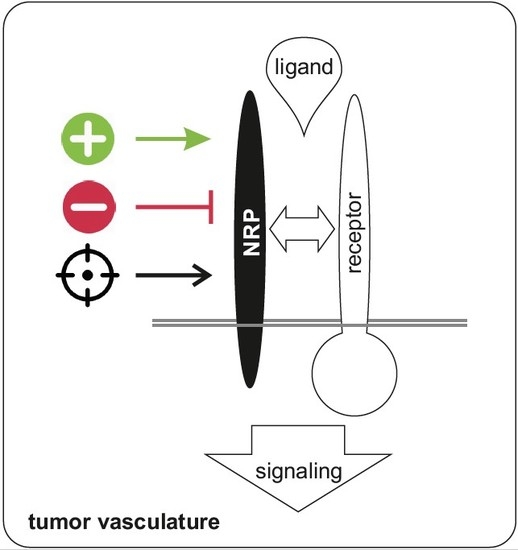
Neuropilins in the Context of Tumor Vasculature
Abstract
:1. Introduction
2. Molecular Structure of NRPs
3. Tissue Distribution of NRP1 and NRP2
4. Potential NRP Interaction Partners: Extracellular Soluble Ligands and Trimeric Complexes with Signal Receptors
4.1. NRP1 Interaction with VEGFs and VEGFRs
4.2. NRP1 Interaction with Other Growth Factor Receptors
4.3. Interaction of NRPs with Semaphorins and Plexins
4.4. NRP1 Interaction with Integrins
4.5. NRP1 Interaction with Other Molecules
4.6. NRP1 Can Trans-Interact with Ligands on Neighboring Cells
5. Signaling and (Patho) Physiological Functions of NRP
5.1. NRP Modulates Receptor Tyrosine Kinase Signaling
5.2. NRP Modulates TGF-β Receptor Signaling
5.3. NRP Modulates Semaphorin/Plexin Signaling
5.4. NRP Modulates Signaling of Integrins
5.5. NRP Modulates Signaling of Other Extracellular Ligands
5.6. Effects of Intracellular Partners and PDZ-Binding Proteins on NRP Signaling
5.7. NRP Signaling is Regulated by Endocytosis
5.8. NRP Regulates Hedgehog and Wnt/β-Catenin Pathways
5.9. Soluble NRPs Act as Decoy Receptors
5.10. Regulation of NRP Expression as a Potential Feedback Loop of NRP Signaling
6. NRP in the Tumor Biological Setting
6.1. Tumor Cells and Tumor Microenvironment
6.2. Origin and Structure of the Tumor Vasculature
6.3. NRP on Tumor Vessels
6.4. NRP-Dependent Effects of Tumor Cells on Endothelial Cells
7. NRP as a Therapeutic Target
7.1. Soluble NRP in Tumor Therapy
7.2. NRP-Directed Antibodies
7.3. Targeting NRP with Peptides/Small Molecule Inhibitors
7.4. Natural Compounds That Target NRP
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 3′-UTR | 3′ untranslated region |
| ADAM | A disintegrin and metalloproteinase |
| AGO | Argonaute |
| AKT | Protein kinase B |
| ALK | Activin receptor-like kinase |
| BMP | Bone Morphogenetic Protein 1 |
| BRAF | Rat/rapidly accelerated fibrosarcoma, isoform B |
| CAF | cancer-associated fibroblasts |
| CD | Cluster of differentiation |
| CendR | Carboxy-terminal end rule |
| CSC | Cancer stem cell |
| CUB domain | Cubilin homology domain |
| Dlg domain | Discs-large domain |
| EC | Endothelial cell |
| ECM | Extracellular matrix |
| EGF(R) | Epidermal growth factor (receptor) |
| EMT | Epithelial to mesenchymal transition |
| ErbB | Erythroblasotsis oncogene B |
| ERK | Extracellular-signal-regulated kinase |
| FGF(R) | Fibroblast growth factor (receptor) |
| EphA2 | Erythropoietin-producing human hepatocellular (EPH) receptor A2 |
| FAK | Focal adhesion kinase |
| Frzb | Frizzled-related protein |
| GAIP | G alpha interacting protein |
| GAP | GTPase activation protein |
| GIPC | GAIP interacting protein, C terminus |
| GIPC1 | GIPC PDZ domain containing family member 1, synectin |
| GLUT1CBP | Glucose transporter 1 C-terminal binding protein |
| Gq | Guanine nucleotide-binding protein, q polypeptide |
| GLI1 | Glioma-associated oncogene homolog 1 |
| Her2 | Human epidermal growth factor receptor 2 |
| HGF(R) | Hepatocyte growth factor (receptor) |
| HH | Hedgehog |
| IIP1 | insulin-like growth factor-1 receptor-interacting protein 1 |
| Jnk | c-Jun N-terminal kinase |
| L1CAM | L1 cell adhesion molecule |
| LAMC2 | Laminin subunit γ2 |
| LRP5 | Low-density lipoprotein receptor related protein 5 |
| MAM domain | meprin/A5-protein/PTPmu |
| MAP(K) | Mitogen-activated protein (kinase) |
| MET | Mesenchymal-epithelial transition factor (MET) proto-oncogene, Hepatocyte growth factor receptor, HGFR |
| miR | microRNA |
| MMP | Matrix metalloproteinase |
| NIP | Neuropilin-1 interacting protein |
| NRP | Neuropilin |
| p130Cas | CRK associated substrate |
| PDGF(R) | Platelet-derived growth factor(receptor) |
| PD-L1 | Programmed cell death 1 ligand 1, CD274 |
| PDZ bd | Post synaptic density/Disks large/Zonula occludens-1 binding domain |
| PlGF(R) | Placenta growth factor (receptor) |
| PI3K | Phosphoinositide 3-kinase |
| PKC | Protein kinase C |
| PSD-95 domain | postsynaptic density protein 95 domain |
| PTEN | Phosphatase and tensin homolog |
| PTPmu | receptor-type protein tyrosine phosphatase µ |
| RAS | Rat sarcoma |
| RhoGEF | Rho guanine nucleotide exchange factor 1 |
| RTK | Receptor tyrosine kinase |
| sNRP | Soluble neuropilin |
