The Effects of Resveratrol in the Treatment of Metabolic Syndrome
Abstract
1. Introduction
2. The Synthesis and Functions of Resveratrol
3. Metabolic Syndrome
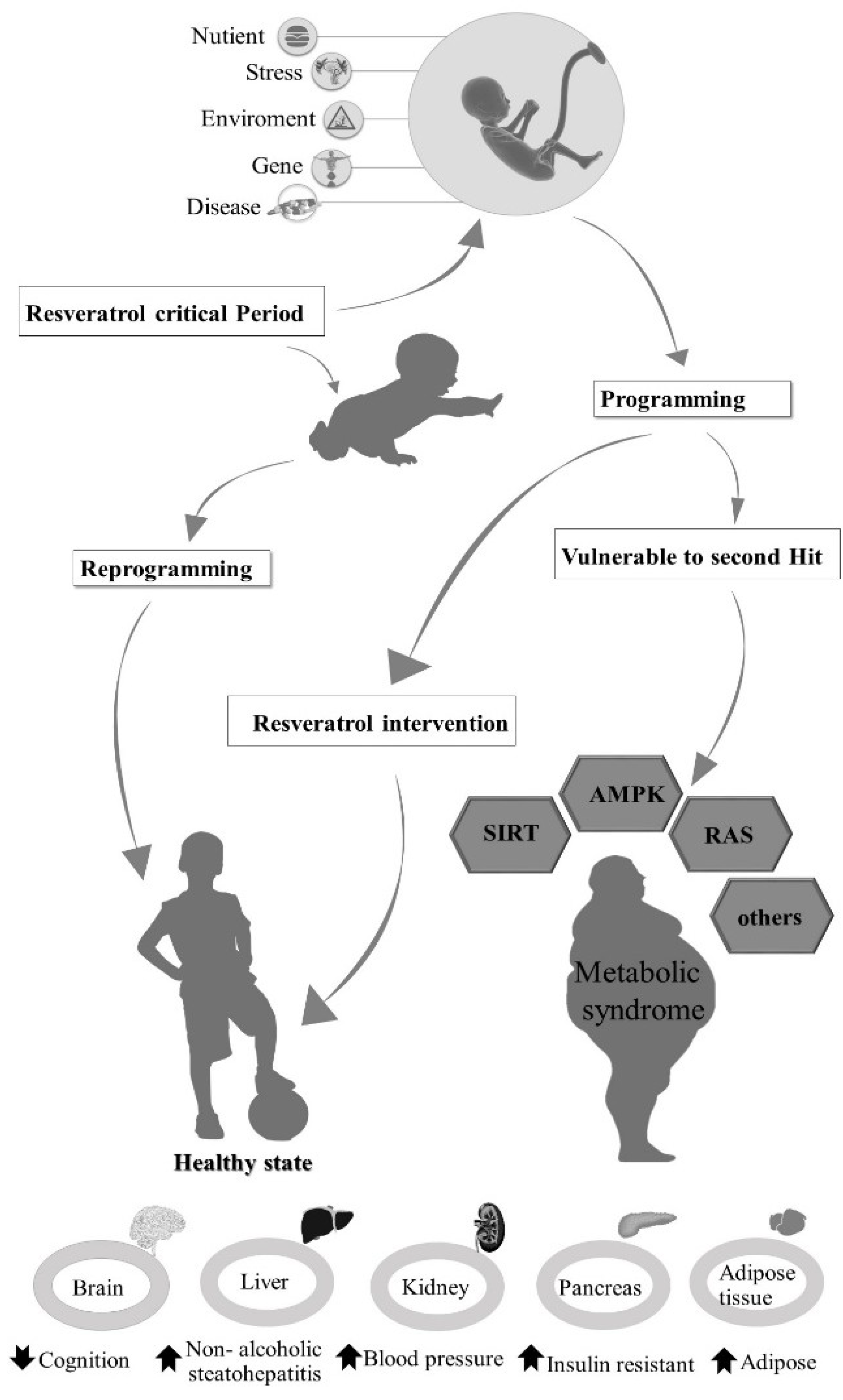
4. Mechanisms of Resveratrol Action in the Treatment of Metabolic Syndrome
4.1. SIRT1
4.2. AMPK
4.3. Renin-Angiotensin System
4.4. Others
5. Treatment of Metabolic Syndrome
5.1. Reprogramming of Metabolic Syndrome by Resveratrol
5.2. Treatment of Metabolic Syndrome by Resveratrol
5.2.1. Clinical Studies
5.2.2. Preclinical Studies
6. Resveratrol Derivatives
7. Conclusions
Funding
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase |
| AT1R | Angiotensin II Type I Receptors |
| AT2R | Angiotensin type 2 receptor |
| Bax | BCL2 Associated X, Apoptosis Regulator |
| Bcl-2 | B-cell lymphoma 2 |
| BDNF | brain-derived neurotrophic factor |
| BMI | Body Mass Index |
| DOHaD | Developmental origins of health and disease |
| FOXO | Forkhead box class O |
| MasR | receptor Mas |
| MKRN1 | Makorin ring finger protein 1 |
| NAFLD | nonalcoholic fatty liver disease |
| NMDA receptor | N-Methyl-d-Aspartate receptor |
| Nrf2 | Nuclear factor (erythroid-derived 2)-like 2 |
| PAI-1 | plasminogen activator inhibitor-1 |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator-1α |
| RAS | Renin-angiotensin system |
| SIRT1 | Sirtuins 1 |
| VSMCs | vascular smooth muscle cells |
References
- Frémont, L. Biological effects of resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef]
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat. Res. /Rev. Mutat. Res. 2012, 750, 60–82. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.Z.; Liu, R.X.; Wang, D.P.; Wang, X.; Dai, C.C. Biocatalysis and biotransformation of resveratrol in microorganisms. Biotechnol. Lett. 2015, 37, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, B.; Liu, H.; Guo, X.; Yuan, Y. Profiling influences of gene overexpression on heterologous resveratrol production in Saccharomyces cerevisiae. Front. Chem. Sci. Eng. 2017, 11, 117–125. [Google Scholar] [CrossRef]
- Chastang, T.; Pozzobon, V.; Taidi, B.; Courot, E.; Clément, C.; Pareau, D. Resveratrol production by grapevine cells in fed-batch bioreactor: Experiments and modelling. Biochem. Eng. J. 2018, 131, 9–16. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Nonomura, S.; Kanagawa, H.; Makimoto, A. Chemical Constituents of Polygonaceous Plants. I. Studies on the Components of KO-J O-KON. (Polygonum Cuspidatum Sieb. ET ZUCC.). Yakugaku Zasshi J. Pharm. Soc. Jpn. 1963, 83, 988–990. [Google Scholar] [CrossRef]
- Becker, J.; Armstrong, G.; Vandermerwe, M.; Lambrechts, M.; Vivier, M.; Pretorius, I. Metabolic engineering of for the synthesis of the wine-related antioxidant resveratrol. FEMS Yeast Res. 2003, 4, 79–85. [Google Scholar] [CrossRef]
- Oh, W.Y.; Shahidi, F. Antioxidant activity of resveratrol ester derivatives in food and biological model systems. Food Chem. 2018, 261, 267–273. [Google Scholar] [CrossRef]
- Donnelly, L.E.; Newton, R.; Kennedy, G.E.; Fenwick, P.S.; Leung, R.H.F.; Ito, K.; Russell, R.E.K.; Barnes, P.J. Anti-inflammatory effects of resveratrol in lung epithelial cells: Molecular mechanisms. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L774–L783. [Google Scholar] [CrossRef]
- Amri, A.; Chaumeil, J.C.; Sfar, S.; Charrueau, C. Administration of resveratrol: What formulation solutions to bioavailability limitations? J. Control. Release 2012, 158, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Kursvietiene, L.; Staneviciene, I.; Mongirdiene, A.; Bernatoniene, J. Multiplicity of effects and health benefits of resveratrol. Medicina 2016, 52, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Fabjanowicz, M.; Płotka-Wasylka, J.; Namieśnik, J. Detection, identification and determination of resveratrol in wine. Problems and challenges. TrAC Trends Anal. Chem. 2018, 103, 21–33. [Google Scholar] [CrossRef]
