Developing Gold Nanoparticles-Conjugated Aflatoxin B1 Antifungal Strips
Abstract
:1. Introduction
2. Results
2.1. H-NMR Spectra of AuNP Synthesis
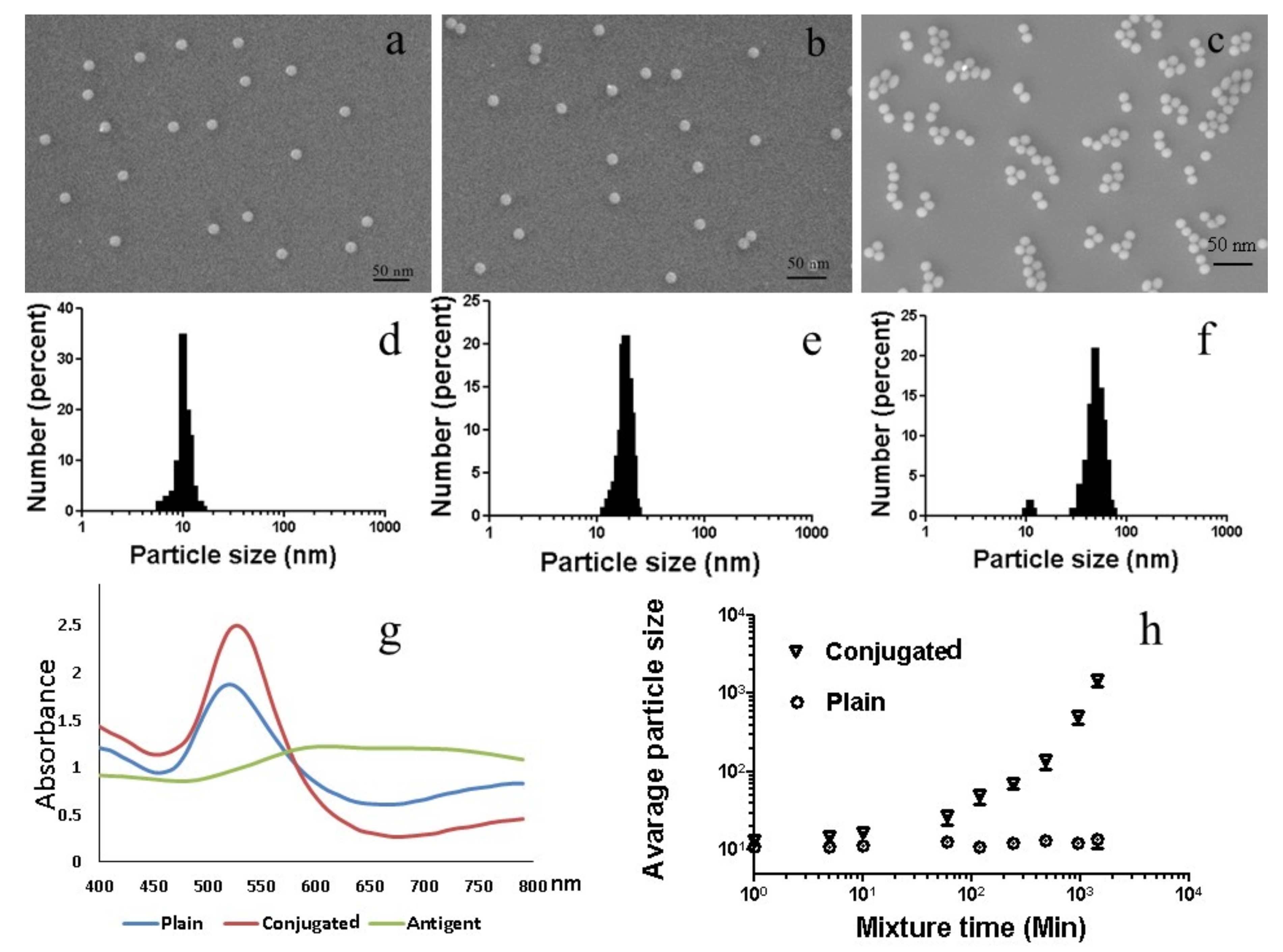
2.2. Confirmation of Physical Characteristics of AuNPs
2.3. AFB1 antibody–BSA–AuNPs in 96-well Plate
2.4. Comparison between Strips and HPLC for AFB1 Detection in Food Samples
3. Discussion
3.1. Confirming Conjugation of AuNPs Using TLC, Gel Electrophoresis and NMR
3.2. Define the LOD of Conjugated AuNPs in 96-Well Plate
3.3. Comparison between HPLC and Immunochromatographic Strip for the Detection of AFLs
4. Materials and Methods
4.1. Material
4.2. Synthesis of AFB1-conjugated AuNPs
4.3. NMR
4.4. Thin layer Chromatography (TLC)
4.5. Characterisation of AuNPs
4.6. SEM of AuNPs
4.7. Layout of AuNPs and Concentration of Antigen in 96-well Plates
4.8. HPLC
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AFLs/AFL | Aflatoxin(s) |
| AFB1 | Aflatoxin B1 |
| AuNPs | Gold nanoparticles |
| LFIAs | lateral flow immunochromatographic assays |
| SERS | Surface enhanced ramen-scattering |
| POC | Point of care |
| TLC | Thin layer chromatography |
| HPLC | High performance liquid chromatography |
| NMR | Nuclear magnetic resonance |
| Ppt | Parts per trillion |
| BSA | Bovine serum albumin |
| SPR | Surface plasmon resonance |
| NHS | N-hydroxysuccinimide |
| EDC | 1-Ethyl3-(3-dimethylaminopropyl)carbodiimide |
| DCC | N,N’- Dicyclohexylcarbodiimide |
| TFA | Trifluoroacetic |
| CMO | Carboxymethyloxime |
References
- Montesano, R.; Hainaut, P.; Wild, C. Hepatocellular carcinoma: From gene to public health. J. Natl. Cancer Inst. 1997, 89, 1844–1851. [Google Scholar] [CrossRef] [Green Version]
- Eaton, D.L.; Gallagher, E.P. Mechanisms of aflatoxin carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 1994, 34, 135–172. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.H.; Bosch, F.X.; Troxell, T.C.; Bolger, P.M. Reducing liver cancer—Global control of aflatoxin. Science 1999, 286, 2453–2454. [Google Scholar] [CrossRef] [PubMed]
- Wogan, G.; Goldblatt, L. Aflatoxin–Scientific Background, Control and Implications; Academic Press: New York, NY, USA; London, UK, 1969; pp. 151–186. [Google Scholar]
- Ingenbleek, L.; Sulyok, M.; Adegboye, A.; Hossou, S.E.; Koné, A.Z.; Oyedele, A.D.; Kisito, C.S.K.; Dembélé, Y.K.; Eyangoh, S.; Verger, P. Regional sub-saharan africa total diet study in benin, cameroon, mali and nigeria reveals the presence of 164 mycotoxins and other secondary metabolites in foods. Toxins 2019, 11, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battilani, P.; Toscano, P.; Van der Fels-Klerx, H.; Moretti, A.; Leggieri, M.C.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B 1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brysse, K.; Oreskes, N.; O’Reilly, J.; Oppenheimer, M. Climate change prediction: Erring on the side of least drama? Glob. Environ. Chang. 2013, 23, 327–337. [Google Scholar] [CrossRef]
