable silencing of endogenous genes by hit-and-run targeted epigenetic editing. Cell 2016, 167, 219–232. [Google Scholar] [CrossRef] [Green Version]
- Yeo, N.C.; Chavez, A.; Lance-Byrne, A.; Chan, Y.; Menn, D.; Milanova, D.; Kuo, C.-C.; Guo, X.; Sharma, S.; Tung, A.; et al. An enhanced CRISPR repressor for targeted mammalian gene regulation. Nat. Methods 2018, 15, 611. [Google Scholar] [CrossRef]
- Kearns, N.A.; Pham, H.; Tabak, B.; Genga, R.M.; Silverstein, N.J.; Garber, M.; Maehr, R. Functional annotation of native enhancers with a Cas9-histone demethylase fusion. Nat. Methods 2015, 12, 401–403. [Google Scholar] [CrossRef] [Green Version]
- Hilton, I.B.; Vockley, C.M.; Pratiksha, I.; Crawford, G.E.; Reddy, T.E.; Gersbach, C.A. CRISPR Acetyltransferase Activates Genes From Promoters and Enhancers. Nat. Biotechnol. 2015, 33, 510–517. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, L.A.; Larson, M.H.; Morsut, L.; Liu, Z.; Brar, G.A.; Torres, S.E.; Stern-Ginossar, N.; Brandman, O.; Whitehead, E.H.; Doudna, J.A. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 2013, 154, 442–451. [Google Scholar] [CrossRef] [Green Version]
- Konermann, S.; Brigham, M.D.; Trevino, A.E.; Joung, J.; Abudayyeh, O.O.; Barcena, C.; Hsu, P.D.; Habib, N.; Gootenberg, J.S.; Nishimasu, H. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature 2015, 517, 583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Pinera, P.; Kocak, D.D.; Vockley, C.M.; Adler, A.F.; Kabadi, A.M.; Polstein, L.R.; Thakore, P.I.; Glass, K.A.; Ousterout, D.G.; Leong, K.W.; et al. RNA-guided gene activation by CRISPR-Cas9-based transcription factors. Nat. Methods 2013, 10, 973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeder, M.L.; Linder, S.J.; Cascio, V.M.; Fu, Y.; Ho, Q.H.; Joung, J.K. CRISPR RNA—guided activation of endogenous human genes. Nat. Methods 2013, 10, 977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, A.W.; Wang, H.; Yang, H.; Shi, L.; Katz, Y.; Theunissen, T.W.; Rangarajan, S.; Shivalila, C.S.; Dadon, D.B.; Jaenisch, R. Multiplexed activation of endogenous genes by CRISPR-on, an RNA-guided transcriptional activator system. Cell Res. 2013, 23, 1163. [Google Scholar] [CrossRef] [PubMed]
- Chavez, A.; Scheiman, J.; Vora, S.; Pruitt, B.W.; Tuttle, M.; Iyer, E.P.R.; Lin, S.; Kiani, S.; Guzman, C.D.; Wiegand, D.J. Highly efficient Cas9-mediated transcriptional programming. Nat. Methods 2015, 12, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, L.; Hung, S.S.C.; Yek, J.; El Wazan, L.; Nguyen, T.; Khan, S.; Lim, S.Y.; Hewitt, A.W.; Wong, R.C.B. A Simple Cloning-free Method to Efficiently Induce Gene Expression Using CRISPR/Cas9. Mol. Ther. 2019, 14, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Chavez, A.; Tuttle, M.; Pruitt, B.W.; Ewen-Campen, B.; Chari, R.; Ter-Ovanesyan, D.; Haque, S.J.; Cecchi, R.J.; Kowal, E.J.K.; Buchthal, J. Comparison of Cas9 activators in multiple species. Nat. Methods 2016, 13, 563. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Liu, J.; Zhou, C.; Gao, N.; Rao, Z.; Li, H.; Hu, X.; Li, C.; Yao, X.; Shen, X.; et al. In vivo simultaneous transcriptional activation of multiple genes in the brain using CRISPR-dCas9-activator transgenic mice. Nat. Neurosci. 2018, 21, 440. [Google Scholar] [CrossRef]
- Tanenbaum, M.E.; Gilbert, L.A.; Qi, L.S.; Weissman, J.S.; Vale, R.D. A Protein-Tagging System for Signal Amplification in Gene Expression and Fluorescence Imaging. Cell 2014, 159, 635–646. [Google Scholar] [CrossRef] [Green Version]
- Zalatan, J.G.; Lee, M.E.; Almeida, R.; Gilbert, L.A.; Whitehead, E.H.; La Russa, M.; Tsai, J.C.; Weissman, J.S.; Dueber, J.E.; Qi, L.S. Engineering complex synthetic transcriptional programs with CRISPR RNA scaffolds. Cell 2015, 160, 339–350. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.W.; Jillette, N.; Lee, P.; Plaskon, D.; Fujiwara, Y.; Wang, W.; Taghbalout, A.; Wang, H. Casilio: A versatile CRISPR-Cas9-Pumilio hybrid for gene regulation and genomic labeling. Cell Res. 2016, 26, 254–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunii, A.; Hara, Y.; Takenaga, M.; Hattori, N.; Fukazawa, T.; Ushijima, T.; Yamamoto, T.; Sakuma, T. Three-Component Repurposed Technology for Enhanced Expression: Highly Accumulable Transcriptional Activators via Branched Tag Arrays. CRISPR J. 2018, 1, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gao, J.; Dai, W.; Wang, D.; Wu, J.; Wang, J. Gene activation by a CRISPR-assisted trans enhancer. Elife 2019, 8, e45973. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.A.; Horlbeck, M.A.; Adamson, B.; Villalta, J.E.; Chen, Y.; Whitehead, E.H.; Guimaraes, C.; Panning, B.; Ploegh, H.L.; Bassik, M.C.; et al. Genome-Scale CRISPR-Mediated Control of Gene Repression and Activation. Cell 2014, 159, 647–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, R.; Gebhard, C.; Miguel-Escalada, I.; Hoof, I.; Bornholdt, J.; Boyd, M.; Chen, Y.; Zhao, X.; Schmidl, C.; Suzuki, T.; et al. An atlas of active enhancers across human cell types and tissues. Nature 2014, 507, 455. [Google Scholar] [CrossRef] [PubMed]
- Kuscu, C.; Arslan, S.; Singh, R.; Thorpe, J.; Adli, M. Genome-wide analysis reveals characteristics of off-target sites bound by the Cas9 endonuclease. Nat. Biotechnol. 2014, 32, 677. [Google Scholar] [CrossRef] [PubMed]