| SAPK1 | Stress-activated protein kinase 1 |
| SEMA | Semaphorin |
| SEMCAP1 | Semaphorin 4C (SEMA4C)-interacting protein 1 |
| Src | Sarcoma |
| Syx | Synectin-binding GEF |
| TAM | Tumor-associated macrophage |
| TEC | Tumor endothelial cell |
| TFPI1 | Tissue factor pathway inhibitor |
| TGF-β(R) | Transforming growth factor-β (receptor) |
| TIE | Tyrosine kinase with immunoglobulin-like and EGF-like domains |
| TIP2 | Tax-interacting protein 2 |
| TORC2 | rapamycin-sensitive TOR complex 2 |
| Treg | Regulatory T Cell |
| uPA | urokinase plasminogen activator |
| VCAM-1 | Vascular adhesion protein-1 |
| VEGF(R) | Vascular endothelial growth factor (receptor) |
| VM | Vasculogenic mimicry |
| WIF1 | Wnt inhibitory factor 1 |
| Wnt | Wingless-related integration site |
| YAP1 | Yes-associated protein 1 |
| ZO-1 domain | Zonula occludens-1 domain |
References
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A., Jr.; Kinzler, K.W. Cancer genome landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Rizzolio, S.; Tamagnone, L. Multifaceted role of neuropilins in cancer. Curr. Med. Chem. 2011, 18, 3563–3575. [Google Scholar] [CrossRef] [PubMed]
- Rizzolio, S.; Rabinowicz, N.; Rainero, E.; Lanzetti, L.; Serini, G.; Norman, J.; Neufeld, G.; Tamagnone, L. Neuropilin-1-dependent regulation of EGF-receptor signaling. Cancer Res. 2012, 72, 5801–5811. [Google Scholar] [CrossRef] [PubMed]
- Wild, J.R.; Staton, C.A.; Chapple, K.; Corfe, B.M. Neuropilins: Expression and roles in the epithelium. Int. J. Exp. Pathol. 2012, 93, 81–103. [Google Scholar] [CrossRef] [PubMed]
- Tata, M.; Ruhrberg, C.; Fantin, A. Vascularisation of the central nervous system. Mech. Dev. 2015, 138 Pt 1, 26–36. [Google Scholar] [CrossRef]
- Roy, S.; Bag, A.K.; Singh, R.K.; Talmadge, J.E.; Batra, S.K.; Datta, K. Multifaceted role of neuropilins in the immune system: Potential targets for immunotherapy. Front. Immunol. 2017, 8, 1228. [Google Scholar] [CrossRef] [PubMed]
- Mecollari, V.; Nieuwenhuis, B.; Verhaagen, J. A perspective on the role of class III semaphorin signaling in central nervous system trauma. Front. Cell. Neurosci. 2014, 8, 328. [Google Scholar] [CrossRef] [PubMed]
- Soker, S.; Miao, H.Q.; Nomi, M.; Takashima, S.; Klagsbrun, M. VEGF165 mediates formation of complexes containing VEGFR-2 and neuropilin-1 that enhance VEGF165-receptor binding. J. Cell. Biochem. 2002, 85, 357–368. [Google Scholar] [CrossRef]
- Hamerlik, P.; Lathia, J.D.; Rasmussen, R.; Wu, Q.; Bartkova, J.; Lee, M.; Moudry, P.; Bartek, J., Jr.; Fischer, W.; Lukas, J.; et al. Autocrine VEGF-VEGFR2-Neuropilin-1 signaling promotes glioma stem-like cell viability and tumor growth. J. Exp. Med. 2012, 209, 507–520. [Google Scholar] [CrossRef]
- Bagci, T.; Wu, J.K.; Pfannl, R.; Ilag, L.L.; Jay, D.G. Autocrine semaphorin 3A signaling promotes glioblastoma dispersal. Oncogene 2009, 28, 3537–3550. [Google Scholar] [CrossRef]
- He, Z.G.; Tessier-Lavigne, M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell 1997, 90, 739–751. [Google Scholar] [CrossRef]
- Kolodkin, A.L.; Levengood, D.V.; Rowe, E.G.; Tai, Y.T.; Giger, R.J.; Ginty, D.D. Neuropilin is a semaphorin III receptor. Cell 1997, 90, 753–762. [Google Scholar] [CrossRef]
- Soker, S.; Takashima, S.; Miao, H.Q.; Neufeld, G.; Klagsbrun, M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 1998, 92, 735–745. [Google Scholar] [CrossRef]
- Kitsukawa, T.; Shimono, A.; Kawakami, A.; Kondoh, H.; Fujisawa, H. Overexpression of a membrane protein, neuropilin, in chimeric mice causes anomalies in the cardiovascular system, nervous system and limbs. Development 1995, 121, 4309–4318. [Google Scholar] [PubMed]
- Kawasaki, T.; Kitsukawa, T.; Bekku, Y.; Matsuda, Y.; Sanbo, M.; Yagi, T.; Fujisawa, H. A requirement for neuropilin-1 in embryonic vessel formation. Development 1999, 126, 4895–4902. [Google Scholar]
- Nakamura, F.; Goshima, Y. Structural and functional relation of neuropilins. Adv. Exp. Med. Biol. 2002, 515, 55–69. [Google Scholar] [PubMed]
- Takagi, S.; Hirata, T.; Agata, K.; Mochii, M.; Eguchi, G.; Fujisawa, H. The A5 antigen, a candidate for the neuronal recognition molecule, has homologies to complement components and coagulation factors. Neuron 1991, 7, 295–307. [Google Scholar] [CrossRef]
