Lab-On-A-Chip for the Development of Pro-/Anti-Angiogenic Nanomedicines to Treat Brain Diseases
Abstract
:1. Introduction
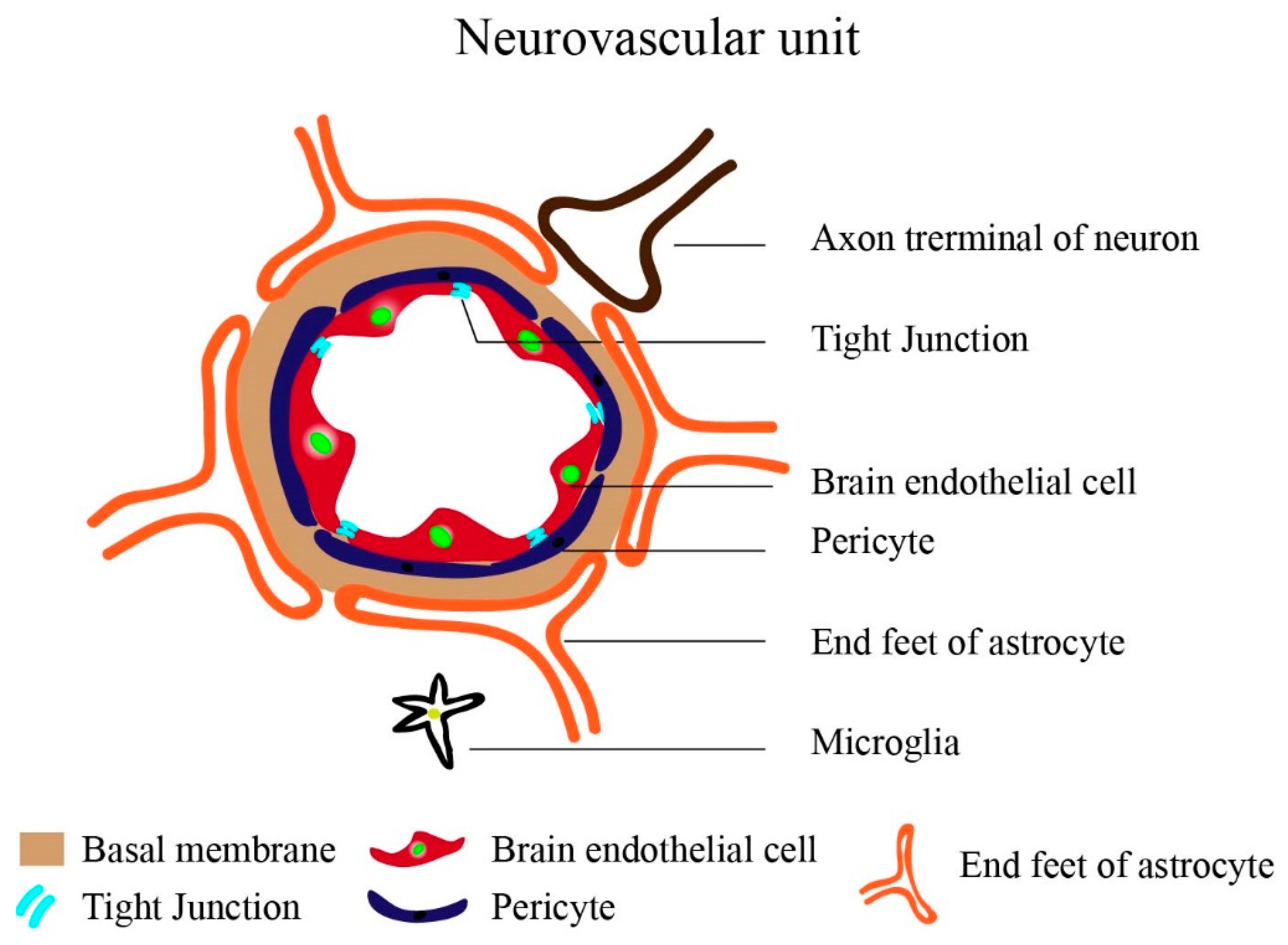
The Brain Vasculature: General Background
2. Nano-Drug Delivery for Treating Brain Diseases
2.1. Pro-Angiogenic Nanomedicine for Brain Diseases
2.2. Anti-Angiogenic Nanomedicine for Brain Diseases
3. Lab-on-a-Chip—A Model for Angiogenic Brain Diseases
3.1. Angiogenic Nanomedicine Screening in LOCs
3.2. Design and Fabrication of Brain-Angiogenesis LOCs
3.3. Establishing Brain Vasculature on Chips
3.4. Important Features of Vascularized LOCs Vs. Petri Dish Models
3.4.1. Dynamic Flow and Shear Stress in Brain-Angiogenesis LOCs
3.4.2. Lumen Perfusability in Brain-Angiogenesis LOCs
3.4.3. Compartmentalization in Brain-Angiogenesis LOCs
3.5. LOCs for the Synthesis of NMs
4. Conclusions
Funding
Conflicts of Interest
Abbreviations
| BBB | Blood brain barrier |
| LOC | Lab-on-a-chip |
| TEER | Transendothelial Electrical Resistance |
| NMs | Nanomedicines |
| SLNs | Solid lipid nanoparticles |
| NPs | Nanoparticles |
| CSF | Cerebrospinal fluid |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| HD | Huntington’s disease |
| BVD | Brain vessel density |
| BMECs | Brain microvascular endothelial cells |
| VEGF | Vascular endothelial growth factor |
| NO | Nitric oxide |
| CsA | Cyclosporine A |
| mPEG-PLGA | Methoxy poly(ethylene glycol)-b-poly(lactic-co-glycolic acid) |
| BVZ | Bevacizumab |
| TMZ | Temozolomide |
| DOX | Doxorubicin |
| SFN | Sorafenib |
| GBM | Glioblastoma multiforme |
| ApoE | Apolipoprotein E |
| MTX | Methotrexate |
| CTX | Chlorotoxin |
| PC | Parallel channel |
| HT | hollow-microtube |
| CR | Concentric-ring |
| PMA | Phorbol 12-myristate 13-acetate |
| S1P | Sphingosine-1-phosphate |
| GFAP | Glial fibrillary acidic protein |
| PDMS | Polydimethylsiloxane |
| P-gp | Permeability glycoprotein |
| HUVEC | Human Umbilical Vein Endothelial Cell |
| hCMEC | Human Cerebral Microvascular Endothelial Cell |
| iPS-BMVEC | Induced pluripotent stem cell-derived human brain microvascular endothelial cell |
| hBMVEC | Human brain microvascular endothelial cells |
| RBE4 | Rat brain endothelial cell 4 |
| b.End3 | Mice brain endothelial cell |
| hA | Human Astrocyte |
| hP | Human brain Pericyte |
| hNSC | Human neuronal stem cell |
| HIP-009 | Human Hippocampal Neural Stem |
| C8D1A | Mice astrocyte cell |
| LF | Human Lung fibroblast |
References
- Boruah, S.; Henderson, K.; Subit, D.; Salzar, R.S.; Shender, B.S.; Paskoff, G. Response of human skull bone to dynamic compressive loading. In Proceedings of the 2013 IRCOBI Conference Proceedings—International Research Council on the Biomechanics of Injury, Gothenburg, Sweden, 11–13 September 2013; pp. 497–508. [Google Scholar]
- Park, T.-E.; Mustafaoglu, N.; Herland, A.; Hasselkus, R.M.; Mannix, R.; FitzGerald, E.A.; Prantil-Baun, R.; Watters, A.; Henry, O.; Benz, M.; et al. Hypoxia-enhanced Blood-Brain Barrier Chip recapitulates human barrier function, drug penetration, and antibody shuttling properties. bioRxiv 2018, 10, 2621. [Google Scholar]
- Flühmann, B.; Ntai, I.; Borchard, G.; Simoens, S.; Mühlebach, S. Nanomedicines: The magic bullets reaching their target? Eur. J. Pharm. Sci. 2019, 128, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, L.; Gallarate, M.; Peira, E.; Chirio, D.; Solazzi, I.; Giordano, S.M.A.; Gigliotti, C.L.; Riganti, C.; Dianzani, C. Bevacizumab loaded solid lipid nanoparticles prepared by the coacervation technique: Preliminary in vitro studies. Nanotechnology 2015, 26, 255102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahiminejad, A.; Dinarvand, R.; Johari, B.; Nodooshan, S.J.; Rashti, A.; Rismani, E.; Mahdaviani, P.; Saltanatpour, Z.; Rahiminejad, S.; Raigani, M.; et al. Preparation and investigation of indirubin-loaded SLN nanoparticles and their anti-cancer effects on human glioblastoma U87MG cells. Cell Biol. Int. 2019, 43, 2–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battaglia, L.; Muntoni, E.; Chirio, D.; Peira, E.; Annovazzi, L.; Schiffer, D.; Mellai, M.; Riganti, C.; Salaroglio, I.C.; Lanotte, M.; et al. Solid lipid nanoparticles by coacervation loaded with a methotrexate prodrug: Preliminary study for glioma treatment. Nanomedicine 2017, 12, 639–656. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; Queiroz, J.F.; Lima, S.A.C.; Reis, S. Apo E-Functionalization of Solid Lipid Nanoparticles Enhances Brain Drug Delivery: Uptake Mechanism and Transport Pathways. Bioconjug. Chem. 2017, 28, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; De, K.; Mukherjee, D.; Dey, G.; Chattopadhyay, S.; Mukherjee, M.; Mandal, M.; Bandyopadhyay, A.K.; Gupta, A.; Ganguly, S.; et al. Paclitaxel-loaded solid lipid nanoparticles modified with Tyr-3-octreotide for enhanced anti-angiogenic and anti-glioma therapy. Acta Biomater. 2016, 38, 69–81. [Google Scholar] [CrossRef]
- Hwang, H.; Jeong, H.S.; Oh, P.S.; Na, K.S.; Kwon, J.; Kim, J.; Lim, S.; Sohn, M.H.; Jeong, H.J. Improving cerebral blood flow through liposomal delivery of angiogenic peptides: Potential of 18F-fdg pet imaging in ischemic stroke treatment. J. Nucl. Med. 2015, 56, 1106–1111. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Jiang, Y.; Lv, W.; Wang, Z.; Lv, L.; Wang, B.; Liu, X.; Liu, Y.; Hu, Q.; Sun, W.; et al. Dual targeted nanocarrier for brain ischemic stroke treatment. J. Control. Release 2016, 233, 64–71. [Google Scholar] [CrossRef]
- Campos-Martorell, M.; Cano-Sarabia, M.; Simats, A.; Hernández-Guillamon, M.; Rosell, A.; Maspoch, D.; Montaner, J. Charge effect of a liposomal delivery system encapsulating simvastatin to treat experimental ischemic stroke in rats. Int. J. Nanomed. 2016, 11, 3035–3048. [Google Scholar]
- Partoazar, A.; Nasoohi, S.; Rezayat, S.M.; Gilani, K.; Mehr, S.E.; Amani, A.; Rahimi, N.; Dehpour, A.R. Nanoliposome containing cyclosporine A reduced neuroinflammation responses and improved neurological activities in cerebral ischemia/reperfusion in rat. Fundam. Clin. Pharmacol. 2017, 31, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Xia, J.; Cai, T.; Du, J.; Chen, J.; Li, P.; Shen, Y.; Zhang, A.; Fu, B.; et al. Neuronal PirB upregulated in cerebral ischemia acts as an attractive theranostic target for ischemic stroke. J. Am. Heart Assoc. 2018, 7, e007197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, P.M.; Cardoso, A.L.; Custódia, C.; Cunha, P.; de Almeida, L.P.; de Lima, M.C.P. MiRNA-21 silencing mediated by tumor-targeted nanoparticles combined with sunitinib: A new multimodal gene therapy approach for glioblastoma. J. Control. Release 2015, 207, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Clavreul, A.; Roger, E.; Pourbaghi-Masouleh, M.; Lemaire, L.; Tétaud, C.; Menei, P. Development and characterization of sorafenib-loaded lipid nanocapsules for the treatment of glioblastoma. Drug Deliv. 2018, 25, 1756–1765. [Google Scholar] [CrossRef] [Green Version]
- Bredlau, A.L.; Motamarry, A.; Chen, C.; McCrackin, M.A.; Helke, K.; Armeson, K.E.; Bynum, K.; Broome, A.M.; Haemmerich, D. Localized delivery of therapeutic doxorubicin dose across the canine blood-brain barrier with hyperthermia and temperature sensitive liposomes. Drug Deliv. 2018, 25, 973–984. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wu, J.; Wu, J.; Fan, Q.; Zhou, J.; Wu, J.; Liu, S.; Zang, J.; Ye, J.; Xiao, M.; et al. Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in mice. J. Nanobiotechnol. 2019, 17, 29. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Segura, T. Cell-Demanded VEGF Release via Nanocapsules Elicits Different Receptor Activation Dynamics and Enhanced Angiogenesis. Ann. Biomed. Eng. 2016, 44, 1983–1992. [Google Scholar] [CrossRef] [Green Version]
- Fang, C.; Wang, K.; Stephen, Z.R.; Mu, Q.; Kievit, F.M.; Chiu, D.T.; Press, O.W.; Zhang, M. Temozolomide nanoparticles for targeted glioblastoma therapy. ACS Appl. Mater. Interfaces 2015, 7, 6674–6682. [Google Scholar] [CrossRef] [Green Version]
- Sousa, F.; Cruz, A.; Pinto, I.M.; Sarmento, B. Nanoparticles provide long-term stability of bevacizumab preserving its antiangiogenic activity. Acta Biomater. 2018, 78, 285–295. [Google Scholar] [CrossRef]
- Yang, C.; Hwang, H.H.; Jeong, S.; Seo, D.; Jeong, Y.; Lee, D.Y.; Lee, K. Inducing angiogenesis with the controlled release of nitric oxide from biodegradable and biocompatible copolymeric nanoparticles. Int. J. Nanomed. 2018, 13, 6517–6530. [Google Scholar] [CrossRef] [Green Version]