- Radzisheuskaya, A.; Shlyueva, D.; Muller, I.; Helin, K. Optimizing sgRNA position markedly improves the efficiency of CRISPR/dCas9-mediated transcriptional repression. Nucleic Acids Res. 2016, 44, e141. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Xu, J.; Cheng, M.; Liao, X.; Peng, S. Review of CRISPR/Cas9 sgRNA Design Tools. Interdiscip. Sci. 2018, 10, 455–465. [Google Scholar] [CrossRef]
- Gasperini, M.; Hill, A.J.; McFaline-Figueroa, J.L.; Martin, B.; Kim, S.; Zhang, M.D.; Jackson, D.; Leith, A.; Schreiber, J.; Noble, W.S.; et al. A Genome-wide Framework for Mapping Gene Regulation via Cellular Genetic Screens. Cell 2019, 176, 377. [Google Scholar] [CrossRef] [Green Version]
- Klann, T.S.; Black, J.B.; Chellappan, M.; Safi, A.; Song, L.; Hilton, I.B.; Crawford, G.E.; Reddy, T.E.; Gersbach, C.A. CRISPR-Cas9 epigenome editing enables high-throughput screening for functional regulatory elements in the human genome. Nat. Biotechnol. 2017, 35, 561. [Google Scholar] [CrossRef]
- Bogerd, H.P.; Kornepati, A.V.R.; Marshall, J.B.; Kennedy, E.M.; Cullen, B.R. Specific induction of endogenous viral restriction factors using CRISPR/Cas-derived transcriptional activators. Proc. Natl. Acad. Sci. USA 2015, 112, E7249–E7256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saayman, S.M.; Lazar, D.C.; Scott, T.A.; Hart, J.R.; Takahashi, M.; Burnett, J.C.; Planelles, V.; Morris, K.V.; Weinberg, M.S. Potent and Targeted Activation of Latent HIV-1 Using the CRISPR/dCas9 Activator Complex. Mol. Ther. 2016, 24, 488–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limsirichai, P.; Gaj, T.; Schaffer, D.V. CRISPR-mediated Activation of Latent HIV-1 Expression. Mol. Ther. 2016, 24, 499–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Yin, C.; Zhang, T.; Li, F.; Yang, W.; Kaminski, R.; Fagan, P.R.; Putatunda, R.; Young, W.-B.; Khalili, K.; et al. CRISPR/gRNA-directed synergistic activation mediator (SAM) induces specific, persistent and robust reactivation of the HIV-1 latent reservoirs. Sci. Rep. 2015, 5, 16277. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Jiang, Z.; Lu, P.; Ma, L.; Liz, C.; Pan, H.; Fill, Z.; Qui, X.; Wang, P.; Deng, J.; et al. Specific Reactivation of Latent HIV-1 by dCas9-SunTag-VP64-mediated Guide RNA Targeting the HIV-1 Promoter. Mol. Ther. 2016, 24, 508–521. [Google Scholar] [CrossRef] [Green Version]
- Kostiushev, D.; Brezgin, S.; Kostyusheva, A.; Zarifyan, D.; Chulanov, V. A novel CRISPR/Cas9-based approach to transient activation of intracellular host restriction factors results in strong suppression of hepatitis B virus and degradation of cccDNA. J. Viral Hepat. 2018, 25, 16–17. [Google Scholar]
- Kostyushev, D.; Brezgin, S.; Kostyusheva, A.; Zarifyan, D.; Goptar, I.; Chulanov, V. Orthologous CRISPR/Cas9 systems for specific and efficient degradation of covalently closed circular DNA of hepatitis B virus. Cell Mol. Life Sci. 2019, 76, 1779–1794. [Google Scholar] [CrossRef]
- Kostyushev, D.; Kostyusheva, A.; Brezgin, S.; Zarifyan, D.; Utkina, A.; Goptar, I.; Chulanov, V. Suppressing the NHEJ pathway by DNA-PKcs inhibitor NU7026 prevents degradation of HBV cccDNA cleaved by CRISPR/Cas9. Sci. Rep. 2019, 9, 1847. [Google Scholar] [CrossRef] [Green Version]
- Orchard, R.; Sullender, M.E.; Dunlap, B.F.; Balce, D.R.; Doench, J.G.; Virgin, H.W. Identification of anti-norovirus genes in mouse and human cells using genome-wide CRISPR activation screening. BioRxiv 2018, 350090. [Google Scholar] [CrossRef] [Green Version]
- Matharu, N.; Rattanasopha, S.; Tamura, S.; Maliskova, L.; Wang, Y.; Bernard, A.; Hardin, A.; Eckalbar, W.L.; Vaisse, C.; Ahituv, N. CRISPR-mediated activation of a promoter or enhancer rescues obesity caused by haploinsufficiency. Science 2019, 363, 246. [Google Scholar] [CrossRef]
- Colasante, G.; Lignani, G.; Brusco, S.; Di Berardino, C.; Carpenter, J.; Giannelli, S.; Valassina, N.; Bido, S.; Ricci, R.; Castoldi, V. dCas9-based Scn1a gene activation restores inhibitory interneuron excitability and attenuates seizures in Dravet syndrome mice. Mol. Ther. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, R.; Du, Z.; Bai, L.; Li, L.; Cui, J.; Li, W.; Hoffman, A.R.; Hu, J.-F. Epigenetic Targeting of Granulin in Hepatoma cells by Synthetic CRISPR dCas9 Epi-suppressors. Mol. Ther. Nucl. Acids 2018, 11, 23–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moses, C.; Nugent, F.; Waryah, C.B.; Garcia-Bloj, B.; Harvey, A.R.; Blancafort, P. Activating PTEN Tumor Suppressor Expression with the CRISPR/dCas9 System. Mol. Ther. Nucl. Acids 2019, 14, 287–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, C.J.; Bruno, P.M.; Horlbeck, M.A.; Gilbert, L.A.; Weissman, J.S.; Hemann, M.T. Versatile in vivo regulation of tumor phenotypes by dCas9-mediated transcriptional perturbation. Proc. Natl. Acad. Sci. USA 2016, 113, E3892–E3900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kardooni, H.; Gonzalez-Gualda, E.; Stylianakis, E.; Saffaran, S.; Waxman, J.; Kypta, R.M. CRISPR-Mediated Reactivation of DKK3 Expression Attenuates TGF-beta Signaling in Prostate Cancer. Cancers 2018, 10, 165. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Chow, R.D.; Bai, Z.; Zhu, L.; Errami, Y.; Dai, X.; Dong, M.B.; Ye, L.; Zhang, X.; Renauer, P.A. Multiplexed activation of endogenous genes by CRISPRa elicits potent antitumor immunity. Nat. Immunol. 2019, 20, 1494–1505. [Google Scholar] [CrossRef]
