Rice Protein Exerts Anti-Inflammatory Effect in Growing and Adult Rats via Suppressing NF-κB Pathway
Abstract
:1. Introduction
2. Results
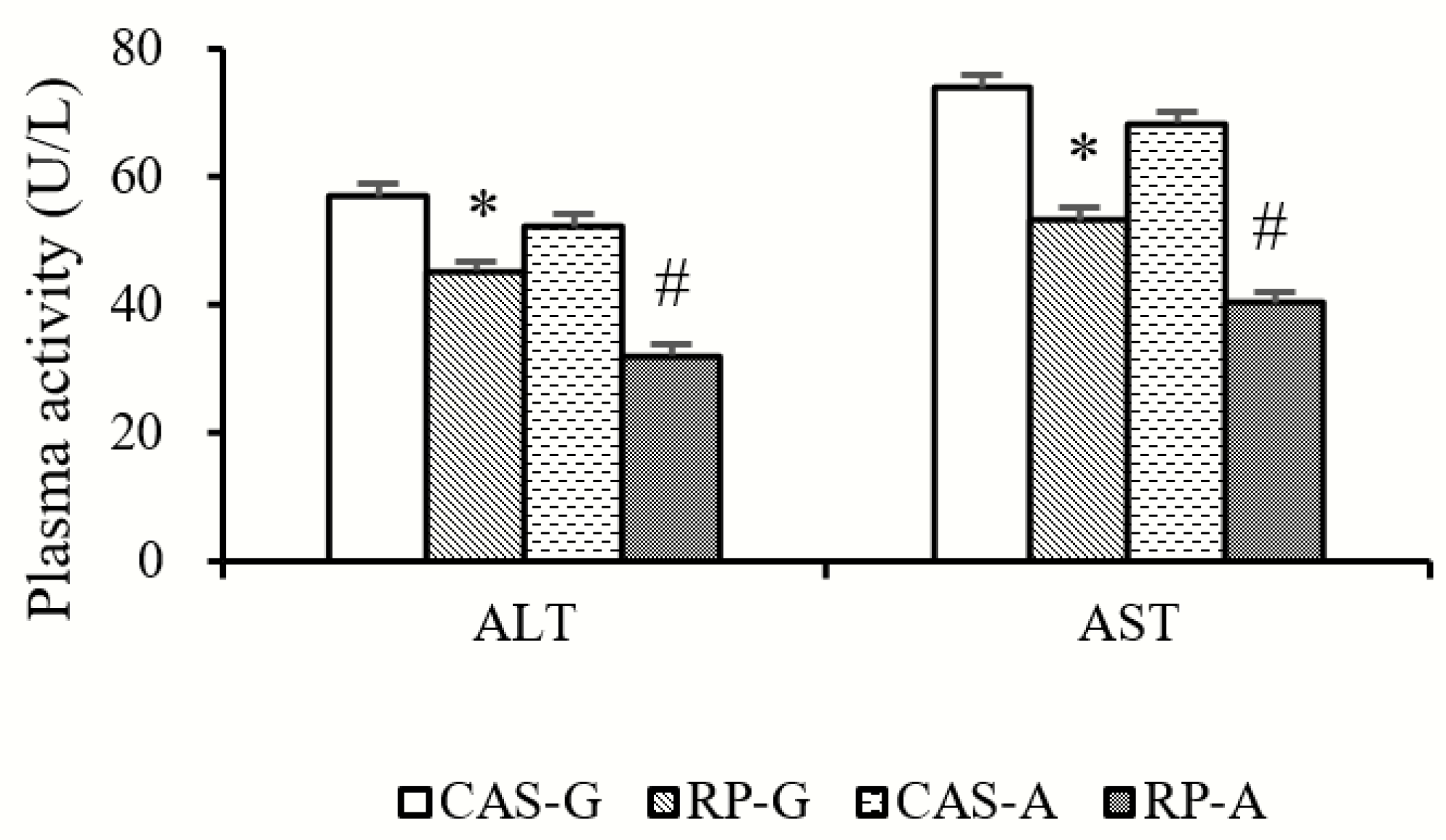
2.1. Plasma ALT and AST Activities
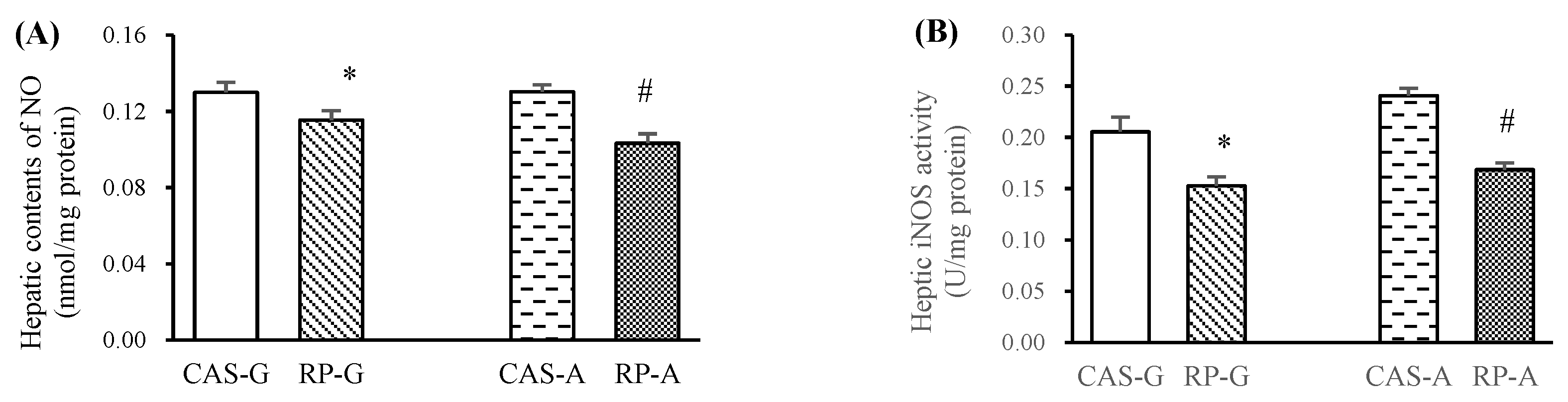
2.2. Hepatic NO Levels and iNOS Activity
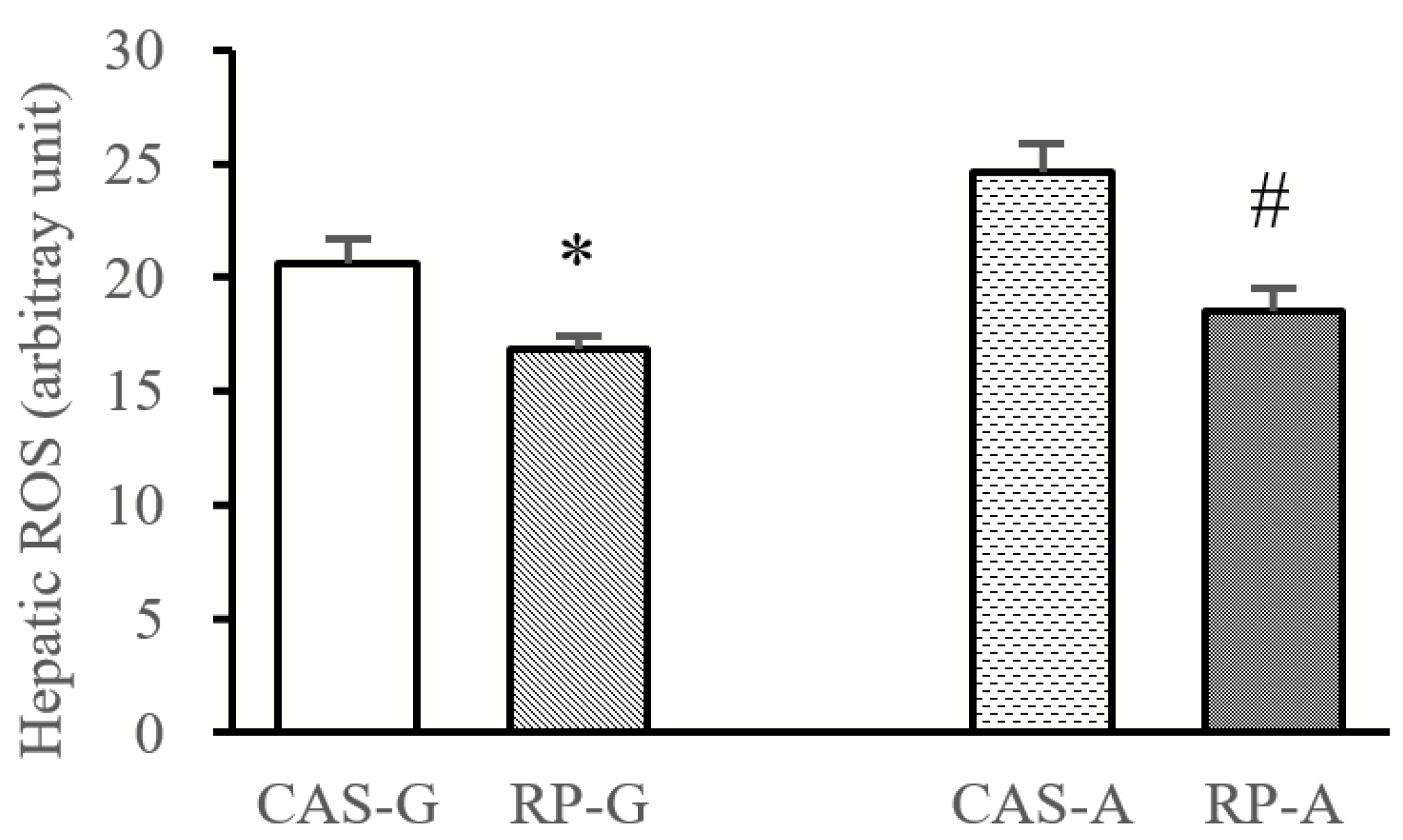
2.3. Hepatic ROS Accumulation
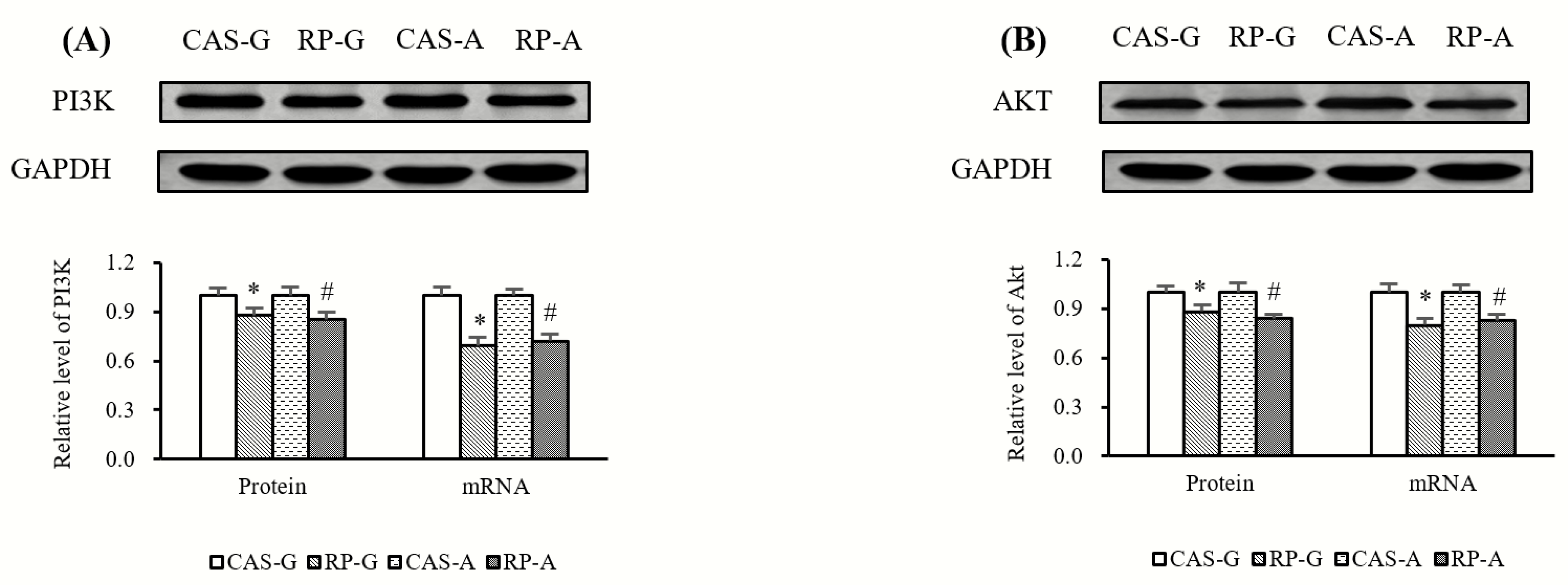
2.4. Expressions of PI3K and AKT
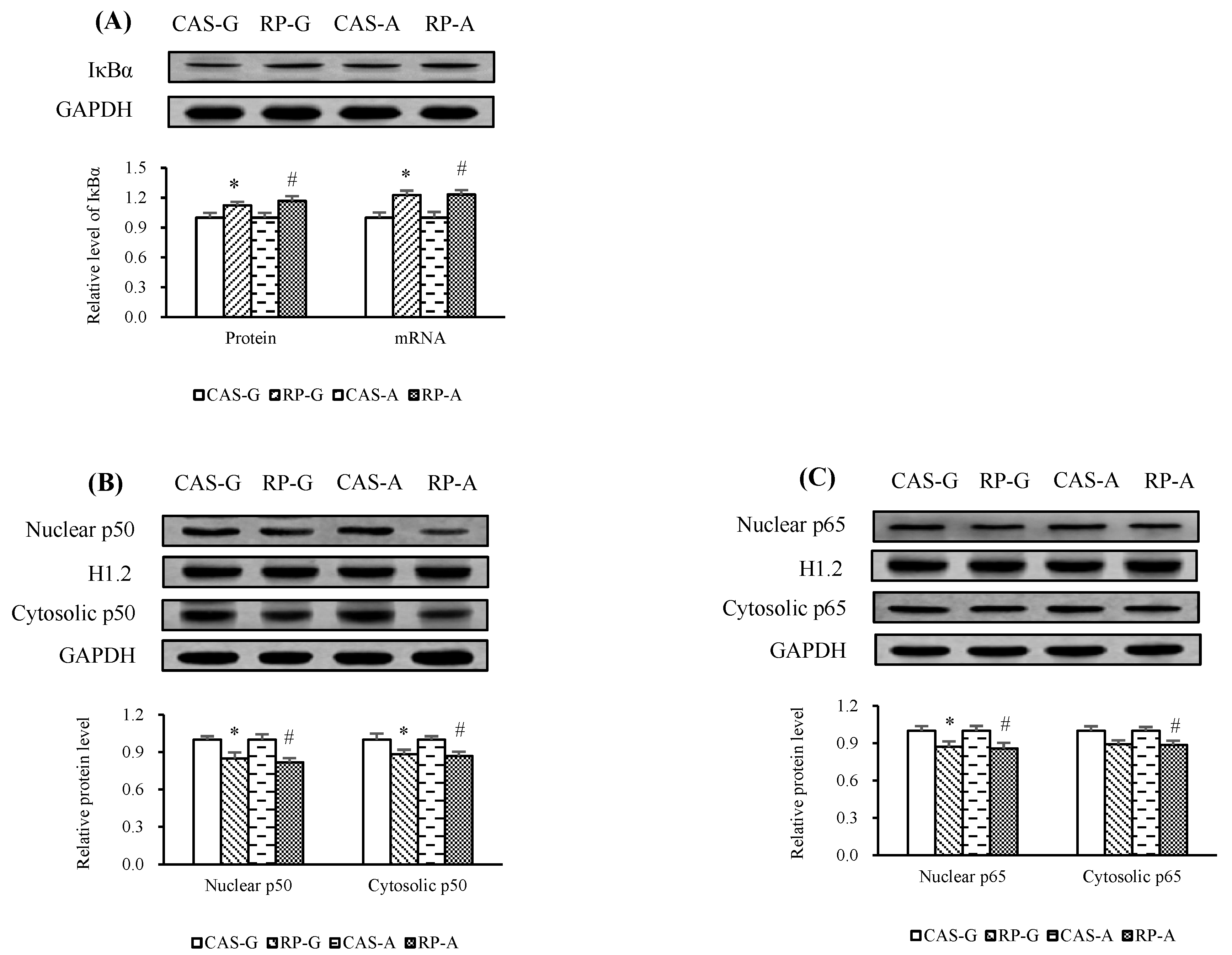
2.5. Expressions of NF-κB
2.6. NF-κB Activation
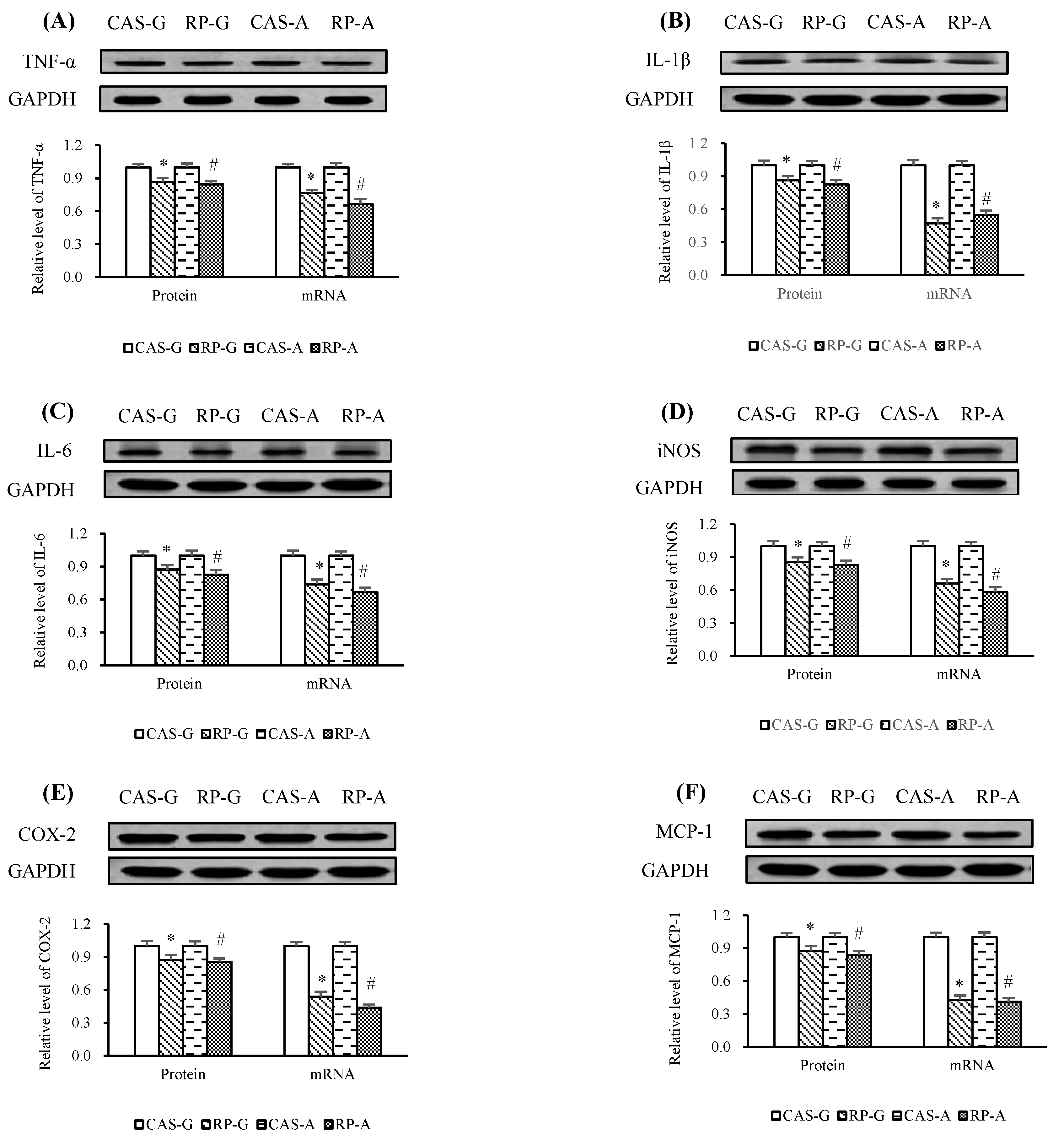
2.7. Expressions of Inflammatory Mediators
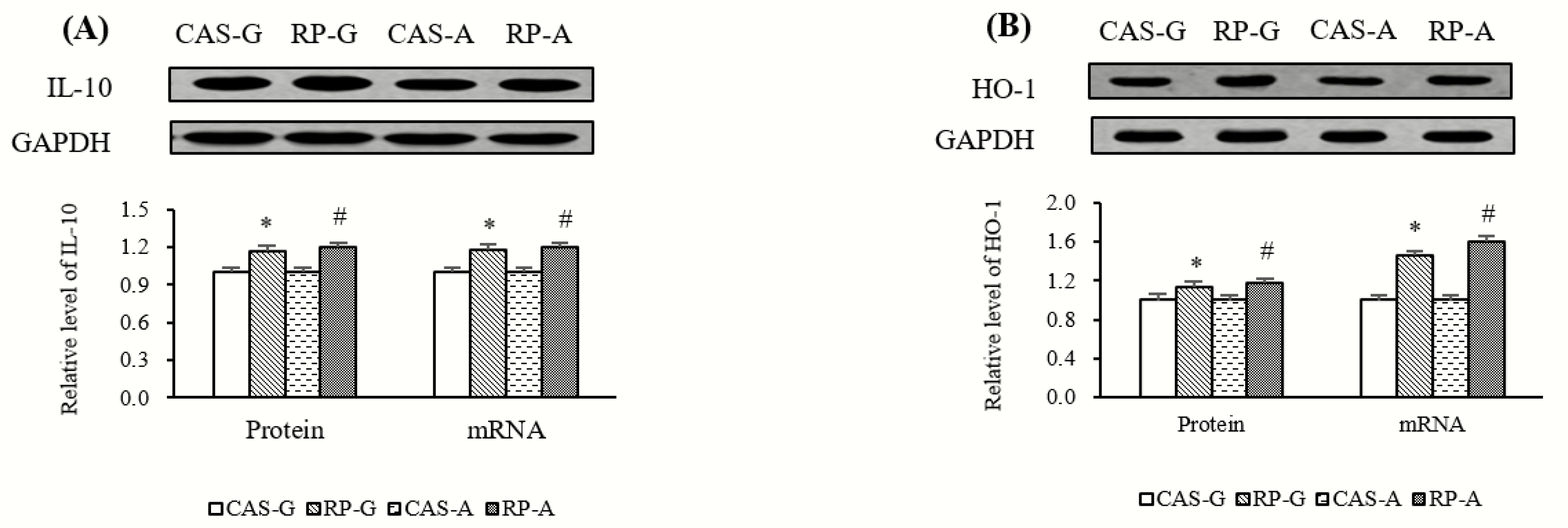
2.8. Expressions of Anti-Inflammatory Mediators
3. Discussion
4. Materials and Methods
4.1. Protein Sources
4.2. Animals Experiments
4.3. Measurement of Plasma Enzyme Activity
4.4. Analyses of Hepatic NO Level and iNOS Activity
4.5. Determination of Hepatic ROS
4.6. Quantitative Real-Time PCR
4.7. Western Blotting Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, S.; Kitamura, M. Bidirectional regulation of NF-κB by reactive oxygen species: A role of unfolded protein response. Free Radic. Biol. Med. 2013, 65, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, J.; Xu, T.; Qiu, W.; Zhang, Y.; Zhang, L.; Xu, F.; Liu, H. Rice protein extracted by different methods affects cholesterol metabolism in rats due to its lower digestibility. Int. J. Mol. Sci. 2011, 12, 7594–7608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Yang, L.; Yang, H.