- Sack-Zschauer, M.; Bader, S.; Brenneisen, P. Cerium Oxide Nanoparticles as Novel Tool in Glioma Treatment: An In vitro Study. J. Nanomed. Nanotechnol. 2017, 8, 474. [Google Scholar] [CrossRef] [Green Version]
- Moustafa, E.M.; Mohamed, M.A.; Thabet, N.M. Gallium nanoparticle-mediated reduction of brain specific serine protease-4 in an experimental metastatic cancer model. Asian Pacific J. Cancer Prev. 2017, 18, 895–903. [Google Scholar]
- Cheng, R.; Huang, W.; Huang, L.; Yang, B.; Mao, L.; Jin, K.; Zhuge, Q.; Zhao, Y. Acceleration of tissue plasminogen activator-mediated thrombolysis by magnetically powered nanomotors. ACS Nano 2014, 8, 7746–7754. [Google Scholar] [CrossRef] [PubMed]
- Gilert, A.; MacHluf, M. Nano to micro delivery systems: Targeting angiogenesis in brain tumors. J. Angiogenes. Res. 2010, 2, 20. [Google Scholar] [CrossRef] [Green Version]
- Gribkoff, V.K.; Kaczmarek, L.K. The need for new approaches in CNS drug discovery: Why drugs have failed, and what can be done to improve outcomes. Neuropharmacology 2017, 120, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Long, Q.; Xu, Y.; Xing, J. Evaluating nanoparticles in preclinical research using microfluidic systems. Micromachines 2019, 10, 414. [Google Scholar] [CrossRef] [Green Version]
- Osborne, N.; Avey, M.T.; Anestidou, L.; Ritskes-Hoitinga, M.; Griffin, G. Improving animal research reporting standards. EMBO Rep. 2018, 19, e46069. [Google Scholar] [CrossRef]
- Achberger, K.; Probst, C.; Haderspeck, J.; Bolz, S.; Rogal, J.; Chuchuy, J.; Nikolova, M.; Cora, V.; Antkowiak, L.; Haq, W.; et al. Merging organoid and organ-on-a-chip technology to generate complex multi-layer tissue models in a human retina-on-a-chip platform. Elife 2019, 8, e46188. [Google Scholar] [CrossRef]
- Sriram, G.; Alberti, M.; Dancik, Y.; Wu, B.; Wu, R.; Feng, Z.; Ramasamy, S.; Bigliardi, P.L.; Bigliardi-Qi, M.; Wang, Z. Full-thickness human skin-on-chip with enhanced epidermal morphogenesis and barrier function. Mater. Today 2018, 21, 326–340. [Google Scholar] [CrossRef]
- Abaci, H.E.; Coffman, A.; Doucet, Y.; Chen, J.; Jacków, J.; Wang, E.; Guo, Z.; Shin, J.U.; Jahoda, C.A.; Christiano, A.M. Tissue engineering of human hair follicles using a biomimetic developmental approach. Nat. Commun. 2018, 9, 5301. [Google Scholar] [CrossRef]
- Marturano-Kruik, A.; Nava, M.M.; Yeager, K.; Chramiec, A.; Hao, L.; Robinson, S.; Guo, E.; Raimondi, M.T.; Vunjak-Novakovic, G. Human bone perivascular niche-on-a-chip for studying metastatic colonization. Proc. Natl. Acad. Sci. USA 2018, 115, 1256–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulanova, E.A.; Koudan, E.V.; Degosserie, J.; Heymans, C.; Pereira, F.D.A.S.; Parfenov, V.A.; Sun, Y.; Wang, Q.; Akhmedova, S.A.; Sviridova, I.K.; et al. Bioprinting of a functional vascularized mouse thyroid gland construct. Biofabrication 2017, 9, 034105. [Google Scholar] [CrossRef] [PubMed]
- Jastrzebska, E.; Tomecka, E.; Jesion, I. Heart-on-a-chip based on stem cell biology. Biosens. Bioelectron. 2016, 75, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Yuan Hsin, H.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [Green Version]
- Homan, K.A.; Gupta, N.; Kroll, K.T.; Kolesky, D.B.; Skylar-Scott, M.; Miyoshi, T.; Mau, D.; Valerius, M.T.; Ferrante, T.; Bonventre, J.V.; et al. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat. Methods 2019, 16, 255–262. [Google Scholar] [CrossRef]
- Nguyen, D.-H.T.; Lee, E.; Alimperti, S.; Norgard, R.J.; Wong, A.; Lee, J.J.-K.; Eyckmans, J.; Stanger, B.Z.; Chen, C.S. A biomimetic pancreatic cancer on-chip reveals endothelial ablation via ALK7 signaling. Sci. Adv. 2019, 5, eaav6789. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.B.; Park, J.S.; Shin, Y.M.; Lee, D.H.; Yoon, J.K.; Kim, D.H.; Ko, U.H.; Kim, Y.T.; Bae, S.H.; Sung, H.J. Implantable Vascularized Liver Chip for Cross-Validation of Disease Treatment with Animal Model. Adv. Funct. Mater. 2019, 29, 1900075. [Google Scholar] [CrossRef]
- Carvalho, M.R.; Barata, D.; Teixeira, L.M.; Giselbrecht, S.; Reis, R.L.; Oliveira, J.M.; Truckenmüller, R.; Habibovic, P. Colorectal tumor-on-a-chip system: A 3D tool for precision onco-nanomedicine. Sci. Adv. 2019, 5, eaaw1317. [Google Scholar] [CrossRef] [Green Version]