- Carleton, J.B.; Berrett, K.C.; Gertz, J. Multiplex Enhancer Interference Reveals Collaborative Control of Gene Regulation by Estrogen Receptor alpha-Bound Enhancers. Cell Syst. 2017, 5, 333. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Shen, W.; Zhang, J.; Yang, B.; Liu, Y.-N.; Qi, H.; Yu, X.; Lul, S.-Y.; Chen, Y.; Xu, Y.-Z.; et al. CRISPR interference-based specific and efficient gene inactivation in the brain. Nat. Neurosci. 2018, 21, 447. [Google Scholar] [CrossRef]
- Savell, K.E.; Bach, V.S.; Zipperly, M.E.; Revanna, J.S.; Goska, N.A.; Tuscher, J.J.; Duke, C.G.; Sultan, F.A.; Burke, J.N.; Williams, D.; et al. A Neuron-Optimized CRISPR/dCas9 Activation System for Robust and Specific Gene Regulation. eNeuro 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Baeumler, T.A.; Ahmed, A.A.; Fulga, T.A. Engineering Synthetic Signaling Pathways with Programmable dCas9-Based Chimeric Receptors. Cell Rep. 2017, 20, 2639–2653. [Google Scholar] [CrossRef] [Green Version]
- Kipniss, N.H.; Dingal, P.C.D.P.; Abbott, T.R.; Gao, Y.; Wang, H.; Dominguez, A.A.; Labanieh, L.; Qi, L.S. Engineering cell sensing and responses using a GPCR-coupled CRISPR-Cas system. Nat. Commun. 2017, 8, 2212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- 92. Sanson, K.R.; Hanna, R.E.; Hegde, M.; Donovan, K.F.; Strand, C.; Sullender, M.E.; Vaimberg, E.W.; Goodale, A.; Root, D.E.; Piccioni, F.; et al. Optimized libraries for CRISPR-Cas9 genetic screens with multiple modalities. Nat. Commun. 2018, 9, 5416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Law, C.-T.; Wei, L.; Tsang, F.H.-C.; Chan, C.Y.-K.; Xu, I.M.-J.; Lai, R.K.-H.; Ho, D.W.-H.; Lee, J.M.-F.; Wong, C.C.-L.; Ng, I.O.-L.; et al. HELLS Regulates Chromatin Remodeling and Epigenetic Silencing of Multiple Tumor Suppressor Genes in Human Hepatocellular Carcinoma. Hepatology 2019, 69, 2013–2030. [Google Scholar] [CrossRef]
- Bester, A.C.; Lee, J.D.; Chavez, A.; Lee, Y.-R.; Nachmani, D.; Vora, S.; Victor, J.; Sauvageau, M.; Monteleone, E.; Rinn, J.L.; et al. An Integrated Genome-wide CRISPRa Approach to Functionalize lncRNAs in Drug Resistance. Cell 2018, 173, 649. [Google Scholar] [CrossRef] [PubMed]
- Joung, J.; Engreitz, J.M.; Konermann, S.; Abudayyeh, O.O.; Verdine, V.K.; Aguet, F.; Gootenberg, J.S.; Sanjana, N.E.; Wright, J.B.; Fulco, C.P. Genome-scale activation screen identifies a lncRNA locus regulating a gene neighbourhood. Nature 2017, 548, 343. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.J.; Horlbeck, M.A.; Cho, S.W.; Birk, H.S.; Malatesta, M.; He, D.; Attenello, F.J.; Villalta, J.E.; Cho, M.Y.; Chen, Y. CRISPRi-based genome-scale identification of functional long noncoding RNA loci in human cells. Science 2017, 355, eaah7111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wangensteen, K.J.; Wang, Y.J.; Dou, Z.; Wang, A.W.; Mosleh-Shirazi, E.; Horlbeck, M.A.; Gilbert, L.A.; Weissman, J.S.; Berger, S.L.; Kaestner, K.H. Combinatorial genetics in liver repopulation and carcinogenesis with a in vivo CRISPR activation platform. Hepatology 2018, 68, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Lou, K.; Steri, V.; Ge, A.Y.; Hwang, Y.C.; Yogodzinski, C.H.; Shkedi, A.R.; Choi, A.L.M.; Mitchell, D.C.; Swaney, D.L.; Hann, B.; et al. KRAS(G12C) inhibition produces a driver-limited state revealing collateral dependencies. Sci. Signal 2019, 12, eaaw9450. [Google Scholar] [CrossRef]
- Zhang, Y.; Ozono, S.; Yao, W.; Tobiume, M.; Yamaoka, S.; Kishigami, S.; Fujita, H.; Tokunaga, K. CRISPR-mediated activation of endogenous BST-2/tetherin expression inhibits wild-type HIV-1 production. Sci. Rep. 2019, 9, 3134. [Google Scholar] [CrossRef] [Green Version]
- Farhang, N.; Brunger, J.M.; Stover, J.D.; Thakore, P.I.; Lawrence, B.; Guilak, F.; Gersbach, C.A.; Setton, L.A.; Bowles, R.D. CRISPR-Based Epigenome Editing of Cytokine Receptors for the Promotion of Cell Survival and Tissue Deposition in Inflammatory Environments. Tissue Eng. Part A 2017, 23, 738–749. [Google Scholar] [CrossRef]
- Farhang, N.; Ginley-Hidinger, M.; Berrett, K.C.; Gertz, J.; Lawrence, B.; Bowles, R.D. Lentiviral CRISPR Epigenome Editing of Inflammatory Receptors as a Gene Therapy Strategy for Disc Degeneration. Hum. Gene Ther. 2019. [Google Scholar] [CrossRef] [PubMed]
- Stover, J.D.; Farhang, N.; Lawrence, B.; Bowles, R.D. Multiplex Epigenome Editing of Dorsal Root Ganglion Neuron Receptors Abolishes Redundant Interleukin 6, Tumor Necrosis Factor Alpha, and Interleukin 1 beta Signaling by the Degenerative Intervertebral Disc. Hum. Gene Ther. 2019. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-D.; Zeldich, E.; Li, Y.; Yuste, A.; Abraham, C.R. Activation of the Anti-Aging and Cognition-Enhancing Gene Klotho by CRISPR-dCas9 Transcriptional Effector Complex. J. Mol. Neurosci. 2018, 64, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Thakore, P.I.; Kwon, J.B.; Nelson, C.E.; Rouse, D.C.; Gemberling, M.P.; Oliver, M.L.; Gersbach, C.A. RNA-guided transcriptional silencing in vivo with S. aureus CRISPR-Cas9 repressors. Nat. Commun. 2018, 9, 1674. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.-K.; Hatanaka, F.; Araoka, T.; Reddy, P.; Wu, M.-Z.; Sui, Y.; Yamauchi, T.; Sakurai, M.; O’Keefe, D.D.; Nunez-Delicado, E.; et al. In Vivo Target Gene Activation via CRISPR/Cas9-Mediated Trans-epigenetic Modulation. Cell 2017, 171, 1495. [Google Scholar] [CrossRef] [Green Version]
