Contribution of Mucins towards the Physical Properties of the Tear Film: A Modern Update
Abstract
1. Introduction
2. Contributions of Secretory Mucins to the Stability of Human Tear Film
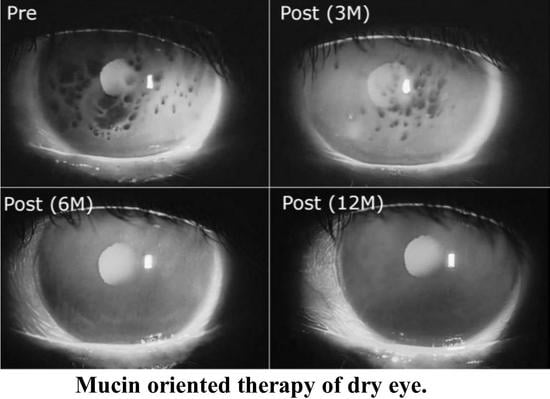
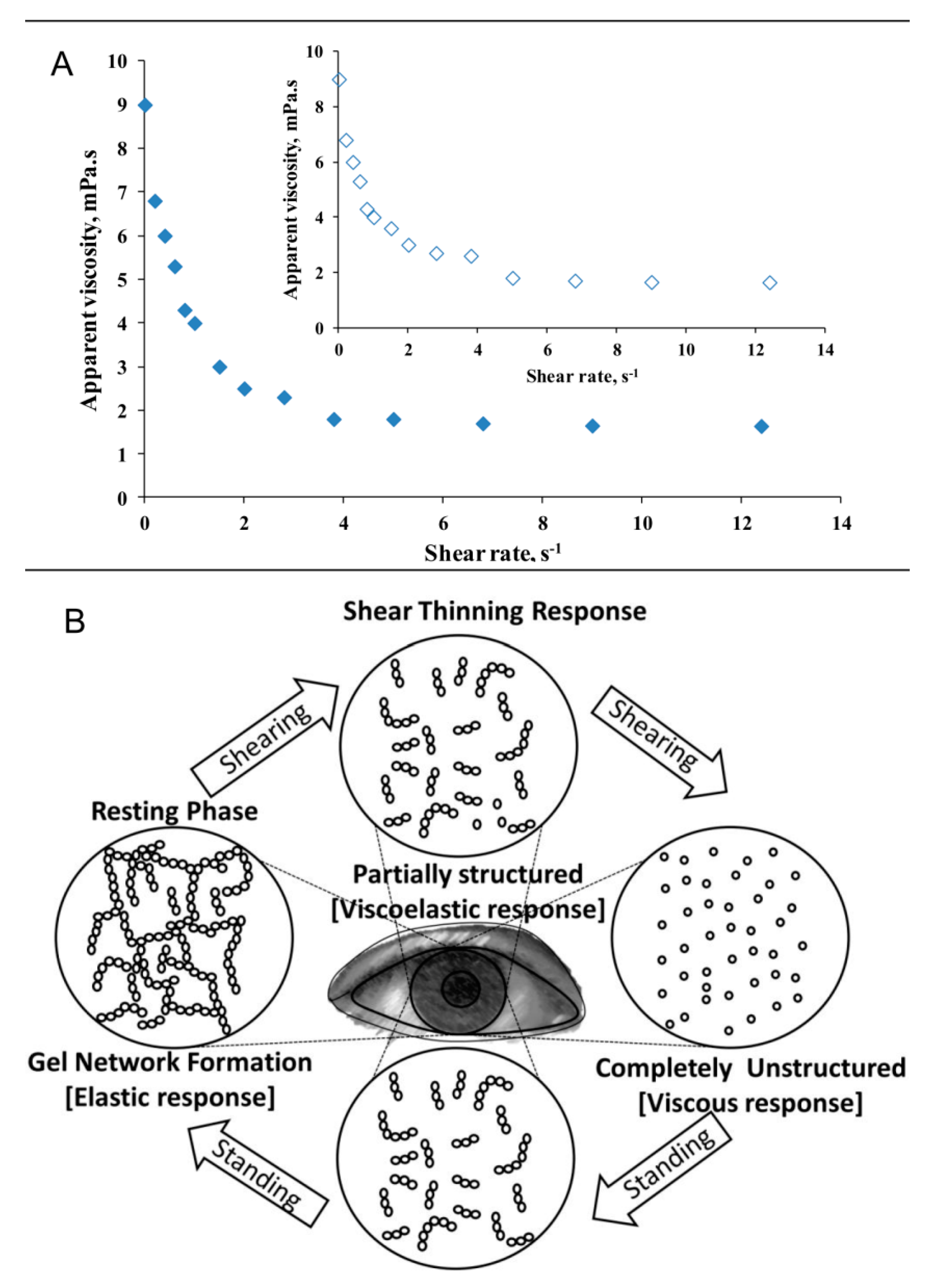
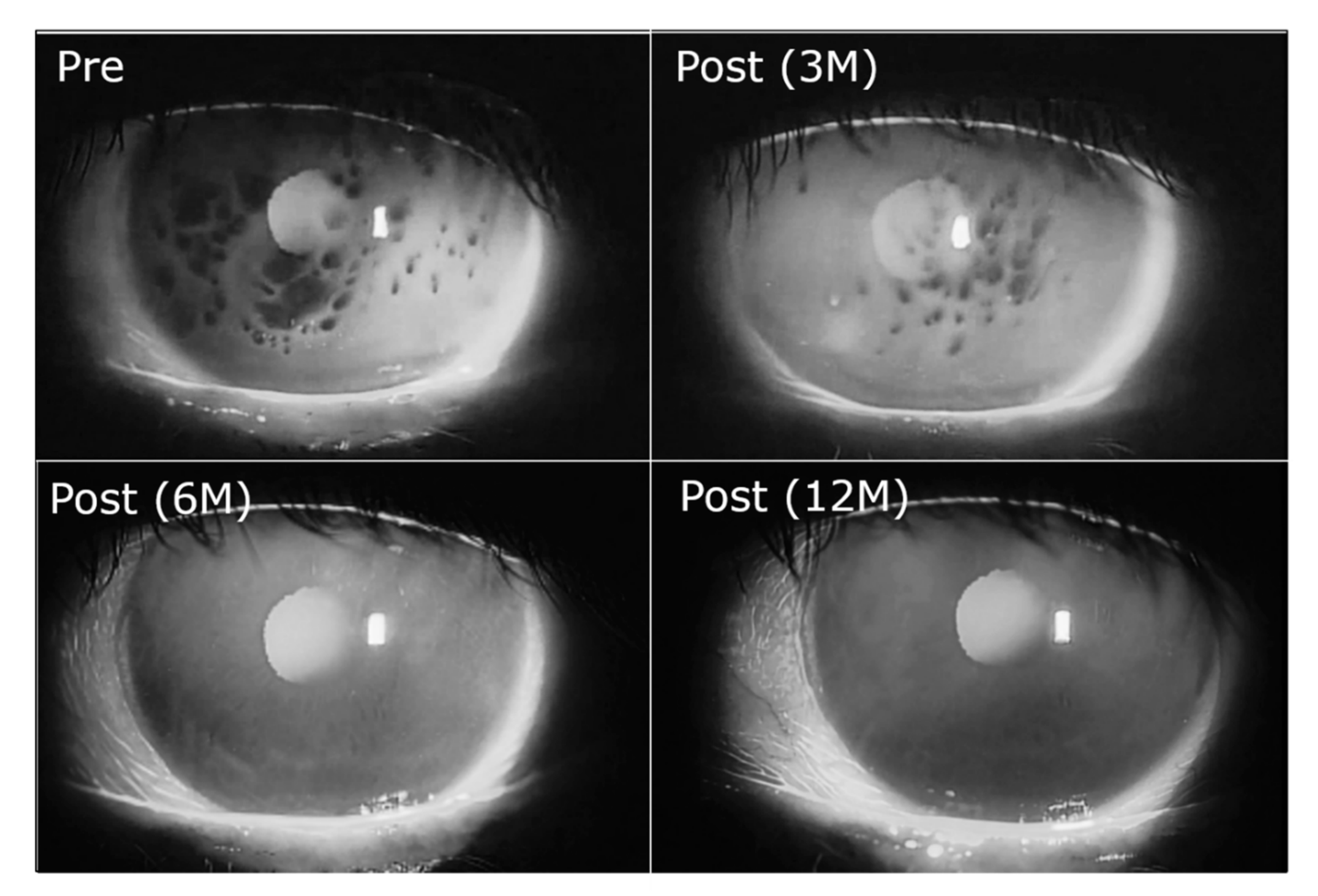
2.1. Shear Thinning Property of Whole Human Tears
2.2. Aqueous Tear Gel as a Surface Chemical Protection of the Ocular Surface
2.3. Secretory Mucins as Spreading Agents for TFLL
3. Contributions of Membrane Associated Mucins to the Stability of Human Tear Film
3.1. MAM and Corneal Wettability
3.2. MAMs and the Ocular Surface Lubrication
3.3. On the Interplay of Secretory Mucins and MAM
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AT | Aqueous tear |
| BAM | Brewster angle microscopy |
| DES | Dry eye syndrome |
| HA | Hyaluronic acid |
| HCEC | Human corneal epithelium cells |
| MAM | Membrane associated mucins |
| MAG | Muco-aqueous gel |
| MGD | Meibomian gland dysfunction |
| NAC | N-acetylcysteine |
| NIBUT | Non-invasive breakup time |
| SM | Secretory mucins |
| TF | Tear film |
| TFLL | Tear film lipid layer |
References
- Willcox, M.D.P.; Argueso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U.; et al. TFOS DEWS II Tear Film Report. Ocul. Surf. 2017, 15, 366–403. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, G.A.; Eftimov, P.; Yokoi, N. Structure-function relationship of tear film lipid layer: A contemporary perspective. Exp. Eye Res. 2017, 163, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Gayton, J.L. Etiology, prevalence, and treatment of dry eye disease. Clin. Ophthalmol. 2009, 3, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Asche, C.V.; Fairchild, C.J. The economic burden of dry eye disease in the United States: A decision tree analysis. Cornea 2011, 30, 379–387. [Google Scholar] [CrossRef]
- McDonald, M.; Patel, D.A.; Keith, M.S.; Snedecor, S.J. Economic and Humanistic Burden of Dry Eye Disease in Europe, North America, and Asia: A Systematic Literature Review. Ocul. Surf. 2016, 14, 144–167. [Google Scholar] [CrossRef]
- Guzman-Aranguez, A.; Argueso, P. Structure and biological roles of mucin-type O-glycans at the ocular surface. Ocul. Surf. 2010, 8, 8–17. [Google Scholar] [CrossRef]
- Mantelli, F.; Argueso, P. Functions of ocular surface mucins in health and disease. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 477–483. [Google Scholar] [CrossRef]