- Novak, R.; Ingram, M.; Clauson, S.; Das, D.; Delahanty, A.; Herland, A.; Maoz, B.M.; Jeanty, S.S.F.; Somayaji, M.R.; Burt, M.; et al. A robotic platform for fluidically-linked human body-on-chips experimentation. bioRxiv 2019, 569541. [Google Scholar] [CrossRef]
- Phan, D.T.T.; Wang, X.; Craver, B.M.; Sobrino, A.; Zhao, D.; Chen, J.C.; Lee, L.Y.N.; George, S.C.; Lee, A.P.; Hughes, C.C.W. A vascularized and perfused organ-on-a-chip platform for large-scale drug screening applications. Lab Chip 2017, 17, 511–520. [Google Scholar] [CrossRef]
- Bang, S.; Lee, S.R.; Ko, J.; Son, K.; Tahk, D.; Ahn, J.; Im, C.; Jeon, N.L. A Low Permeability Microfluidic Blood-Brain Barrier Platform with Direct Contact between Perfusable Vascular Network and Astrocytes. Sci. Rep. 2017, 7, 8083. [Google Scholar] [CrossRef] [PubMed]
- Van Duinen, V.; Zhu, D.; Ramakers, C.; van Zonneveld, A.J.; Vulto, P.; Hankemeier, T. Perfused 3D angiogenic sprouting in a high-throughput in vitro platform. Angiogenesis 2019, 22, 157–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osaki, T.; Sivathanu, V.; Kamm, R.D. Engineered 3D vascular and neuronal networks in a microfluidic platform. Sci. Rep. 2018, 8, 5168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.; Kasuya, J.; Jeon, J.; Chung, S.; Kamm, R.D. A quantitative microfluidic angiogenesis screen for studying anti-angiogenic therapeutic drugs. Lab Chip 2015, 15, 301–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, J.; Ahn, J.; Kim, S.; Lee, Y.; Lee, J.; Park, D.; Jeon, N.L. Tumor spheroid-on-a-chip: A standardized microfluidic culture platform for investigating tumor angiogenesis. Lab Chip 2019, 19, 2822–2833. [Google Scholar] [CrossRef]
- Cho, H.; Seo, J.H.; Wong, K.H.K.; Terasaki, Y.; Park, J.; Bong, K.; Arai, K.; Lo, E.H.; Irimia, D. Three-dimensional blood-brain barrier model for in vitro studies of neurovascular pathology. Sci. Rep. 2015, 5, 15222. [Google Scholar] [CrossRef] [Green Version]
- Vatine, G.D.; Barrile, R.; Workman, M.J.; Sances, S.; Barriga, B.K.; Rahnama, M.; Barthakur, S.; Kasendra, M.; Lucchesi, C.; Kerns, J.; et al. Human iPSC-Derived Blood-Brain Barrier Chips Enable Disease Modeling and Personalized Medicine Applications. Cell Stem Cell 2019, 24, 995–1005. [Google Scholar] [CrossRef]
- Wevers, N.R.; Kasi, D.G.; Gray, T.; Wilschut, K.J.; Smith, B.; Vught, R.; Shimizu, F.; Sano, Y.; Kanda, T.; Marsh, G.; et al. A perfused human blood-brain barrier on-a-chip for high-throughput assessment of barrier function and antibody transport. Fluids Barriers CNS 2018, 15, 23. [Google Scholar] [CrossRef] [Green Version]
- Herland, A.; Van Der Meer, A.D.; FitzGerald, E.A.; Park, T.E.; Sleeboom, J.J.F.; Ingber, D.E. Distinct contributions of astrocytes and pericytes to neuroinflammation identified in a 3D human blood-brain barrier on a chip. PLoS ONE 2016, 11, e0150360. [Google Scholar] [CrossRef] [Green Version]
- Bonakdar, M.; Graybill, P.M.; Davalos, R.V. A microfluidic model of the blood-brain barrier to study permeabilization by pulsed electric fields. RSC Adv. 2017, 7, 42811–42818. [Google Scholar] [CrossRef]
- Brown, T.D.; Nowak, M.; Bayles, A.V.; Prabhakarpandian, B.; Karande, P.; Lahann, J.; Helgeson, M.E.; Mitragotri, S. A microfluidic model of human brain (μHuB) for assessment of blood brain barrier. Bioeng. Transl. Med. 2019, 4, e10126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booth, R.; Kim, H. Characterization of a microfluidic in vitro model of the blood-brain barrier (μBBB). Lab Chip 2012, 12, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.G.; Jeong, Y.H.; Kim, Y.; Choi, Y.J.; Moon, H.E.; Park, S.H.; Kang, K.S.; Bae, M.; Jang, J.; Youn, H.; et al. A bioprinted human-glioblastoma-on-a-chip for the identification of patient-specific responses to chemoradiotherapy. Nat. Biomed. Eng. 2019, 3, 509–519. [Google Scholar] [CrossRef] [PubMed]
- O’Cearbhaill, E. 3D bioprinting chips away at glioblastomal resistance. Sci. Transl. Med. 2019, 11, eaax1724. [Google Scholar] [CrossRef]