- Kawakami, A.; Kitsukawa, T.; Takagi, S.; Fujisawa, H. Developmentally regulated expression of a cell surface protein, neuropilin, in the mouse nervous system. J. Neurobiol. 1996, 29, 1–17. [Google Scholar] [CrossRef]
- Fujisawa, H.; Kitsukawa, T.; Kawakami, A.; Takagi, S.; Shimizu, M.; Hirata, T. Roles of a neuronal cell-surface molecule, neuropilin, in nerve fiber fasciculation and guidance. Cell Tissue Res. 1997, 290, 465–470. [Google Scholar] [CrossRef]
- Chen, H.; Chedotal, A.; He, Z.; Goodman, C.S.; Tessier-Lavigne, M. Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron 1997, 19, 547–559. [Google Scholar] [CrossRef]
- Rossignol, M.; Beggs, A.H.; Pierce, E.A.; Klagsbrun, M. Human neuropilin-1 and neuropilin-2 map to 10p12 and 2q34, respectively. Genomics 1999, 57, 459–460. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, M.; Gagnon, M.L.; Klagsbrun, M. Genomic organization of human neuropilin-1 and neuropilin-2 genes: Identification and distribution of splice variants and soluble isoforms. Genomics 2000, 70, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, M.L.; Bielenberg, D.R.; Gechtman, Z.; Miao, H.Q.; Takashima, S.; Soker, S.; Klagsbrun, M. Identification of a natural soluble neuropilin-1 that binds vascular endothelial growth factor: In vivo expression and antitumor activity. Proc. Natl. Acad. Sci. USA 2000, 97, 2573–2578. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y. Exogenous clustered neuropilin 1 enhances vasculogenesis and angiogenesis. Blood 2001, 97, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Spring, S.C.; Terman, B.I. Characterization of a new alternatively spliced neuropilin-1 isoform. Angiogenesis 2003, 6, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Cackowski, F.C.; Xu, L.; Hu, B.; Cheng, S.Y. Identification of two novel alternatively spliced Neuropilin-1 isoforms. Genomics 2004, 84, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Kiedzierska, A.; Smietana, K.; Czepczynska, H.; Otlewski, J. Structural similarities and functional diversity of eukaryotic discoidin-like domains. Biochim. Biophys. Acta 2007, 1774, 1069–1078. [Google Scholar] [CrossRef]
- Lee, C.C.; Kreusch, A.; McMullan, D.; Ng, K.; Spraggon, G. Crystal structure of the human neuropilin-1 b1 domain. Structure 2003, 11, 99–108. [Google Scholar] [CrossRef]
- Shintani, Y.; Takashima, S.; Asano, Y.; Kato, H.; Liao, Y.; Yamazaki, S.; Tsukamoto, O.; Seguchi, O.; Yamamoto, H.; Fukushima, T.; et al. Glycosaminoglycan modification of neuropilin-1 modulates VEGFR2 signaling. EMBO J. 2006, 25, 3045–3055. [Google Scholar] [CrossRef]
- Frankel, P.; Pellet-Many, C.; Lehtolainen, P.; D’Abaco, G.M.; Tickner, M.L.; Cheng, L.; Zachary, I.C. Chondroitin sulphate-modified neuropilin 1 is expressed in human tumour cells and modulates 3D invasion in the U87MG human glioblastoma cell line through a p130Cas-mediated pathway. EMBO Rep. 2008, 9, 983–989. [Google Scholar] [CrossRef]
- Pellet-Many, C.; Frankel, P.; Evans, I.M.; Herzog, B.; Junemann-Ramirez, M.; Zachary, I.C. Neuropilin-1 mediates PDGF stimulation of vascular smooth muscle cell migration and signalling via p130Cas. Biochem. J. 2011, 435, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Curreli, S.; Arany, Z.; Gerardy-Schahn, R.; Mann, D.; Stamatos, N.M. Polysialylated neuropilin-2 is expressed on the surface of human dendritic cells and modulates dendritic cell-T lymphocyte interactions. J. Biol. Chem. 2007, 282, 30346–30356. [Google Scholar] [CrossRef] [PubMed]
- Bhide, G.P.; Fernandes, N.R.; Colley, K.J. Sequence Requirements for Neuropilin-2 Recognition by ST8SiaIV and Polysialylation of Its O-Glycans. J. Biol. Chem. 2016, 291, 9444–9457. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.; Fields, L.; Evans, I.M.; Yamaji, M.; Pellet-Many, C.; Jones, T.; Mahmoud, M.; Zachary, I. VEGF (vascular endothelial growth factor) induces NRP1 (neuropilin-1) cleavage via ADAMs (a disintegrin and metalloproteinase) 9 and 10 to generate novel carboxy-terminal NRP1 fragments that regulate angiogenic signaling. Arterioscler. Thromb. Vasc. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Geretti, E.; Shimizu, A.; Klagsbrun, M. Neuropilin structure governs VEGF and semaphorin binding and regulates angiogenesis. Angiogenesis 2008, 11, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Roth, L.; Nasarre, C.; Dirrig-Grosch, S.; Aunis, D.; Cremel, G.; Hubert, P.; Bagnard, D. Transmembrane domain interactions control biological functions of neuropilin-1. Mol. Biol. Cell 2008, 19, 646–654. [Google Scholar] [CrossRef]