- Luo, L.; Li, D.Q.; Corrales, R.M.; Pflugfelder, S.C. Hyperosmolar saline is a proinflammatory stress on the mouse ocular surface. Eye Contact Lens 2005, 31, 186–193. [Google Scholar] [CrossRef]
- Dey, M.; Vivek, A.S.; Dixit, H.N.; Richhariya, A.; Feng, J.J. A model of tear-film breakup with continuous mucin concentration and viscosity profiles. J. Fluid Mech. 2018, 858, 352–376. [Google Scholar] [CrossRef]
- Jones, M.B.; Please, C.P.; McElwain, D.L.; Fulford, G.R.; Roberts, A.P.; Collins, M.J. Dynamics of tear film deposition and draining. Math. Med. Biol. 2005, 22, 265–288. [Google Scholar] [CrossRef]
- Sharma, A. Breakup and dewetting of the corneal mucus layer. An update. Adv. Exp. Med. Biol. 1998, 438, 273–280. [Google Scholar] [PubMed]
- Tiffany, J.M. The viscosity of human tears. Int. Ophthalmol. 1991, 15, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Tiffany, J.M. Viscoelastic Properties of Human Tears and Polymer Solutions. In Lacrimal Gland, Tear Film, and Dry Eye Syndromes: Basic Science and Clinical Relevance; Sullivan, D.A., Ed.; Springer: Boston, MA, USA, 1994; pp. 267–270. [Google Scholar]
- Tiffany, J.M. Measurement of wettability of the corneal epithelium. II. Contact angle method. Acta Ophthalmol. 1990, 68, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Tiffany, J.M.; Pandit, J.C.; Bron, A.J. Soluble mucin and the physical properties of tears. Adv. Exp. Med. Biol. 1998, 438, 229–234. [Google Scholar] [PubMed]
- Pandit, J.C.; Nagyova, B.; Bron, A.J.; Tiffany, J.M. Physical properties of stimulated and unstimulated tears. Exp. Eye Res. 1999, 68, 247–253. [Google Scholar] [CrossRef]
- Sharma, A.; Tiwari, S.; Khanna, R.; Tiffany, J.M. Hydrodynamics of meniscus-induced thinning of the tear film. Adv. Exp. Med. Biol. 1998, 438, 425–431. [Google Scholar]
- Creech, J.L.; Do, L.T.; Fatt, I.; Radke, C.J. In vivo tear-film thickness determination and implications for tear-film stability. Curr. Eye Res. 1998, 17, 1058–1066. [Google Scholar] [CrossRef]
- Fatt, I. Observations of tear film break up on model eyes. CLAO J. 1991, 17, 267–281. [Google Scholar]
- Jyoti, B.V.S.; Baek, S.W. Formulation and Comparative Study of Rheological Properties of Loaded and Unloaded Ethanol-Based Gel Propellants. J. Energetic Mater. 2015, 33, 125–139. [Google Scholar] [CrossRef]
- McDonnell, A.; Lee, J.H.; Makrai, E.; Yeo, L.Y.; Downie, L.E. Tear Film Extensional Viscosity Is a Novel Potential Biomarker of Dry Eye Disease. Ophthalmology 2019, 126, 1196–1198. [Google Scholar] [CrossRef]