- Argentino, C.A. Anexo Mercosur, en Codigo Alimentario Argentino Actualizado; De La Canal & Asoc. SRL: Buenos Aires, Argentina, 1995; p. 26. [Google Scholar]
- Burdaspal, P.; Legarda, T.M. Estudio de propección sobre” aflatoxinas en cacahuetes y pistachos”. Comparación con datos publicados en 1989 en España. Aliment. Rev. Tecnol. Hig. Aliment. 1998, 297, 151–156. [Google Scholar]
- Kiarie, G.; Dominguez-Salas, P.; Kang’ethe, S.; Grace, D.; Lindahl, J. Aflatoxin exposure among young children in urban low-income areas of Nairobi and association with child growth. Afr. J. Food Agric. Nutr. Dev. 2016, 16, 10967–10990. [Google Scholar] [CrossRef]
- Kaittanis, C.; Santra, S.; Perez, J.M. Emerging nanotechnology-based strategies for the identification of microbial pathogenesis. Adv. Drug Deliv. Rev. 2010, 62, 408–423. [Google Scholar] [CrossRef] [Green Version]
- Sajid, M.; Kawde, A.-N.; Daud, M. Designs, formats and applications of lateral flow assay: A literature review. J. Saudi Chem. Soc. 2015, 19, 689–705. [Google Scholar] [CrossRef] [Green Version]
- Sanguansri, P.; Augustin, M.A. Nanoscale materials development—A food industry perspective. Trends Food Sci. Technol. 2006, 17, 547–556. [Google Scholar] [CrossRef]
- Delmulle, B.S.; De Saeger, S.M.; Sibanda, L.; Barna-Vetro, I.; Van Peteghem, C.H. Development of an immunoassay-based lateral flow dipstick for the rapid detection of aflatoxin B1 in pig feed. J. Agric. Food Chem. 2005, 53, 3364–3368. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.-Y.; Li, H. Lateral flow immunodipstick for visual detection of aflatoxin B 1 in food using immuno-nanoparticles composed of a silver core and a gold shell. Microchim. Acta 2010, 171, 289–295. [Google Scholar] [CrossRef]
- Anfossi, L.; Baggiani, C.; Giovannoli, C.; D’Arco, G.; Giraudi, G. Lateral-flow immunoassays for mycotoxins and phycotoxins: A review. Anal. Bioanal. Chem. 2013, 405, 467–480. [Google Scholar] [CrossRef]
- Anfossi, L.; D’Arco, G.; Calderara, M.; Baggiani, C.; Giovannoli, C.; Giraudi, G. Development of a quantitative lateral flow immunoassay for the detection of aflatoxins in maize. Food Addit. Contam. 2011, 28, 226–234. [Google Scholar] [CrossRef]
