Biofilms Positively Contribute to Bacillus amyloliquefaciens 54-induced Drought Tolerance in Tomato Plants
Abstract
:1. Introduction
2. Results
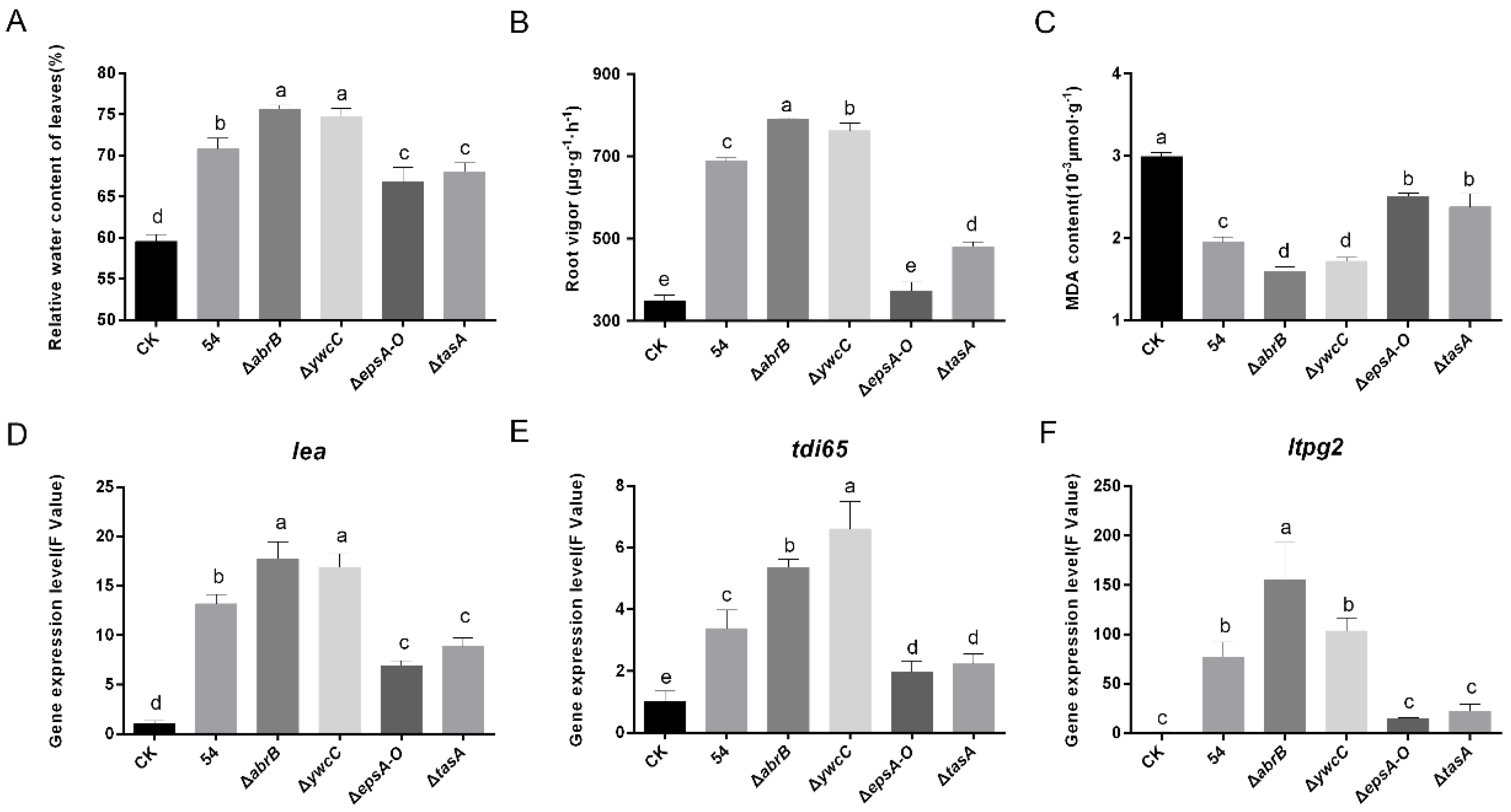
2.1. Beneficial Rhizobacteria Significantly Improve Drought Tolerance
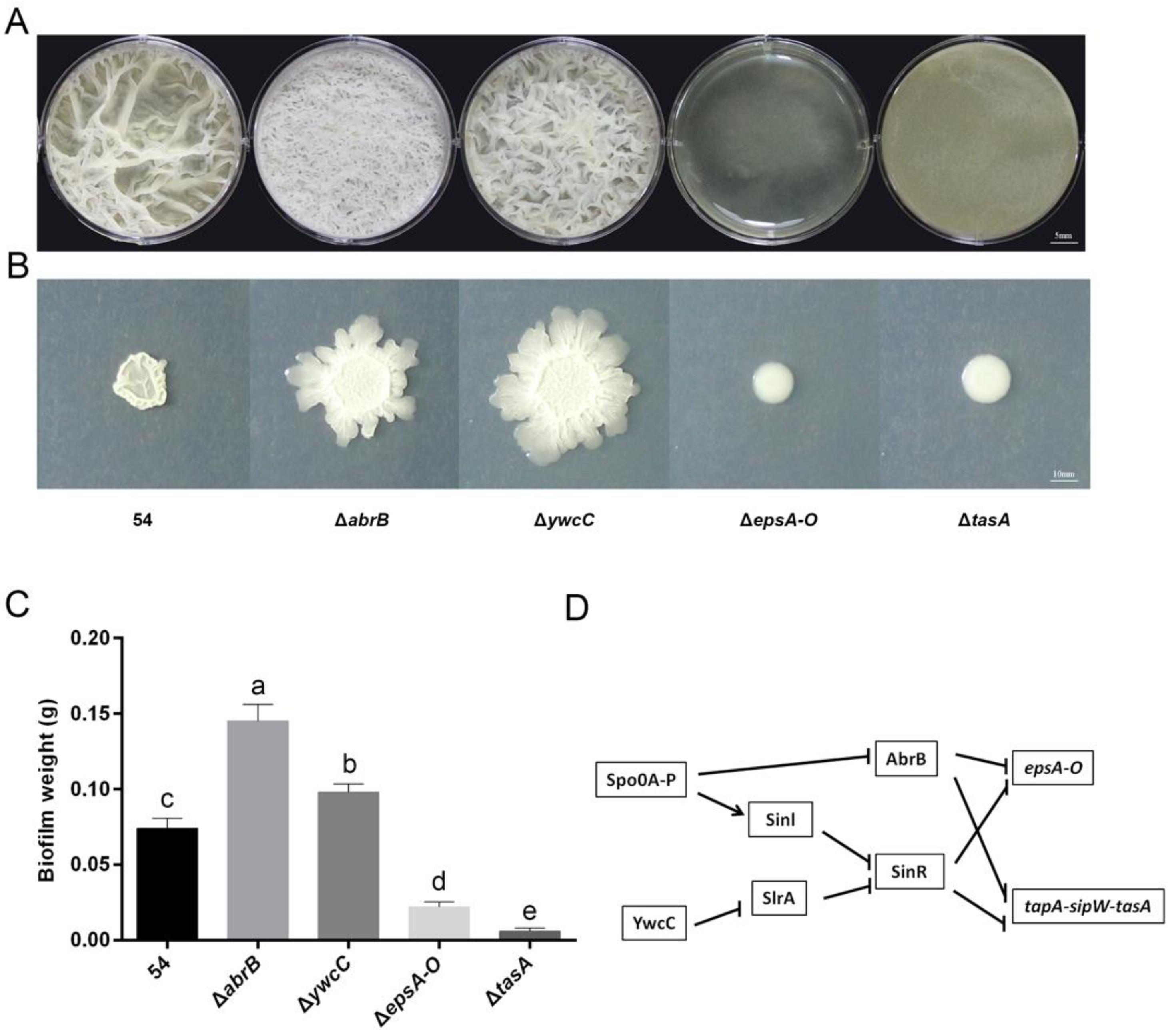
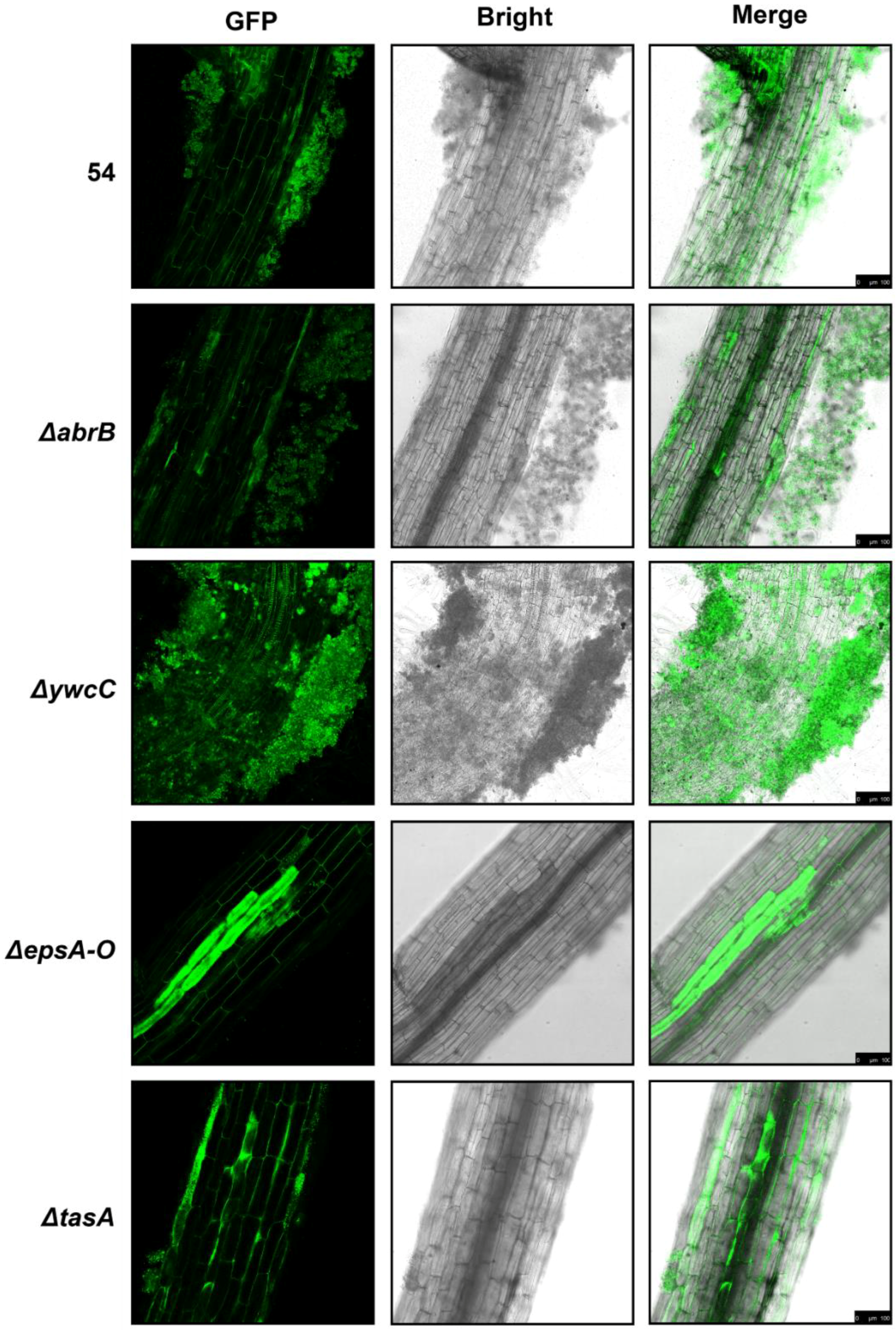
2.2. B. Amyloliquefaciens 54 Forms Robust Biofilms Both in LBGM Medium and on The Surface of Tomato Roots
2.3. Biofilm Formation Contributed to B. Amyloliquefaciens 54-Induced Drought Tolerance
2.4. Biofilm Formation Was Conductive to B. Amyloliquefaciens 54-Regulated Stress-Related Genes
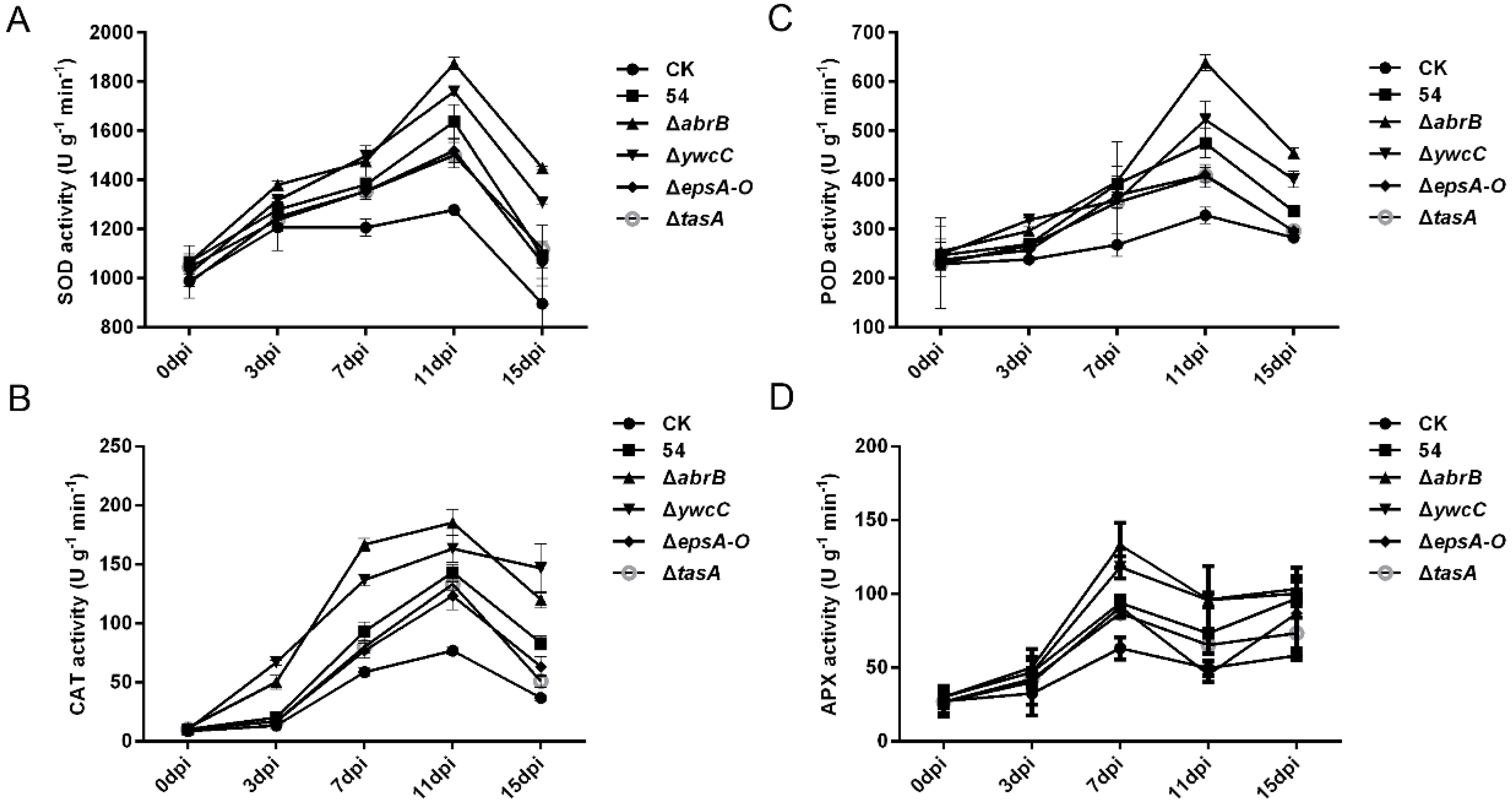
2.5. Biofilm Formation Was Involved in B. Amyloliquefaciens 54-Mediated Antioxidant Enzyme Activities
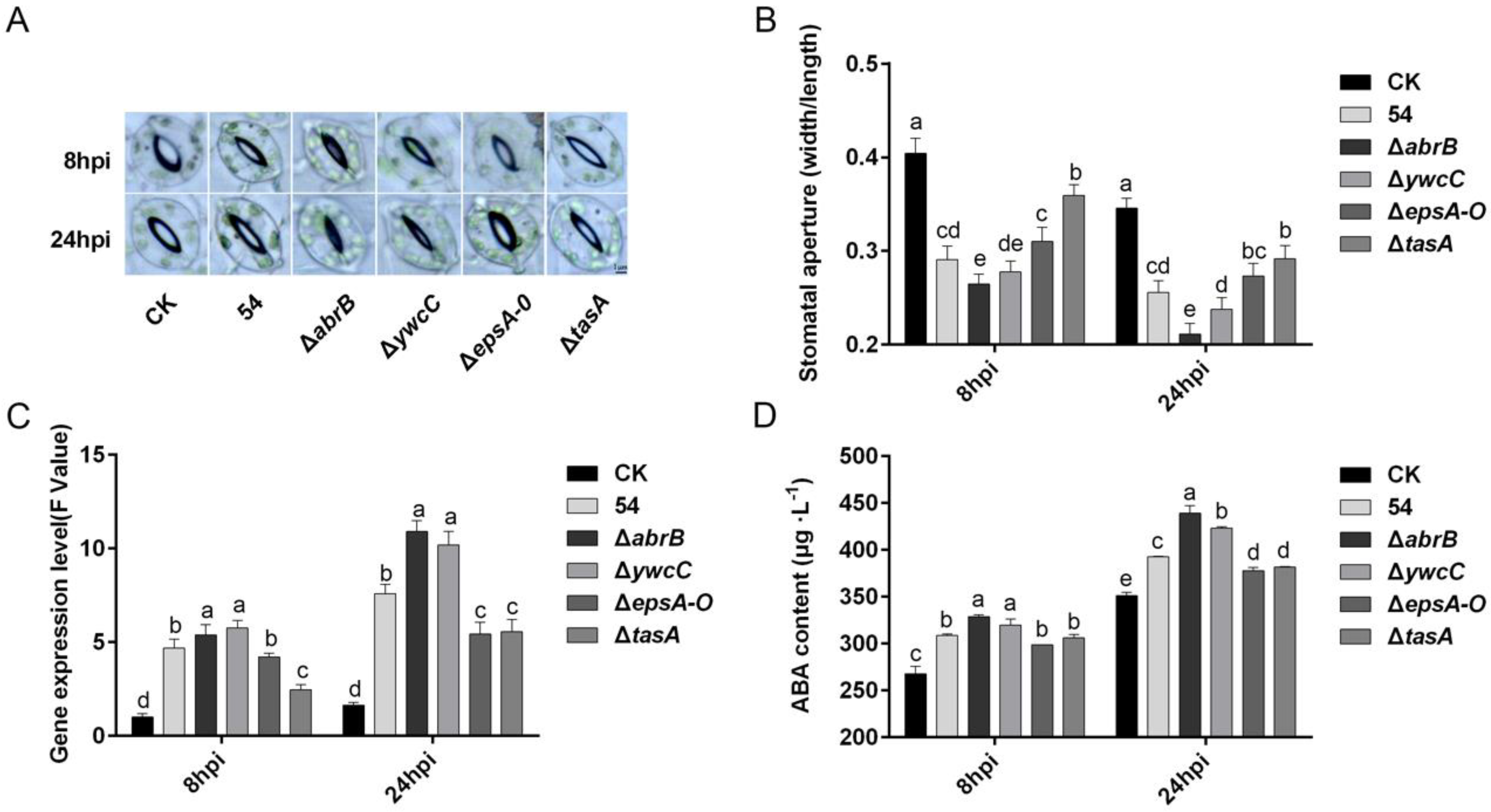
2.6. Biofilm Formation Was Involved in B. Amyloliquefaciens 54-Induced Stomatal Closure in Tomato Plants
2.7. ABA Is Required for B. Amyloliquefaciens 54-Mediated Stomatal Closure
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. Plant Growth and Treatments
4.3. Biofilm Formation Assay
4.4. Biofilm Formation of B. Amyloliquefaciens 54 and Its Biofilm Mutants on Tomato Roots
4.5. Stomata Assays
4.6. Leaf ABA Content Determination
4.7. RNA Isolation, RT-PCR, and qRT-PCR
4.8. Analysis of Antioxidant Enzyme Activities, Malondialdehyde (MDA), Relative Water Content, and Root Vigor
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Misra, A.K. Climate change and challenges of water and food security. Int. J. Sustain. Built Environ. 2014, 3, 153–165. [Google Scholar] [CrossRef] [Green Version]
- Finkelstein, R.R.; Gibson, S.I. ABA and sugar interactions regulating development: Cross-talk or voices in a crowd? Curr. Opin. Plant Biol. 2002, 5, 26–32. [Google Scholar] [CrossRef]
- Loukehaich, R.; Wang, T.; Ouyang, B.; Ziaf, K.; Li, H.; Zhang, J.; Lu, Y.; Ye, Z. SpUSP, an annexin-interacting universal stress protein, enhances drought tolerance in tomato. J. Exp. Bot. 2012, 63, 5593–5606. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.J.; Yang, W.; Wang, C.; Gu, C.; Niu, D.D.; Liu, H.X.; Wang, Y.P.; Guo, J.H. Induction of drought tolerance in cucumber plants by a consortium of three plant growth-promoting rhizobacterium strains. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [Green Version]
- Rai, G.K.; Rai, N.P.; Rathaur, S.; Kumar, S.; Singh, M. Expression of rd29A::ATDREB1A/CBF3 in tomato alleviates drought-induced oxidative stress by regulating key enzymatic and non-enzymatic antioxidants. Plant Physiol. Biochem. 2013, 69, 90–100. [Google Scholar] [CrossRef]