-K.; Sun, S.-H.; Liu, H.-B.; Wu, Q.; Chen, J.-H.; Zhuang, T.-C. Rice protein regulates HDL metabolism-related gene expression and enzyme activity in adult rats. Food Biosci. 2014, 8, 1–7. [Google Scholar] [CrossRef]
- Yang, L.; Chen, J.; Zhang, H.; Qiu, W.; Liu, Q.; Peng, X.; Li, Y.; Yang, H. Alikali treatment affects in vitro digestibility and bile acid binding activity of rice protein due to varying its ratio of arginine to lysine. Food Chem. 2012, 132, 925–930. [Google Scholar] [CrossRef]
- Yang, L.; Chen, J.; Xu, T.; Nie, M.; Yang, H. Hypocholesterolemic efect of rice protein is due to regulating hepatic cholesterol metabolism in adult rats. Gene 2013, 512, 470–476. [Google Scholar] [CrossRef]
- Cai, J.; Yang, L.; He, H.; Xu, T.; Liu, H.; Wu, Q.; Ma, Y.; Liu, Q.; Nie, M. Antioxidant capacity responsible for a hypocholesterolemia is independent of dietary cholesterol in adult rats fed rice protein. Gene 2014, 533, 57–66. [Google Scholar] [CrossRef]
- Yang, L.; Chen, J.; Xu, T.; Zhou, A.; Yang, H. Rice protein improves oxidative stress by regulating glutathione metabolism and attenuating oxidative damage to lipids and proteins in rats. Life Sci. 2012, 91, 389–394. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Li, H.; Liang, M.; Yang, L. In vitro antioxidant activity of rice protein affected by alkaline degree and gastrointestinal protease digestion. J. Sci. Food Agric. 