- Bostanghadiri, N.; Pormohammad, A.; Chirani, A.S.; Pouriran, R.; Erfanimanesh, S.; Hashemi, A. Comprehensive review on the antimicrobial potency of the plant polyphenol Resveratrol. Biomed. Pharmacother. 2017, 95, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Ji, H.-F. Reciprocal Interactions Between Resveratrol and Gut Microbiota Deepen Our Understanding of Molecular Mechanisms Underlying Its Health Benefits. Trends Food Sci. Technol. 2018. [Google Scholar] [CrossRef]
- Cottart, C.H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J.L. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 2010, 54, 7–16. [Google Scholar] [CrossRef] [PubMed]
- La Porte, C.; Voduc, N.; Zhang, G.; Seguin, I.; Tardiff, D.; Singhal, N.; Cameron, D.W. Steady-State pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects. Clin. Pharmacokinet. 2010, 49, 449–454. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A. The developmental origins of the metabolic syndrome. Trends Endocrinol. Metab. 2004, 15, 183–187. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef]
- Bremer, A.A. Resveratrol use in metabolic syndrome. Metab. Syndr. Relat. Disord. 2014, 12, 493–495. [Google Scholar] [CrossRef]
- Elibol, B.; Kilic, U. High Levels of SIRT1 Expression as a Protective Mechanism Against Disease-Related Conditions. Front. Endocrinol. 2018, 9, 614. [Google Scholar] [CrossRef] [PubMed]
- Rabi, T.; Bishayee, A. d-Limonene sensitizes docetaxel-induced cytotoxicity in human prostate cancer cells: Generation of reactive oxygen species and induction of apoptosis. J. Carcinog. 2009, 8, 9. [Google Scholar] [PubMed]
- Diaz-Gerevini, G.T.; Repossi, G.; Dain, A.; Tarres, M.C.; Das, U.N.; Eynard, A.R. Beneficial action of resveratrol: How and why? Nutrition 2016, 32, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.S.; Canto, C. The molecular targets of resveratrol. Biochim. Biophys. Acta 2015, 1852, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, S.; Higurashi, T.; Nakajima, A. AMPK: Therapeutic Target for Diabetes and Cancer Prevention. Curr. Pharm. Des. 2017, 23, 3629–3644. [Google Scholar] [CrossRef] [PubMed]
- Bullon, P.; Marin-Aguilar, F.; Roman-Malo, L. AMPK/Mitochondria in Metabolic Diseases. Exp. Suppl. 2016, 107, 129–152. [Google Scholar] [PubMed]
- Tamaki, N.; Cristina Orihuela-Campos, R.; Inagaki, Y.; Fukui, M.; Nagata, T.; Ito, H.-O. Resveratrol improves oxidative stress and prevents the progression of periodontitis via the activation of the Sirt1/AMPK and the Nrf2/antioxidant defense pathways in a rat periodontitis model. Free Radic. Biol. Med. 2014, 75, 222–229. [Google Scholar] [CrossRef]
- Jang, I.A.; Kim, E.N.; Lim, J.H.; Kim, M.Y.; Ban, T.H.; Yoon, H.E.; Park, C.W.; Chang, Y.S.; Choi, B.S. Effects of Resveratrol on the Renin-Angiotensin System in the Aging Kidney. Nutrients 2018, 10, 1741. [Google Scholar] [CrossRef]
- Kim, E.N.; Kim, M.Y.; Lim, J.H.; Kim, Y.; Shin, S.J.; Park, C.W.; Kim, Y.S.; Chang, Y.S.; Yoon, H.E.; Choi, B.S. The protective effect of resveratrol on vascular aging by modulation of the renin-angiotensin system. Atherosclerosis 2018, 270, 123–131. [Google Scholar] [CrossRef]
- Chaplin, A.; Carpene, C.; Mercader, J. Resveratrol, Metabolic Syndrome, and Gut Microbiota. Nutrients 2018, 10, 1651. [Google Scholar] [CrossRef]
- De Oliveira, M.R.; Nabavi, S.F.; Manayi, A.; Daglia, M.; Hajheydari, Z.; Nabavi, S.M. Resveratrol and the mitochondria: From triggering the intrinsic apoptotic pathway to inducing mitochondrial biogenesis, a mechanistic view. Biochim. Biophys. Acta (BBA) Gen. Subj. 2016, 1860, 727–745. [Google Scholar] [CrossRef] [PubMed]