- Gimenez, C.A.; Ielpi, M.; Mutto, A.; Grosembacher, L.; Argibay, P.; Pereyra-Bonnet, F. CRISPR-on system for the activation of the endogenous human INS gene. Gene Ther. 2016, 23, 543–547. [Google Scholar] [CrossRef]
- Pinto, B.S.; Saxena, T.; Oliveira, R.; Mendez-Gomez, H.R.; Cleary, J.D.; Denes, L.T.; McConnell, O.; Arboleda, J.; Xia, G.; Swanson, M.S.; et al. Impeding Transcription of Expanded Microsatellite Repeats by Deactivated Cas9. Mol. Cell 2017, 68, 479. [Google Scholar] [CrossRef] [Green Version]
- Kemaladewi, D.U.; Bassi, P.S.; Erwood, S.; Al-Basha, D.; Gawlik, K.I.; Lindsay, K.; Hyatt, E.; Kember, R.; Place, K.M.; Marks, R.M.; et al. A mutation-independent approach for muscular dystrophy via upregulation of a modifier gene. Nature 2019, 572, 125. [Google Scholar] [CrossRef]
- Dai, W.; Xu, X.; Wang, D.; Wu, J.; Wang, J. Cancer therapy with a CRISPR-assisted telomerase-activating gene expression system. Oncogene 2019, 38, 4110–4124. [Google Scholar] [CrossRef]
- Xiong, K.; Zhou, Y.; Blichfeld, K.A.; Hyttel, P.; Bolund, L.; Freude, K.K.; Luo, Y. RNA-Guided Activation of Pluripotency Genes in Human Fibroblasts. Cell. Reprogram. 2017, 19, 189–198. [Google Scholar] [CrossRef]
- Hu, J.; Lei, Y.; Wong, W.-K.; Liu, S.; Lee, K.-C.; He, X.; You, W.; Zhou, R.; Guo, J.-T.; Chen, X.; et al. Direct activation of human and mouse Oct4 genes using engineered TALE and Cas9 transcription factors. Nucleic Acids Res. 2014, 42, 4375–4390. [Google Scholar] [CrossRef] [PubMed]
- Weltner, J.; Balboa, D.; Katayama, S.; Bespalov, M.; Krjutskov, K.; Jouhilahti, E.-M.; Trokovic, R.; Kere, J.; Otonkoski, T. Human pluripotent reprogramming with CRISPR activators. Nat. Commun. 2018, 9, 2643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Ma, D.; Huang, R.; Ming, J.; Ye, M.; Kee, K.; Xie, Z.; Na, J. An inducible CRISPR-ON system for controllable gene activation in human pluripotent stem cells. Protein Cell 2017, 8, 379–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuhata, Y.; Nihongaki, Y.; Sato, M.; Yoshimoto, K. Control of Adipogenic Differentiation in Mesenchymal Stem cells via Endogenous Gene Activation Using CRISPR-Cas9. ACS Synth. Biol. 2017, 6, 2191–2197. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, C.; Daley, T.P.; Wang, F.; Cao, W.S.; Bhate, S.; Lin, X.; Still, I.I.C.; Liu, H.; Zhao, D.; et al. CRISPR Activation Screens Systematically Identify Factors that Drive Neuronal Fate and Reprogramming. Cell Stem Cell 2018, 23, 758. [Google Scholar] [CrossRef] [Green Version]
- Balboa, D.; Weltner, J.; Eurola, S.; Trokovic, R.; Wartiovaara, K.; Otonkoski, T. Conditionally Stabilized dCas9 Activator for Controlling Gene Expression in Human Cell Reprogramming and Differentiation. Stem Cell Rep. 2015, 5, 448–459. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhang, Y.; Chen, Y.; Li, M.; Zhou, F.; Li, K.; Cao, H.; Ni, M.; Liu, Y.; Gu, Z.; et al. In Situ Capture of Chromatin Interactions by Biotinylated dCas9. Cell 2017, 170, 1028. [Google Scholar] [CrossRef] [Green Version]
- Schmidtmann, E.; Anton, T.; Rombaut, P.; Herzog, F.; Leonhardt, H. Determination of local chromatin composition by CasID. Nucleus 2016, 7, 476–484. [Google Scholar] [CrossRef] [Green Version]
- Myers, S.A.; Wright, J.; Peckner, R.; Kalish, B.T.; Zhang, F.; Carr, S.A. Discovery of proteins associated with a predefined genomic locus via dCas9-APEX-mediated proximity labeling. Nat. Methods 2018, 15, 437. [Google Scholar] [CrossRef]
- Gao, X.D.; Tu, L.-C.; Mir, A.; Rodriguez, T.; Ding, Y.; Leszyk, J.; Dekker, J.; Shaffer, S.A.; Zhu, L.J.; Wolfe, S.A.; et al. C-BERST: Defining subnuclear proteomic landscapes at genomic elements with dCas9-APEX2. Nat. Methods 2018, 15, 433. [Google Scholar] [CrossRef]
- Drier, Y.; Cotton, M.J.; Williamson, K.E.; Gillespie, S.M.; Ryan, R.J.H.; Kluk, M.J.; Carey, C.D.; Rodig, S.J.; Sholl, L.M.; Afrogheh, A.H.; et al. An oncogenic MYB feedback loop drives alternate cell fates in adenoid cystic carcinoma. Nat. Genet. 2016, 48, 265–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, S.L.; Mariano, N.C.; Bermudez, A.; Arruda, N.L.; Wu, F.; Luo, Y.; Shankar, G.; Jia, L.; Chen, H.; Hu, J.-F.; et al. Manipulation of nuclear architecture through CRISPR-mediated chromosomal looping. Nat. Commun. 2017, 8, 15993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daub, H.; Specht, K.; Ullrich, A. Strategies to overcome resistance to targeted protein kinase inhibitors. Nat. Rev. Drug Discov. 2004, 3, 1001–1010. [Google Scholar] [CrossRef]
- Wang, H.; Yang, H.; Shivalila, C.S.; Dawlaty, M.M.; Cheng, A.W.; Zhang, F.; Jaenisch, R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 2013, 153, 910–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabel, C.S.; Jia, H.; Ye, Y.; Shen, L.; Goldschmidt, H.L.; Stivers, J.T.; Zhang, Y.; Kohli, R.M. AID/APOBEC deaminases disfavor modified cytosines implicated in DNA demethylation. Nat. Chem. Biol. 2012, 8, 751–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navaratnam, N.; Sarwar, R. An overview of cytidine deaminases. Int. J. Hematol. 2006, 83, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Rees, H.A.; Liu, D.R. Base editing: Precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 2018, 19, 770–788. [Google Scholar] [CrossRef]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016, 533, 420. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, J.; Wang, Y.; Yang, B.; Wei, J.; Wu, J.; Wang, R.; Huang, X.; Chen, J.; Yang, L. Efficient base editing in methylated regions with a human APOBEC3A-Cas9 fusion. Nat. Biotechnol. 2018, 36, 946. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, J.; Yin, W.; Zhang, Z.; Song, Y.; Chang, X. Targeted AID-mediated mutagenesis (TAM) enables efficient genomic diversification in mammalian cells. Nat. Methods 2016, 13, 1029. [Google Scholar] [CrossRef]