2016, 96, 4940–4950. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Li, H.; Yang, L. Rice proteins, extracted by alkali and α-amylase, differently affect in vitro antioxidant activity. Food Chem. 2016, 206, 137–145. [Google Scholar] [CrossRef]
- Li, H.; He, H.; Wang, Z.; Cai, J.; Sun, B.; Wu, Q.; Zhang, Y.; Zhou, G.; Yang, L. Rice protein suppresses ROS generation and stimulates antioxidant gene expression via Nrf2 activation in adult rats. Gene 2016, 585, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, H.; Liang, M.; Yang, L. Glutelin and prolamin, different components of rice protein, exert differently in vitro antioxidant activities. J. Cereal Sci. 2016, 72, 108–116. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.; Liang, M.; Cai, L.; Yang, L. Methionine augments antioxidant activity of rice protein during gastrointestinal digestion. Int. J. Mol. Sci. 2019, 20, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, L.; Chen, Y.; Zhang, L.; Yu, H.; Xu, Z.; You, H.; Cheng, Y. Rice protein hydrolysates (RPHs) inhibit the LPS-stimulated inflammatory response and phagocytosis in RAW264.7 macrophages by regulating the NF-κB signaling pathway. RSC Adv. 2016, 6, 71295–71304. [Google Scholar] [CrossRef]