- Aci-Seche, S.; Sawma, P.; Hubert, P.; Sturgis, J.N.; Bagnard, D.; Jacob, L.; Genest, M.; Garnier, N. Transmembrane recognition of the semaphorin co-receptors neuropilin 1 and plexin A1: Coarse-grained simulations. PLoS ONE 2014, 9, e97779. [Google Scholar] [CrossRef]
- Herzog, B.; Pellet-Many, C.; Britton, G.; Hartzoulakis, B.; Zachary, I.C. VEGF binding to NRP1 is essential for VEGF stimulation of endothelial cell migration, complex formation between NRP1 and VEGFR2, and signaling via FAK Tyr407 phosphorylation. Mol. Biol. Cell 2011, 22, 2766–2776. [Google Scholar] [CrossRef]
- Herzog, Y.; Kalcheim, C.; Kahane, N.; Reshef, R.; Neufeld, G. Differential expression of neuropilin-1 and neuropilin-2 in arteries and veins. Mech. Dev. 2001, 109, 115–119. [Google Scholar] [CrossRef]
- Teesalu, T.; Sugahara, K.N.; Kotamraju, V.R.; Ruoslahti, E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc. Natl. Acad. Sci. USA 2009, 106, 16157–16162. [Google Scholar] [CrossRef]
- Yuan, L.; Moyon, D.; Pardanaud, L.; Breant, C.; Karkkainen, M.J.; Alitalo, K.; Eichmann, A. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development 2002, 129, 4797–4806. [Google Scholar] [PubMed]
- Stalmans, I.; Ng, Y.S.; Rohan, R.; Fruttiger, M.; Bouche, A.; Yuce, A.; Fujisawa, H.; Hermans, B.; Shani, M.; Jansen, S.; et al. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J. Clin. Investig. 2002, 109, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Rodriguez, E.R.; Reimert, D.V.; Shu, T.; Fritzsch, B.; Richards, L.J.; Kolodkin, A.L.; Ginty, D.D. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev. Cell 2003, 5, 45–57. [Google Scholar] [CrossRef]
- Tordjman, R.; Ortega, N.; Coulombel, L.; Plouet, J.; Romeo, P.H.; Lemarchandel, V. Neuropilin-1 is expressed on bone marrow stromal cells: A novel interaction with hematopoietic cells? Blood 1999, 94, 2301–2309. [Google Scholar] [PubMed]
- Deckers, M.M.; Karperien, M.; van der Bent, C.; Yamashita, T.; Papapoulos, S.E.; Lowik, C.W. Expression of vascular endothelial growth factors and their receptors during osteoblast differentiation. Endocrinology 2000, 141, 1667–1674. [Google Scholar] [CrossRef]
- Miao, H.Q.; Klagsbrun, M. Neuropilin is a mediator of angiogenesis. Cancer Metast. Rev. 2000, 19, 29–37. [Google Scholar] [CrossRef]
- Gammill, L.S.; Gonzalez, C.; Gu, C.; Bronner-Fraser, M. Guidance of trunk neural crest migration requires neuropilin 2/semaphorin 3F signaling. Development 2006, 133, 99–106. [Google Scholar] [CrossRef]
- Karpanen, T.; Heckman, C.A.; Keskitalo, S.; Jeltsch, M.; Ollila, H.; Neufeld, G.; Tamagnone, L.; Alitalo, K. Functional interaction of VEGF-C and VEGF-D with neuropilin receptors. FASEB J. 2006, 20, 1462–1472. [Google Scholar] [CrossRef]
- Jubb, A.M.; Sa, S.M.; Ratti, N.; Strickland, L.A.; Schmidt, M.; Callahan, C.A.; Koeppen, H. Neuropilin-2 expression in cancer. Histopathology 2012, 61, 340–349. [Google Scholar] [CrossRef]
- Bielenberg, D.R.; Pettaway, C.A.; Takashima, S.; Klagsbrun, M. Neuropilins in neoplasms: Expression, regulation, and function. Exp. Cell Res. 2006, 312, 584–593. [Google Scholar] [CrossRef]
- Takashima, S.; Kitakaze, M.; Asakura, M.; Asanuma, H.; Sanada, S.; Tashiro, F.; Niwa, H.; Miyazaki Ji, J.; Hirota, S.; Kitamura, Y.; et al. Targeting of both mouse neuropilin-1 and neuropilin-2 genes severely impairs developmental yolk sac and embryonic angiogenesis. Proc. Natl. Acad. Sci. USA 2002, 99, 3657–3662. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Bagri, A.; Zupicich, J.A.; Zou, Y.; Stoeckli, E.; Pleasure, S.J.; Lowenstein, D.H.; Skarnes, W.C.; Chedotal, A.; Tessier-Lavigne, M. Neuropilin-2 regulates the development of selective cranial and sensory nerves and hippocampal mossy fiber projections. Neuron 2000, 25, 43–56. [Google Scholar] [CrossRef]