- Shim, W.-B.; Kim, M.J.; Mun, H.; Kim, M.-G. An aptamer-based dipstick assay for the rapid and simple detection of aflatoxin B1. Biosens. Bioelectron. 2014, 62, 288–294. [Google Scholar] [CrossRef]
- Lu, W.; Wang, K.; Xiao, K.; Qin, W.; Hou, Y.; Xu, H.; Yan, X.; Chen, Y.; Cui, D.; He, J. Dual immunomagnetic nanobeads-based lateral flow test strip for simultaneous quantitative detection of carcinoembryonic antigen and neuron specific enolase. Sci. Rep. 2017, 7, 42414. [Google Scholar] [CrossRef]
- Jianchun, M.; Qing, Y.; Wenyuan, Z.; Wangwei, H.; Jianguo, T. Development and study of lateral flow test strip reader based on embedded system. In Proceedings of the IEEE 2011 10th International Conference on Electronic Measurement & Instruments, Chengdu, China, 16–19 August 2011; pp. 201–204. [Google Scholar]
- Hou, Y.; Wang, K.; Xiao, K.; Qin, W.; Lu, W.; Tao, W.; Cui, D. Smartphone-based dual-modality imaging system for quantitative detection of color or fluorescent lateral flow immunochromatographic strips. Nanoscale Res. Lett. 2017, 12, 291. [Google Scholar] [CrossRef]
- Marquina, C.; De Teresa, J.; Serrate, D.; Marzo, J.; Cardoso, F.; Saurel, D.; Cardoso, S.; Freitas, P.; Ibarra, M. GMR sensors and magnetic nanoparticles for immuno-chromatographic assays. J. Magn. Magn. Mater. 2012, 324, 3495–3498. [Google Scholar] [CrossRef] [Green Version]
- Oncescu, V.; O’Dell, D.; Erickson, D. Smartphone based health accessory for colorimetric detection of biomarkers in sweat and saliva. Lab Chip 2013, 13, 3232–3238. [Google Scholar] [CrossRef]
- Feng, S.; Caire, R.; Cortazar, B.; Turan, M.; Wong, A.; Ozcan, A. Immunochromatographic diagnostic test analysis using Google Glass. ACS Nano 2014, 8, 3069–3079. [Google Scholar] [CrossRef] [PubMed]
- You, D.J.; San Park, T.; Yoon, J.-Y. Cell-phone-based measurement of TSH using Mie scatter optimized lateral flow assays. Biosens. Bioelectron. 2013, 40, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Mudanyali, O.; Dimitrov, S.; Sikora, U.; Padmanabhan, S.; Navruz, I.; Ozcan, A. Integrated rapid-diagnostic-test reader platform on a cellphone. Lab Chip 2012, 12, 2678–2686. [Google Scholar] [CrossRef] [Green Version]
- Yeo, S.-J.; Huong, D.T.; Hong, N.N.; Li, C.-Y.; Choi, K.; Yu, K.; Choi, D.-Y.; Chong, C.-K.; Choi, H.S.; Mallik, S.K. Rapid and quantitative detection of zoonotic influenza A virus infection utilizing coumarin-derived dendrimer-based fluorescent immunochromatographic strip test (FICT). Theranostics 2014, 4, 1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Wang, K.; Zhang, J.; Qin, W.; Yan, X.; Shen, G.; Gao, G.; Pan, F.; Cui, D. Simultaneous quantitative detection of Helicobacter pylori based on a rapid and sensitive testing platform using quantum dots-labeled immunochromatiographic test strips. Nanoscale Res. Lett. 2016, 11, 62. [Google Scholar] [CrossRef] [Green Version]