- Sun, W.H.; Wang, Y.; He, H.G.; Li, X.; Song, W.; Du, B.; Meng, Q.W. Reduction of methylviologen-mediated oxidative stress tolerance in antisense transgenic tobacco seedlings through restricted expression of StAPX. J. Zhejiang Univ. Sci. B 2013, 14, 578–585. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Meng, X.; Yang, D.; Ma, N.; Wang, G.; Meng, Q. Overexpression of tomato GDP-l-galactose phosphorylase gene in tobacco improves tolerance to chilling stress. Plant Cell Rep. 2014, 33, 1441–1451. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef]
- Liu, F.; Xing, S.; Ma, H.; Du, Z.; Ma, B. Plant growth-promoting rhizobacteria affect the growth and nutrient uptake of fraxinus americana, container seedlings. Appl. Microbiol. Biotechnol. 2013, 97, 4617–4625. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, S.D. Induction of drought stress resistance by multi-functional PGPR Bacillus licheniformis k11 in pepper. Plant Pathol. J. 2013, 29, 201–208. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; Skz, A. Multifunctional Pseudomonas putida strain fbkv2 from arid rhizosphere soil and its growth promotional effects on maize under drought stress. Rhizosphere 2016. [Google Scholar] [CrossRef]
- Hasna, H.S.; Hossain, K.; Mohd, S.H. Plant growth-promoting rhizobacteria enhance salinity stress tolerance in okra through ROS-scavenging enzymes. BioMed Res. Int. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Gayathri, I.; Smith, D.L. Plant growth promoting rhizobacteria in amelioration of salinity stress: A systems biology perspective. Front. Plant Sci. 2017, 8, 1768. [Google Scholar] [CrossRef]
- Ali, S.Z.; Sandhya, V.; Grover, M.; Kishore, N.; Rao, L.V.; Venkateswarlu, B. Pseudomonas sp. strain AKM-P6 enhances tolerance of sorghum seedlings to elevated temperatures. Biol. Fertil. Soils 2009, 46, 45–55. [Google Scholar] [CrossRef]
- Ali, S.Z.; Sandhya, V.; Grover, M.; Linga, V.R.; Bandi, V. Effect of inoculation with a thermotolerant plant growth promoting Pseudomonas putida strain AKMP7 on growth of wheat (Triticum spp.) under heat stress. J. Plant Interact. 2011, 6, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Fan, S.; Jacquard, C.; Sandra, V.; Jean, M.; Fanja, R.; Clément, C.; Barka, E.A.; Dhondt-Cordelier, S.; Vaillant-Gaveau, N. Burkholderia phytofirmans PSJN reduces impact of freezing temperatures on photosynthesis in Arabidopsis thaliana. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Turk, H.; Erdal, S.; Genisel, M.; Atici, O.; Demir, Y.; Yanmis, D. The regulatory effect of melatonin on physiological, biochemical and molecular parameters in cold-stressed wheat seedlings. Plant Growth Regul. 2014, 74, 139–152. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Bakker, P.A.H.M.; Pieterse, C.M.J. Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 1998, 36, 453–483. [Google Scholar] [CrossRef] [Green Version]