- Verlinden, L.; Kriebitzsch, C.; Beullens, I.; Tan, B.K.; Carmeliet, G.; Verstuyf, A. Nrp2 deficiency leads to trabecular bone loss and is accompanied by enhanced osteoclast and reduced osteoblast numbers. Bone 2013, 55, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, L.; Vanderschueren, D.; Verstuyf, A. Semaphorin signaling in bone. Mol. Cell. Endocrinol. 2016, 432, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hao, J.; Duan, X.; Wu, N.; Zhou, Z.; Yang, F.; Li, J.; Zhao, Z.; Huang, S. The Role of Semaphorin 3A in Bone Remodeling. Front. Cell. Neurosci. 2017, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Romeo, P.H.; Lemarchandel, V.; Tordjman, R. Neuropilin-1 in the immune system. Adv. Exp. Med. Biol. 2002, 515, 49–54. [Google Scholar]
- Schellenburg, S.; Schulz, A.; Poitz, D.M.; Muders, M.H. Role of neuropilin-2 in the immune system. Mol. Immunol. 2017, 90, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Mizui, M.; Kikutani, H. Neuropilin-1: The glue between regulatory T cells and dendritic cells? Immunity 2008, 28, 302–303. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liao, X.; Kang, Y. Tregs: Where We Are and What Comes Next? Front. Immunol. 2017, 8, 1578. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Hou, L.; Li, J.; Shao, S.; Huang, S.; Meng, D.; Liu, L.; Feng, L.; Xia, P.; Qin, T.; et al. VEGF/NRP-1axis promotes progression of breast cancer via enhancement of epithelial-mesenchymal transition and activation of NF-kappaB and beta-catenin. Cancer Lett. 2016, 373, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, Y.; Zhang, J.; Xing, B.; Xuan, W.; Wang, H.; Huang, H.; Yang, J.; Tang, J. NRP-2 in tumor lymphangiogenesis and lymphatic metastasis. Cancer Lett. 2018, 418, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Grandclement, C.; Borg, C. Neuropilins: A new target for cancer therapy. Cancers 2011, 3, 1899–1928. [Google Scholar] [CrossRef] [PubMed]
- Serini, G.; Bussolino, F.; Maione, F.; Giraudo, E. Class 3 semaphorins: Physiological vascular normalizing agents for anti-cancer therapy. J. Intern. Med. 2013, 273, 138–155. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, B.; Khaled, Y.S.; Ammori, B.J.; Elkord, E. Neuropilin 1: Function and therapeutic potential in cancer. Cancer Immunol. Immunother. 2014, 63, 81–99. [Google Scholar] [CrossRef] [PubMed]
- Stasikowska-Kanicka, O.; Wagrowska-Danilewicz, M.; Danilewicz, M. Immunohistochemical study on neuropilin 1 (NRP1) immunoexpression in oral squamous cell carcinoma. Folia Histochem. Cytobiol. 2018, 1, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhou, J.; Wang, S.; Li, Y.; Mi, Y.; Gao, S.; Xu, Y.; Chen, Y.; Yan, J. Anti-neuropilin-1 monoclonal antibody suppresses the migration and invasion of human gastric cancer cells via Akt dephosphorylation. Exp. Ther. Med. 2018, 16, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Matkar, P.N.; Jong, E.D.; Ariyagunarajah, R.; Prud’homme, G.J.; Singh, K.K.; Leong-Poi, H. Jack of many trades: Multifaceted role of neuropilins in pancreatic cancer. Cancer Med. 2018, 7, 5036–5046. [Google Scholar] [CrossRef]
- Morin, E.; Sjoberg, E.; Tjomsland, V.; Testini, C.; Lindskog, C.; Franklin, O.; Sund, M.; Ohlund, D.; Kiflemariam, S.; Sjoblom, T.; et al. VEGF receptor-2/neuropilin 1 trans-complex formation between endothelial and tumor cells is an independent predictor of pancreatic cancer survival. J. Pathol. 2018, 246, 311–322. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, Y.; Wu, J.; Li, L.; Chen, N.; Ni, P.; Song, L.; Liu, X. Neuropilin 1 (NRP1) is a novel tumor marker in hepatocellular carcinoma. Clin. Chim. Acta 2018, 485, 158–165. [Google Scholar] [CrossRef]
- Onsurathum, S.; Haonon, O.; Pinlaor, P.; Pairojkul, C.; Khuntikeo, N.; Thanan, R.; Roytrakul, S.; Pinlaor, S. Proteomics detection of S100A6 in tumor tissue interstitial fluid and evaluation of its potential as a biomarker of cholangiocarcinoma. Tumour Biol. 2018, 40, 1010428318767195. [Google Scholar] [CrossRef]
- Zhu, H.; Jiang, X.; Zhou, X.; Dong, X.; Xie, K.; Yang, C.; Jiang, H.; Sun, X.; Lu, J. Neuropilin-1 regulated by miR-320 contributes to the growth and metastasis of cholangiocarcinoma cells. Liver Int. 2018, 38, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Tomida, C.; Yamagishi, N.; Nagano, H.; Uchida, T.; Ohno, A.; Hirasaka, K.; Nikawa, T.; Teshima-Kondo, S. VEGF pathway-targeting drugs induce evasive adaptation by activation of neuropilin-1/cMet in colon cancer cells. Int. J. Oncol. 2018, 52, 1350–1362. [Google Scholar] [CrossRef] [PubMed]