- Nishida, K.; Arazoe, T.; Yachie, N.; Banno, S.; Kakimoto, M.; Tabata, M.; Mochizuki, M.; Miyabe, A.; Araki, M.; Hara, K.Y. Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems. Science 2016, 353, aaf8729. [Google Scholar] [CrossRef] [PubMed]
- Hess, G.T.; Frésard, L.; Han, K.; Lee, C.H.; Li, A.; Cimprich, K.A.; Montgomery, S.B.; Bassik, M.C. Directed evolution using dCas9-targeted somatic hypermutation in mammalian cells. Nat. Methods 2016, 13, 1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komor, A.C.; Zhao, K.T.; Packer, M.S.; Gaudelli, N.M.; Waterbury, A.L.; Koblan, L.W.; Kim, Y.B.; Badran, A.H.; Liu, D.R. Improved base excision repair inhibition and bacteriophage Mu Gam protein yields C: G-to-T: A base editors with higher efficiency and product purity. Sci. Adv. 2017, 3, eaao4774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaudelli, N.M.; Komor, A.C.; Rees, H.A.; Packer, M.S.; Badran, A.H.; Bryson, D.I.; Liu, D.R. Programmable base editing of A• T to G• C in genomic DNA without DNA cleavage. Nature 2017, 551, 464. [Google Scholar] [CrossRef]
- Kim, Y.B.; Komor, A.C.; Levy, J.M.; Packer, M.S.; Zhao, K.T.; Liu, D.R. Increasing the genome-targeting scope and precision of base editing with engineered Cas9-cytidine deaminase fusions. Nat. Biotechnol. 2017, 35, 371. [Google Scholar] [CrossRef]
- Nishimasu, H.; Shi, X.; Ishiguro, S.; Gao, L.; Hirano, S.; Okazaki, S.; Noda, T.; Abudayyeh, O.O.; Gootenberg, J.S.; Mori, H.; et al. Engineered CRISPR-Cas9 nuclease with expanded targeting space. Science 2018, 361, 1259–1262. [Google Scholar] [CrossRef]
- Zhou, C.; Sun, Y.; Yan, R.; Liu, Y.; Zuo, E.; Gu, C.; Han, L.; Wei, Y.; Hu, X.; Zeng, R. Off-target RNA mutation induced by DNA base editing and its elimination by mutagenesis. Nature 2019, 1, 275–278. [Google Scholar] [CrossRef]
- Grunewald, J.; Zhou, R.; Garcia, S.P.; Iyer, S.; Lareau, C.A.; Aryee, M.J.; Joung, J.K. Transcriptome-wide off-target RNA editing induced by CRISPR-guided DNA base editors. Nature 2019, 569, 433. [Google Scholar] [CrossRef]
- Grünewald, J.; Zhou, R.; Iyer, S.; Lareau, C.A.; Garcia, S.P.; Aryee, M.J.; Joung, J.K. CRISPR DNA base editors with reduced RNA off-target and self-editing activities. Nat. Biotechnol. 2019, 37, 1–8. [Google Scholar] [CrossRef]
- Rees, H.A.; Wilson, C.; Doman, J.L.; Liu, D.R. Analysis and minimization of cellular RNA editing by DNA adenine base editors. Sci. Adv. 2019, 5, eaax5717. [Google Scholar] [CrossRef] [Green Version]
- Thuronyi, B.W.; Koblan, L.W.; Levy, J.M.; Yeh, W.-H.; Zheng, C.; Newby, G.A.; Wilson, C.; Bhaumik, M.; Shubina-Oleinik, O.; Holt, J.R. Continuous evolution of base editors with expanded target compatibility and improved activity. Nat. Biotechnol. 2019, 1, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.B.T.; Gootenberg, J.S.; Abudayyeh, O.O.; Franklin, B.; Kellner, M.J.; Joung, J.; Zhang, F. RNA editing with CRISPR-Cas13. Science 2017, 358, 1019–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abudayyeh, O.O.; Gootenberg, J.S.; Franklin, B.; Koob, J.; Kellner, M.J.; Ladha, A.; Joung, J.; Kirchgatterer, P.; Cox, D.B.T.; Zhang, F. A cytosine deaminase for programmable single-base RNA editing. Science 2019, 365, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Kuscu, C.; Parlak, M.; Tufan, T.; Yang, J.; Szlachta, K.; Wei, X.; Mammadov, R.; Adli, M. CRISPR-STOP: Gene silencing through base-editing-induced nonsense mutations. Nat. Methods 2017, 14, 710. [Google Scholar] [CrossRef] [PubMed]
- Billon, P.; Bryant, E.E.; Joseph, S.A.; Nambiar, T.S.; Hayward, S.B.; Rothstein, R.; Ciccia, A. CRISPR-Mediated Base Editing Enables Efficient Disruption of Eukaryotic Genes through Induction of STOP Codons. Mol. Cell 2017, 67, 1068. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Feng, S.; Huang, S.; Yu, W.; Li, G.; Yang, G.; Liu, Y.; Zhang, Y.; Zhang, L.; Hou, Y.; et al. BE-PLUS: A new base editing tool with broadened editing window and enhanced fidelity. Cell Res. 2018, 28, 855–861. [Google Scholar] [CrossRef] [Green Version]
- Zafra, M.P.; Schatoff, E.M.; Katti, A.; Foronda, M.; Breinig, M.; Schweitzer, A.Y.; Simon, A.; Han, T.; Goswami, S.; Montgomery, E.; et al. Optimized base editors enable efficient editing in cells, organoids and mice. Nat. Biotechnol. 2018, 36, 888. [Google Scholar] [CrossRef]
- Kim, K.; Ryu, S.-M.; Kim, S.-T.; Baek, G.; Kim, D.; Lim, K.; Chung, E.; Kim, S.; Kim, J.-S. Highly efficient RNA-guided base editing in mouse embryos. Nat. Biotechnol. 2017, 35, 435. [Google Scholar] [CrossRef]
- Liang, P.; Sun, H.; Zhang, X.; Xie, X.; Zhang, J.; Bai, Y.; Ouyang, X.; Zhi, S.; Xiong, Y.; Ma, W. Effective and precise adenine base editing in mouse zygotes. Protein Cell 2018, 9, 808–813. [Google Scholar] [CrossRef] [Green Version]