- Canto, C.; Auwerx, J. Targeting sirtuin 1 to improve metabolism: All you need is NAD(+)? Pharm. Rev. 2012, 64, 166–187. [Google Scholar] [CrossRef] [PubMed]
- Palsamy, P.; Subramanian, S. Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2-Keap1 signaling. Biochim. Biophys. Acta 2011, 1812, 719–731. [Google Scholar] [CrossRef]
- De Sá Coutinho, D.; Pacheco, T.M.; Frozza, L.R.; Bernardi, A. Anti-Inflammatory Effects of Resveratrol: Mechanistic Insights. Int. J. Mol. Sci. 2018, 19, 1812. [Google Scholar] [CrossRef] [PubMed]
- Cherniack, E.P. Polyphenols: Planting the seeds of treatment for the metabolic syndrome. Nutrition 2011, 27, 617–623. [Google Scholar] [CrossRef]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Sata, F. Developmental Origins of Health and Disease (DOHaD) and Epidemiology. Nihon Eiseigaku Zasshi. Jpn. J. Hyg. 2016, 71, 41–46. [Google Scholar] [CrossRef]
- Nüsken, E.; Dötsch, J.; Weber, L.T.; Nüsken, K.-D. Developmental Programming of Renal Function and Re-Programming Approaches. Front. Pediatr. 2018, 6, 36. [Google Scholar] [CrossRef]
- Tain, Y.-L.; Huang, L.-T.; Hsu, C.-N. Developmental Programming of Adult Disease: Reprogramming by Melatonin? Int. J. Mol. Sci. 2017, 18, 426. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Developmental Programming of the Metabolic Syndrome: Can We Reprogram with Resveratrol? Int. J. Mol. Sci. 2018, 19, 2584. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, S.; Gao, K.; Zhou, Z.; Wang, C.; Shen, Z.; Guo, Y.; Li, Z.; Wan, Z.; Liu, C.; et al. Resveratrol protects against spinal cord injury by activating autophagy and inhibiting apoptosis mediated by the SIRT1/AMPK signaling pathway. Neuroscience 2017, 348, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Quintela, A.; Carpene, C.; Fernandez, M.; Aguirre, L.; Milton-Laskibar, I.; Contreras, J.; Portillo, M.P. Anti-obesity effects of resveratrol: Comparison between animal models and humans. J. Physiol. Biochem. 2016, 73, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Pasinetti, G.M.; Wang, J.; Marambaud, P.; Ferruzzi, M.; Gregor, P.; Knable, L.A.; Ho, L. Neuroprotective and metabolic effects of resveratrol: Therapeutic implications for Huntington’s disease and other neurodegenerative disorders. Exp. Neurol. 2011, 232, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mendez-del Villar, M.; Gonzalez-Ortiz, M.; Martinez-Abundis, E.; Perez-Rubio, K.G.; Lizarraga-Valdez, R. Effect of resveratrol administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab. Syndr. Relat. Disord. 2014, 12, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Korsholm, A.S.; Kjaer, T.N.; Ornstrup, M.J.; Pedersen, S.B. Comprehensive Metabolomic Analysis in Blood, Urine, Fat, and Muscle in Men with Metabolic Syndrome: A Randomized, Placebo-Controlled Clinical Trial on the Effects of Resveratrol after Four Months’ Treatment. Int. J. Mol. Sci. 2017, 18, 554. [Google Scholar] [CrossRef] [PubMed]
- Amiot, M.J.; Riva, C.; Vinet, A. Effects of dietary polyphenols on metabolic syndrome features in humans: A systematic review. Obes. Rev. 2016, 17, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, R.; Tamtaji, O.R.; Lankarani, K.B.; Mirhosseini, N.; Akbari, M.; Dadgostar, E.; Peymani, P.; Asemi, Z. The effects of resveratrol supplementation on biomarkers of inflammation and oxidative stress among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Food Funct. 2018, 9, 6116–6128. [Google Scholar] [CrossRef] [PubMed]
- Gunstad, J.; Lhotsky, A.; Wendell, C.R.; Ferrucci, L.; Zonderman, A.B. Longitudinal examination of obesity and cognitive function: Results from the Baltimore longitudinal study of aging. Neuroepidemiology 2010, 34, 222–229. [Google Scholar] [CrossRef]