- Zhao, H.; Jumblatt, J.E.; Wood, T.O.; Jumblatt, M.M. Quantification of MUC5AC protein in human tears. Cornea 2001, 20, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Moschos, M.M.; Rouvas, A.A.; Papadimitriou, S.; Kotsolis, A.; Sitaras, N.; Apostolopoulos, M. Quantitative determination of glycosaminoglycans in tears of diabetic patients. Clin Ophthalmol. 2008, 2, 581–584. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ablamowicz, A.F.; Nichols, J.J. Concentrations of MUC16 and MUC5AC using three tear collection methods. Mol. Vis. 2017, 23, 529–537. [Google Scholar]
- Miyake, H.; Mori, N.; Mano, H.; Imanaka, T.; Nakamura, M. Development of a highly sensitive and reliable enzyme-linked immunosorbent assay for MUC5AC in human tears extracted from Schirmer strips. Clin. Ophthalmol. 2018, 12, 1571–1580. [Google Scholar] [CrossRef]
- Lemp, M.A. Corneal desiccation despite normal tear volume. Ann. Ophthalmol. 1970, 2, 258–284. [Google Scholar]
- Lemp, M.A. The mucin-deficient dry eye. Int. Ophthalmol. Clin. 1973, 13, 185–189. [Google Scholar] [CrossRef]
- Lemp, M.A.; Dohlman, C.H.; Kuwabara, T.; Holly, F.J.; Carroll, J.M. Dry eye secondary to mucus deficiency. Trans. Am. Acad. Ophthalmol. Otolaryngol. 1971, 75, 1223–1227. [Google Scholar]
- Lemp, M.A.; Hamill, J.R., Jr. Factors affecting tear film breakup in normal eyes. Arch. Ophthalmol. 1973, 89, 103–105. [Google Scholar] [CrossRef]
- Hori, Y. Secreted Mucins on the Ocular Surface. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES151–DES156. [Google Scholar] [CrossRef]
- Dohlman, C.H.; Friend, J.; Kalevar, V.; Yagoda, D.; Balazs, E. The glycoprotein (mucus) content of tears from normals and dry eye patients. Exp. Eye Res. 1976, 22, 359–365. [Google Scholar] [CrossRef]
- Baudouin, C.; Rolando, M.; Benitez Del Castillo, J.M.; Messmer, E.M.; Figueiredo, F.C.; Irkec, M.; Van Setten, G.; Labetoulle, M. Reconsidering the central role of mucins in dry eye and ocular surface diseases. Prog. Retin. Eye Res. 2019, 71, 68–87. [Google Scholar] [CrossRef] [PubMed]
- Viau, S.; Maire, M.A.; Pasquis, B.; Gregoire, S.; Fourgeux, C.; Acar, N.; Bretillon, L.; Creuzot-Garcher, C.P.; Joffre, C. Time course of ocular surface and lacrimal gland changes in a new scopolamine-induced dry eye model. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 246, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.C.; Ahn, K.Y.; Choi, W.; Li, Z.; Choi, J.S.; Lee, S.H.; Park, S.H. Tear production and ocular surface changes in experimental dry eye after elimination of desiccating stress. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7267–7273. [Google Scholar] [CrossRef] [PubMed]