- Zangheri, M.; Cevenini, L.; Anfossi, L.; Baggiani, C.; Simoni, P.; Di Nardo, F.; Roda, A. A simple and compact smartphone accessory for quantitative chemiluminescence-based lateral flow immunoassay for salivary cortisol detection. Biosens. Bioelectron. 2015, 64, 63–68. [Google Scholar] [CrossRef]
- Ozkan, H.; Kayhan, O.S. A novel automatic rapid diagnostic test reader platform. Comput. Math. Methods Med. 2016, 2016, 7498217. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.; Patnaik, R.; Kuhlmann, K.; Rai, A.J.; Sia, S.K. Smartphone dongle for simultaneous measurement of hemoglobin concentration and detection of HIV antibodies. Lab Chip 2015, 15, 3514–3520. [Google Scholar] [CrossRef]
- Di Nardo, F.; Baggiani, C.; Giovannoli, C.; Spano, G.; Anfossi, L. Multicolor immunochromatographic strip test based on gold nanoparticles for the determination of aflatoxin B1 and fumonisins. Microchim. Acta 2017, 184, 1295–1304. [Google Scholar] [CrossRef]
- Di Nardo, F.; Alladio, E.; Baggiani, C.; Cavalera, S.; Giovannoli, C.; Spano, G.; Anfossi, L. Colour-encoded lateral flow immunoassay for the simultaneous detection of aflatoxin B1 and type-B fumonisins in a single Test line. Talanta 2019, 192, 288–294. [Google Scholar] [CrossRef]
- Aizpurua, J.; Hillenbrand, R. Localized surface plasmons: Basics and applications in field-enhanced spectroscopy. In Plasmonics; Springer: Berlin/Heidelberg, Germany, 2012; pp. 151–176. [Google Scholar]
- Sojinrin, T.; Conde, J.; Liu, K.; Curtin, J.; Byrne, H.J.; Cui, D.; Tian, F. Plasmonic gold nanoparticles for detection of fungi and human cutaneous fungal infections. Anal. Bioanal. Chem. 2017, 409, 4647–4658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cervino, C.; Knopp, D.; Weller, M.; Niessner, R. Novel aflatoxin derivatives and protein conjugates. Molecules 2007, 12, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Phong, T.Q.; Phạm, N.T.; Nguyen, H.D.; Nguyen, A.T.N. Efficient conjugation of aflatoxin b1 with bovine serum albumin applying for development of aflatoxin b1 quick test. Vietnam J. Sci. Technol. 2018, 56, 190. [Google Scholar] [CrossRef] [Green Version]
- Asis, R.; Paola, R.D.D.; Aldao, M.A. Determination of aflatoxin B 1 in highly contaminated peanut samples using HPLC and ELISA. Food Agric. Immunol. 2002, 14, 201–208. [Google Scholar] [CrossRef]