- Ryu, S.-M.; Koo, T.; Kim, K.; Lim, K.; Baek, G.; Kim, S.-T.; Kim, H.S.; Kim, D.; Lee, H.; Chung, E.; et al. Adenine base editing in mouse embryos and an adult mouse model of Duchenne muscular dystrophy. Nat. Biotechnol. 2018, 36, 536–539. [Google Scholar] [CrossRef]
- Park, D.-S.; Yoon, M.; Kweon, J.; Jang, A.-H.; Kim, Y.; Choi, S.-C. Targeted Base Editing via RNA-Guided Cytidine Deaminases in Xenopus laevis Embryos. Mol. Cells 2017, 40, 823–827. [Google Scholar] [PubMed]
- Liu, Z.; Chen, M.; Chen, S.; Deng, J.; Song, Y.; Lai, L.; Li, Z. Highly efficient RNA-guided base editing in rabbit. Nat. Commun. 2018, 9, 2717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaguri, H.; Nagata, K.; Sekiguchi, M.; Fujioka, R.; Matsuba, Y.; Hashimoto, S.; Sato, K.; Kurup, D.; Yokota, T.; Saido, T.C. Introduction of pathogenic mutations into the mouse Psen1 gene by Base Editor and Target-AID. Nat. Commun. 2018, 9, 2892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehrke, J.M.; Cervantes, O.; Clement, M.K.; Wu, Y.; Zeng, J.; Bauer, D.E.; Pinello, L.; Joung, J.K. An APOBEC3A-Cas9 base editor with minimized bystander and off-target activities. Nat. Biotechnol. 2018, 36, 977. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Ding, C.; Sun, H.; Xie, X.; Xu, Y.; Zhang, X.; Sun, Y.; Xiong, Y.; Ma, W.; Liu, Y.; et al. Correction of beta-thalassemia mutant by base editor in human embryos. Protein Cell 2017, 8, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Li, J.; Li, G.; Huang, S.; Yu, W.; Zhang, Y.; Chen, D.; Chen, J.; Liu, J.; Huang, X. Correction of the Marfan Syndrome Pathogenic FBN1 Mutation by Base Editing in Human Cells and Heterozygous Embryos. Mol. Ther. 2018, 26, 2631–2637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villiger, L.; Grisch-Chan, H.M.; Lindsay, H.; Ringnalda, F.; Pogliano, C.B.; Allegri, G.; Fingerhut, R.; Haberle, J.; Matos, J.; Robinson, M.D.; et al. Treatment of a metabolic liver disease by in vivo genome base editing in adult mice. Nat. Med. 2018, 24, 1519. [Google Scholar] [CrossRef]
- Rossidis, A.C.; Stratigis, J.D.; Chadwick, A.C.; Hartman, H.A.; Ahn, N.J.; Li, H.; Singh, K.; Coons, B.E.; Li, L.; Lv, W.; et al. In utero CRISPR-mediated therapeutic editing of metabolic genes. Nat. Med. 2018, 24, 1513. [Google Scholar] [CrossRef]
- Chadwick, A.C.; Wang, X.; Musunuru, K. In Vivo Base Editing of PCSK9 (Proprotein Convertase Subtilisin/Kexin Type 9) as a Therapeutic Alternative to Genome Editing. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1741. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Jo, D.H.; Hwang, G.-H.; Yu, J.; Kim, J.H.; Park, S.; Kim, J.-S.; Kim, J.H.; Bae, S. CRISPR-pass: Gene rescue of nonsense mutations using adenine base editors. Mol. Ther. 2019. [Google Scholar] [CrossRef] [Green Version]
- Gapinske, M.; Luu, A.; Winter, J.; Woods, W.S.; Kostan, K.A.; Shiva, N.; Song, J.S.; Perez-Pinera, P. CRISPR-SKIP: Programmable gene splicing with single base editors. Genome Biol. 2018, 19, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, G.-H.; Park, J.; Lim, K.; Kim, S.; Yu, J.; Yu, E.; Kim, S.-T.; Eils, R.; Kim, J.-S.; Bae, S. Web-based design and analysis tools for CRISPR base editing. BMC Bioinformatics 2018, 19, 542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Gao, R.; Wu, J.; Xiong, Y.-C.; Wei, J.; Zhang, S.; Yang, B.; Chen, J.; Yang, L. Comparison of cytosine base editors and development of the BEable-GPS database for targeting pathogenic SNVs. Genome Biol. 2019, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Campa, C.C.; Weisbach, N.R.; Santinha, A.J.; Incarnato, D.; Platt, R.J. Multiplexed genome engineering by Cas12a and CRISPR arrays encoded on single transcripts. Nat. Methods 2019, 16, 887. [Google Scholar] [CrossRef] [PubMed]
- Klompe, S.E.; Vo, P.L.H.; Halpin-Healy, T.S.; Sternberg, S.H. Transposon-encoded CRISPR–Cas systems direct RNA-guided DNA integration. Nature 2019, 571, 219–225. [Google Scholar] [CrossRef]
- Strecker, J.; Ladha, A.; Gardner, Z.; Schmid-Burgk, J.L.; Makarova, K.S.; Koonin, E.V.; Zhang, F. RNA-guided DNA insertion with CRISPR-associated transposases. Science 2019, 365, 48–53. [Google Scholar] [CrossRef]
- Liu, X.-M.; Zhou, J.; Mao, Y.; Ji, Q.; Qian, S.-B. Programmable RNA N-6-methyladenosine editing by CRISPR-Cas9 conjugates. Nat. Chem. Biol. 2019, 15, 865. [Google Scholar] [CrossRef]
- Ma, H.; Tu, L.-C.; Naseri, A.; Huisman, M.; Zhang, S.; Grunwald, D.; Pederson, T. Multiplexed labeling of genomic loci with dCas9 and engineered sgRNAs using CRISPRainbow. Nat. Biotechnol. 2016, 34, 528. [Google Scholar] [CrossRef] [Green Version]
- Duan, J.; Lu, G.; Hong, Y.; Hu, Q.; Mai, X.; Guo, J.; Si, X.; Wang, F.; Zhang, Y. Live imaging and tracking of genome regions in CRISPR/dCas9 knock-in mice. Genome Biol. 2018, 19, 192. [Google Scholar] [CrossRef]
