Kidney Cells Regeneration: Dedifferentiation of Tubular Epithelium, Resident Stem Cells and Possible Niches for Renal Progenitors
Abstract
:1. Introduction
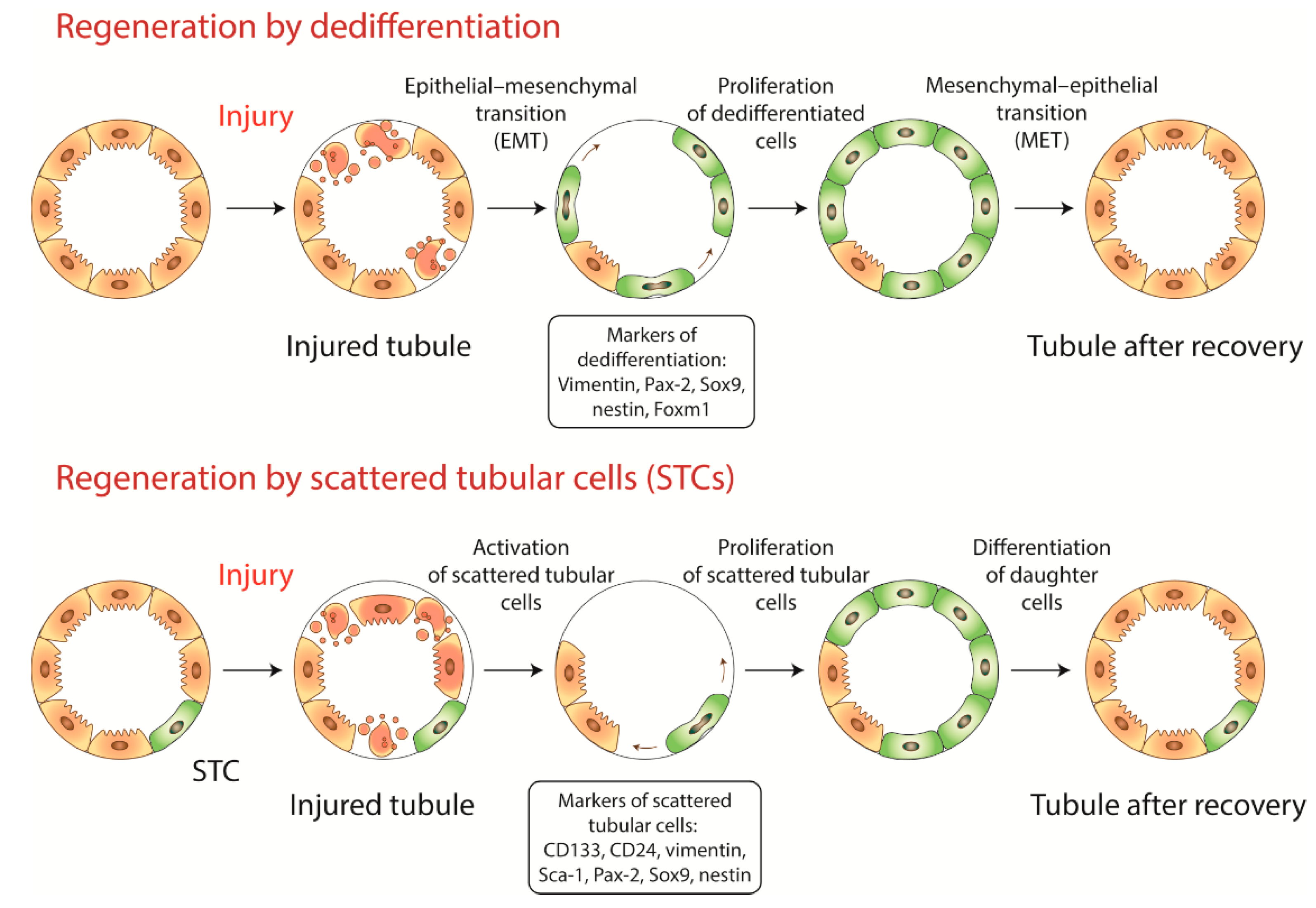
2. Dedifferentiation or Recruitment of Progenitor Cells?
2.1. Dedifferentiation
2.2. Progenitor Cells
2.2.1. Progenitor Cells in Rodent Kidneys
2.2.2. Progenitor Cells in Human Kidneys
2.3. State of the Art
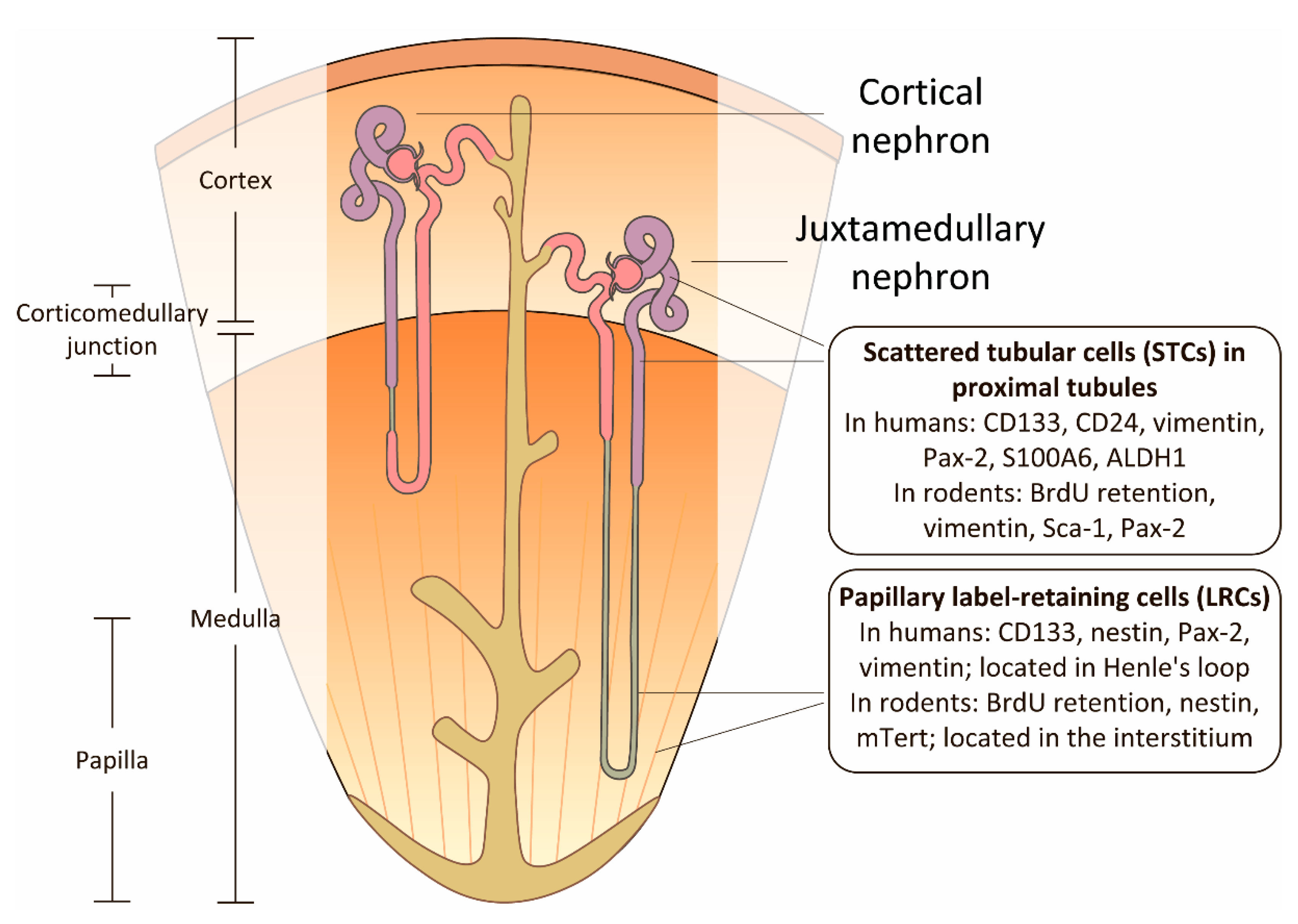
3. Renal Papilla as a Niche for Progenitor Cells
4. Potential Approaches Affecting Kidney Regeneration
5. Summary
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| I/R | Ischemia/reperfusion |
| AKI | Acute kidney injury |
| STCs | Scattered tubular cells |
| LRCs | Label-retaining cells |
References
- Little, M.H.; Kairath, P. Does Renal Repair Recapitulate Kidney Development? J. Am. Soc. Nephrol. 2017, 28, 34–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huling, J.; Yoo, J.J. Comparing adult renal stem cell identification, characterization and applications. J. Biomed. Sci. 2017, 24, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eymael, J.; Smeets, B. Origin and fate of the regenerating cells of the kidney. Eur. J. Pharmacol. 2016, 790, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Kramann, R.; Kusaba, T.; Humphreys, B.D. Who regenerates the kidney tubule? Nephrol. Dial. Transplant. 2015, 30, 903–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonventre, J. V Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J. Am. Soc. Nephrol. 2003, 14 (Suppl. 1), S55–S61. [Google Scholar] [CrossRef] [Green Version]
- Ledda-Columbano, G.M.; Columbano, A.; Coni, P.; Curto, M.; Faa, G.; Pani, P. Cell proliferation in rat kidney induced by 1,2-dibromoethane. Toxicol. Lett. 1987, 37, 85–90. [Google Scholar] [CrossRef]
- Iatropoulos, M.J.; Williams, G.M. Proliferation markers. Exp. Toxicol. Pathol. 1996, 48, 175–181. [Google Scholar] [CrossRef]
- Humphreys, B.D.; Czerniak, S.; DiRocco, D.P.; Hasnain, W.; Cheema, R.; Bonventre, J. V Repair of injured proximal tubule does not involve specialized progenitors. Proc. Natl. Acad. Sci. USA 2011, 108, 9226–9231. [Google Scholar] [CrossRef] [Green Version]
- Witzgall, R.; Brown, D.; Schwarz, C.; Bonventre, J.V. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J. Clin. Invest. 1994, 93, 2175–2188. [Google Scholar] [CrossRef] [Green Version]
- Barker, N.; Rookmaaker, M.B.; Kujala, P.; Ng, A.; Leushacke, M.; Snippert, H.; van de Wetering, M.; Tan, S.; Van Es, J.H.; Huch, M.; et al. Lgr5+ve Stem/Progenitor Cells Contribute to Nephron Formation during Kidney Development. Cell Rep. 2012, 2, 540–552. [Google Scholar] [CrossRef] [Green Version]
- Imgrund, M.; Gröne, E.; Gröne, H.J.; Kretzler, M.; Holzman, L.; Schlöndorff, D.; Rothenpieler, U.W. Re-expression of the developmental gene Pax-2 during experimental acute tubular necrosis in mice. Kidney Int. 1999, 56, 1423–1431. [Google Scholar]
- Poché, R.A.; Furuta, Y.; Chaboissier, M.C.; Schedl, A.; Behringer, R.R. Sox9 is expressed in mouse multipotent retinal progenitor cells and functions in Müller Glial cell development. J. Comp. Neurol. 2008, 510, 237–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeshima, A.; Yamashita, S.; Nojima, Y. Identification of renal progenitor-like tubular cells that participate in the regeneration processes of the kidney. J. Am. Soc. Nephrol. 2003, 14, 3138–3146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitamura, S.; Yamasaki, Y.; Kinomura, M.; Sugaya, T.; Sugiyama, H.; Maeshima, Y.; Makino, H. Establishment and characterization of renal progenitor like cells from S3 segment of nephron in rat adult kidney. FASEB J. 2005, 19, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Challen, G.A.; Bertoncello, I.; Deane, J.A.; Ricardo, S.D.; Little, M.H. Kidney Side Population Reveals Multilineage Potential and Renal Functional Capacity but also Cellular Heterogeneity. J. Am. Soc. Nephrol. 2006, 17, 1896–1912. [Google Scholar] [CrossRef]
- Smeets, B.; Boor, P.; Dijkman, H.; Sharma, S.V.; Jirak, P.; Mooren, F.; Berger, K.; Bornemann, J.; Gelman, I.H.; Floege, J.; et al. Proximal tubular cells contain a phenotypically distinct, scattered cell population involved in tubular regeneration. J. Pathol. 2013, 229, 645–659. [Google Scholar] [CrossRef]
- Angelotti, M.L.; Ronconi, E.; Ballerini, L.; Peired, A.; Mazzinghi, B.; Sagrinati, C.; Parente, E.; Gacci, M.; Carini, M.; Rotondi, M.; et al. Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cells 2012, 30, 1714–1725. [Google Scholar] [CrossRef] [Green Version]
