Engineering Translation Components Improve Incorporation of Exotic Amino Acids
Abstract
:1. Introduction
2. EF-Tu Engineering to Compensate for Poor Affinity to nPAA-tRNAs
3. Ribosome Engineering to Improve Single Incorporation of Exotic Amino Acids
4. tRNA Engineering to Improve Multiple and Consecutive Incorporations of Exotic Amino Acids
5. Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EF-Tu | Elongation factor-thermo unstable |
| EF-P | Elongation factor-P |
| EF-G | Elongation factor-G |
| EF4 | Elongation factor 4 |
References
- Fahnestock, S.; Rich, A. Ribosome-catalyzed polyester formation. Science 1971, 173, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Forster, A.C.; Blacklow, S.C.; Cornish, V.W. Amino acid backbone specificity of the Escherichia coli translation machinery. J. Am. Chem. Soc. 2004, 126, 12752–12753. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Murakami, H.; Suga, H. Messenger RNA-programmed incorporation of multiple N-methyl-amino acids into linear and cyclic peptides. Chem. Biol. 2008, 15, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Fujino, T.; Goto, Y.; Suga, H.; Murakami, H. Reevaluation of the d-amino acid compatibility with the elongation event in translation. J. Am. Chem. Soc. 2013, 135, 1830–1837. [Google Scholar] [CrossRef] [PubMed]
- Fujino, T.; Goto, Y.; Suga, H.; Murakami, H. Ribosomal synthesis of peptides with multiple β-amino acids. J. Am. Chem. Soc. 2016, 138, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Ohta, A.; Murakami, H.; Higashimura, E.; Suga, H. Synthesis of polyester by means of genetic code reprogramming. Chem. Biol. 2007, 14, 1315–1322. [Google Scholar] [CrossRef]
- LaRiviere, F.J.; Wolfson, A.D.; Uhlenbeck, O.C. Uniform binding of aminoacyl-tRNAs to elongation factor Tu by thermodynamic compensation. Science 2001, 294, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, J.; Jahnz, M.; Bethge, L.; Paal, K.; Jung, M.; Schuster, M.; Albrecht, R.; Jarosch, F.; Nierhaus, K.H.; Klussmann, S. Outwitting EF-Tu and the ribosome: Translation with d-amino acids. Nucleic Acids Res. 2015, 43, 5687–5698. [Google Scholar] [CrossRef]
- Schrader, J.M.; Chapman, S.J.; Uhlenbeck, O.C. Understanding the sequence specificity of tRNA binding to elongation factor Tu using tRNA mutagenesis. J. Mol. Biol. 2009, 386, 1255–1264. [Google Scholar] [CrossRef]
- Dale, T.; Sanderson, L.E.; Uhlenbeck, O.C. The affinity of elongation factor Tu for an aminoacyl-tRNA is modulated by the esterified amino acid. Biochemistry 2004, 43, 6159–6166. [Google Scholar] [CrossRef]