- Nelles, D.A.; Fang, M.Y.; O’Connell, M.R.; Xu, J.L.; Markmiller, S.J.; Doudna, J.A.; Yeo, G.W. Programmable RNA Tracking in Live cells with CRISPR/Cas9. Cell 2016, 165, 488–496. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.; Liu, D.R. Rewritable multi-event analog recording in bacterial and mammalian cells. Science 2018, 360, eaap8992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farzadfard, F.; Gharaei, N.; Higashikuni, Y.; Jung, G.; Cao, J.; Lu, T.K. Single-Nucleotide-Resolution Computing and Memory in Living Cells. Mol. Cell 2019, 75, 769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qian, L.; Wei, W.; Wang, Y.; Wang, B.; Lin, P.; Liu, W.; Xu, L.; Li, X.; Liu, D.; et al. Paired Design of dCas9 as a Systematic Platform for the Detection of Featured Nucleic Acid Sequences in Pathogenic Strains. ACS Synth. Biol. 2017, 6, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.-Y.; Zhu, L.-Y.; Zhu, C.-S.; Ma, J.-X.; Hou, T.; Wu, X.-M.; Xie, S.-S.; Min, L.; Tan, D.-A.; Zhang, D.-Y.; et al. Highly Effective and Low-Cost MicroRNA Detection with CRISPR-Cas9. ACS Synth. Biol. 2018, 7, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.; Kim, D.; Kweon, J.; Jin, C.E.; Kim, S.-H.; Kim, Y.; Shin, Y. CRISPR/dCas9-mediated biosensor for detection of tick-borne diseases. Sens. Actuators B Chem. 2018, 273, 316–321. [Google Scholar] [CrossRef]
- Kosicki, M.; Tomberg, K.; Bradley, A. Repair of double-strand breaks induced by CRISPR-Cas9 leads to large deletions and complex rearrangements. Nat. Biotechnol. 2018, 36, 765. [Google Scholar] [CrossRef] [PubMed]
- Ihry, R.J.; Worringer, K.A.; Salick, M.R.; Frias, E.; Ho, D.; Theriault, K.; Kommineni, S.; Chen, J.; Sondey, M.; Ye, C.; et al. p53 inhibits CRISPR-Cas9 engineering in human pluripotent stem cells. Nat. Med. 2018, 24, 939. [Google Scholar] [CrossRef]
- Tycko, J.; Myer, V.E.; Hsu, P.D. Methods for optimizing CRISPR-Cas9 genome editing specificity. Mol. Cell 2016, 63, 355–370. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Yang, Z.; Xu, J.; Sun, J.; Mao, D.; Hu, Y.; Yang, S.-J.; Qiao, H.-H.; Wang, X.; Hu, Q. Enhanced specificity and efficiency of the CRISPR/Cas9 system with optimized sgRNA parameters in Drosophila. Cell Rep. 2014, 9, 1151–1162. [Google Scholar] [CrossRef] [Green Version]
- Kocak, D.D.; Josephs, E.A.; Bhandarkar, V.; Adkar, S.S.; Kwon, J.B.; Gersbach, C.A. Increasing the specificity of CRISPR systems with engineered RNA secondary structures. Nat. Biotechnol. 2019, 37, 657. [Google Scholar] [CrossRef]
- Slaymaker, I.M.; Gao, L.; Zetsche, B.; Scott, D.A.; Yan, W.X.; Zhang, F. Rationally engineered Cas9 nucleases with improved specificity. Science 2016, 351, 84–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinstiver, B.P.; Pattanayak, V.; Prew, M.S.; Tsai, S.Q.; Nguyen, N.T.; Zheng, Z.; Keith Joung, J. High-fidelity CRISPR–Cas9 nucleases with no detectable genome-wide off-target effects. Nature 2016, 529, 490–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casini, A.; Olivieri, M.; Petris, G.; Montagna, C.; Reginato, G.; Maule, G.; Lorenzin, F.; Prandi, D.; Romanel, A.; Demichelis, F. A highly specific SpCas9 variant is identified by in vivo screening in yeast. Nat. Biotechnol. 2018, 36, 265. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Dagdas, Y.S.; Kleinstiver, B.P.; Welch, M.M.; Sousa, A.A.; Harrington, L.B.; Sternberg, S.H.; Joung, J.K.; Yildiz, A.; Doudna, J.A. Enhanced proofreading governs CRISPR–Cas9 targeting accuracy. Nature 2017, 550, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinstiver, B.P.; Prew, M.S.; Tsai, S.Q.; Topkar, V.V.; Nguyen, N.T.; Zheng, Z.; Gonzales, A.P.W.; Li, Z.; Peterson, R.T.; Yeh, J.-R.J.; et al. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature 2015, 523, 481–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agudelo, D.; Carter, S.; Velimirovic, M.; Duringer, A.; Levesque, S.; Rivest, J.-F.; Loehr, J.; Mouchiroud, M.; Cyr, D.; Waters, P.J. Versatile and robust genome editing with Streptococcus thermophilus CRISPR1-Cas9. BioRxiv 2019, 321208. [Google Scholar] [CrossRef] [Green Version]
- Ran, F.A.; Cong, L.; Yan, W.X.; Scott, D.A.; Gootenberg, J.S.; Kriz, A.J.; Zetsche, B.; Shalem, O.; Wu, X.; Makarova, K.S.; et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 2015, 520, 186–191. [Google Scholar] [CrossRef]
- Tsai, S.Q.; Wyvekens, N.; Khayter, C.; Foden, J.A.; Thapar, V.; Reyon, D.; Goodwin, M.J.; Aryee, M.J.; Joung, J.K. Dimeric CRISPR RNA-guided FokI nucleases for highly specific genome editing. Nat. Biotechnol. 2014, 32, 569. [Google Scholar] [CrossRef] [Green Version]
- Zuris, J.A.; Thompson, D.B.; Shu, Y.; Guilinger, J.P.; Bessen, J.L.; Hu, J.H.; Maeder, M.L.; Joung, J.K.; Chen, Z.-Y.; Liu, D.R. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat. Biotechnol. 2015, 33, 73. [Google Scholar] [CrossRef] [Green Version]

| Target | Modification | Effect on Gene Transcription | System | Efficacy |
|---|---|---|---|---|
| DNA | Methylation | Supression | DNMT3A [6,7,8] | + |
| DNMT3A-3L [9] | +++ | |||
| SunTag-DNMT3A [10]; | +++ | |||
| Demethylation | Activation | dCas9-TET1 [8,11] | + | |
| dCas9-SunTag-TET1 [12] | +++ | |||
| dCas9/MS2/MCP-TET1 [13] | +++ | |||
| Chromatin | Histone demethylation | Supression | dCas9-LSD1 [48] | ++ |
| Histone acetylation | Activation | p300Core [49] | ++ | |
| Transcriptional factor recruitment | Activation | VP64 [50,52,53] | + | |
| VP160/VP192 [54] | ++ | |||
| p65/p65-HSF1 [50,51] | +/++ | |||
| SunTag-VP64 [59] | +++ | |||
| VPR [55,56] | +++ | |||
| SunTag-p65-HSF1 [58] | ++++ | |||
| SAM [51,60] | ++++ | |||
| TREE [62] | ++++ | |||
| Casilio [61] | +++ | |||
| Scaffold [60] | +++ | |||
| Supression | dCas9-KRAB [43] | ++ | ||
| dCas9-KRAB-MeCP2 [47] | +++ | |||
| dCas9-EZH2 [34] | ++ | |||
| Exogenic promoter recruitment | Activation | CMV [63] | ++++ |