- Fujitaka, K.; Otani, H.; Jo, F.; Jo, H.; Nomura, E.; Iwasaki, M.; Nishikawa, M.; Iwasaka, T.; Das, D.K. Modified resveratrol Longevinex improves endothelial function in adults with metabolic syndrome receiving standard treatment. Nutr. Res. 2011, 31, 842–847. [Google Scholar] [CrossRef]
- Kjaer, T.N.; Ornstrup, M.J.; Poulsen, M.M.; Stodkilde-Jorgensen, H.; Jessen, N.; Jorgensen, J.O.L.; Richelsen, B.; Pedersen, S.B. No Beneficial Effects of Resveratrol on the Metabolic Syndrome: A Randomized Placebo-Controlled Clinical Trial. J. Clin. Endocrinol. Metab. 2017, 102, 1642–1651. [Google Scholar] [CrossRef]
- Da Silva, I.V.; Rodrigues, J.S.; Rebelo, I.; Miranda, J.P.G.; Soveral, G. Revisiting the metabolic syndrome: The emerging role of aquaglyceroporins. Cell. Mol. Life Sci. CMLS 2018, 75, 1973–1988. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Sun, J.; Xia, S.; Tang, X.; Shi, Y.; Le, G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014, 5, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, D.; Zambonin, L.; Dalla Sega, F.V.; Hrelia, S. Polyphenols as Modulators of Aquaporin Family in Health and Disease. Oxid. Med. Cell. Longev. 2015, 2015, 196914. [Google Scholar] [CrossRef] [PubMed]
- Cournot, M.; Marquié, J.C.; Ansiau, D.; Martinaud, C.; Fonds, H.; Ferrières, J.; Ruidavets, J.B. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology 2006, 67, 1208. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Seo, H.I.; Cha, H.Y.; Yang, Y.J.; Kwon, S.H.; Yang, S.J. Diabetes and Alzheimer’s Disease: Mechanisms and Nutritional Aspects. Clin. Nutr. Res. 2018, 7, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Heyward, F.D.; Gilliam, D.; Coleman, M.A.; Gavin, C.F.; Wang, J.; Kaas, G.; Trieu, R.; Lewis, J.; Moulden, J.; Sweatt, J.D. Obesity Weighs down Memory through a Mechanism Involving the Neuroepigenetic Dysregulation of Sirt1. J. Neurosci. 2016, 36, 1324–1335. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Wang, J.; Xu, M.; Wang, Y.; Zhang, M.; Zhou, Y. Resveratrol Improves Cognitive Impairment by Regulating Apoptosis and Synaptic Plasticity in Streptozotocin-Induced Diabetic Rats. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2016, 40, 1670–1677. [Google Scholar] [CrossRef]
- Li, S.W.; Yu, H.R.; Sheen, J.M.; Tiao, M.M.; Tain, Y.L.; Lin, I.C.; Lin, Y.J.; Chang, K.A.; Tsai, C.C.; Huang, L.T. A maternal high-fat diet during pregnancy and lactation, in addition to a postnatal high-fat diet, leads to metabolic syndrome with spatial learning and memory deficits: Beneficial effects of resveratrol. Oncotarget 2017, 8, 111998–112013. [Google Scholar] [CrossRef]
- Sheen, J.M.; Yu, H.R.; Tain, Y.L.; Tsai, W.L.; Tiao, M.M.; Lin, I.C.; Tsai, C.C.; Lin, Y.J.; Huang, L.T. Combined maternal and postnatal high-fat diet leads to metabolic syndrome and is effectively reversed by resveratrol: A multiple-organ study. Sci. Rep. 2018, 8, 5607. [Google Scholar] [CrossRef]
- Robich, M.P.; Osipov, R.M.; Chu, L.M.; Han, Y.; Feng, J.; Nezafat, R.; Clements, R.T.; Manning, W.J.; Sellke, F.W. Resveratrol modifies risk factors for coronary artery disease in swine with metabolic syndrome and myocardial ischemia. Eur. J. Pharmacol. 2011, 664, 45–53. [Google Scholar] [CrossRef]
- Poulsen, M.M.; Larsen, J.O.; Hamilton-Dutoit, S.; Clasen, B.F.; Jessen, N.; Paulsen, S.K.; Kjaer, T.N.; Richelsen, B.; Pedersen, S.B. Resveratrol up-regulates hepatic uncoupling protein 2 and prevents development of nonalcoholic fatty liver disease in rats fed a high-fat diet. Nutr. Res. 2012, 32, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Lin, Y.J.; Sheen, J.M.; Lin, I.C.; Yu, H.R.; Huang, L.T.; Hsu, C.N. Resveratrol prevents the combined maternal plus postweaning high-fat-diets-induced hypertension in male offspring. J. Nutr. Biochem. 