- Floyd, A.M.; Zhou, X.; Evans, C.; Rompala, O.J.; Zhu, L.; Wang, M.; Chen, Y. Mucin deficiency causes functional and structural changes of the ocular surface. PLoS ONE 2012, 7, e50704. [Google Scholar] [CrossRef]
- Marko, C.K.; Tisdale, A.S.; Spurr-Michaud, S.; Evans, C.; Gipson, I.K. The ocular surface phenotype of Muc5ac and Muc5b null mice. Investig. Ophthalmol. Vis. Sci. 2014, 55, 291–300. [Google Scholar] [CrossRef]
- Inaba, T.; Tanaka, Y.; Tamaki, S.; Ito, T.; Ntambi, J.M.; Tsubota, K. Compensatory increases in tear volume and mucin levels associated with meibomian gland dysfunction caused by stearoyl-CoA desaturase-1 deficiency. Sci. Rep. 2018, 8, 3358. [Google Scholar] [CrossRef]
- Choi, K.E.; Song, J.S.; Kang, B.; Eom, Y.; Kim, H.M. Immediate Effect of 3% Diquafosol Ophthalmic Solution on Tear MUC5AC Concentration and Corneal Wetting Ability in Normal and Experimental Keratoconjunctivitis Sicca Rat Models. Curr. Eye Res. 2017, 42, 666–671. [Google Scholar] [CrossRef]
- Millar, T.J.; Tragoulias, S.T.; Anderton, P.J.; Ball, M.S.; Miano, F.; Dennis, G.R.; Mudgil, P. The surface activity of purified ocular mucin at the air-liquid interface and interactions with meibomian lipids. Cornea 2006, 25, 91–100. [Google Scholar] [CrossRef]
- Georgiev, G.A.; Yokoi, N.; Ivanova, S.; Dimitrov, T.; Andreev, K.; Krastev, R.; Lalchev, Z. Surface chemistry study of the interactions of hyaluronic acid and benzalkonium chloride with meibomian and corneal cell lipids. Soft Matter 2013, 9, 10841–10856. [Google Scholar] [CrossRef]
- Rosenfeld, L.; Cerretani, C.; Leiske, D.L.; Toney, M.F.; Radke, C.J.; Fuller, G.G. Structural and rheological properties of meibomian lipid. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2720–2732. [Google Scholar] [CrossRef]
- Svitova, T.F.; Lin, M.C. Dynamic interfacial properties of human tear-lipid films and their interactions with model-tear proteins in vitro. Adv. Colloid Interface Sci. 2016, 233, 4–24. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sang, X.; Yang, L.; Wang, X.R.; Liu, J.H.; He, X.J.; Liu, Y.; Lu, X.H.; Wang, Z.C. Low concentration of sodium hyaluronate temporarily elevates the tear film lipid layer thickness in dry eye patients with lipid deficiency. Int. J. Ophthalmol. 2018, 11, 389–394. [Google Scholar] [PubMed]