- Dale, T.; Uhlenbeck, O.C. Amino acid specificity in translation. Trends Biochem. Sci. 2005, 30, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y.; Ohtsuki, T.; Shimizu, Y.; Ueda, T.; Sisido, M. Elongation factor Tu mutants expand amino acid tolerance of protein biosynthesis system. J. Am. Chem. Soc. 2007, 129, 14458–14462. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Hohn, M.J.; Umehara, T.; Guo, L.T.; Osborne, E.M.; Benner, J.; Noren, C.J.; Rinehart, J.; Soll, D. Expanding the genetic code of Escherichia coli with phosphoserine. Science 2011, 333, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Oh, S.; Yang, A.; Kim, J.; Soll, D.; Lee, D.; Park, H.S. A facile strategy for selective incorporation of phosphoserine into histones. Angew. Chem. Int. Ed. Engl. 2013, 52, 5771–5775. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Ip, K.; Soll, D. Expanding the genetic code of Escherichia coli with phosphotyrosine. FEBS Lett. 2016, 590, 3040–3047. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Soll, D.; Sevostyanova, A. Challenges of site-specific selenocysteine incorporation into proteins by Escherichia coli. RNA Biol. 2018, 15, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Haruna, K.; Alkazemi, M.H.; Liu, Y.; Soll, D.; Englert, M. Engineering the elongation factor Tu for efficient selenoprotein synthesis. Nucleic Acids Res. 2014, 42, 9976–9983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dedkova, L.M.; Fahmi, N.E.; Golovine, S.Y.; Hecht, S.M. Enhanced d-amino acid incorporation into protein by modified ribosomes. J. Am. Chem. Soc. 2003, 125, 6616–6617. [Google Scholar] [CrossRef] [PubMed]
- Dedkova, L.M.; Fahmi, N.E.; Golovine, S.Y.; Hecht, S.M. Construction of modified ribosomes for incorporation of d-amino acids into proteins. Biochemistry 2006, 45, 15541–15551. [Google Scholar] [CrossRef]
- Dedkova, L.M.; Fahmi, N.E.; Paul, R.; del Rosario, M.; Zhang, L.; Chen, S.; Feder, G.; Hecht, S.M. β-Puromycin selection of modified ribosomes for in vitro incorporation of β-amino acids. Biochemistry 2012, 51, 401–415. [Google Scholar] [CrossRef]
- Maini, R.; Nguyen, D.T.; Chen, S.; Dedkova, L.M.; Chowdhury, S.R.; Alcala-Torano, R.; Hecht, S.M. Incorporation of β-amino acids into dihydrofolate reductase by ribosomes having modifications in the peptidyltransferase center. Bioorg. Med. Chem. 2013, 21, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Czekster, C.M.; Robertson, W.E.; Walker, A.S.; Soll, D.; Schepartz, A. In vivo biosynthesis of a β-amino acid-containing protein. J. Am. Chem. Soc. 2016, 138, 5194–5197. [Google Scholar] [CrossRef] [PubMed]