- Lindgren, D.; Boström, A.K.; Nilsson, K.; Hansson, J.; Sjölund, J.; Möller, C.; Jirström, K.; Nilsson, E.; Landberg, G.; Axelson, H.; et al. Isolation and characterization of progenitor-like cells from human renal proximal tubules. Am. J. Pathol. 2011, 178, 828–837. [Google Scholar] [CrossRef] [Green Version]
- Berger, K.; Moeller, M.J. Mechanisms of epithelial repair and regeneration after acute kidney injury. Semin. Nephrol. 2014, 34, 394–403. [Google Scholar] [CrossRef] [Green Version]
- Romagnani, P. Family portrait: Renal progenitor of Bowman’s capsule and its tubular brothers. Am. J. Pathol. 2011, 178, 490–493. [Google Scholar] [CrossRef]
- Meyer-Schwesinger, C. The Role of Renal Progenitors in Renal Regeneration. Nephron 2016, 132, 101–109. [Google Scholar] [CrossRef]
- Gupta, S.; Rosenberg, M.E. Do stem cells exist in the adult kidney? Am. J. Nephrol. 2008, 28, 607–613. [Google Scholar] [CrossRef] [PubMed]
- McCampbell, K.K.; Wingert, R.A. Renal stem cells: fact or science fiction? Biochem. J. 2012, 444, 153–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcheque, J.; Bussolati, B.; Csete, M.; Perin, L. Concise Reviews: Stem Cells and Kidney Regeneration: An Update. Stem Cells Transl. Med. 2019, 8, 82–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brossa, A.; Papadimitriou, E.; Collino, F.; Incarnato, D.; Oliviero, S.; Camussi, G.; Bussolati, B. Role of CD133 Molecule in Wnt Response and Renal Repair. Stem Cells Transl. Med. 2018, 7, 283–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeshima, A.; Sakurai, H.; Nigam, S.K. Adult kidney tubular cell population showing phenotypic plasticity, tubulogenic capacity, and integration capability into developing kidney. J. Am. Soc. Nephrol. 2006, 17, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, K.M.; Han, D.J.; Yu, E.; Cho, Y.M. Adult stem cell-like tubular cells reside in the corticomedullary junction of the kidney. Int. J. Clin. Exp. Pathol. 2008, 1, 232–241. [Google Scholar]
- Kim, J.; Kim, J.I.; Na, Y.K.; Park, K.M. Intra-renal slow cell-cycle cells contribute to the restoration of kidney tubules injured by ischemia/reperfusion. Anat. Cell Biol. 2011, 44, 186. [Google Scholar] [CrossRef] [Green Version]
- Sallustio, F.; De Benedictis, L.; Castellano, G.; Zaza, G.; Loverre, A.; Costantino, V.; Grandaliano, G.; Schena, F.P. TLR2 plays a role in the activation of human resident renal stem/progenitor cells. FASEB J. 2009, 24, 514–525. [Google Scholar] [CrossRef]
- Grange, C.; Moggio, A.; Tapparo, M.; Porta, S.; Camussi, G.; Bussolati, B. Protective effect and localization by optical imaging of human renal CD133+ progenitor cells in an acute kidney injury model. Physiol. Rep. 2014, 2, e12009. [Google Scholar] [CrossRef]
- Hansson, J.; Hultenby, K.; Cramnert, C.; Pontén, F.; Jansson, H.; Lindgren, D.; Axelson, H.; Johansson, M.E. Evidence for a morphologically distinct and functionally robust cell type in the proximal tubules of human kidney. Hum. Pathol. 2014, 45, 382–393. [Google Scholar] [CrossRef] [Green Version]
- Bussolati, B.; Bruno, S.; Grange, C.; Buttiglieri, S.; Deregibus, M.C.; Cantino, D.; Camussi, G. Isolation of renal progenitor cells from adult human kidney. Am. J. Pathol. 2005, 166, 545–555. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Verfaillie, C.; Chmielewski, D.; Kren, S.; Eidman, K.; Connaire, J.; Heremans, Y.; Lund, T.; Blackstad, M.; Jiang, Y.; et al. Isolation and characterization of kidney-derived stem cells. J. Am. Soc. Nephrol. 2006, 17, 3028–3040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loverre, A.; Capobianco, C.; Ditonno, P.; Battaglia, M.; Grandaliano, G.; Schena, F.P. Increase of proliferating renal progenitor cells in acute tubular necrosis underlying delayed graft function. Transplantation 2008, 85, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, S.; Sakurai, H.; Makino, H. Single adult kidney stem/progenitor cells reconstitute three-dimensional nephron structures in vitro. Stem Cells 2015, 33, 774–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hishikawa, K.; Marumo, T.; Miura, S.; Nakanishi, A.; Matsuzaki, Y.; Shibata, K.; Ichiyanagi, T.; Kohike, H.; Komori, T.; Takahashi, I.; et al. Musculin/MyoR is expressed in kidney side population cells and can regulate their function. J. Cell Biol. 2005, 169, 921–928. [Google Scholar] [CrossRef] [Green Version]