- Ayuso, J.M.; Monge, R.; Martínez-González, A.; Virumbrales-Muñoz, M.; Llamazares, G.A.; Berganzo, J.; Hernández-Laín, A.; Santolaria, J.; Doblaré, M.; Hubert, C.; et al. Glioblastoma on a microfluidic chip: Generating pseudopalisades and enhancing aggressiveness through blood vessel obstruction events. Neuro. Oncol. 2017, 19, 503–513. [Google Scholar] [CrossRef] [Green Version]
- Maoz, B.M.; Herland, A.; Fitzgerald, E.A.; Grevesse, T.; Vidoudez, C.; Pacheco, A.R.; Sheehy, S.P.; Park, T.E.; Dauth, S.; Mannix, R.; et al. A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nat. Biotechnol. 2018, 36, 865–874. [Google Scholar] [CrossRef]
- Akay, M.; Hite, J.; Avci, N.G.; Fan, Y.; Akay, Y.; Lu, G.; Zhu, J.J. Drug Screening of Human GBM Spheroids in Brain Cancer Chip. Sci. Rep. 2018, 8, 15423. [Google Scholar] [CrossRef] [Green Version]
- Mauleon, G.; Fall, C.P.; Eddington, D.T. Precise spatial and temporal control of oxygen within in vitro brain slices via microfluidic gas channels. PLoS ONE 2012, 7, e43309. [Google Scholar] [CrossRef] [Green Version]
- Jorfi, M.; D’Avanzo, C.; Tanzi, R.E.; Kim, D.Y.; Irimia, D. Human Neurospheroid Arrays for In Vitro Studies of Alzheimer’s Disease. Sci. Rep. 2018, 8, 2450. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, J.T.S.; Chutna, O.; Chu, V.; Conde, J.P.; Outeiro, T.F. A novel microfluidic cell co-culture platform for the study of the molecular mechanisms of Parkinson’s disease and other synucleinopathies. Front. Neurosci. 2016, 10, 511. [Google Scholar] [CrossRef] [Green Version]
- Virlogeux, A.; Moutaux, E.; Christaller, W.; Genoux, A.; Bruyère, J.; Fino, E.; Charlot, B.; Cazorla, M.; Saudou, F. Reconstituting Corticostriatal Network on-a-Chip Reveals the Contribution of the Presynaptic Compartment to Huntington’s Disease. Cell Rep. 2018, 22, 110–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajal, C.; Campisi, M.; Mattu, C.; Chiono, V.; Kamm, R.D. In vitro models of molecular and nano-particle transport across the blood-brain barrier. Biomicrofluidics 2018, 12, 042213. [Google Scholar] [CrossRef] [PubMed]
- Valencia, P.M.; Farokhzad, O.C.; Karnik, R.; Langer, R. Microfluidic technologies for accelerating the clinical translation of nanoparticles. Nat. Nanotechnol. 2012, 7, 623–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubíková, T.; Kochová, P.; Tomášek, P.; Witter, K.; Tonar, Z. Numerical and length densities of microvessels in the human brain: Correlation with preferential orientation of microvessels in the cerebral cortex, subcortical grey matter and white matter, pons and cerebellum. J. Chem. Neuroanat. 2018, 88, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Bohn, K.A.; Adkins, C.E.; Mittapalli, R.K.; Terrell-Hall, T.B.; Mohammad, A.S.; Shah, N.; Dolan, E.L.; Nounou, M.I.; Lockman, P.R. Semi-automated rapid quantification of brain vessel density utilizing fluorescent microscopy. J. Neurosci. Methods 2016, 270, 124–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ando, Y.; Okada, H.; Takemura, G.; Suzuki, K.; Takada, C.; Tomita, H.; Zaikokuji, R.; Hotta, Y.; Miyazaki, N.; Yano, H.; et al. Brain-Specific Ultrastructure of Capillary Endothelial Glycocalyx and Its Possible Contribution for Blood Brain Barrier. Sci. Rep. 2018, 8, 17523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Worzfeld, T.; Schwaninger, M. Apicobasal polarity of brain endothelial cells. J. Cereb. Blood Flow Metab. 2016, 36, 340–362. [Google Scholar] [CrossRef] [Green Version]
- Brown, L.S.; Foster, C.G.; Courtney, J.M.; King, N.E.; Howells, D.W.; Sutherland, B.A. Pericytes and neurovascular function in the healthy and diseased brain. Front. Cell. Neurosci. 2019, 13, 282. [Google Scholar] [CrossRef]
- Michinaga, S.; Koyama, Y. Dual roles of astrocyte-derived factors in regulation of blood-brain barrier function after brain damage. Int. J. Mol. Sci. 2019, 20, 571. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Eyo, U.B.; Murugan, M.; Wu, L.J. Microglial interactions with the neurovascular system in physiology and pathology. Dev. Neurobiol. 2018, 78, 604–617. [Google Scholar] [CrossRef]