- Fukuoka, S.; Arita, R. Increase in tear film lipid layer thickness after instillation of 3% diquafosol ophthalmic solution in healthy human eyes. Ocul. Surf. 2017, 15, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Spurr-Michaud, S.; Russo, C.L.; Argueso, P.; Gipson, I.K. Differential regulation of membrane-associated mucins in the human ocular surface epithelium. Investig. Ophthalmol. Vis. Sci. 2004, 45, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A. Vitamin A Deficiency and Xerophthalmia. JAMA Ophthalmol. 1990, 108, 343–344. [Google Scholar] [CrossRef]
- Shanker, R.M.; Ahmed, I.; Bourassa, P.A.; Carola, K.V. An in vitro technique for measuring contact angles on the corneal surface and its application to evaluate corneal wetting properties of water soluble polymers. Int. J. Pharm. 1995, 119, 149–163. [Google Scholar] [CrossRef]
- Georgiev, G.A.; Yokoi, N.; Eftimov, P.; Stefanova, N.; Ivanova, S. Impact of membrane associated mucin and diquafosol on the wettability of human corneal epithelium cell layers. Investig. Ophthalmol. Vis. Sci. 2015, 56, 294. [Google Scholar]
- Yanez-Soto, B.; Leonard, B.C.; Raghunathan, V.K.; Abbott, N.L.; Murphy, C.J. Effect of Stratification on Surface Properties of Corneal Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 8340–8348. [Google Scholar] [CrossRef]
- Blalock, T.D.; Spurr-Michaud, S.J.; Tisdale, A.S.; Heimer, S.R.; Gilmore, M.S.; Ramesh, V.; Gipson, I.K. Functions of MUC16 in corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4509–4518. [Google Scholar] [CrossRef]
- Danjo, Y.; Watanabe, H.; Tisdale, A.S.; George, M.; Tsumura, T.; Abelson, M.B.; Gipson, I.K. Alteration of mucin in human conjunctival epithelia in dry eye. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2602–2609. [Google Scholar]
- Liotet, S.; Van Bijsterveld, O.P.; Kogbe, O.; Laroche, L. A new hypothesis on tear film stability. Ophthalmologica 1987, 195, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kang, B.; Woo, I.H.; Eom, Y.; Lee, H.K.; Kim, H.M.; Song, J.S. Effects of Topical Mucolytic Agents on the Tears and Ocular Surface: A Plausible Animal Model of Mucin-Deficient Dry Eye. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3104–3114. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, N.; Georgiev, G.A.; Kato, H.; Komuro, A.; Sonomura, Y.; Sotozono, C.; Tsubota, K.; Kinoshita, S. Classification of Fluorescein Breakup Patterns: A Novel Method of Differential Diagnosis for Dry Eye. Am. J. Ophthalmol. 2017, 180, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Bitton, E.; Lovasik, J.V. Longitudinal analysis of precorneal tear film rupture patterns. Adv. Exp. Med. Biol. 1998, 438, 381–389. [Google Scholar]