- Niu, D.D.; Liu, H.X.; Jiang, C.H.; Wang, Y.P.; Wang, Q.Y.; Jin, H.L.; Guo, J.H. The plant growth-promoting rhizobacterium Bacillus cereus AR156 induces systemic resistance in Arabidopsis thaliana by simultaneously activating salicylate-and jasmonate/ethylene-dependent signaling pathways. Mol. Plant Microbe Interact. 2011, 24, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Niu, D.D.; Wang, C.J.; Guo, Y.H.; Jiang, C.H.; Zhang, W.Z.; Wang, Y.P.; Guo, J.H. The plant growth-promoting rhizobacterium Bacillus cereus AR156 induces resistance in tomato with induction and priming of defence response. Biocontrol Sci. Technol. 2012, 22, 991–1004. [Google Scholar] [CrossRef]
- Maji, S.; Chakrabartty, P.K. Biocontrol of bacterial wilt of tomato caused by Ralstonia Solanacearum by isolates of plant growth promoting rhizobacteria. Aust. J. Crop Sci. 2014, 8, 208–214. [Google Scholar]
- Jiang, C.H.; Liao, M.J.; Wang, H.K.; Zheng, M.Z.; Xu, J.J.; Guo, J.H. Bacillus velezensis, a potential and efficient biocontrol agent in control of pepper gray mold caused by Botrytis cinerea. Biol. Control 2018, 126, 147–157. [Google Scholar] [CrossRef]
- Nie, P.; Li, X.; Wang, S.; Guo, J.; Zhao, H.; Niu, D.D. Induced systemic resistance against Botrytis cinerea by Bacillus cereus AR156 through a JA/ET-and NPR1-dependent signaling pathway and activates PAMP-triggered immunity in Arabidopsis. Front. Plant Sci. 2017, 8, 238. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [Green Version]
- Ryu, C.-M.; Farag, M.A.; Hu, C.-H.; Reddy, M.S.; Wei, H.-X.; Paré, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 100, 4927–4932. [Google Scholar] [CrossRef] [Green Version]
- Farag, M.A.; Ryu, C.-M.; Sumner, L.W.; Paré, P.W. GC–MS SPME profiling of rhizobacterial volatiles reveals prospective inducers of growth promotion and induced systemic resistance in plants. Phytochemistry 2006, 67, 2262–2268. [Google Scholar] [CrossRef]
- Tiwari, S.; Prasad, V.; Chauhan, P.; Lata, C. Bacillus amyloliquefaciens confers tolerance to various abiotic stresses and modulates plant response to phytohormones through osmoprotection and gene expression regulation in Rice. Front. Plant Sci. 2017, 8, 1510. [Google Scholar] [CrossRef] [Green Version]
- Davey, M.E.; O’Toole, G.A. Microbial biofilms: From ecology to molecular genetics. Microbiol. Mol. Biol. R. 2000, 64, 847–867. [Google Scholar] [CrossRef] [Green Version]