- Buzhor, E.; Omer, D.; Harari-Steinberg, O.; Dotan, Z.; Vax, E.; Pri-Chen, S.; Metsuyanim, S.; Pleniceanu, O.; Goldstein, R.S.; Dekel, B. Reactivation of NCAM1 defines a subpopulation of human adult kidney epithelial cells with clonogenic and stem/progenitor properties. Am. J. Pathol. 2013, 183, 1621–1633. [Google Scholar] [CrossRef]
- Langworthy, M.; Zhou, B.; de Caestecker, M.; Moeckel, G.; Baldwin, H. NFATc1 identifies a population of proximal tubule cell progenitors. J. Am. Soc. Nephrol. 2009, 20, 311–321. [Google Scholar] [CrossRef] [Green Version]
- Abedin, M.J.; Imai, N.; Rosenberg, M.E.; Gupta, S. Identification and characterization of Sall1-expressing cells present in the adult mouse kidney. Nephron. Exp. Nephrol. 2011, 119, e75–e82. [Google Scholar] [CrossRef]
- Iwatani, H.; Ito, T.; Imai, E.; Matsuzaki, Y.; Suzuki, A.; Yamato, M.; Okabe, M.; Hori, M. Hematopoietic and nonhematopoietic potentials of Hoechst low /side population cells isolated from adult rat kidney. Kidney Int. 2004, 65, 1604–1614. [Google Scholar]
- Da Sacco, S.; Thornton, M.E.; Petrosyan, A.; Lavarreda-Pearce, M.; Sedrakyan, S.; Grubbs, B.H.; De Filippo, R.E.; Perin, L. Direct Isolation and Characterization of Human Nephron Progenitors. Stem Cells Transl. Med. 2017, 6, 419–433. [Google Scholar] [CrossRef]
- Chang-Panesso, M.; Kadyrov, F.F.; Lalli, M.; Wu, H.; Ikeda, S.; Kefaloyianni, E.; Abdelmageed, M.M.; Herrlich, A.; Kobayashi, A.; Humphreys, B.D. FOXM1 drives proximal tubule proliferation during repair from acute ischemic kidney injury. J. Clin. Invest. 2019, 129. [Google Scholar] [CrossRef]
- Kirita, Y.; Chang-Panesso, M.; Humphreys, B.D. Recent Insights into Kidney Injury and Repair from Transcriptomic Analyses. Nephron 2019. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Wang, B.; Jiang, X.; Hu, W.; Feng, J.; Li, H.; Jin, M.; Ying, Y.; Wang, W.; Mao, X.; et al. Proliferative capacity of stem/progenitor-like cells in the kidney may associate with the outcome of patients with acute tubular necrosis. Hum. Pathol. 2011, 42, 1132–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, D.; Ni, L.; You, L.; Zhang, L.; Gu, Y.; Hao, C.M.; Chen, J. Upregulation of nestin in proximal tubules may participate in cell migration during renal repair. Am. J. Physiol. Ren. Physiol. 2012, 303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazzeri, E.; Angelotti, M.L.; Peired, A.; Conte, C.; Marschner, J.A.; Maggi, L.; Mazzinghi, B.; Lombardi, D.; Melica, M.E.; Nardi, S.; et al. Endocycle-related tubular cell hypertrophy and progenitor proliferation recover renal function after acute kidney injury. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Moran, A.; Igarashi, P. Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J. Clin. Invest. 2005, 115, 1756–1764. [Google Scholar] [CrossRef] [PubMed]
- Kusaba, T.; Lalli, M.; Kramann, R.; Kobayashi, A.; Humphreys, B.D. Differentiated kidney epithelial cells repair injured proximal tubule. Proc. Natl. Acad. Sci. USA 2014, 111, 1527–1532. [Google Scholar] [CrossRef] [Green Version]
- Villanueva, S.; Céspedes, C.; Vio, C.P. Ischemic acute renal failure induces the expression of a wide range of nephrogenic proteins. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R861–R870. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.M.; Huang, S.; Reidy, K.; Han, S.H.; Chinga, F.; Susztak, K. Sox9-Positive Progenitor Cells Play a Key Role in Renal Tubule Epithelial Regeneration in Mice. Cell Rep. 2016. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Liu, J.; Pang, P.; Krautzberger, A.M.; Reginensi, A.; Akiyama, H.; Schedl, A.; Humphreys, B.D.; McMahon, A.P. Sox9 Activation Highlights a Cellular Pathway of Renal Repair in the Acutely Injured Mammalian Kidney. Cell Rep. 2015, 12, 1325–1338. [Google Scholar] [CrossRef] [Green Version]
- Fujigaki, Y.; Goto, T.; Sakakima, M.; Fukasawa, H.; Miyaji, T.; Yamamoto, T.; Hishida, A. Kinetics and characterization of initially regenerating proximal tubules in S3 segment in response to various degrees of acute tubular injury. Nephrol. Dial. Transplant. 2006, 21, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Gröne, H.J.; Weber, K.; Gröne, E.; Helmchen, U.; Osborn, M. Coexpression of keratin and vimentin in damaged and regenerating tubular epithelia of the kidney. Am. J. Pathol. 1987, 129, 1–8. [Google Scholar] [PubMed]