- Kriete, A.; Mayo, K. Atypical pathways of NF-κB activation and aging. Exp. Gerontol. 2009, 44, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Yang, J.; Cho, Y.; Woo, H.; Kwon, H.; Kim, D.; Park, M.; Moon, C.; Yeon, M.; Kim, H.; et al. Inhibitory effects of menadione on Helicobacter pylori growth and Helicobacter pylori-induced inflammation via NF-κB inhibition. Int. J. Mol. Sci. 2019, 20, 1169. [Google Scholar] [CrossRef] [Green Version]
- Fei, W.; Xuan, Y.; Jian, X.; Yue, W.; Yuejun, Y.; Yu, J.; Huifang, X.; Yuancai, L.; Yifu, Y.; Xiangwei, Z. One new phenolic compound from Castanea mollissima shells and its suppression of HepatomaCell proliferation and inflammation by inhibiting NF-κB pathway. Int. J. Mol. Sci. 2019, 20, 466. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Xie, W.; Luo, Y.; Lu, S.; Dai, Z.; Wang, R.; Sun, G.; Sun, X. Protective effects of total saponins of Aralia elata (Miq.) on endothelial cell injury induced by TNF-α via modulation of the PI3K/Akt and NF-κB signalling pathways. Int. J. Mol. Sci. 2019, 20, 36. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, Y.; Lee, H.; Tsao, S.; Lu, M.; Yang, S. Monocyte chemoattractant protein-1, a possible biomarker of multiorgan failure and mortality in ventilator-associated pneumonia. Int. J. Mol. Sci. 2019, 20, 2218. [Google Scholar] [CrossRef] [Green Version]