- Katoh, T.; Iwane, Y.; Suga, H. Logical engineering of D-arm and T-stem of tRNA that enhances d-amino acid incorporation. Nucleic Acids Res. 2017, 45, 12601–12610. [Google Scholar] [CrossRef] [PubMed]
- Katoh, T.; Suga, H. Ribosomal incorporation of consecutive β-amino acids. J. Am. Chem. Soc. 2018, 140, 12159–12167. [Google Scholar] [CrossRef] [PubMed]
- Ude, S.; Lassak, J.; Starosta, A.; Kraxenberger, T.; Wilson, D.; Jung, K. Translation elongation factor EF-P alleviates ribosome stalling at polyproline stretches. Science 2013, 339, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Doerfel, L.K.; Wohlgemuth, I.; Kothe, C.; Peske, F.; Urlaub, H.; Rodnina, M.V. EF-P is essential for rapid synthesis of proteins containing consecutive proline residues. Science 2013, 339, 85–88. [Google Scholar] [CrossRef]
- Katoh, T.; Wohlgemuth, I.; Nagano, M.; Rodnina, M.V.; Suga, H. Essential structural elements in tRNA(Pro) for EF-P-mediated alleviation of translation stalling. Nat. Commun. 2016, 7, 11657. [Google Scholar] [CrossRef] [PubMed]
- Huter, P.; Arenz, S.; Bock, L.V.; Graf, M.; Frister, J.O.; Heuer, A.; Peil, L.; Starosta, A.L.; Wohlgemuth, I.; Peske, F.; et al. Structural basis for polyproline-mediated ribosome stalling and rescue by the translation elongation factor EF-P. Mol. Cell 2017, 68, 515–527.e6. [Google Scholar] [CrossRef]
- Katoh, T.; Tajima, K.; Suga, H. Consecutive elongation of d-amino acids in translation. Cell Chem. Biol. 2017, 24, 1–9. [Google Scholar] [CrossRef]
- Murakami, H.; Ohta, A.; Ashigai, H.; Suga, H. A highly flexible tRNA acylation method for non-natural polypeptide synthesis. Nat. Methods 2006, 3, 357–359. [Google Scholar] [CrossRef]
- Goto, Y.; Katoh, T.; Suga, H. Flexizymes for genetic code reprogramming. Nat. Protoc. 2011, 6, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Katoh, T. Engineering the ribosomal translation system to introduce non-proteinogenic amino acids into peptides. Mol. Technol. Life Innov. 2018, 2, 87–111. [Google Scholar]
- Wang, L.; Brock, A.; Herberich, B.; Schultz, P.G. Expanding the genetic code of Escherichia coli. Science 2001, 292, 498–500. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.W.; Cropp, T.A.; Anderson, J.C.; Mukherji, M.; Zhang, Z.; Schultz, P.G. An expanded eukaryotic genetic code. Science 2003, 301, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Schultz, P.G. Adding new chemistries to the genetic code. Annu. Rev. Biochem. 2010, 79, 413–444. [Google Scholar] [CrossRef] [PubMed]