- Patey, A.; Sharman, M.; Gilbert, J. Determination of aflatoxin levels in peanut butter using HPLC and ELISA procedures: Inter-laboratory comparison. Mycotoxin Res. 1990, 6, 2–6. [Google Scholar] [CrossRef]
- Azer, M.; Cooper, C. Determination of aflatoxins in foods using HPLC and a commercial ELISA system. J. Food Prot. 1991, 54, 291–294. [Google Scholar] [CrossRef]
- Moon, J.; Kim, G.; Lee, S. A gold nanoparticle and aflatoxin B1-BSA conjugates based lateral flow assay method for the analysis of aflatoxin B1. Materials 2012, 5, 634–643. [Google Scholar] [CrossRef] [Green Version]
- Asharani, P.; Lianwu, Y.; Gong, Z.; Valiyaveettil, S. Comparison of the toxicity of silver, gold and platinum nanoparticles in developing zebrafish embryos. Nanotoxicology 2011, 5, 43–54. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A.; Rao, R.A. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnology 2018, 16, 14. [Google Scholar] [CrossRef]
- Ahamed, M.; Karns, M.; Goodson, M.; Rowe, J.; Hussain, S.M.; Schlager, J.J.; Hong, Y. DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol. Appl. Pharmacol. 2008, 233, 404–410. [Google Scholar] [CrossRef]
- Liu, K.; He, Z.; Byrne, H.; Curtin, J.; Tian, F. Investigating the Role of Gold Nanoparticle Shape and Size in Their Toxicities to Fungi. Int. J. Environ. Res. Public Health 2018, 15, 998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, F.; Clift, M.J.; Casey, A.; Del Pino, P.; Pelaz, B.; Conde, J.; Byrne, H.J.; Rothen-Rutishauser, B.; Estrada, G.; De La Fuente, J.M. Investigating the role of shape on the biological impact of gold nanoparticles in vitro. Nanomedicine 2015, 10, 2643–2657. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| 1H | BSA | AFB1 Antibody | AFB1 Antibody–BSA–AuNPs ppm |
|---|---|---|---|
| a | 0.8 (J = 0.6 Hz) | 0.8 (J = 2 Hz) | |
| b | 1.1 (J = 1.2 Hz) | 1.1 (J = 1.2 Hz) | |
| c | 1.25 (J = 0.8 Hz) | 1.25 (J = 2 Hz) | |
| d | 2.55 (J = 2 Hz) | 2.55 (J = 0.2 Hz) | |
| e | 2.69 (J = 2 Hz) | 2.64 (J = 2.1 Hz) | |
| f | 3.34 (J = 2 Hz) | 3.34 (J = 2 Hz) | |
| g | 3.97 (J = 6 Hz) | 3.95 (J = 6 Hz) | |
| h | 4.05 (J = 2 Hz) | ||
| i | 4.22 (J = 2 Hz) | ||
| j | 4.76 (J = 2.1 Hz) | 4.76 (J = 2.1 Hz) | |
| k | 5.47 (J = 2.4 Hz) | 5.47 (J = 2 Hz) | |
| l | 6.42 (J = 2.7 Hz) | 6.33 (J = 2.4 Hz) | |
| m | 6.49 (J = 2 Hz) | 6.52 (J = 2.1 Hz) | |
| n | 6.80 (J = 2.3 Hz) |
| Sample | LFIAs | HPLC (ng/g) | |
|---|---|---|---|
| Strip Visual (+,−) | Smartphone (ng/g) | ||
| Peanut | −,−,− | 5.38 ± 0.05 | 5.70 ± 0.55 |
| −,−,− | 4.38 ± 0.15 | 4.07 ± 0.83 | |
| −,−,− | 5.17 ± 0.12 | 5.05 ± 1.19 | |
| +,+,+ | Over10 | 42.53 ± 7.76 | |
| −,−,− | 5.18 ± 0.04 | 5.28 ± 1.36 | |