- Salminen, A.; Huuskonen, J.; Ojala, J.; Kauppinen, A.; Kaarniranta, K.; Suuronen, T. Activation of innate immunity system during aging: NF-κB signaling is the molecular culprit of inflamm-aging. Ageing Res. Rev. 2008, 7, 83–105. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K. Insulin/IGF-1 paradox of aging: Regulating via AKT/IKK/NF-κB signaling. Cell Signal. 2010, 22, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Mu, K.; Yu, S.; Kitts, D.D. The role of nitric oxide in regulating intestinal redox status and intestinal epithelial cell functionality. Int. J. Mol. Sci. 2019, 20, 1755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Wang, G.; Li, C.; Zhang, M.; Zhao, H.; Sheng, J.; Shi, W. Pu-erh tea reduces nitric oxide levels in rats by inhibiting inducible nitric oxide synthase expression through Toll-like receptor 4. Int. J. Mol. Sci. 2012, 13, 7174–7185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, M.; Li, H.; Wang, Z.; Cai, L.; Yang, L. Rice protein reduces DNA damage by activating the p53 pathway and stimulating endogenous antioxidant response in growing and adult rats. J. Sci. Food Agric. 2019, 99, 6097–6107. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, X.; Liu, Z.; Peng, Y.; Qiu, Y. Interleukin-10 protection against lipopolysaccharide-induced neuro-inflammation and neurotoxicity in ventral mesencephalic cultures. Int. J. Mol. Sci. 2016, 17, 25. [Google Scholar] [CrossRef] [Green Version]