- Holly, F.J. Basic Aspects of Tear Film Formation and Stability. In Physicochemical Hydrodynamics: Interfacial Phenomena; Velarde, M.G., Ed.; Springer: Boston, MA, USA, 1988; pp. 401–410. [Google Scholar]
- Argüeso, P. Glycobiology of the ocular surface: Mucins and lectins. Jpn. J. Ophthalmol. 2013, 57, 150–155. [Google Scholar] [CrossRef][Green Version]
- King-Smith, P.E.; Reuter, K.S.; Braun, R.J.; Nichols, J.J.; Nichols, K.K. Tear film breakup and structure studied by simultaneous video recording of fluorescence and tear film lipid layer images. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4900–4909. [Google Scholar] [CrossRef]
- Yokoi, N.; Kato, H.; Kinoshita, S. Facilitation of tear fluid secretion by 3% diquafosol ophthalmic solution in normal human eyes. Am. J. Ophthalmol. 2014, 157, 85–92. [Google Scholar] [CrossRef]
- Yokoi, N.; Kato, H.; Kinoshita, S. The increase of aqueous tear volume by diquafosol sodium in dry-eye patients with Sjogren’s syndrome: A pilot study. Eye 2016, 30, 857–864. [Google Scholar] [CrossRef]
- Shigeyasu, C.; Yamada, M.; Akune, Y. Influence of Ophthalmic Solutions on Tear Components. Cornea 2016, 35 (Suppl. 1), S71–S77. [Google Scholar] [CrossRef]
- Takaoka-Shichijo, Y.; Nakamura, M. Stimulatory effect of diquafosol tetrasodium on the expression of membrane-binding mucin genes in cultured human corneal epithelial cells. J. Eye 2011, 28, 425–429. [Google Scholar]
- Yokoi, N.; Sonomura, Y.; Kato, H.; Komuro, A.; Kinoshita, S. Three percent diquafosol ophthalmic solution as an additional therapy to existing artificial tears with steroids for dry-eye patients with Sjogren’s syndrome. Eye 2015, 29, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M. Diquafosol ophthalmic solution 3%: A review of its use in dry eye. Drugs 2015, 75, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.; Aeschlimann, R.; Tosatti, S.; Toubouti, Y.; Kakkassery, J.; Osborn Lorenz, K. Coefficient of Friction of Human Corneal Tissue. Cornea 2015, 34, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Pitenis, A.A.; Urueña, J.M.; Hormel, T.T.; Bhattacharjee, T.; Niemi, S.R.; Marshall, S.L.; Hart, S.M.; Schulze, K.D.; Angelini, T.E.; Sawyer, W.G. Corneal cell friction: Survival, lubricity, tear films, and mucin production over extended duration in vitro studies. Biotribology 2017, 11, 77–83. [Google Scholar] [CrossRef]
- Pult, H.; Tosatti, S.G.; Spencer, N.D.; Asfour, J.M.; Ebenhoch, M.; Murphy, P.J. Spontaneous Blinking from a Tribological Viewpoint. Ocul. Surf. 2015, 13, 236–249. [Google Scholar] [CrossRef]
- Klein, J. Hydration lubrication. Friction 2013, 1, 1–23. [Google Scholar] [CrossRef]