- Andreone, B.J.; Lacoste, B.; Gu, C. Neuronal and Vascular Interactions. Annu. Rev. Neurosci. 2015, 38, 25–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berndt, P.; Winkler, L.; Cording, J.; Breitkreuz-Korff, O.; Rex, A.; Dithmer, S.; Rausch, V.; Blasig, R.; Richter, M.; Sporbert, A.; et al. Tight junction proteins at the blood–brain barrier: Far more than claudin-5. Cell. Mol. Life Sci. 2019, 76, 1987–2002. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Kisler, K.; Montagne, A.; Toga, A.W.; Zlokovic, B.V. The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci. 2018, 21, 1318–1331. [Google Scholar] [CrossRef] [PubMed]
- Szade, A.; Grochot-Przeczek, A.; Florczyk, U.; Jozkowicz, A.; Dulak, J. Cellular and molecular mechanisms of inflammation-induced angiogenesis. IUBMB Life 2015, 67, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Xia, R.; Jiang, Y.; Wang, L.; Gao, F. Vascular endothelial growth factors enhance the permeability of the mouse blood-brain barrier. PLoS ONE 2014, 9, e86407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janelidze, S.; Hertze, J.; Nägga, K.; Nilsson, K.; Nilsson, C.; Wennström, M.; van Westen, D.; Blennow, K.; Zetterberg, H.; Hansson, O. Increased blood-brain barrier permeability is associated with dementia and diabetes but not amyloid pathology or APOE genotype. Neurobiol. Aging 2017, 51, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Ceña, V.; Játiva, P. Nanoparticle crossing of blood-brain barrier: A road to new therapeutic approaches to central nervous system diseases. Nanomedicine 2018, 13, 1513–1516. [Google Scholar] [CrossRef] [Green Version]
- Shilo, M.; Sharon, A.; Baranes, K.; Motiei, M.; Lellouche, J.P.M.; Popovtzer, R. The effect of nanoparticle size on the probability to cross the blood-brain barrier: An in-vitro endothelial cell model. J. Nanobiotechnol. 2015, 13, 19. [Google Scholar] [CrossRef] [Green Version]
- Trickler, W.J.; Lantz-Mcpeak, S.M.; Robinson, B.L.; Paule, M.G.; Slikker, W.; Biris, A.S.; Schlager, J.J.; Hussain, S.M.; Kanungo, J.; Gonzalez, C.; et al. Porcine brain microvessel endothelial cells show pro-inflammatory response to the size and composition of metallic nanoparticles. Drug Metab. Rev. 2014, 46, 224–231. [Google Scholar] [CrossRef]
- Lockman, P.R.; Koziara, J.M.; Mumper, R.J.; Allen, D. Nanoparticle surface charges alter blood-brain barrier integrity and permeability. J. Drug Target. 2004, 12, 635–641. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the blood-brain barrier with nanoparticles. J. Control. Release 2018, 270, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chang, J.; Fu, X.; Liang, C.; Liang, S.; Yan, R.; Li, A. Nano-sized cationic polymeric magnetic liposomes significantly improves drug delivery to the brain in rats. J. Drug Target. 2012, 20, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhu, J.; Ma, J.; Yang, Y.; Cui, X. Functionalized Bletilla striata polysaccharide micelles for targeted intracellular delivery of Doxorubicin: In vitro and in vivo evaluation. Int. J. Pharm. 2019, 567, 118436. [Google Scholar] [CrossRef] [PubMed]
- Karim, R.; Palazzo, C.; Evrard, B.; Piel, G. Nanocarriers for the treatment of glioblastoma multiforme: Current state-of-the-art. J. Control. Release 2016, 227, 23–37. [Google Scholar] [CrossRef]
- Hohman, T.J.; Bell, S.P.; Jefferson, A.L. The role of vascular endothelial growth factor in neurodegeneration and cognitive decline: Exploring interactions with biomarkers of Alzheimer disease. JAMA Neurol. 2015, 72, 520–529. [Google Scholar] [CrossRef]
- Zhao, H.; Bao, X.J.; Wang, R.Z.; Li, G.L.; Gao, J.; Ma, S.H.; Wei, J.J.; Feng, M.; Zhao, Y.J.; Ma, W.B.; et al. Postacute ischemia vascular endothelial growth factor transfer by transferrin-targeted liposomes attenuates ischemic brain injury after experimental stroke in rats. Hum. Gene Ther. 2011, 22, 207–215. [Google Scholar] [CrossRef]
- Dulak, J.; Jozkowicz, A. Anti-Angiogenic and Anti-Inflammatory Effects of Statins: Relevance to Anti-Cancer Therapy. Curr. Cancer Drug Targets 2005, 5, 579–594. [Google Scholar] [CrossRef]
- Moore, T.L.; Urban, D.A.; Rodriguez-Lorenzo, L.; Milosevic, A.; Crippa, F.; Spuch-Calvar, M.; Balog, S.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle administration method in cell culture alters particle-cell interaction. Sci. Rep. 2019, 9, 900. [Google Scholar] [CrossRef]
- DeStefano, J.G.; Xu, Z.S.; Williams, A.J.; Yimam, N.; Searson, P.C. Effect of shear stress on iPSC-derived human brain microvascular endothelial cells (dhBMECs). Fluids Barriers CNS 2017, 14, 20. [Google Scholar] [CrossRef]