- Kasim, W.A.; Gaafar, R.M.; Abou-Ali, R.M.; Omar, M.N.; Hewait, H.M. Effect of biofilm forming plant growth promoting rhizobacteria on salinity tolerance in barley. Ann. Agric. Sci. 2016. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Yan, F.; Chai, Y.; Liu, H.; Kolter, R.; Losick, R.; Guo, J.H. Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation. Environ. Microbiol. 2013, 15, 848–864. [Google Scholar] [CrossRef] [Green Version]
- Shank, E.A.; Klepac-Ceraj, V.; Collado-Torres, L.; Powers, G.E.; Losick, R.; Kolter, R. Interspecies interactions that result in Bacillus subtilis forming biofilms are mediated mainly by members of its own genus. Proc. Natl. Acad. Sci. USA 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beauregard, P.B.; Chai, Y.; Vlamakis, H.; Losick, R.; Kolter, R. Bacillus subtilis biofilm induction by plant polysaccharides. Proc. Natl. Acad. Sci. USA 2013, 110, E1621–E1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar, C.; Eichwald, C.; Eberl, L. Multicellularity in bacteria: From division of labor to biofilm formation. Evol. Transit. Multicell. Life 2015, 79–95. [Google Scholar] [CrossRef]
- Kearns, D.B.; Chu, F.; Branda, S.S.; Kolter, R.; Losick, R. A master regulator for biofilm formation by Bacillus subtilis. Mol. Microbiol. 2005, 55, 739–749. [Google Scholar] [CrossRef]
- Hu, G.; Tylzanowski, P.; Inoue, H.; Veis, A. Targets of the master regulator of biofilm formation in Bacillus subtilis. Mol. Microbiol. 2010, 59, 1216–1228. [Google Scholar] [CrossRef]
- Bai, U.; Mandic-Mulec, I.; Smith, I. SinI modulates the activity of SinR, a developmental switch protein of Bacillus subtilis, by protein-protein interaction. Genes Dev. 1993, 7, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Newman, J.A.; Rodrigues, C.; Lewis, R.J. Molecular basis of the activity of SinR, the master regulator of biofilm formation in Bacillus subtilis. J. Biol. Chem. 2013, 288, 10766–10778. [Google Scholar] [CrossRef] [Green Version]
- Chai, Y.; Norman, T.; Kolter, R.; Losick, R. Evidence that metabolism and chromosome copy number control mutually exclusive cell fates in Bacillus subtilis. EMBO J. 2011, 30, 1402–1413. [Google Scholar] [CrossRef] [Green Version]
- Burbulys, D.; Trach, K.A.; Hoch, J.A. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell 1991, 64, 545–552. [Google Scholar] [CrossRef]
- Ireton, K.; Rudner, D.Z.; Siranosian, K.J.; Grossman, A.D. Integration of multiple developmental signals in Bacillus subtilis through the Spo0A transcription factor. Genes Dev. 1993, 7, 283–294. [Google Scholar] [CrossRef] [Green Version]