- Humphreys, B.D.; Valerius, M.T.; Kobayashi, A.; Mugford, J.W.; Soeung, S.; Duffield, J.S.; McMahon, A.P.; Bonventre, J.V. Intrinsic Epithelial Cells Repair the Kidney after Injury. Cell Stem Cell 2008, 2, 284–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver, J.A.; Maarouf, O.; Cheema, F.H.; Martens, T.P.; Al-Awqati, Q. The renal papilla is a niche for adult kidney stem cells. J. Clin. Invest. 2004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, D.C.; Oxburgh, L. The long-term label retaining population of the renal papilla arises through divergent regional growth of the kidney. Am. J. Physiol. Physiol. 2009, 297, F809–F815. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Liu, H.; Sun, L.; Chen, Z.; Nie, H.; Sun, A.; Liu, G.; Guan, G. The role of long-term label-retaining cells in the regeneration of adult mouse kidney after ischemia/reperfusion injury. Stem Cell Res. Ther. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Oliver, J.A.; Klinakis, A.; Cheema, F.H.; Friedlander, J.; Sampogna, R.V.; Martens, T.P.; Liu, C.; Efstratiadis, A.; Al-Awqati, Q. Proliferation and migration of label-retaining cells of the kidney papilla. J. Am. Soc. Nephrol. 2009, 20, 2315–2327. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Czerniak, S.; Wang, T.; Ying, W.; Carlone, D.L.; Breault, D.T.; Humphreys, B.D. Characterization and fate of telomerase-expressing epithelia during kidney repair. J. Am. Soc. Nephrol. 2011, 22, 2256–2265. [Google Scholar] [CrossRef] [Green Version]
- Ward, H.H.; Romero, E.; Welford, A.; Pickett, G.; Bacallao, R.; Gattone, V.H.; Ness, S.A.; Wandinger-Ness, A.; Roitbak, T. Adult human CD133/1(+) kidney cells isolated from papilla integrate into developing kidney tubules. Biochim. Biophys. Acta 2011, 1812, 1344–1357. [Google Scholar] [CrossRef] [Green Version]
- Bussolati, B.; Moggio, A.; Collino, F.; Aghemo, G.; D’Armento, G.; Grange, C.; Camussi, G. Hypoxia modulates the undifferentiated phenotype of human renal inner medullary CD133+ progenitors through Oct4/miR-145 balance. Am. J. Physiol. Renal Physiol. 2012, 302, F116–F128. [Google Scholar] [CrossRef] [Green Version]
- Patschan, D.; Michurina, T.; Shi, H.K.; Dolff, S.; Brodsky, S.V.; Vasilieva, T.; Cohen-Gould, L.; Winaver, J.; Chander, P.N.; Enikolopov, G.; et al. Normal distribution and medullary-to-cortical shift of Nestin-expressing cells in acute renal ischemia. Kidney Int. 2007, 71, 744–754. [Google Scholar] [CrossRef] [Green Version]
- Dekel, B.; Zangi, L.; Shezen, E.; Reich-Zeliger, S.; Eventov-Friedman, S.; Katchman, H.; Jacob-Hirsch, J.; Amariglio, N.; Rechavi, G.; Margalit, R.; et al. Isolation and characterization of nontubular Sca-1+Lin-multipotent stem/progenitor cells from adult mouse kidney. J. Am. Soc. Nephrol. 2006, 17, 3300–3314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schutgens, F.; Rookmaaker, M.B.; Blokzijl, F.; Van Boxtel, R.; Vries, R.; Cuppen, E.; Verhaar, M.C.; Clevers, H. Troy/TNFRSF19 marks epithelial progenitor cells during mouse kidney development that continue to contribute to turnover in adult kidney. Proc. Natl. Acad. Sci. USA 2017, 114, E11190–E11198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver, J.A.; Sampogna, R.V.; Jalal, S.; Zhang, Q.-Y.; Dahan, A.; Wang, W.; Shen, T.H.; Al-Awqati, Q. A Subpopulation of Label-Retaining Cells of the Kidney Papilla Regenerates Injured Kidney Medullary Tubules. Stem Cell Rep. 2016, 6, 757–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, S.; Moggio, A.; Bussolati, B. Concise Review: Stem/Progenitor Cells for Renal Tissue Repair: Current Knowledge and Perspectives. Stem Cells Transl. Med. 2013, 2, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Pleniceanu, O.; Omer, D.; Harari-Steinberg, O.; Dekel, B. Renal lineage cells as a source for renal regeneration. Pediatr. Res. 2018, 83, 267–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houghton, D.C.; Hartnett, M.; Campbell-Boswell, M.; Porter, G.; Bennett, W. A light and electron microscopic analysis of gentamicin nephrotoxicity in rats. Am. J. Pathol. 1976, 82, 589. [Google Scholar]
- Vogetseder, A.; Karadeniz, A.; Kaissling, B.; Hir, M. Le Tubular cell proliferation in the healthy rat kidney. Histochem. Cell Biol. 2005, 124, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Vogetseder, A.; Palan, T.; Bacic, D.; Kaissling, B.; Le Hir, M. Proximal tubular epithelial cells are generated by division of differentiated cells in the healthy kidney. Am. J. Physiol. Cell Physiol. 2007, 292, C807–C813. [Google Scholar] [CrossRef] [Green Version]
- Molitoris, B.A.; Hoilien, C.A.; Dahl, R.; Ahnen, D.J.; Wilson, P.D.; Kim, J. Characterization of ischemia-induced loss of epithelial polarity. J. Membr. Biol. 1988, 106, 233–242. [Google Scholar] [CrossRef]