- Ye, M.; Sanchez, H.M.; Hultz, M.; Yang, Z.; Bogorad, M.; Wong, A.D.; Searson, P.C. Brain microvascular endothelial cells resist elongation due to curvature and shear stress. Sci. Rep. 2014, 4, 4681. [Google Scholar] [CrossRef] [Green Version]
- Tanimoto, R.; Hiraiwa, T.; Nakai, Y.; Shindo, Y.; Oka, K.; Hiroi, N.; Funahashi, A. Detection of Temperature Difference in Neuronal Cells. Sci. Rep. 2016, 6, 22071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cochrane, A.; Albers, H.J.; Passier, R.; Mummery, C.L.; van den Berg, A.; Orlova, V.V.; van der Meer, A.D. Advanced in vitro models of vascular biology: Human induced pluripotent stem cells and organ-on-chip technology. Adv. Drug Deliv. Rev. 2018, 1, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Nzou, G.; Wicks, R.T.; Wicks, E.E.; Seale, S.A.; Sane, C.H.; Chen, A.; Murphy, S.V.; Jackson, J.D.; Atala, A.J. Human cortex spheroid with a functional blood brain barrier for high-throughput neurotoxicity screening and disease modeling. Sci. Rep. 2018, 8, 7413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicha, I. Strategies to enhance nanoparticle-endothelial interactions under flow. J. Cell. Biotechnol. 2016, 1, 191–208. [Google Scholar] [CrossRef] [Green Version]
- Nashimoto, Y.; Hayashi, T.; Kunita, I.; Nakamasu, A.; Torisawa, Y.S.; Nakayama, M.; Takigawa-Imamura, H.; Kotera, H.; Nishiyama, K.; Miura, T.; et al. Integrating perfusable vascular networks with a three-dimensional tissue in a microfluidic device. Integr. Biol. 2017, 9, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Baer, D.R. The Chameleon Effect: Characterization Challenges Due to the Variability of Nanoparticles and Their Surfaces. Front. Chem. 2018, 6, 145. [Google Scholar] [CrossRef] [PubMed]
- Badilescu, S.; Packirisamy, M. Microfluidics-nano-integration for synthesis and sensing. Polymers 2012, 4, 1278–1310. [Google Scholar] [CrossRef] [Green Version]
- Deshpande, S.; Dekker, C. On-chip microfluidic production of cell-sized liposomes. Nat. Protoc. 2018, 13, 856–874. [Google Scholar] [CrossRef]
- Pessoa, A.C.S.N.; Sipoli, C.C.; De La Torre, L.G. Effects of diffusion and mixing pattern on microfluidic-assisted synthesis of chitosan/ATP nanoparticles. Lab Chip 2017, 17, 2281–2293. [Google Scholar] [CrossRef] [Green Version]
- Van Ballegooie, C.; Man, A.; Andreu, I.; Gates, B.D.; Yapp, D. Using a microfluidics system to reproducibly synthesize protein nanoparticles: Factors contributing to size, homogeneity, and stability. Processes 2019, 7, 290. [Google Scholar] [CrossRef] [Green Version]
- Kimura, N.; Maeki, M.; Sato, Y.; Note, Y.; Ishida, A.; Tani, H.; Harashima, H.; Tokeshi, M. Development of the iLiNP Device: Fine Tuning the Lipid Nanoparticle Size within 10 nm for Drug Delivery. ACS Omega 2018, 3, 5044–5051. [Google Scholar] [CrossRef] [PubMed]
- Capretto, L.; Carugo, D.; Mazzitelli, S.; Nastruzzi, C.; Zhang, X. Microfluidic and lab-on-a-chip preparation routes for organic nanoparticles and vesicular systems for nanomedicine applications. Adv. Drug Deliv. Rev. 2013, 65, 1496–1532. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Pilkington, E.H.; Sun, Y.; Davis, T.P.T.; Ke, P.C.; Ding, F. Modulating protein amyloid aggregation with nanomaterials. Environ. Sci. Nano 2017, 4, 1772–1783. [Google Scholar] [CrossRef] [PubMed]


| S.No. | NM Formulation * | Particle Size (nm) | Zeta Potential (mV) | PDI | EE% | LC% | Disease Model | Ref. |
|---|---|---|---|---|---|---|---|---|
| Pro-angiogenic NMs | ||||||||
| 1 | microRNA-210-Exosome-c(RGDyK) peptide a | ~140 | - | - | - | - | Ischemic brain, A | 2019, [17] |
| 2 | NO donor-Nanocapsule-PEG-PLGA | 200 | 1.59 ± 0.254 | 1.48–1.53 | 70 ± 4 | - | Non-specific, P | 2018, [21] |
| 3 | PirB-Liposome | 100 | - | 0.201 ± 0.034 | - | - | Ischemic stroke, A | 2018, [13] |
| 4 | CsA-Liposome | 81.5 ± 0.75 | −37.1 | 0.056 ± 0.02 | 78.8 ± 0.59 | - | Ischemic neuroinflamation, A | 2017, [12] |
| 5 | ZL006- Liposome-T7-SHp b | 96.24 ± 1.13 | −3.237 ± 0.206 | 0.157 ± 0.015 | 79.12 ± 3.44 | 9.37 ± 0.48 | Ischemic stroke, P | 2016, [10] |
| 6 | Simvastatin-Liposome | 151.85 | −1.01 | 0.15 | 64.37 ± 7.55 | - | Ischemic stroke, A | 2016, [11] |