- Tse, B.W.; Volpert, M.; Ratther, E.; Stylianou, N.; Nouri, M.; McGowan, K.; Lehman, M.L.; McPherson, S.J.; Roshan-Moniri, M.; Butler, M.S.; et al. Neuropilin-1 is upregulated in the adaptive response of prostate tumors to androgen-targeted therapies and is prognostic of metastatic progression and patient mortality. Oncogene 2017, 36, 3417–3427. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.; Al-Yahyaee, A.; Abdullah, N.; Sam, J.E.; Al-Zeheimi, N.; Yaish, M.W.; Adham, S.A. Neuropilin-1 promotes the oncogenic Tenascin-C/integrin beta3 pathway and modulates chemoresistance in breast cancer cells. BMC Cancer 2018, 18, 533. [Google Scholar] [CrossRef] [PubMed]
- Bobinski, M.; Okla, K.; Kotarski, J.; Szumilo, J.; Polak, G.; Sobstyl, M.; Bednarek, W. Neuropilin 1 in uterine leiomyosarcoma. Clinical and pathological analysis. Ginekol. Pol. 2018, 89, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Andersen, L.M.K.; Rofstad, E.K. Metastatic pathway and the microvascular and physicochemical microenvironments of human melanoma xenografts. J. Transl. Med. 2017, 15, 203. [Google Scholar] [CrossRef] [PubMed]
- Ruffini, F.; Levati, L.; Graziani, G.; Caporali, S.; Atzori, M.G.; D’Atri, S.; Lacal, P.M. Platelet-derived growth factor-C promotes human melanoma aggressiveness through activation of neuropilin-1. Oncotarget 2017, 8, 66833–66848. [Google Scholar] [CrossRef]
- Zhao, H.; Hou, C.; Hou, A.; Zhu, D. Concurrent Expression of VEGF-C and Neuropilin-2 Is Correlated with Poor Prognosis in Glioblastoma. Tohoku J. Exp. Med. 2016, 238, 85–91. [Google Scholar] [CrossRef]
- Kwiatkowski, S.C.; Guerrero, P.A.; Hirota, S.; Chen, Z.; Morales, J.E.; Aghi, M.; McCarty, J.H. Neuropilin-1 modulates TGFbeta signaling to drive glioblastoma growth and recurrence after anti-angiogenic therapy. PLoS ONE 2017, 12, e0185065. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, Z.; Sun, M.; Li, H.; Fan, H.; Chen, D.; Zheng, J. Prognostic significance of VEGF-C, semaphorin 3F, and neuropilin-2 expression in oral squamous cell carcinomas and their relationship with lymphangiogenesis. J. Surg. Oncol. 2015, 111, 382–388. [Google Scholar] [CrossRef]
- Fung, T.M.; Ng, K.Y.; Tong, M.; Chen, J.N.; Chai, S.; Chan, K.T.; Law, S.; Lee, N.P.; Choi, M.Y.; Li, B.; et al. Neuropilin-2 promotes tumourigenicity and metastasis in oesophageal squamous cell carcinoma through ERK-MAPK-ETV4-MMP-E-cadherin deregulation. J. Pathol. 2016, 239, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wang, R.; Zhao, Y.F.; Jia, J.; Sun, Z.J.; Chen, X.M. Expression of Neuropilin-2 in salivary adenoid cystic carcinoma: Its implication in tumor progression and angiogenesis. Pathol. Res. Pract. 2010, 206, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Tu, D.G.; Chang, W.W.; Jan, M.S.; Tu, C.W.; Lu, Y.C.; Tai, C.K. Promotion of metastasis of thyroid cancer cells via NRP-2-mediated induction. Oncol. Lett. 2016, 12, 4224–4230. [Google Scholar] [CrossRef] [PubMed]
- Yasuoka, H.; Kodama, R.; Tsujimoto, M.; Yoshidome, K.; Akamatsu, H.; Nakahara, M.; Inagaki, M.; Sanke, T.; Nakamura, Y. Neuropilin-2 expression in breast cancer: Correlation with lymph node metastasis, poor prognosis, and regulation of CXCR4 expression. BMC Cancer 2009, 9, 220. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, P.; Grubinger, M.; Groger, C.; Huber, H.; Sieghart, W.; Peck-Radosavljevic, M.; Mikulits, W. Neuropilin-2 induced by transforming growth factor-beta augments migration of hepatocellular carcinoma cells. BMC Cancer 2015, 15, 909. [Google Scholar] [CrossRef] [PubMed]
- Dallas, N.A.; Gray, M.J.; Xia, L.; Fan, F.; van Buren, G., 2nd; Gaur, P.; Samuel, S.; Lim, S.J.; Arumugam, T.; Ramachandran, V.; et al. Neuropilin-2-mediated tumor growth and angiogenesis in pancreatic adenocarcinoma. Clin. Cancer. Res. 2008, 14, 8052–8060. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.J.; Wei, X.; Peng, Y.; Zha, L.; Zhou, R.B.; Shi, H.; Zhou, Q.; Liang, H.J. Neuropilin-2 mediates lymphangiogenesis of colorectal carcinoma via a VEGFC/VEGFR3 independent signaling. Cancer Lett. 2015, 358, 200–209. [Google Scholar] [CrossRef]