- Solez, K.; Morel-Maroger, L.; Sraer, J.D. The morphology of “acute tubular necrosis” in man: analysis of 57 renal biopsies and a comparison with the glycerol model. Medicine (Baltimore) 1979, 58, 362–376. [Google Scholar] [CrossRef]
- Schena, F.P. Role of growth factors in acute renal failure. Kidney Int. Suppl. 1998, 66, S11-5. [Google Scholar] [PubMed]
- El Sabbahy, M.; Vaidya, V.S. Ischemic kidney injury and mechanisms of tissue repair. Wiley Interdiscip. Rev. Syst. Biol. Med. 2011, 3, 606–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devarajan, P.; Mishra, J.; Supavekin, S.; Patterson, L.T.; Steven Potter, S. Gene expression in early ischemic renal injury: clues towards pathogenesis, biomarker discovery, and novel therapeutics. Mol. Genet. Metab. 2003, 80, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, D.M.; Shanley, P.F.; Molitoris, B.A. Mild ischemia predisposes the S3 segment to gentamicin toxicity. Kidney Int. 1990, 38, 459–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekine, M.; Monkawa, T.; Morizane, R.; Matsuoka, K.; Taya, C.; Akita, Y.; Joh, K.; Itoh, H.; Hayashi, M.; Kikkawa, Y.; et al. Selective depletion of mouse kidney proximal straight tubule cells causes acute kidney injury. Transgenic Res. 2012, 21, 51–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogetseder, A.; Picard, N.; Gaspert, A.; Walch, M.; Kaissling, B.; Le Hir, M. Proliferation capacity of the renal proximal tubule involves the bulk of differentiated epithelial cells. Am. J. Physiol. Physiol. 2008, 294, C22–C28. [Google Scholar] [CrossRef] [Green Version]
- Ichimura, T.; Hung, C.C.; Yang, S.A.; Stevens, J.L.; Bonventre, J.V. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am. J. Physiol. Physiol. 2004, 286, F552–F563. [Google Scholar] [CrossRef]
- Ichimura, T.; Bonventre, J.V.; Bailly, V.; Wei, H.; Hession, C.A.; Cate, R.L.; Sanicola, M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J. Biol. Chem. 1998, 273, 4135–4142. [Google Scholar] [CrossRef] [Green Version]
- Berger, K.; Bangen, J.-M.; Hammerich, L.; Liedtke, C.; Floege, J.; Smeets, B.; Moeller, M.J. Origin of regenerating tubular cells after acute kidney injury. Proc. Natl. Acad. Sci. USA 2014, 111, 1533–1538. [Google Scholar] [CrossRef] [Green Version]
- Romagnani, P.; Rinkevich, Y.; Dekel, B. The use of lineage tracing to study kidney injury and regeneration. Nat. Rev. Nephrol. 2015, 11, 420–431. [Google Scholar] [CrossRef]
- Chaboissier, M.C.; Kobayashi, A.; Vidal, V.I.P.; Lützkendorf, S.; van de Kant, H.J.G.; Wegner, M.; de Rooij, D.G.; Behringer, R.R.; Schedl, A. Functional analysis os Sox8 and Sox9 during sex determination in the mouse. Development 2004, 131, 1891–1901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavanagh, B.L.; Walker, T.; Norazit, A.; Meedeniya, A.C.B. Thymidine analogues for tracking DNA synthesis. Molecules 2011, 16, 7980–7993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piepenhagen, P.A.; Peters, L.L.; Lux, S.E.; Nelson, W.J. Differential expression of Na+-K+-ATPase, ankyrin, fodrin, and E- cadherin along the kidney nephron. Am. J. Physiol. Cell Physiol. 1995, 269, C1417–C1432. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.A.; Polak, L.; Pasolli, H.A.; Fuchs, E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell 2008, 3, 33–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, M.; Briscoe, J. Neural crest development is regulated by the transcription factor Sox9. Development 2003, 130, 5681–5693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, K.; Sato, A.; Asashima, M.; Wang, P.C.; Nishinakamura, R. Mouse homolog of SALL1, a causative gene for Townes-Brocks syndrome, binds to A/T-rich sequences in pericentric heterochromatin via its C-terminal zinc finger domains. Genes Cells 2007, 12, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Shirasawa, T.; Akashi, T.; Sakamoto, K.; Takahashi, H.; Maruyama, N.; Hirokawa, K. Gene expression of CD24 core peptide molecule in developing brain and developing non-neural tissues. Dev. Dyn. 1993, 198, 1–13. [Google Scholar] [CrossRef]
- Mignone, J.L.; Kukekov, V.; Chiang, A.S.; Steindler, D.; Enikolopov, G. Neural Stem and Progenitor Cells in Nestin-GFP Transgenic Mice. J. Comp. Neurol. 2004, 469, 311–324. [Google Scholar] [CrossRef]
- Rinkevich, Y.; Montoro, D.T.; Contreras-Trujillo, H.; Harari-Steinberg, O.; Newman, A.M.; Tsai, J.M.; Lim, X.; Van-Amerongen, R.; Bowman, A.; Januszyk, M.; et al. In vivo clonal analysis reveals lineage-restricted progenitor characteristics in mammalian kidney development, maintenance, and regeneration. Cell Rep. 2014. [Google Scholar] [CrossRef] [Green Version]