- Winkeljann, B.; Boettcher, K.; Balzer, B.N.; Lieleg, O. Mucin Coatings Prevent Tissue Damage at the Cornea–Contact Lens Interface. Adv. Mater. Interfaces 2017, 4, 1700186. [Google Scholar] [CrossRef]
- Sterner, O.; Karageorgaki, C.; Zurcher, M.; Zurcher, S.; Scales, C.W.; Fadli, Z.; Spencer, N.D.; Tosatti, S.G.P. Reducing Friction in the Eye: A Comparative Study of Lubrication by Surface-Anchored Synthetic and Natural Ocular Mucin Analogues. ACS Appl. Mater. Interfaces 2017, 9, 20150–20160. [Google Scholar] [CrossRef]
- Berry, M.; Pult, H.; Purslow, C.; Murphy, P.J. Mucins and ocular signs in symptomatic and asymptomatic contact lens wear. Optom. Vis. Sci. 2008, 85, E930–E938. [Google Scholar] [CrossRef]
- Pitenis, A.A.; Urueña, J.M.; Hart, S.M.; O’Bryan, C.S.; Marshall, S.L.; Levings, P.P.; Angelini, T.E.; Sawyer, W.G. Friction-Induced Inflammation. Tribol. Lett. 2018, 66, 81. [Google Scholar] [CrossRef]
- Bron, A.J.; Argueso, P.; Irkec, M.; Bright, F.V. Clinical staining of the ocular surface: Mechanisms and interpretations. Prog. Retin. Eye Res. 2015, 44, 36–61. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, N.; Georgiev, G.A. Tear Film-Oriented Diagnosis and Tear Film-Oriented Therapy for Dry Eye Based on Tear Film Dynamics. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES13–DES22. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, N.; Georgiev, G.A. Tear-film-oriented diagnosis for dry eye. Jpn. J. Ophthalmol. 2019, 63, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Markoulli, M.; Hui, A. Emerging targets of inflammation and tear secretion in dry eye disease. Drug Discov. Today 2019, 24, 1427–1432. [Google Scholar] [CrossRef]
- Itoh, S.; Itoh, K.; Shinohara, H. Regulation of human corneal epithelial mucins by rebamipide. Curr. Eye Res. 2014, 39, 133–141. [Google Scholar] [CrossRef]
- Ogawa, M.; Simsek, C.; Kojima, T.; Nagata, T.; Igarashi, A.; Kawakita, T.; Dogru, M.; Shimazaki, J.; Tsubota, K. The Effect of Rebamipide Ophthalmic Solution on Cytokine and Mucin Secretion in Culture of Conjunctival Epithelial Cells from the Cu, Zn-Superoxide Dismutase-1 (SOD-1) Knock-Down Mouse. Eye Contact Lens 2019, 45, 93–98. [Google Scholar] [CrossRef]
- Kase, S.; Shinohara, T.; Kase, M.; Ishida, S. Effect of topical rebamipide on goblet cells in the lid wiper of human conjunctiva. Exp. Med. 2017, 13, 3516–3522. [Google Scholar] [CrossRef]
- Karnati, R.; Talla, V.; Peterson, K.; Laurie, G.W. Lacritin and other autophagy associated proteins in ocular surface health. Exp. Eye Res. 2016, 144, 4–13. [Google Scholar] [CrossRef]