- Perego, M.; Hanstein, C.; Welsh, K.M.; Djavakhishvili, T.; Glaser, P.; Hoch, J.A. Multiple protein-aspartate phosphatases provide a mechanism for the integration of diverse signals in the control of development in B. subtilis. Cell 1994, 79, 1047–1055. [Google Scholar] [CrossRef]
- Chu, F.; Kearns, D.B.; Mcloon, A.; Chai, Y.; Kolter, R.; Losick, R. A novel regulatory protein governing biofilm formation in bacillus subtilis. Mol. Microbiol. 2010, 68, 1117–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamon, M.A.; Lazazzera, B.A. The sporulation transcription factor Spo0A is required for biofilm development in Bacillus subtilis. Mol. Microbiol. 2010, 42, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Kolter, R.; Losick, R. Paralogous antirepressors acting on the master regulator for biofilm formation in Bacillus subtilis. Mol. Microbiol. 2010, 74, 876–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morikawa, M. Beneficial biofilm formation by industrial bacteria Bacillus subtilis and related species. J. Biosci. Bioeng. 2006, 101. [Google Scholar] [CrossRef] [Green Version]
- Pandin, C.; Coq, D.L.; Canette, A.; Stéphane, A.; Briandet, R. Should the biofilm mode of life be taken into consideration for microbial biocontrol agents? Microb. Biotechnol. 2017, 10, 719–734. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef]
- Kumar, A.S.; Lakshmanan, V.; Caplan, J.L.; Powell, D.; Czymmek, K.J.; Levia, D.F.; Bais, H.P. Rhizobacteria Bacillus subtilis restricts foliar pathogen entry through stomata. Plant J. 2012, 72, 694. [Google Scholar] [CrossRef]
- Acharya, B.R.; Assmann, S.M. Hormone interactions in stomatal function. Plant Mol. Biol. 2009, 69, 451–462. [Google Scholar] [CrossRef]
- Lim, C.W.; Baek, W.; Jung, J.; Kim, J.H.; Lee, S.C. Function of ABA in stomatal defense against biotic and drought stresses. Int. J. Mol. Sci. 2015, 16, 15251–15270. [Google Scholar] [CrossRef] [Green Version]
- Munemasa, S.; Hauser, F.; Park, J.; Waadt, R.; Brandt, B.; Schroeder, J.I. Mechanisms of abscisic acid-mediated control of stomatal aperture. Curr. Opin. Plant Biol. 2015, 28, 154–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Franklin, M.J.; Williamson, K.S.; Folsom, J.P.; Boegli, L.; James, G.A. Contribution of stress responses to antibiotic tolerance in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2015, 59, 3838–3847. [Google Scholar] [CrossRef] [Green Version]