- Keck, B.; Wach, S.; Taubert, H.; Zeiler, S.; Ott, O.J.; Kunath, F.; Hartmann, A.; Bertz, S.; Weiss, C.; Honscheid, P.; et al. Neuropilin-2 and its ligand VEGF-C predict treatment response after transurethral resection and radiochemotherapy in bladder cancer patients. Int. J. Cancer 2015, 136, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Rushing, E.C.; Stine, M.J.; Hahn, S.J.; Shea, S.; Eller, M.S.; Naif, A.; Khanna, S.; Westra, W.H.; Jungbluth, A.A.; Busam, K.J.; et al. Neuropilin-2: A novel biomarker for malignant melanoma? Hum. Pathol. 2012, 43, 381–389. [Google Scholar] [CrossRef]
- Goel, H.L.; Chang, C.; Pursell, B.; Leav, I.; Lyle, S.; Xi, H.S.; Hsieh, C.C.; Adisetiyo, H.; Roy-Burman, P.; Coleman, I.M.; et al. VEGF/neuropilin-2 regulation of Bmi-1 and consequent repression of IGF-IR define a novel mechanism of aggressive prostate cancer. Cancer Discov. 2012, 2, 906–921. [Google Scholar] [CrossRef]
- Boro, A.; Arlt, M.J.; Lengnick, H.; Robl, B.; Husmann, M.; Bertz, J.; Born, W.; Fuchs, B. Prognostic value and in vitro biological relevance of Neuropilin 1 and Neuropilin 2 in osteosarcoma. Am. J. Transl. Res. 2015, 7, 640–653. [Google Scholar] [PubMed]
- Kawakami, T.; Tokunaga, T.; Hatanaka, H.; Kijima, H.; Yamazaki, H.; Abe, Y.; Osamura, Y.; Inoue, H.; Ueyama, Y.; Nakamura, M. Neuropilin 1 and neuropilin 2 co-expression is significantly correlated with increased vascularity and poor prognosis in nonsmall cell lung carcinoma. Cancer 2002, 95, 2196–2201. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, W.F.; Kim, E.; Gerber, S.A.; Hammers, H.; Alani, R.M. Neuropilin-2 promotes melanoma growth and progression in vivo. Melanoma Res. 2016, 26, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Gemmill, R.M.; Nasarre, P.; Nair-Menon, J.; Cappuzzo, F.; Landi, L.; D’Incecco, A.; Uramoto, H.; Yoshida, T.; Haura, E.B.; Armeson, K.; et al. The neuropilin 2 isoform NRP2b uniquely supports TGFbeta-mediated progression in lung cancer. Sci. Signal. 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Ellis, L.M. The role of neuropilins in cancer. Mol. Cancer Ther. 2006, 5, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Guttmann-Raviv, N.; Shraga-Heled, N.; Varshavsky, A.; Guimaraes-Sternberg, C.; Kessler, O.; Neufeld, G. Semaphorin-3A and semaphorin-3F work together to repel endothelial cells and to inhibit their survival by induction of apoptosis. J. Biol. Chem. 2007, 282, 26294–26305. [Google Scholar] [CrossRef]
- Giger, R.J.; Urquhart, E.R.; Gillespie, S.K.; Levengood, D.V.; Ginty, D.D.; Kolodkin, A.L. Neuropilin-2 is a receptor for semaphorin IV: Insight into the structural basis of receptor function and specificity. Neuron 1998, 21, 1079–1092. [Google Scholar] [CrossRef]
- Gu, C.; Yoshida, Y.; Livet, J.; Reimert, D.V.; Mann, F.; Merte, J.; Henderson, C.E.; Jessell, T.M.; Kolodkin, A.L.; Ginty, D.D. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science 2005, 307, 265–268. [Google Scholar] [CrossRef]
- Chauvet, S.; Cohen, S.; Yoshida, Y.; Fekrane, L.; Livet, J.; Gayet, O.; Segu, L.; Buhot, M.C.; Jessell, T.M.; Henderson, C.E.; et al. Gating of Sema3E/PlexinD1 signaling by neuropilin-1 switches axonal repulsion to attraction during brain development. Neuron 2007, 56, 807–822. [Google Scholar] [CrossRef]
- Mota, F.; Fotinou, C.; Rana, R.R.; Chan, A.W.E.; Yelland, T.; Arooz, M.T.; O’Leary, A.P.; Hutton, J.; Frankel, P.; Zachary, I.; et al. Architecture and hydration of the arginine-binding site of neuropilin-1. FEBS J. 2018, 285, 1290–1304. [Google Scholar] [CrossRef]
- Peng, K.; Bai, Y.; Zhu, Q.; Hu, B.; Xu, Y. Targeting VEGF-neuropilin interactions: A promising antitumor strategy. Drug Discov. Today 2018. [Google Scholar] [CrossRef] [PubMed]
- Peach, C.J.; Mignone, V.W.; Arruda, M.A.; Alcobia, D.C.; Hill, S.J.; Kilpatrick, L.E.; Woolard, J. Molecular Pharmacology of VEGF-A Isoforms: Binding and Signalling at VEGFR2. Int. J. Mol. Sci. 2018, 19, 1264. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.J.; Stringer, S.E. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J. Cell Sci. 2001, 114, 853–865. [Google Scholar] [PubMed]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Klagsbrun, M.; Takashima, S.; Mamluk, R. The role of neuropilin in vascular and tumor biology. Adv. Exp. Med. Biol. 2002, 515, 33–48. [Google Scholar] [PubMed]