2017, 48, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T.; Szkudelska, K. Resveratrol and diabetes: From animal to human studies. Biochim. Biophys. Acta 2015, 1852, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Ahmad, R. Resveratrol mitigate structural changes and hepatic stellate cell activation in N′-nitrosodimethylamine-induced liver fibrosis via restraining oxidative damage. Chem. -Biol. Interact. 2014, 221, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz Demirtas, C.; Bircan, F.S.; Pasaoglu, O.T.; Turkozkan, N. The effects of resveratrol on hepatic oxidative stress in metabolic syndrome model induced by high fructose diet. Bratisl. Lek. Listy 2018, 119, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Bagul, P.K.; Middela, H.; Matapally, S.; Padiya, R.; Bastia, T.; Madhusudana, K.; Reddy, B.R.; Chakravarty, S.; Banerjee, S.K. Attenuation of insulin resistance, metabolic syndrome and hepatic oxidative stress by resveratrol in fructose-fed rats. Pharm. Res 2012, 66, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Burgess, T.A.; Robich, M.P.; Chu, L.M.; Bianchi, C.; Sellke, F.W. Improving glucose metabolism with resveratrol in a swine model of metabolic syndrome through alteration of signaling pathways in the liver and skeletal muscle. Arch. Surg. 2011, 146, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Tiao, M.M.; Lin, Y.J.; Yu, H.R.; Sheen, J.M.; Lin, I.C.; Lai, Y.J.; Tain, Y.L.; Huang, L.T.; Tsai, C.C. Resveratrol ameliorates maternal and post-weaning high-fat diet-induced nonalcoholic fatty liver disease via renin-angiotensin system. Lipids Health Dis. 2018, 17, 178. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Lim, J.H.; Youn, H.H.; Hong, Y.A.; Yang, K.S.; Park, H.S.; Chung, S.; Koh, S.H.; Shin, S.J.; Choi, B.S.; et al. Resveratrol prevents renal lipotoxicity and inhibits mesangial cell glucotoxicity in a manner dependent on the AMPK–SIRT1–PGC1α axis in db/db mice. Diabetologia 2013, 56, 204–217. [Google Scholar] [CrossRef]
- Cao, X.; Luo, T.; Luo, X.; Tang, Z. Resveratrol prevents AngII-induced hypertension via AMPK activation and RhoA/ROCK suppression in mice. Hypertens. Res. 2014, 37, 803. [Google Scholar] [CrossRef]
- Galic, S.; Oakhill, J.S.; Steinberg, G.R. Adipose tissue as an endocrine organ. Mol. Cell. Endocrinol. 2010, 316, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.G., Jr.; Leibel, R.L.; Seeley, R.J.; Schwartz, M.W. Obesity and leptin resistance: Distinguishing cause from effect. Trends Endocrinol. Metab. TEM 2010, 21, 643–651. [Google Scholar] [CrossRef]
- Franco, J.G.; Dias-Rocha, C.P.; Fernandes, T.P.; Albuquerque Maia, L.; Lisboa, P.C.; Moura, E.G.; Pazos-Moura, C.C.; Trevenzoli, I.H. Resveratrol treatment rescues hyperleptinemia and improves hypothalamic leptin signaling programmed by maternal high-fat diet in rats. Eur. J. Nutr. 2016, 55, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, L.; Fernandez-Quintela, A.; Arias, N.; Portillo, M.P. Resveratrol: Anti-obesity mechanisms of action. Molecules 2014, 19, 18632–18655. [Google Scholar] [CrossRef]
- Fernandez-Quintela, A.; Milton-Laskibar, I.; Gonzalez, M.; Portillo, M.P. Antiobesity effects of resveratrol: Which tissues are involved? Ann. N. Y. Acad. Sci. 2017, 1403, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Zagotta, I.; Dimova, E.Y.; Funcke, J.B.; Wabitsch, M.; Kietzmann, T.; Fischer-Posovszky, P. Resveratrol suppresses PAI-1 gene expression in a human in vitro model of inflamed adipose tissue. Oxid. Med. Cell. Longev. 2013, 2013, 793525. [Google Scholar]
- Fischer-Posovszky, P.; Kukulus, V.; Tews, D.; Unterkircher, T.; Debatin, K.M.; Fulda, S.; Wabitsch, M. Resveratrol regulates human adipocyte number and function in a Sirt1-dependent manner. Am. J. Clin. Nutr. 2010, 92, 5–15. [Google Scholar] [CrossRef]