| Fundamental Studies | ||
| Application | CRISPR tool | Target |
| Annotating regulatory elements | dCas9-KRAB dCas9-p300 | Distal regulatory elements [69,70] |
| dCas9-KRAB | Estrogen receptor enhancers [87] | |
| Analyzing gene function | dCas9-KRAB/ dCas9-VPR | Function of Syt1 [88], Bdnf, and Reln [89] in mammalian brain |
| Analyzing cell signaling | dCas9-VPR Scaffolds SAM | Generating chimeric receptors [90,91] |
| Identifying antiviral factors | SAM | Norovirus infection [79] |
| Analyzing human genome | dCas9-KRAB | CRISPRi gRNA libraries [92] |
| SAM | CRISPRa gRNA libraries [92] | |
| Annotating tumor-related factors | SAM SunTag-VP64 dCas9-KRAB | Genes involved in cancer: Hells [93], Egfr [51], lncRNAs [94,95,96], Myc [97], Kras-dependent genes [98] |
| Creating Therapeutic Approaches | ||
| Application | CRISPR tool | Target |
| Treating infectious diseases | SAM; Scaffold (MCP-p65-HSF1) | HIV therapy by activating BST2/tetherin [99], APOBEC3B [71], and APOBEC3G [71] |
| dCas9-p300 | HBV therapy by activating APOBEC3A, APOBEC3B, APOBEC3G, AID [76] | |
| SunTag-VP64; dCas9-VPR; SAM | Reactivating HIV in a “shock-and-kill” therapeutic approach [72,73,74,75] | |
| Treating metabolic and inflammatory diseases | dCas9-KRAB | Repressing TNFR1, IL1R1, IL6st [100,101,102] |
| SAM | Neuro- and nephroprotection by activating Klotho gene [103] | |
| dCas9-KRAB | Repressing Pcsk9 to reduce serum cholesterol levels [104] | |
| SAM dCas9-VP160 | Generating insulin-producing cells by upregulating Pdx1 or Ins [105,106] | |
| Treating genetic disorders | SAM | Treating DMD by activating Utrophin gene [105] |
| dCas9-VP64 | Treating obesity by upregulating Sim1 [80] | |
| dCas9-VP64 | Treating Dravet syndrome by upregulating Scn1a [81] | |
| dCas9 | Correcting myotonic dystrophy types 1 and 2 by blocking transcription of expanded microsatellite repeats [107] | |
| dCas9-VP64 | Treating congenital muscular dystrophy type 1A by upregulating Lama1 [108] | |
| Treating cancer | dCas9-DNMT3A dCas9-KRAB dCas9-Ezh2 | Repressing Granulin proto-oncogene [82] |
| dCas9-VP64 dCas9-VPR | Activating tumor suppressors PTEN [83], CHEK2 [84], DKK3 [85] | |
| dCas9-VP64 | Activating telomere-targeting Cas9 nuclease in cancer cells [109] | |
| SAM | Increased presentation of tumor antigens to immune cells [86] | |
| Stem cell field | dCas9-VP64 SAM dCas9-p300 | Generating iPSCs by inducing KLF4, LIN28, MYC, OCT4, SOX2 [110,111,112] |
| dCas9-VPR | Upregulating NANOG to maintain pluripotency [113] | |
| SAM SunTag-VP64 | Differentiating stem cells into adipocytes [114], neural cells [115], pancreatic cells [116] | |
| SAM SunTag-p65-HSF1 | Neural reprogramming by activating Neurog2, Ascl1, Neurod1, Dkk1, etc. [58] | |
| Aim | Deaminase Domain | Applications |
|---|---|---|
| Disease modeling | AID | Mutating Bcr-Abl gene resulting in imatinib resistance [130] |
| CRISPR-X (dCas/MCP-AID) | Mutating Psmb5 resulting in bortezomib resistance [132] | |
| rAPOBEC1 | Mutating Ctnnb1, Apc, and Pi3kca genes as cancer models [147] | |
| TadA | Introducing SNPs to model hereditary persistence of fetal hemoglobin syndrome and hereditary haemochromatosis [134] | |
| rAPOBEC1 TadA | Modeling DMD and albinism by mutating Dmd and Tyr genes [148,149,150] | |
| Target-AID rAPOBEC1 | Modeling amyloidosis by mutating Psen1 gene [153] | |
| rAPOBEC1 TadA | Modeling hereditary diseases by mutating Tia1, Lmna, and Dmd genes [152] | |
| Developing new therapies | APOBEC3A rAPOBEC1 | Correcting β-thalassemia-linked mutations [154,155] |
| rAPOBEC1 | Correcting phenylketonuria-linked mutations [157] | |
| rAPOBEC1 | Introducing stop codons in Pcsk9 gene to treat atherosclerosis [158,159] | |
| ADAR2 | Correcting mutations in Avpr2 and Fancc mRNAs to treat X-linked nephrogenic diabetes and Fanconi anemia [142] | |
| rAPOBEC1 | Treating Marfan syndrome by correcting pathogenic mutation Fbn1T7489C [156] |
| System | Change | Activity at Methylated Sites | Target | Base Editing Window |
|---|---|---|---|---|
| dCas9-rAPOBEC1 | C→T | Weak | DNA | 13–17 nt from PAM [128] |
| dCas9-APOBEC3A | C→T | Potent | DNA | 13–18 nt from PAM [129] |
| dCas9-AID | C→T | Weak | DNA | 16–19 nt from PAM [131] |
| dCas9-TadA | A→G | - | DNA | 14–16 nt from PAM [134] |
| dCas13b-ADAR2 (RESCUE) | A→I C→U | - | RNA | - |
| dCas13b-ADAR2 (REPAIR) | A→I | - | RNA | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brezgin, S.; Kostyusheva, A.; Kostyushev, D.; Chulanov, V. Dead Cas Systems: Types, Principles, and Applications. Int. J. Mol. Sci. 2019, 20, 6041. https://doi.org/10.3390/ijms20236041
Brezgin S, Kostyusheva A, Kostyushev D, Chulanov V. Dead Cas Systems: Types, Principles, and Applications. International Journal of Molecular Sciences. 2019; 20(23):6041. https://doi.org/10.3390/ijms20236041
Chicago/Turabian StyleBrezgin, Sergey, Anastasiya Kostyusheva, Dmitry Kostyushev, and Vladimir Chulanov. 2019. "Dead Cas Systems: Types, Principles, and Applications" International Journal of Molecular Sciences 20, no. 23: 6041. https://doi.org/10.3390/ijms20236041
APA StyleBrezgin, S., Kostyusheva, A., Kostyushev, D., & Chulanov, V. (2019). Dead Cas Systems: Types, Principles, and Applications. International Journal of Molecular Sciences, 20(23), 6041. https://doi.org/10.3390/ijms20236041