- Kuan, Y.; Huang, F.; Li, Y.; Chang, Y. Proinflammatory activation of macrophages by bisphenol A-glycidyl-methacrylate involved NFκB activation via PI3K/Akt pathway. Food Chem. Toxicol. 2012, 50, 4003–4009. [Google Scholar] [CrossRef]
- Madrid, L.V.; Mayo, M.W.; Reuther, J.Y.; Baldwin, A.S., Jr. Akt stimulates the transactivation potential of the RelA/p65 subunit of NF-κB through utilization of the IκB kinase and activation of the mitogen-activated protein kinase p38. J. Biol. Chem. 2001, 276, 18934–18940. [Google Scholar] [CrossRef] [Green Version]
- Majumder, K.; Mine, Y.; Wu, J. The potential of food protein-derived anti-inflammatory peptides against various chronic inflammatory diseases. J. Sci. Food Agric. 2016, 96, 2303–2311. [Google Scholar] [CrossRef]
- Liang, M.; Wang, Z.; Li, H.; Cai, L.; Pan, J.; He, H.; Wu, Q.; Tang, Y.; Ma, J.; Yang, L. l-Arginine induces antioxidant response to prevent oxidative stress via stimulation of glutathione synthesis and activation of Nrf2 pathway. Food Chem. Toxicol. 2018, 115, 315–328. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, M.; Li, H.; Cai, L.; He, H.; Wu, Q.; Yang, L. l-Methionine activates Nrf2-ARE pathway to induce endogenous antioxidant activity for depressing ROS-derived oxidative stress in growing rats. J. Sci. Food Agric. 2019, 99, 4849–4862. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Li, W.; Go, Y.; Oh, Y. Atractylodis rhizoma alba attenuates neuroinflammation in BV2 microglia upon LPS stimulation by inducing HO-1 activity and inhibiting NF-κB and MAPK. Int. J. Mol. Sci. 2019, 20, 4015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bronte, V.; Zanovello, P. Regulation of immune responses by l-arginine metabolism. Nat. Rev. Immunol. 2005, 5, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Suliburska, J.; Bogdanskj, P.; Krejpcio, Z.; Pupek-Musialik, D.; Jablecka, A. The effects of l-arginine, alone and combined with vitamin C, on mineral status in relation to its antidiabetic, anti-inflammatory, and antioxidant properties in male rats on a high-fat diet. Biol. Trace Elem. Res. 2014, 157, 67–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salminen, A.; Kaarniranta, K. Glycolysis links p53 function with NF-κB signaling: Impact on cancer and aging process. J. Cell Physiol. 2010, 224, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K. Genetics vs. entropy: Longevity factors suppress the NF-κB-driven entropic aging process. Ageing Res. Rev. 2010, 9, 298–314. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition Ad Hoc Writing Committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]


| Gene | Forward | Reverse |
|---|---|---|
| GAPDH | ACAGCAACAGGGTGGTGGAC | TTTGAGGGTGCAGCGAACTT |
| NF-κB1 | TATGGGCAGGATGGACCTA | TCAGAGCCAAGAAAGGAAGC |
| RelA | TGTGAACCAATTCGCCGAGAAGG | CTCAGCCAGCCAGTGCTTGTC |