- Lee, H.; Jeon, S.; Kim, C.E.; Park, Y.J.; Yang, J. A New Ophthalmic Pharmaceutical Formulation, Topical Sulglycotide, Enhances the Ocular Mucin Secretion in Desiccation Stress-Mediated Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1076–1087. [Google Scholar] [CrossRef]


| Type of Mucins | Contribution to TF Physical Properties and Stability | Alterations in Pathology | Mucin Oriented Therapeutics |
|---|---|---|---|
| Secreted gel forming mucins: MUC5AC (most abundant), MUC5B, MUC2, and MUC19 | The mucus gel ensures the shear thinning property of tears, facilitates the spreading of the lipid layer and acts as surface chemistry trap and barrier against invasion of lipids and exogenous agents towards the corneal surface. | Quantitative abnormalities of MUC5AC are observed in as diverse range of DES subtypes as aqueous tear deficient DE and evaporative DE | Diquafosol tetrasodium, lacritin |
| Membrane associated mucins: MUC1, MUC4, MUC16 | MAM ensure ideal wettability and high lubricity of the ocular surface with MUC16 (having the longest hydrophilic ectodomain) considered especially critical. By ensuring no slip condition of the cornea and viscosity gradient across the TF, MAM might cooperate with secretory mucins on the shear thinning properties of tears. | Altered in decreased wettability DE subtype, characterized with normal AT volume and TFLL, but rapid breakup immediately at or <5 s after eye opening | Diquafosol tetrasodium, rebamipide |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgiev, G.A.; Eftimov, P.; Yokoi, N. Contribution of Mucins towards the Physical Properties of the Tear Film: A Modern Update. Int. J. Mol. Sci. 2019, 20, 6132. https://doi.org/10.3390/ijms20246132
Georgiev GA, Eftimov P, Yokoi N. Contribution of Mucins towards the Physical Properties of the Tear Film: A Modern Update. International Journal of Molecular Sciences. 2019; 20(24):6132. https://doi.org/10.3390/ijms20246132
Chicago/Turabian StyleGeorgiev, Georgi As., Petar Eftimov, and Norihiko Yokoi. 2019. "Contribution of Mucins towards the Physical Properties of the Tear Film: A Modern Update" International Journal of Molecular Sciences 20, no. 24: 6132. https://doi.org/10.3390/ijms20246132
APA StyleGeorgiev, G. A., Eftimov, P., & Yokoi, N. (2019). Contribution of Mucins towards the Physical Properties of the Tear Film: A Modern Update. International Journal of Molecular Sciences, 20(24), 6132. https://doi.org/10.3390/ijms20246132