- Ai, H.W. Biochemical analysis with the expanded genetic lexicon. Anal. Bioanal. Chem. 2012, 403, 2089–2102. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.M.; Alford, B.L.; Kuroda, Y.; Kitano, S. “Chemical aminoacylation” of tRNA’s. J. Biol. Chem. 1978, 253, 4517–4520. [Google Scholar] [PubMed]
- Heckler, T.G.; Zama, Y.; Naka, T.; Hecht, S.M. Dipeptide formation with misacylated tRNAPhes. J. Biol. Chem. 1983, 258, 4492–4495. [Google Scholar] [PubMed]
- Robertson, S.A.; Noren, C.J.; Anthony-Cahill, S.J.; Griffith, M.C.; Schultz, P.G. The use of 5′-phospho-2 deoxyribocytidylylriboadenosine as a facile route to chemical aminoacylation of tRNA. Nucleic Acids Res. 1989, 17, 9649–9660. [Google Scholar] [CrossRef]
- Gite, S.; Mamaev, S.; Olejnik, J.; Rothschild, K. Ultrasensitive fluorescence-based detection of nascent proteins in gels. Anal. Biochem. 2000, 279, 218–225. [Google Scholar] [CrossRef]
- Merryman, C.; Green, R. Transformation of aminoacyl tRNAs for the in vitro selection of “drug-like” molecules. Chem. Biol. 2004, 11, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Forster, A.C.; Cornish, V.W.; Blacklow, S.C. Pure translation display. Anal. Biol. 2004, 333, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Mamaev, S.; Olejnik, J.; Olejnik, E.K.; Rothschild, K.J. Cell-free N-terminal protein labeling using initiator suppressor tRNA. Anal. Biochem. 2004, 326, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, Y.; Shoji, I.; Miyagawa, S.; Kawakami, T.; Katoh, T.; Goto, Y.; Suga, H. Natural product-like macrocyclic N-methyl-peptide inhibitors against a ubiquitin ligase uncovered from a ribosome-expressed de novo library. Chem. Biol. 2011, 18, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Morimoto, J.; Suga, H. In vitro selection of anti-Akt2 thioether-macrocyclic peptides leading to isoform-selective inhibitors. ACS Chem. Biol. 2012, 7, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, J.; Hayashi, Y.; Suga, H. Discovery of macrocyclic peptides ARMED with a mechanism-based warhead: Isoform-selective inhibition of human deacetylase SIRT2. Angew. Chem. Int. Ed. Eng. 2012, 51, 3423–3427. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Hipolito, C.J.; Maturana, A.D.; Ito, K.; Kuroda, T.; Higuchi, T.; Katoh, T.; Kato, H.E.; Hattori, M.; Kumazaki, K.; et al. Structural basis for the drug extrusion mechanism by a MATE multidrug transporter. Nature 2013, 496, 247–251. [Google Scholar] [CrossRef]