- Ding, S.; Jiang, J.; Wang, Z.; Zhang, G.; Yin, J.; Wang, X.; Wang, S.; Yu, Z. Resveratrol reduces the inflammatory response in adipose tissue and improves adipose insulin signaling in high-fat diet-fed mice. PeerJ 2018, 6, e5173. [Google Scholar] [CrossRef]
- Kornicka, K.; Szlapka-Kosarzewska, J.; Smieszek, A.; Marycz, K. 5-Azacytydine and resveratrol reverse senescence and ageing of adipose stem cells via modulation of mitochondrial dynamics and autophagy. J. Cell. Mol. Med. 2018. [Google Scholar] [CrossRef]
- Peredo-Escarcega, A.E.; Guarner-Lans, V.; Perez-Torres, I.; Ortega-Ocampo, S.; Carreon-Torres, E.; Castrejon-Tellez, V.; Diaz-Diaz, E.; Rubio-Ruiz, M.E. The Combination of Resveratrol and Quercetin Attenuates Metabolic Syndrome in Rats by Modifying the Serum Fatty Acid Composition and by Upregulating SIRT 1 and SIRT 2 Expression in White Adipose Tissue. Evid. Based Complement. Altern. Med. 2015, 2015, 474032. [Google Scholar] [CrossRef]
- Bird, J.K.; Raederstorff, D.; Weber, P.; Steinert, R.E. Cardiovascular and Antiobesity Effects of Resveratrol Mediated through the Gut Microbiota. Adv. Nutr. 2017, 8, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Silvester, A.J.; Aseer, K.R.; Yun, J.W. Dietary polyphenols and their roles in fat browning. J. Nutr. Biochem. 2018, 64, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.R.; Tain, Y.L.; Tiao, M.M.; Chen, C.C.; Sheen, J.M.; Lin, I.C.; Li, S.W.; Tsai, C.C.; Lin, Y.J.; Hsieh, K.S.; et al. Prenatal dexamethasone and postnatal high-fat diet have a synergistic effect of elevating blood pressure through a distinct programming mechanism of systemic and adipose renin-angiotensin systems. Lipids Health Dis. 2018, 17, 50. [Google Scholar] [CrossRef] [PubMed]
- Suganami, T.; Ogawa, Y. Adipose tissue macrophages: Their role in adipose tissue remodeling. J. Leukoc. Biol. 2010, 88, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xu, S.; Qian, Y.; Xiao, Q. Resveratrol regulates microglia M1/M2 polarization via PGC-1alpha in conditions of neuroinflammatory injury. Brain Behav. Immun. 2017, 64, 162–172. [Google Scholar] [CrossRef]
- Carreras, A.; Zhang, S.X.; Almendros, I.; Wang, Y.; Peris, E.; Qiao, Z.; Gozal, D. Resveratrol attenuates intermittent hypoxia-induced macrophage migration to visceral white adipose tissue and insulin resistance in male mice. Endocrinology 2015, 156, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Lee, Y.R.; Park, H.G.; Lee, W.L. The effects of either resveratrol or exercise on macrophage infiltration and switching from M1 to M2 in high fat diet mice. J. Exerc. Nutr. Biochem. 2015, 19, 65–72. [Google Scholar] [CrossRef]
- Chen, S.; Li, J.; Zhang, Z.; Li, W.; Sun, Y.; Zhang, Q.; Feng, X.; Zhu, W. Effects of resveratrol on the amelioration of insulin resistance in KKAy mice. Can. J. Physiol. Pharmacol. 2012, 90, 237–242. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Sabe, A.A.; Sadek, A.A.; Elmadhun, N.Y.; Dalal, R.S.; Robich, M.P.; Bianchi, C.; Sellke, F.W. Investigating the Effects of Resveratrol on Chronically Ischemic Myocardium in a Swine Model of Metabolic Syndrome: A Proteomics Analysis. J. Med. Food 2014, 18, 60–66. [Google Scholar] [CrossRef]
- Jeong, H.; Samdani, K.J.; Yoo, D.H.; Lee, D.W.; Kim, N.H.; Yoo, I.-S.; Lee, J.H. Resveratrol cross-linked chitosan loaded with phospholipid for controlled release and antioxidant activity. Int. J. Biol. Macromol. 2016, 93, 757–766. [Google Scholar] [CrossRef]
- Lu, L.; Zhu, S.; Zhang, H.; Zhang, S. Improvement of antioxidative activity of resveratrol by elongating conjugated chain: A DFT theoretical study. Comput. Theor. Chem. 2013, 1019, 39–47. [Google Scholar] [CrossRef]

| Aim | Treatment | Result | References |
|---|---|---|---|
| Human | |||