- Chang-Panesso, M.; Humphreys, B.D. Cellular plasticity in kidney injury and repair. Nat. Rev. Nephrol. 2017, 13, 39–46. [Google Scholar] [CrossRef]
- Kim, K.; Park, B.-H.; Ihm, H.; Kim, K.M.; Jeong, J.; Chang, J.W.; Cho, Y.M. Expression of stem cell marker CD133 in fetal and adult human kidneys and pauci-immune crescentic glomerulonephritis. Histol. Histopathol. 2011, 26, 223–232. [Google Scholar] [PubMed]
- Lieberthal, W.; Nigam, S.K. Acute renal failure. I. Relative importance of proximal vs. distal tubular injury. Am. J. Physiol. 1998, 275, F623–F632. [Google Scholar] [CrossRef] [PubMed]
- Kemper, K.; Sprick, M.R.; De Bree, M.; Scopelliti, A.; Vermeulen, L.; Hoek, M.; Zeilstra, J.; Pals, S.T.; Mehmet, H.; Stassi, G.; et al. The AC133 epitope, but not the CD133 protein, is lost upon cancer stem cell differentiation. Cancer Res. 2010, 70, 719–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelotti, M.L.; Lazzeri, E.; Lasagni, L.; Romagnani, P. Only anti-CD133 antibodies recognizing the CD133/1 or the CD133/2 epitopes can identify human renal progenitors. Kidney Int. 2010, 78, 620–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbeil, D.; Röper, K.; Hellwig, A.; Tavian, M.; Miraglia, S.; Watt, S.M.; Simmons, P.J.; Peault, B.; Buck, D.W.; Huttner, W.B. The human AC133 hematopoietic stem cell antigen is also expressed in epithelial cells and targeted to plasma membrane protrusions. J. Biol. Chem. 2000, 275, 5512–5520. [Google Scholar] [CrossRef] [Green Version]
- Mizrak, D.; Brittan, M.; Alison, M.R. CD 133: Molecule of the moment. J. Pathol. 2008, 214, 3–9. [Google Scholar] [CrossRef]
- Romagnani, P.; Remuzzi, G. CD133+ renal stem cells always co-express CD24 in adult human kidney tissue. Stem Cell Res. 2014, 12, 828–829. [Google Scholar] [CrossRef] [Green Version]
- Sagrinati, C.; Netti, G.S.; Mazzinghi, B.; Lazzeri, E.; Liotta, F.; Frosali, F.; Ronconi, E.; Meini, C.; Gacci, M.; Squecco, R.; et al. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J. Am. Soc. Nephrol. 2006, 17, 2443–2456. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Luo, Z.; Dong, L.; Tan, Y.; Yang, J.; Feng, G.; Wu, M.; Li, Z.; Wang, H. CD133/prominin-1-mediated autophagy and glucose uptake beneficial for hepatoma cell survival. PLoS ONE 2013, 8, e56878. [Google Scholar] [CrossRef] [Green Version]
- Rehman, J. Empowering self-renewal and differentiation: The role of mitochondria in stem cells. J. Mol. Med. 2010, 88, 981–986. [Google Scholar] [CrossRef] [Green Version]
- Santeramo, I.; Perez, Z.H.; Illera, A.; Taylor, A.; Kenny, S.; Murray, P.; Wilm, B.; Gretz, N. Human kidney-derived cells ameliorate acute kidney injury without engrafting into renal tissue. Stem Cells Transl. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, P.; Lasagni, L.; Remuzzi, G. Renal progenitors: An evolutionary conserved strategy for kidney regeneration. Nat. Rev. Nephrol. 2013, 9, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Shankland, S.J.; Freedman, B.S.; Pippin, J.W. Can podocytes be regenerated in adults? Curr. Opin. Nephrol. Hypertens. 2017, 26, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, B.D. Kidney injury, stem cells and regeneration. Curr. Opin. Nephrol. Hypertens. 2014, 23, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Forbes, M.S.; Thornhill, B.A.; Galarreta, C.I.; Chevalier, R.L. A population of mitochondrion-rich cells in the pars recta of mouse kidney. Cell Tissue Res. 2016, 363, 791–803. [Google Scholar] [CrossRef] [Green Version]
- Bussolati, B.; Camussi, G. Therapeutic use of human renal progenitor cells for kidney regeneration. Nat. Rev. Nephrol. 2015. [Google Scholar] [CrossRef]
- Romagnani, P. Of mice and men: The riddle of tubular regeneration. J. Pathol. 2013, 229, 641–644. [Google Scholar] [CrossRef]
- Vanslambrouck, J.; Li, J.; Little, M.H. The renal papilla: An enigma in damage and repair. J. Am. Soc. Nephrol. 2011, 22, 2145–2147. [Google Scholar] [CrossRef] [Green Version]
- Burmeister, D.M.; McIntyre, M.K.; Montgomery, R.K.; Gómez, B.I.; Dubick, M.A. Isolation and Characterization of Multipotent CD24+ Cells From the Renal Papilla of Swine. Front. Med. 2018, 5. [Google Scholar] [CrossRef] [Green Version]
- Pannabecker, T.L.; Layton, A.T. Targeted delivery of solutes and oxygen in the renal medulla: role of microvessel architecture. Am. J. Physiol. Renal Physiol. 2014, 307, F649–F655. [Google Scholar] [CrossRef] [Green Version]