| Bread | −,−,− | 2.03 ± 0.44 | 2.02 ± 0.56 |
| −,−,− | 5.18 ± 0.04 | 5.21 ± 0.23 | |
| +,+,+ | Over10 | 27.67 ± 6.43 | |
| −,−,− | 4.01 ± 0.30 | 4.05 ± 0.61 | |
| −,−,− | 5.59 ± 0.06 | 5.71 ± 0.55 | |
| Corn | +,+,+ | Over10 | 19.16 ± 4.25 |
| −,−,− | 0.37 ± 0.01 | 0.31 ± 0.15 | |
| −,−,− | 2.27 ± 0.97 | 2.50 ± 0.54 | |
| −,−,− | 4.79 ± 0.29 | 5.03 ± 0.66 | |
| −,−,− | 5.38 ± 0.53 | 5.32 ± 0.99 | |
| Rice | −,−,− | 2.94 ± 0.81 | 3.00 ± 1.15 |
| −,−,− | 0.42 ± 0.09 | 0.36 ± 0.20 | |
| −,−,− | 0.45 ± 0.16 | 0.49 ± 0.22 | |
| −,−,− | 4.46 ± 0.08 | 4.86 ± 0.95 | |
| +,+,+ | Over10 | 15.49 ± 5.12 | |
| Author | Strip Type | Materials | Sample | Limit of Detection | Time | Image | Ref. |
|---|---|---|---|---|---|---|---|
| Moon et al. | one-dot | 40 nm AuNPs | buffer | 10 μg/mL | 10 min | [41] | |
| Phong et al. | line | AuNPs | buffer | ND | ND | [37] | |
| Delmulle et al. | line | 40 nm AuNPs | pig feed | 5 μg/kg | 10 min | [14] | |
| Liao and Li | line | 20 nm silver core AuNPs | rice, wheat, sunflower, cotton, chillies and almond | 0.3 ng/mL | 10 min | [15] | |
| Shim et al. | Dot aptamer | (Cy5)-modified a single strand-DNA | buffer/corn | 0.1/0.3 ng/g | >30 min | Fluorescent apparatus | [18] |
| Anfossi et al. | line | 40 nm AuNPs | maize | 1 ng/g | 10 min | OpticSlim + Labtop | [17] |
| Di Nardo | Single line | 30 nm and 72 nm AuNPs | maize | 2 ng/g AFB1 1 μg/g fumonisins | 10 min | [32] | |
| Di Nardo | Single line | 30 nm and 75 nm AuNPs | wheat, pasta, pastry | 1 and 50 ng/g | 10 min | smartphone | [33] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sojinrin, T.; Liu, K.; Wang, K.; Cui, D.; J. Byrne, H.; Curtin, J.F.; Tian, F. Developing Gold Nanoparticles-Conjugated Aflatoxin B1 Antifungal Strips. Int. J. Mol. Sci. 2019, 20, 6260. https://doi.org/10.3390/ijms20246260
Sojinrin T, Liu K, Wang K, Cui D, J. Byrne H, Curtin JF, Tian F. Developing Gold Nanoparticles-Conjugated Aflatoxin B1 Antifungal Strips. International Journal of Molecular Sciences. 2019; 20(24):6260. https://doi.org/10.3390/ijms20246260
Chicago/Turabian StyleSojinrin, Tobiloba, Kangze Liu, Kan Wang, Daxiang Cui, Hugh J. Byrne, James F. Curtin, and Furong Tian. 2019. "Developing Gold Nanoparticles-Conjugated Aflatoxin B1 Antifungal Strips" International Journal of Molecular Sciences 20, no. 24: 6260. https://doi.org/10.3390/ijms20246260
APA StyleSojinrin, T., Liu, K., Wang, K., Cui, D., J. Byrne, H., Curtin, J. F., & Tian, F. (2019). Developing Gold Nanoparticles-Conjugated Aflatoxin B1 Antifungal Strips. International Journal of Molecular Sciences, 20(24), 6260. https://doi.org/10.3390/ijms20246260