- Hipolito, C.J.; Tanaka, Y.; Katoh, T.; Nureki, O.; Suga, H. A macrocyclic peptide that serves as a cocrystallization ligand and inhibits the function of a MATE family transporter. Molecules 2013, 18, 10514–10530. [Google Scholar] [CrossRef]
- Ito, K.; Sakai, K.; Suzuki, Y.; Ozawa, N.; Hatta, T.; Natsume, T.; Matsumoto, K.; Suga, H. Artificial human Met agonists based on macrocycle scaffolds. Nat. Commun. 2015, 6, 6373. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, K.; Goto, Y.; Katoh, T.; Yamashita, T.; Kaneko, S.; Suga, H. A fluorescent imaging probe based on a macrocyclic scaffold that binds to cellular EpCAM. J. Mol. Evol. 2015, 81, 210–217. [Google Scholar] [CrossRef]
- Matsunaga, Y.; Bashiruddin, N.K.; Kitago, Y.; Takagi, J.; Suga, H. Allosteric inhibition of a semaphorin 4D Receptor plexin B1 by a high-affinity macrocyclic peptide. Cell Chem. Biol. 2016, 23, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Jongkees, S.A.K.; Caner, S.; Tysoe, C.; Brayer, G.D.; Withers, S.G.; Suga, H. Rapid discovery of potent and selective glycosidase-inhibiting de novo peptides. Cell Chem. Biol. 2017, 24, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Dranchak, P.; Li, Z.; MacArthur, R.; Munson, M.S.; Mehzabeen, N.; Baird, N.J.; Battalie, K.P.; Ross, D.; Lovell, S.; et al. Macrocycle peptides delineate locked-open inhibition mechanism for microorganism phosphoglycerate mutases. Nat. Commun. 2017, 8, 14932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawamura, A.; Munzel, M.; Kojima, T.; Yapp, C.; Bhushan, B.; Goto, Y.; Tumber, A.; Katoh, T.; King, O.N.; Passioura, T.; et al. Highly selective inhibition of histone demethylases by de novo macrocyclic peptides. Nat. Commun. 2017, 8, 14773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, X.; Lu, L.Y.; Passioura, T.; Suga, H. Macrocyclic peptide inhibitors for the protein-protein interaction of Zaire Ebola virus protein 24 and karyopherin alpha 5. Org. Biomol. Chem. 2017, 15, 5155–5160. [Google Scholar] [CrossRef] [PubMed]
- Nishio, K.; Belle, R.; Katoh, T.; Kawamura, A.; Sengoku, T.; Hanada, K.; Ohsawa, N.; Shirouzu, M.; Yokoyama, S.; Suga, H. Thioether macrocyclic peptides selected against TET1 compact catalytic domain inhibit TET1 catalytic activity. Chembiochem 2018, 19, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Passioura, T.; Bhushan, B.; Tumber, A.; Kawamura, A.; Suga, H. Structure-activity studies of a macrocyclic peptide inhibitor of histone lysine demethylase 4A. Bioorg. Med. Chem. 2018, 26, 1225–1231. [Google Scholar] [CrossRef]