- Hazan, R.; Que, Y.A.; Maura, D.; Strobel, B.; Majcherczyk, P.A.; Hopper, L.R.; David, J.; Wilbur, D.J.; Hreha, T.N.; Barquera, B.; et al. Auto poisoning of the respiratory chain by a quorum-sensing-regulated molecule favors biofilm formation and antibiotic tolerance. Curr. Biol. 2016, 26, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Timmusk, S.; Kim, S.B.; Nevo, E.; Abd El Daim, I.; Ek, B.; Bergquist, J.; Behers, L. Sfp-type PPTase inactivation promotes bacterial biofilm formation and ability to enhance wheat drought tolerance. Front. Microbiol. 2015, 6, 387. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Liu, S.F.; Yue, L.; Zhao, X.; Zhang, Y.B.; Xie, Z.K.; Wang, R.Y. Epsc involved in the encoding of exopolysaccharides produced by Bacillus amyloliquefaciens FZB42 act to boost the drought tolerance of Arabidopsis thaliana. Int. J. Mol. Sci. 2018, 19, 3795. [Google Scholar] [CrossRef] [Green Version]
- Soltys-Kalina, D.; Plich, J.; Strzelczyk-Żyta, D.; Śliwka, J.; Marczewski, W. The effect of drought stress on the leaf relative water content and tuber yield of a half-sib family of ‘Katahdin’-derived potato cultivars. Breed. Sci. 2016, 66, 328–331. [Google Scholar] [CrossRef] [Green Version]
- Agami, R.A.; Medani, R.A.; Abd El-Mola, I.A.; Taha, R.S. Exogenous application with plant growth promoting rhizobacteria (PGPR) or proline induces stress tolerance in basil plants (Ocimum basilicum L.) exposed to water stress. Int. J. Environ. Agric. Res. 2016, 2, 78. [Google Scholar]
- Su, A.Y.; Niu, S.Q.; Liu, Y.Z.; He, A.L.; Zhao, Q.; Paré, P.; Li, M.F.; Han, Q.Q.; Ali, K.S.; Zhang, J.L. Synergistic effects of Bacillus amyloliquefaciens (GB03) and water retaining agent on drought tolerance of perennial ryegrass. Int. J. Mol. Sci. 2017, 18, 2651. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.F.; Zhu, Y.H.; Sun, H.J. Determination of drought tolerance using root activities in Robinia pseudoacacia ‘Idaho’ transformed with mtl-D gene. For. Stud. China 2006, 8, 75–81. [Google Scholar]
- Huang, S.; Van Aken, O.; Schwarzländer, M.; Belt, K.; Millar, A.H. Roles of mitochondrial reactive oxygen species in cellular signalling and stress response in plants. Plant Physiol. 2016, 171, 1551–1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietz, K.J.; Mittler, R.; Noctor, G. Recent progress in understanding the role of reactive oxygen species in plant cell signaling. Plant Physiol. 2016, 171, 1535–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, B.; Cao, J.; Ge, K.; Li, L. The site of water stress governs the pattern of ABA synthesis and transport in peanut. Sci. Rep. 2016, 6, 32143. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Peng, F.; Xue, X.; You, Q.; Zhang, W.; Wang, T.; Huang, C.H. The growth promotion of two salt-tolerant plant groups with PGPR inoculation: A meta-analysis. Sustainability 2019, 11, 378. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.P.; Jha, P.N. The multifarious PGPR Serratia marcescens CDP-13 augments induced systemic resistance and enhanced salinity tolerance of wheat (Triticum aestivum L.). PLoS ONE 2016, 11, e0155026. [Google Scholar] [CrossRef]