| Modified resveratrol Longevinex improves endothelial function in adults with metabolic syndrome receiving standard treatment | Thirty-four patients who had been treated for MetS and lifestyle-related disease were randomly assigned to group A and B. A: Longevinex was administered for 3 months and then discontinued for 3 months. B: Longevinex was administered between 3 and 6 months. (The patients received 1 capsule of Longevinex containing 100 mg trans resveratrol daily after dinner). | Flow-mediated dilatation significantly increased during the administration of Longevinex but decreased to baseline 3 months after the discontinuation of Longevinex in the group A patients. Conversely, in the group B patients, flow-mediated dilatation remained unchanged for the first 3 months without Longevinex but was significantly increased 3 months after the treatment with Longevinex. | [49] |
| Effects of Resveratrol on the Metabolic Syndrome | Middle-aged community-dwelling men (n = 74) with MetS, 66 of whom completed all visits (age, 49.5 ± 0.796 years). Daily oral supplementation with 1000 mg RSV (RSVhigh), 150 mg RSV, or placebo, for 16 weeks. | RSVhigh treatment significantly increased total cholesterol (p < 0.002), low-density lipoprotein (LDL) cholesterol (p < 0.006), and fructosamine (p < 0.013) levels compared with placebo. | [50] |
| Animal | |||
| The effects of resveratrol on hepatic oxidative stress in metabolic syndrome model induced by high fructose diet | Male adult rats were induced by MetS, fructose solution (20% in drinking water). Resveratrol (10 mg/kg/day) was given by oral gavage. | Fructose-fed rats displayed statistically significant increases in TOS levels and decrease in PON activity compared to the control group, and prevented the decrease in liver PON activity caused by fructose. | [65] |
| Resveratrol improves oxidative stress and prevents the progression of periodontitis via the activation/antioxidant defense pathways in a rat periodontitis model | Animals in the periodontitis group underwent ligature-induced periodontitis and were given pure water. The melinjo resveratrol was administered orally at a dose of 10 mg/kg body weight. | Oral administration of melinjo resveratrol may prevent the progression of ligature-induced periodontitis and improve systemic oxidative and nitrosative stress. | [27] |
| The Combination of Resveratrol and Quercetin Attenuates Metabolic Syndrome in Rats | Each group of rats (control or MetS) received orally in drinking water or sucrose solution a mixture of RSV and QRC daily for 4 week. (1) RSV (10 mg/kg/day) + QRC (0.19 mg/Kg/day). (2) RSV (50 mg/kg/day) + QRC (0.95 mg/kg/day). | RSV + QRC administration improved the serum health parameters modified by MetS and upregulate SIRT 1 and SIRT 2 expression in white abdominal tissue in MetS animals. | [80] |
| Attenuation of insulin resistance, metabolic syndrome and hepatic oxidative stress by resveratrol in fructose-fed rats | Fructose-fed insulin resistant group (Dia) animals were fed 65% fructose for a period of 8 weeks, whereas control group animals were fed 65% cornstarch. Resveratrol, 10 mg/kg/day (Dia+Resv) or metformin 300 mg/kg/day (Dia+Met) were administered orally to the 65% fructose-fed rats. | Attenuation of hepatic oxidative stress in fructose-fed rat liver after resveratrol administration was associated with significant increase in the nuclear level of NRF2, and more effective than metformin in improving insulin sensitivity and attenuating metabolic syndrome and hepatic oxidative stress in fructose-fed rats. | [66] |