| IκBα | GAAGGACGAGGATTACGAGCAGATG | ATGGTCAGTGTCTTCTCTTCATGGATG |
| Akt | ACTCATTCCAGACCCACGAC | AGCCCGAAGTCCGTTATCTT |
| PI3K | TATTGCGAGGGAAACGAGAT | CCAGGGAGGTGTGTTGGTAA |
| TNF-α | TGCCTCAGCCTCTTCTCATT | GCTTGGTGGTTTGCTACGAC |
| IL-1β | TCACAGCAGCATCTCGACAA | GGTCCTCATCCTGGAAGCTC |
| IL-6 | TCCGTTTCTACCTGGAGTTTG | GTTGGATGGTCTTGGTCCTT |
| iNOS | GATGTGCTGCCTCTGGTCCT | GAGCTCCTGGAACCACTCGT |
| COX-2 | AGCGACTGTTCCAAACCAGC | CCTCTTGGCGAGGGAGATGG |
| MCP-1 | GGCCTGTTGTTCACAGTTGC | GTTCTCCAGCCGACTCATTG |
| IL-10 | GCACTGCTATGTTGCCTGCT | TCAGCTCTCGGAGCATGTG |
| HO-1 | GCCCTGGAAGAGGAGATAGAG | TAGTGCTGTGTGGCTGGTGT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Liang, M.; Li, H.; Cai, L.; Yang, L. Rice Protein Exerts Anti-Inflammatory Effect in Growing and Adult Rats via Suppressing NF-κB Pathway. Int. J. Mol. Sci. 2019, 20, 6164. https://doi.org/10.3390/ijms20246164
Wang Z, Liang M, Li H, Cai L, Yang L. Rice Protein Exerts Anti-Inflammatory Effect in Growing and Adult Rats via Suppressing NF-κB Pathway. International Journal of Molecular Sciences. 2019; 20(24):6164. https://doi.org/10.3390/ijms20246164
Chicago/Turabian StyleWang, Zhengxuan, Mingcai Liang, Hui Li, Liang Cai, and Lin Yang. 2019. "Rice Protein Exerts Anti-Inflammatory Effect in Growing and Adult Rats via Suppressing NF-κB Pathway" International Journal of Molecular Sciences 20, no. 24: 6164. https://doi.org/10.3390/ijms20246164