- Passioura, T.; Watashi, K.; Fukano, K.; Shimura, S.; Saso, W.; Morishita, R.; Ogasawara, Y.; Tanaka, Y.; Mizokami, M.; Sureau, C.; et al. De novo macrocyclic peptide inhibitors of hepatitis B virus cellular entry. Cell Chem. Biol. 2018, 25, 906–915.e5. [Google Scholar] [CrossRef]
- Passioura, T.; Katoh, T.; Goto, Y.; Suga, H. Selection-based discovery of druglike macrocyclic peptides. Annu. Rev. Biochem. 2014, 83, 727–752. [Google Scholar] [CrossRef]
- Obexer, R.; Walport, L.J.; Suga, H. Exploring sequence space: Harnessing chemical and biological diversity towards new peptide leads. Curr. Opin. Chem. Biol. 2017, 38, 52–61. [Google Scholar] [CrossRef]
- Walport, L.J.; Obexer, R.; Suga, H. Strategies for transitioning macrocyclic peptides to cell-permeable drug leads. Curr. Opin. Biotechnol. 2017, 48, 242–250. [Google Scholar] [CrossRef] [PubMed]

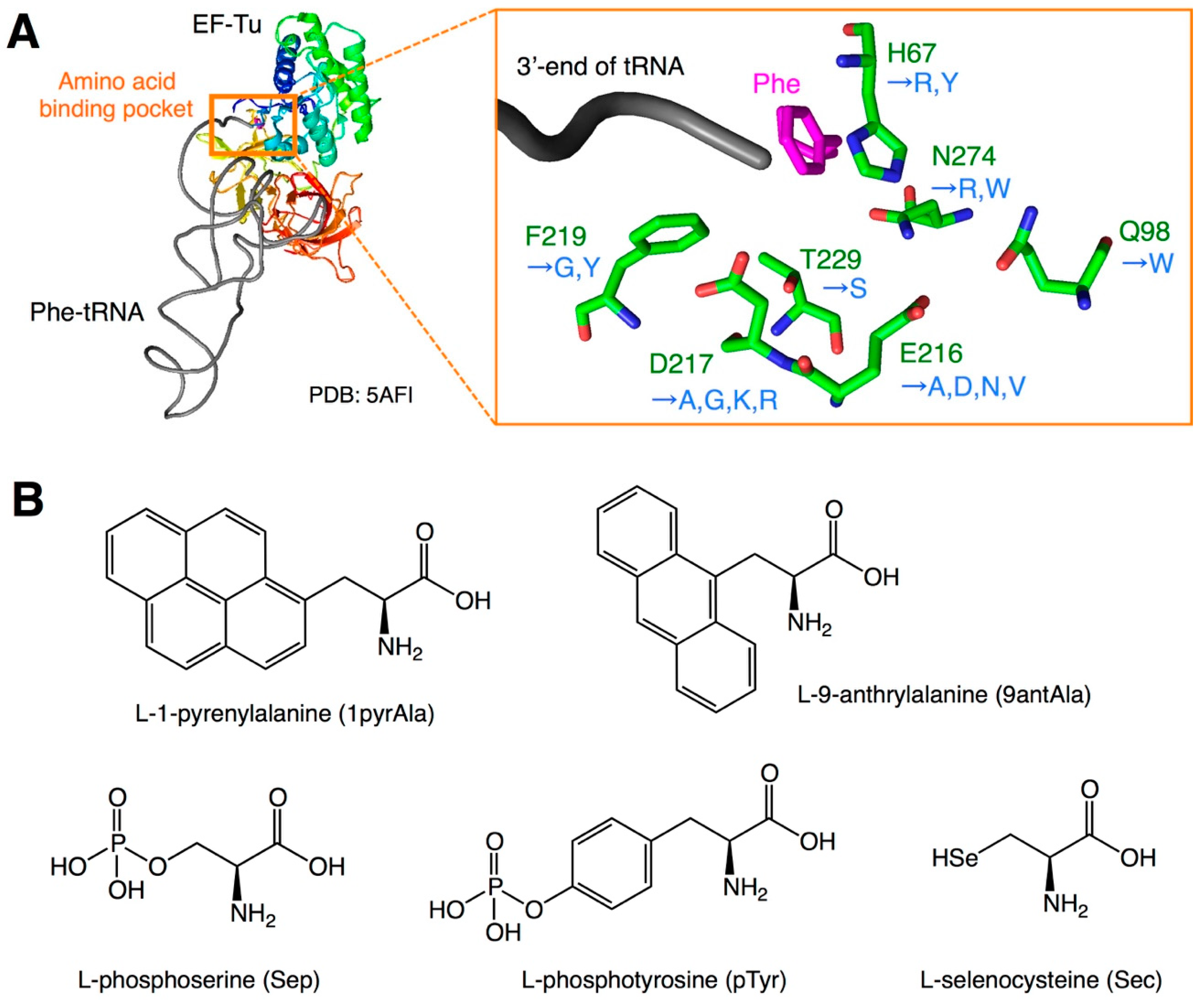

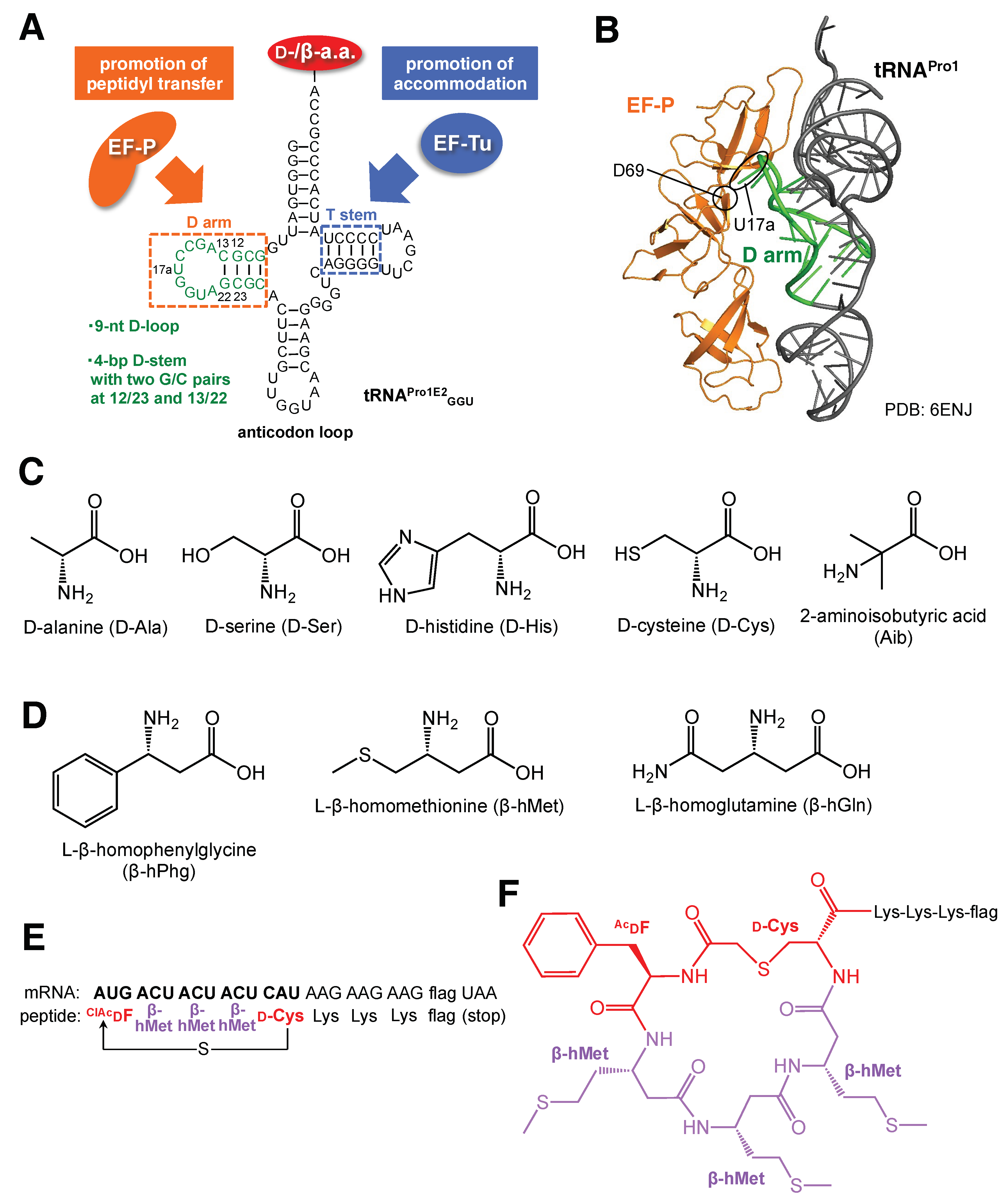
| Variant Name | Mutation | Amino Acid | Reference |
|---|---|---|---|
| E215A | E216A * | bulky a.a. | Doi et al. [12] |
| D216A | D217 * | bulky a.a. | Doi et al. [12] |
| EF-Sep | H67R, E216N, D217G, F219Y, T229S, N274W | Sep | Park et al. [13] |
| EF-Sep21 | H67R, E216V, D217G, F219Y, T229S, N274W | Sep | Lee et al. [14] |
| EF-pY | E216V, D217G, F219G | pTyr | Fan et al. [15] |
| EF-R1 | H67Y, E216D, D217R, N274R | Sec | Haruna et al. [17] |
| EF-Sel1 | H67R, Q98W, E216N, D217K, N274R | Sec | Haruna et al. [17] |
| Variant Name | Mutation | Amino Acid | Reference |
|---|---|---|---|
| A4 | 2447UGGC2450 | D-Phe, D-Met | Dedkova et al. [18] |
| B25 | 2457GCUGAU2462 | D-Phe, D-Met | Dedkova et al. [19] |
| 040329 | 2057AGCGUGA2063, 2502UGGCAG2507 | β-hGly, β-hAla, β, β-dimethyl-β-hGly, β-hPhg, β-(p-Br)hPhg | Dedkova et al. [20] Maini et al. [21] |
| P7A7 | 2057AGCGUGA2063, 2502UGACUU2507 | β-(p-Br)hPhg | Czekster et al. [22] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katoh, T.; Suga, H. Engineering Translation Components Improve Incorporation of Exotic Amino Acids. Int. J. Mol. Sci. 2019, 20, 522. https://doi.org/10.3390/ijms20030522
Katoh T, Suga H. Engineering Translation Components Improve Incorporation of Exotic Amino Acids. International Journal of Molecular Sciences. 2019; 20(3):522. https://doi.org/10.3390/ijms20030522
Chicago/Turabian StyleKatoh, Takayuki, and Hiroaki Suga. 2019. "Engineering Translation Components Improve Incorporation of Exotic Amino Acids" International Journal of Molecular Sciences 20, no. 3: 522. https://doi.org/10.3390/ijms20030522