| A high-fat diet, leads to metabolic syndrome with spatial learning and memory deficits: beneficial effects of resveratrol | Receive high-fat hypercaloric diet. A therapeutic group with resveratrol on maternal high-fat diet/postnatal high-fat diet was raised for comparison (HHR). 50 mg/kg/day, for 16 weeks. | Treatment with resveratrol is able to rescue that maternal obesity/high-fat diet interacts with a postnatal high-fat diet to induce features of metabolic syndrome include alter biochemical profiles in the dorsal hippocampus, and lead to cognitive deficits. | [58] |
| Combined maternal and postnatal high-fat diet leads to metabolic syndrome and is effectively reversed by resveratrol: a multiple-organ study | Receive high-fat hypercaloric diet. Resveratrol 50 mg/kg/day, for 16 weeks. | Resveratrol ameliorated most of the features of metabolic syndrome and molecular alterations. | [59] |
| Resveratrol ameliorates maternal and post-weaning high-fat diet-induced nonalcoholic fatty liver disease via renin-angiotensin system | Receive high-fat hypercaloric diet. Resveratrol 50 mg/kg/day for offspring from post-weaning to postnatal day (PND) 120. | Resveratrol administration mediated a protective effect on rats on HF/HF by regulating lipid metabolism, reducing oxidative stress and apoptosis, restoring nutrient-sensing pathways by increasing Sirt1 and leptin expression, and mediating the renin-angiotensin system (RAS) to decrease angiotensinogen, renin, ACE1, and AT1R levels and increased ACE2, AT2R and MAS1 levels compared to those in the OHF group. | [68] |
| Improving glucose metabolism with resveratrol in a swine model of metabolic syndrome through alteration of signaling pathways in the liver and skeletal muscle | The swine developed metabolic syndrome by consuming a high-calorie, high–fat/cholesterol diet for 11 weeks. A hypercholesterolemic group diet with supplemental resveratrol (100 mg/kg/day). | Immunoblotting in the liver showed increased levels of mammalian target of rapamycin, insulin receptor substrate 1, and phosphorylated AKT in the HCRV group. Immunoblotting in skeletal muscle tissue demonstrated increased glucose transporter type 4 (Glut 4), peroxisome proliferating activation receptor coactivator 1α, peroxisome proliferator-activated receptor α, peroxisome proliferator-activated receptor, and phosphorylated AKT at threonine 308 expression as well as decreased retinol binding protein 4 in the HCRV group. | [67] |
| Investigating the Effects of Resveratrol on Chronically Ischemic Myocardium in a Swine Model of Metabolic Syndrome | The high-cholesterol diet, experimental group were supplemented with daily oral resveratrol (HCRV; 100 mg/kg/day, n = 6) for 11 weeks. | In a swine model with metabolic syndrome and chronic myocardial ischemia, we demonstrate that resveratrol supplementation significantly alters the levels of several key proteins involved in metabolism, cell death, and structural remodeling and decreases myocardial fibrosis. | [90] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, C.-Y.; Tain, Y.-L.; Yu, H.-R.; Huang, L.-T. The Effects of Resveratrol in the Treatment of Metabolic Syndrome. Int. J. Mol. Sci. 2019, 20, 535. https://doi.org/10.3390/ijms20030535
Hou C-Y, Tain Y-L, Yu H-R, Huang L-T. The Effects of Resveratrol in the Treatment of Metabolic Syndrome. International Journal of Molecular Sciences. 2019; 20(3):535. https://doi.org/10.3390/ijms20030535
Chicago/Turabian StyleHou, Chih-Yao, You-Lin Tain, Hong-Ren Yu, and Li-Tung Huang. 2019. "The Effects of Resveratrol in the Treatment of Metabolic Syndrome" International Journal of Molecular Sciences 20, no. 3: 535. https://doi.org/10.3390/ijms20030535
APA StyleHou, C.-Y., Tain, Y.-L., Yu, H.-R., & Huang, L.-T. (2019). The Effects of Resveratrol in the Treatment of Metabolic Syndrome. International Journal of Molecular Sciences, 20(3), 535. https://doi.org/10.3390/ijms20030535