- Ali, S.; Chaudhary, A.; Rizwan, M.; Anwar, H.T.; Adrees, M.; Farid, M.; Irshad, M.K.; Hayat, T.; Anjum, S.A. Alleviation of chromium toxicity by glycinebetaine is related to elevated antioxidant enzymes and suppressed chromium uptake and oxidative stress in wheat (Triticum aestivum L.). Environ. Sci. Pollut. R. 2015, 22, 10669–10678. [Google Scholar] [CrossRef]
- Li, W.; Nguyen, K.H.; Chu, H.D.; Van Ha, C.; Watanabe, Y.; Osakabe, Y.; Leyva-González, M.A.; Sato, M.; Toyooka, K.; Voges, L. The karrikin receptor KAI2 promotes drought resistance in Arabidopsis thaliana. PLoS Genet. 2017, 13, e1007076. [Google Scholar] [CrossRef] [Green Version]
- Bang, S.W.; Lee, D.K.; Jung, H.; Chung, P.J.; Kim, Y.S.; Choi, Y.D.; Suh, J.W.; Kim, J.K. Overexpression of Os TF 1L, a rice HD-Zip transcription factor, promotes lignin biosynthesis and stomatal closure that improves drought tolerance. Plant Biotechnol. J. 2019, 17, 118–131. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Huang, Z.; Li, X.; Ma, L.; Gu, Q.; Wu, H.; Liu, J.; Borriss, R.; Wu, Z.; Gao, X.W. Stomatal closure and SA-, JA/ET-signaling pathways are essential for Bacillus amyloliquefaciens FZB42 to restrict leaf disease caused by Phytophthora nicotianae in Nicotiana benthamiana. Front. Microbiol. 2018, 9, 847. [Google Scholar] [CrossRef] [Green Version]
- Bright, J.; Desikan, R.; Hancock, J.T.; Weir, I.S.; Neill, S.J. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 2006, 45, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.J.; Farquhar, G.D. The effect of exogenous abscisic acid on stomatal development, stomatal mechanics, and leaf gas exchange in Tradescantia virginiana. Plant Physiol. 2001, 125, 935–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bresson, J.; Varoquaux, F.; Bontpart, T.; Touraine, B.; Vile, D. The PGPR strain Phyllobacterium brassicacearum STM196 induces a reproductive delay and physiological changes that result in improved drought tolerance in Arabidopsis. New Phytol. 2013, 200, 558–569. [Google Scholar] [CrossRef]
- Chen, Y.; Chai, Y.; Guo, J.H.; Losick, R. Evidence for cyclic di-GMP-mediated signaling in Bacillus subtilis. J. Bacteriol. 2012, 194, 5080–5090. [Google Scholar] [CrossRef]