| 7 | VEGF-Nanocapsule- peptide c | 22 ± 3 | - | - | - | - | Non-specific, P & A | 2016, [18] |
| 8 | L-Peptide- Liposome | 127.6 ± 48.0 | - | - | - | 62.1 | Ischemic stroke, A | 2015 [9] |
| Anti-angiogenic NMs | ||||||||
| 1 | Indirubin-SLN | 118 | −16.3 ± 8.11 | 0.104 | 99.73 | 0.054 | GBM, P | 2019, [5] |
| 2 | BVZ-Nano-scaffold-PLGA, trehalose | 208–238 | −6.37 | 0.09–0.14 | 84.7 ± 0.3 | - | Non-specific, P | 2018, [20] |
| 3 | SFN-nano-capsule | 54 ± 1 | −7.8 ± 0.6 | 0.15 ± 0.01 | >90 | - | GBM, P & A | 2018, [15] |
| 4 | MTX-SLN-ApoE | 338.0±10.0 | −7.18 ± 1.92 | ~0.287 | 89 | 1.4 | GBM, P & A | 2017, [6] |
| 5 | SLN-ApoE, Palmitate | 174 ± 10.3 | −11.46 | 0.156 ± 0.092 | - | - | Non-specific, P | 2017, [7] |
| 6 | Palcitexel-SLN | 80–90 | −17.4 to −24.8 | 0.19 ± 0.02 | ~88 | 5.18 ± 0.14 | GBM, P & A | 2016, [8] |
| 7 | TMZ-Nano-capsule-CTX | ~67.2 | −1.8 ± 4.3 | - | - | 4.9 ± 0.5 | GBM, P & A | 2015, [19] |
| 8 | microRNA-21-Liposome-CTX | 190 | Neutral | < 0.3 | 85–95 | - | GBM, P & A | 2015, [14] |
| 9 | BVZ-SLN-stearic acid | 515.6 ± 113.6 | - | 0.191 | 29.8 ± 4.4 | 30.0 ± 5.0 | GBM, P | 2015 [4] |
| 10 | Dox-Liposome | 111 ± 5.3 | - | - | - | - | GBM, A | 2013, [16] |
| Others | ||||||||
| 1 | QD-Angiopep-2 | 20 | - | - | - | - | LOC | 2018, [2] |
| 2 | Cerium oxide NP | 1–10 | - | - | - | - | P | 2017, [22] |
| 3 | Gallium NP | 5–7 | - | - | - | - | A | 2017 [23] |
| S.No. | Model | Drug Screening | Dynamic Flow | Lumen perfusability | Vessel Dia. | Endothelial Cells | Brain Cells | Other Cells | TEER | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | GBM (spheroid)-angiogenesis (PC) | BVZ, Sunitinib, Cetuximab | Y | Y | - | HUVEC | U87MG | hLF | NA | 2019, [46] |
| 2 | BBB (HT) | - | Y | Y | W = 200 μm, H = 100 μm | hCMEC/D3 | hA | - | NA | 2019, [52] |
| 3 | GBM-angiogenesis (CR) | TMZ | Static | N | - | HUVEC | U87MG | - | NA | 2019, [54] |
| 4 | BBB (HT) | Dox, Cetuximab, Q-dot-Angiopep-2 | Y | Y | W = 1000 μm, H = 200 μm | iPS-BMVEC | hP, hA | - | Impedance, ~25,000 Ω | 2018, [2] |
| 5 | Angiogenesis 3D (PC) | - | Y | Y | D = 25 μm | HUVEC | - | - | NA | 2018, [43] |
| 6 | BBB (HT) | Antibody MEM-189 | Y | Y | NA | TY10 | hBPCT*, hA | - | NA | 2018, [49] |
| 7 | GBM spheroid (Microwell) | TMZ, BEV | Static | N | NA | - | GBM cell* | - | NA | 2018, [58] |
| 8 | Vasculogenesis (PC) | - | Static | Y | - | HUVEC | E17-brain cells | hLF | NA | 2017, [42] |
| 9 | Vasculogenesis (spheroid) (PC) | - | Y | Y | D = 60 μm | HUVEC, iPS-EC | hNSC | - | NA | 2017, [44] |
| 10 | Hybrid-Brain (others) | Methamphetamine | Y | N | NA | hBMVEC | hP, HIP-009, hA | - | NA | 2017. [57] |
| 11 | BBB (HT) | - | Y | Y | D = 600–800 μm | hBMVEC | hP, hA | - | NA | 2016, [50] |
| 12 | Angiogenesis (PC) | Bortezomib | Y | Y | - | HUVEC | - | NA | 2015, [45] | |
| 13 | BBB (HT) | - | Y | Y | H = 50 μm | RBE4 | - | NA | 2015, [47] | |
| 14 | BBB (others) | Mannitol | Y | N | NA | b.End3 | C8D1A | - | Resistance, 250 Ω cm2 | 2012, [53] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subramaniyan Parimalam, S.; Badilescu, S.; Sonenberg, N.; Bhat, R.; Packirisamy, M. Lab-On-A-Chip for the Development of Pro-/Anti-Angiogenic Nanomedicines to Treat Brain Diseases. Int. J. Mol. Sci. 2019, 20, 6126. https://doi.org/10.3390/ijms20246126
Subramaniyan Parimalam S, Badilescu S, Sonenberg N, Bhat R, Packirisamy M. Lab-On-A-Chip for the Development of Pro-/Anti-Angiogenic Nanomedicines to Treat Brain Diseases. International Journal of Molecular Sciences. 2019; 20(24):6126. https://doi.org/10.3390/ijms20246126
Chicago/Turabian StyleSubramaniyan Parimalam, Subhathirai, Simona Badilescu, Nahum Sonenberg, Rama Bhat, and Muthukumaran Packirisamy. 2019. "Lab-On-A-Chip for the Development of Pro-/Anti-Angiogenic Nanomedicines to Treat Brain Diseases" International Journal of Molecular Sciences 20, no. 24: 6126. https://doi.org/10.3390/ijms20246126
APA StyleSubramaniyan Parimalam, S., Badilescu, S., Sonenberg, N., Bhat, R., & Packirisamy, M. (2019). Lab-On-A-Chip for the Development of Pro-/Anti-Angiogenic Nanomedicines to Treat Brain Diseases. International Journal of Molecular Sciences, 20(24), 6126. https://doi.org/10.3390/ijms20246126