- Mohyeldin, A.; Garzón-Muvdi, T.; Quiñones-Hinojosa, A. Oxygen in stem cell biology: A critical component of the stem cell niche. Cell Stem Cell 2010, 7, 150–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphreys, B.D. Slow-Cycling Cells in Renal Papilla: Stem Cells Awaken? J. Am. Soc. Nephrol. 2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tumbar, T.; Guasch, G.; Greco, V.; Blanpain, C.; Lowry, W.E.; Rendl, M.; Fuchs, E. Defining the epithelial stem cell niche in skin. Science 2004, 303, 359–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiese, C.; Rolletschek, A.; Kania, G.; Blyszczuk, P.; Tarasov, K.V.; Tarasova, Y.; Wersto, R.P.; Boheler, K.R.; Wobus, A.M. Nestin expression-A property of multi-lineage progenitor cells? Cell. Mol. Life Sci. 2004. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Boyle, S.; Zhao, M.; Su, W.; Takahashi, K.; Davis, L.; DeCaestecker, M.; Takahashi, T.; Brever, M.D.; Hao, C.M. Differential expression of the intermediate filament protein nestin during renal development and its localization in adult podocytes. J. Am. Soc. Nephrol. 2006, 17, 1283–1291. [Google Scholar] [CrossRef]
- Kirik, O.V.; Korzhevskii, D.E. Expression of neural stem cell marker nestin in the kidney of rats and humans. Bull. Exp. Biol. Med. 2009, 147, 539–541. [Google Scholar] [CrossRef]
- Zou, J.; Yaoita, E.; Watanabe, Y.; Yoshida, Y.; Nameta, M.; Li, H.; Qu, Z.; Yamamoto, T. Upregulation of nestin, vimentin, and desmin in rat podocytes in response to injury. Virchows Arch. 2006, 448, 485–492. [Google Scholar] [CrossRef]
- Perry, J.; Ho, M.; Viero, S.; Zheng, K.; Jacobs, R.; Thorner, P.S. The intermediate filament nestin is highly expressed in normal human podocytes and podocytes in glomerular disease. Pediatr. Dev. Pathol. 2007, 10, 369–382. [Google Scholar] [CrossRef]
- Westhoff, J.H.; Schildhorn, C.; Jacobi, C.; Hömme, M.; Hartner, A.; Braun, H.; Kryzer, C.; Wang, C.; Von Zglinicki, T.; Kränzlin, B.; et al. Telomere shortening reduces regenerative capacity after acute kidney injury. J. Am. Soc. Nephrol. 2010, 21, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell Migration: Integrating Signals from Front to Back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef] [Green Version]
- Solanas, G.; Benitah, S.A. Architecture of the Interfolliculer epidermis. Nat. Rev. Mol. Cell Biol. 2013, 14, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Li, L.; Lengner, C.J. Hierarchy and Plasticity in the Intestinal Stem Cell Compartment. Trends Cell Biol. 2017, 27, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lin, G.; Alwaal, A.; Zhang, X.; Wang, G.; Jia, X.; Banie, L.; Villalta, J.; Lin, C.S.; Lue, T.F. Kinetics of Label Retaining Cells in the Developing Rat Kidneys. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Jadhav, S.H.; Tripathy, N.K.; Nityanand, S. Fetal kidney cells can ameliorate ischemic acute renal failure in rats through their anti-inflammatory, anti-apoptotic and anti-oxidative effects. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.K. Fetal kidney stem cells ameliorate cisplatin induced acute renal failure and promote renal angiogenesis. World J. Stem Cells 2015, 7, 776. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Grange, C.; Iampietro, C.; Camussi, G.; Bussolati, B. Human CD133 + Renal Progenitor Cells Induce Erythropoietin Production and Limit Fibrosis after Acute Tubular Injury. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Chen, C.; Chou, K.; Fang, H.; Hsu, C.; Huang, W.; Huang, C.; Huang, C.; Chen, H.; Lee, P. Progenitor-like cells derived from mouse kidney protect against renal fibrosis in a remnant kidney model via decreased endothelial mesenchymal transition. Stem Cell Res. Ther. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.T.; Lin, H.H.; Jiang, S.T.; Lu, P.J.; Chou, K.J.; Fang, H.C.; Chiou, Y.Y.; Tang, M.J. Mouse kidney progenitor cells accelerate renal regeneration and prolong survival after ischemic injury. Stem Cells 2010, 28, 573–584. [Google Scholar] [CrossRef]
- Lasagni, L.; Angelotti, M.L.; Ronconi, E.; Lombardi, D.; Nardi, S.; Peired, A.; Becherucci, F.; Mazzinghi, B.; Sisti, A.; Romoli, S.; et al. Podocyte Regeneration Driven by Renal Progenitors Determines Glomerular Disease Remission and Can Be Pharmacologically Enhanced. Stem Cell Rep. 2015, 5, 248–263. [Google Scholar] [CrossRef] [Green Version]
- Lasagni, L.; Ballerini, L.; Angelotti, M.L.; Parente, E.; Sagrinati, C.; Mazzinghi, B.; Peired, A.; Ronconi, E.; Becherucci, F.; Bani, D.; et al. Notch activation differentially regulates renal progenitors proliferation and differentiation toward the podocyte lineage in glomerular disorders. Stem Cells 2010, 28, 1673–1685. [Google Scholar] [CrossRef] [Green Version]