- Götz, M.; Gomes, N.C.; Dratwinski, A.; Costa, R.; Berg, G.; Peixoto, R.; Mendonça-Hagler, L.; Smalla, K. Survival of gfp-tagged antagonistic bacteria in the rhizosphere of tomato plants and their effects on the indigenous bacterial community. FEMS Microbiol. Ecol. 2006, 56, 207–218. [Google Scholar] [CrossRef] [Green Version]
- Eisele, J.F.; Florian, F.; Patrick, F.B.; Chaban, C. A rapid and simple method for microscopy-based stomata analyses. PLoS ONE 2016, 11, e0164576. [Google Scholar] [CrossRef]
- Mittova, V.; Guy, M.; Tal, M.; Volokita, M. Salinity up-regulates the antioxidative system in root mitochondria and peroxisomes of the wild salt-tolerant tomato species Lycopersicon pennellii. J. Exp. Bot. 2004, 55, 1105–1113. [Google Scholar] [CrossRef]
- Qiu, Z.B.; Liu, X.; Tian, X.J.; Yue, M. Effects of CO2 laser pretreatment on drought stress resistance in wheat. J. Photochem. Photobiol. B 2008, 90, 17–25. [Google Scholar] [CrossRef]
- Zhou, S.; Hu, W.; Deng, X.; Ma, Z.; Chen, L.; Huang, C.; Wang, C.; Wang, J.; He, Y.Z.; Yang, G.X.; et al. Overexpression of the wheat aquaporin gene, TaAQP7, enhances drought tolerance in transgenic tobacco. PLoS ONE 2012, 7, e52439. [Google Scholar] [CrossRef] [Green Version]
- Dobrá, J.; Vanková, R.; Havlová, M.; Burman, A.J.; Libus, J.; Štorchová, H. Tobacco leaves and roots differ in the expression of proline metabolism-related genes in the course of drought stress and subsequent recovery. J. Plant Physiol. 2011, 168, 1588–1597. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.-C.; Jiang, C.-H.; Zhang, L.-N.; Chen, L.; Zhang, X.-Y.; Guo, J.-H. Biofilms Positively Contribute to Bacillus amyloliquefaciens 54-induced Drought Tolerance in Tomato Plants. Int. J. Mol. Sci. 2019, 20, 6271. https://doi.org/10.3390/ijms20246271
Wang D-C, Jiang C-H, Zhang L-N, Chen L, Zhang X-Y, Guo J-H. Biofilms Positively Contribute to Bacillus amyloliquefaciens 54-induced Drought Tolerance in Tomato Plants. International Journal of Molecular Sciences. 2019; 20(24):6271. https://doi.org/10.3390/ijms20246271
Chicago/Turabian StyleWang, Da-Cheng, Chun-Hao Jiang, Li-Na Zhang, Lin Chen, Xiao-Yun Zhang, and Jian-Hua Guo. 2019. "Biofilms Positively Contribute to Bacillus amyloliquefaciens 54-induced Drought Tolerance in Tomato Plants" International Journal of Molecular Sciences 20, no. 24: 6271. https://doi.org/10.3390/ijms20246271
APA StyleWang, D.-C., Jiang, C.-H., Zhang, L.-N., Chen, L., Zhang, X.-Y., & Guo, J.-H. (2019). Biofilms Positively Contribute to Bacillus amyloliquefaciens 54-induced Drought Tolerance in Tomato Plants. International Journal of Molecular Sciences, 20(24), 6271. https://doi.org/10.3390/ijms20246271