- Migliorini, A.; Angelotti, M.L.; Mulay, S.R.; Kulkarni, O.O.; Demleitner, J.; Dietrich, A.; Sagrinati, C.; Ballerini, L.; Peired, A.; Shankland, S.J.; et al. The antiviral cytokines IFN-α and IFN-β modulate parietal epithelial cells and promote podocyte loss: Implications for IFN toxicity, viral glomerulonephritis, and glomerular regeneration. Am. J. Pathol. 2013, 183, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Pippin, J.W.; Krofft, R.D.; Naito, S.; Liu, Z.H.; Shankland, S.J. Podocyte repopulation by renal progenitor cells following glucocorticoids treatment in experimental FSGS. Am. J. Physiol. Ren. Physiol. 2013, 304, 1375–1389. [Google Scholar] [CrossRef] [PubMed]
- Becherucci, F.; Mazzinghi, B.; Allinovi, M.; Angelotti, M.L.; Romagnani, P. Regenerating the kidney using human pluripotent stem cells and renal progenitors. Expert Opin. Biol. Ther. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lasagni, L.; Romagnani, P. Glomerular Epithelial Stem Cells: The Good, The Bad, and The Ugly. J. Am. Soc. Nephrol. 2010, 21, 1612–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gheisari, Y.; Nassiri, S.M.; Arefian, E.; Ahmadbeigi, N.; Azadmanesh, K.; Jamali, M.; Jahanzad, I.; Zeinali, S.; Vasei, M.; Soleimani, M. Severely damaged kidneys possess multipotent renoprotective stem cells. Cytotherapy 2010, 12. [Google Scholar] [CrossRef]
- Wilson, P.C.; Humphreys, B.D. Kidney and organoid single-cell transcriptomics: the end of the beginning. Pediatr. Nephrol. 2019. [Google Scholar] [CrossRef]
| Marker | Progenitor Cells | Dedifferentiation | ||
|---|---|---|---|---|
| Human | Rodents | |||
| Markers of progenitor cells | ALDH1 | [18,25] | - | - |
| BrdU retention | Not applicable | [13,26,27,28] | - | |
| CD24 | [16,17,18,25,29,30,31] | [15] | - | |
| CD44 | [30,32] | [33] | - | |
| CD73 | [30,32] | - | - | |
| CD133 | [16,17,18,29,30,31,32,34] | Not applicable | - | |
| C-kit | - | [14,35] | - | |
| Musculin | - | [36] | - | |
| NCAM1 | [37] | - | - | |
| NFATc1 | - | [38] | - | |
| S100A6 | [16,18,25] | - | - | |
| Sall1 | [25,37] | [39] | - | |
| Sca-1 | - | [14,15,35,36,40] | - | |
| SIX2 | [37,41] | - | - | |
| Marker of dedifferentiation | Foxm1 | - | - | [42,43] |
| Non-selective markers | Nestin | [44] | [35] | [45] |
| Pax-2 | [25,30,32,34,37,44] | [14,33,35,46] | [8,11,47,48,49] | |
| Sox9 | - | [50] | [42,51] | |
| Vimentin | [16,17,18,25,30,31,44] | [13,14,26,33,35] | [9,42,47,48,52,53] | |
| Marker | The Papilla of Human Kidney | The Papilla of Rodent Kidney |
|---|---|---|
| BrdU retention | Not applicable | [27,54,55,56,57,58,59] |
| CD133 | [60,61] | Not applicable |
| mTert | - | [59] |
| Nestin | [60,61] | [55,62] |
| Oct4 | [60,61] | - |
| Pax-2 | [61] | - |
| Sca-1 | - | [63] |
| Troy/TNFRSF19 | - | [64] |
| Vimentin | [61] | - |
| Zfyve27 | - | [65] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrianova, N.V.; Buyan, M.I.; Zorova, L.D.; Pevzner, I.B.; Popkov, V.A.; Babenko, V.A.; Silachev, D.N.; Plotnikov, E.Y.; Zorov, D.B. Kidney Cells Regeneration: Dedifferentiation of Tubular Epithelium, Resident Stem Cells and Possible Niches for Renal Progenitors. Int. J. Mol. Sci. 2019, 20, 6326. https://doi.org/10.3390/ijms20246326
Andrianova NV, Buyan MI, Zorova LD, Pevzner IB, Popkov VA, Babenko VA, Silachev DN, Plotnikov EY, Zorov DB. Kidney Cells Regeneration: Dedifferentiation of Tubular Epithelium, Resident Stem Cells and Possible Niches for Renal Progenitors. International Journal of Molecular Sciences. 2019; 20(24):6326. https://doi.org/10.3390/ijms20246326
Chicago/Turabian StyleAndrianova, Nadezda V., Marina I. Buyan, Ljubava D. Zorova, Irina B. Pevzner, Vasily A. Popkov, Valentina A. Babenko, Denis N. Silachev, Egor Y. Plotnikov, and Dmitry B. Zorov. 2019. "Kidney Cells Regeneration: Dedifferentiation of Tubular Epithelium, Resident Stem Cells and Possible Niches for Renal Progenitors" International Journal of Molecular Sciences 20, no. 24: 6326. https://doi.org/10.3390/ijms20246326
APA StyleAndrianova, N. V., Buyan, M. I., Zorova, L. D., Pevzner, I. B., Popkov, V. A., Babenko, V. A., Silachev, D. N., Plotnikov, E. Y., & Zorov, D. B. (2019). Kidney Cells Regeneration: Dedifferentiation of Tubular Epithelium, Resident Stem Cells and Possible Niches for Renal Progenitors. International Journal of Molecular Sciences, 20(24), 6326. https://doi.org/10.3390/ijms20246326