- Neufeld, G.; Cohen, T.; Shraga, N.; Lange, T.; Kessler, O.; Herzog, Y. The neuropilins: Multifunctional semaphorin and VEGF receptors that modulate axon guidance and angiogenesis. Trends Cardiovasc. Med. 2002, 12, 13–19. [Google Scholar] [CrossRef]
- Pan, Q.; Chanthery, Y.; Liang, W.C.; Stawicki, S.; Mak, J.; Rathore, N.; Tong, R.K.; Kowalski, J.; Yee, S.F.; Pacheco, G.; et al. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell 2007, 11, 53–67. [Google Scholar] [CrossRef]
- Sarabipour, S.; Mac Gabhann, F. VEGF-A121a binding to Neuropilins—A concept revisited. Cell Adhes. Migr. 2018, 12, 204–214. [Google Scholar] [CrossRef]
- Gluzman-Poltorak, Z.; Cohen, T.; Herzog, Y.; Neufeld, G. Neuropilin-2 is a receptor for the vascular endothelial growth factor (VEGF) forms VEGF-145 and VEGF-165 [corrected]. J. Biol. Chem. 2000, 275, 18040–18045. [Google Scholar] [CrossRef]
- Muller, Y.A.; Li, B.; Christinger, H.W.; Wells, J.A.; Cunningham, B.C.; de Vos, A.M. Vascular endothelial growth factor: Crystal structure and functional mapping of the kinase domain receptor binding site. Proc. Natl. Acad. Sci. USA 1997, 94, 7192–7197. [Google Scholar] [CrossRef]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Baek, D.S.; Kim, J.H.; Kim, Y.J.; Kim, Y.S. Immunoglobulin Fc-Fused Peptide without C-Terminal Arg or Lys Residue Augments Neuropilin-1-Dependent Tumor Vascular Permeability. Mol. Pharm. 2018, 15, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, S.; Driscoll, P.C. Targeting VEGF signalling via the neuropilin co-receptor. Drug Discov. Today 2013, 18, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Tugues, S.; Li, X.; Gualandi, L.; Claesson-Welsh, L. Signal transduction by vascular endothelial growth factor receptors. Biochem. J. 2011, 437, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Chathery, Y.; Wu, Y.; Rathore, N.; Tong, R.K.; Peale, F.; Bagri, A.; Tessier-Lavigne, M.; Koch, A.W.; Watts, R.J. Neuropilin-1 binds to VEGF121 and regulates endothelial cell migration and sprouting. J. Biol. Chem. 2007, 282, 24049–24056. [Google Scholar] [CrossRef] [PubMed]
- Lala, P.K.; Nandi, P.; Majumder, M. Roles of prostaglandins in tumor-associated lymphangiogenesis with special reference to breast cancer. Cancer Metast. Rev. 2018, 37, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Migdal, M.; Huppertz, B.; Tessler, S.; Comforti, A.; Shibuya, M.; Reich, R.; Baumann, H.; Neufeld, G. Neuropilin-1 is a placenta growth factor-2 receptor. J. Biol. Chem. 1998, 273, 22272–22278. [Google Scholar] [CrossRef]
- Mamluk, R.; Gechtman, Z.; Kutcher, M.E.; Gasiunas, N.; Gallagher, J.; Klagsbrun, M. Neuropilin-1 binds vascular endothelial growth factor 165, placenta growth factor-2, and heparin via its b1b2 domain. J. Biol. Chem. 2002, 277, 24818–24825. [Google Scholar] [CrossRef]
- Matsushita, A.; Gotze, T.; Korc, M. Hepatocyte growth factor-mediated cell invasion in pancreatic cancer cells is dependent on neuropilin-1. Cancer Res. 2007, 67, 10309–10316. [Google Scholar] [CrossRef]
- Sulpice, E.; Plouet, J.; Berge, M.; Allanic, D.; Tobelem, G.; Merkulova-Rainon, T. Neuropilin-1 and neuropilin-2 act as coreceptors, potentiating proangiogenic activity. Blood 2008, 111, 2036–2045. [Google Scholar] [CrossRef]
- West, D.C.; Rees, C.G.; Duchesne, L.; Patey, S.J.; Terry, C.J.; Turnbull, J.E.; Delehedde, M.; Heegaard, C.W.; Allain, F.; Vanpouille, C.; et al. Interactions of multiple heparin binding growth factors with neuropilin-1 and potentiation of the activity of fibroblast growth factor-2. J. Biol. Chem. 2005, 280, 13457–13464. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, S.; Nodale, C.; Vescarelli, E.; Pontecorvi, P.; Manganelli, V.; Casella, G.; Onesti, M.G.; Sorice, M.; Romano, F.; Angeloni, A.; et al. Neuropilin 1 Mediates Keratinocyte Growth Factor Signaling in Adipose-Derived Stem Cells: Potential Involvement in Adipogenesis. Stem Cells Int. 2018, 2018, 1075156. [Google Scholar] [CrossRef] [PubMed]
- Ohsaka, A.; Hirota-Komatsu, S.; Araki, M.; Komatsu, N. Platelet-derived growth factor receptors form complexes with neuropilin-1 during megakaryocytic differentiation of thrombopoietin-dependent UT-7/TPO cells. Biochem. Biophys. Res. Commun. 2015, 459, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Muhl, L.; Folestad, E.B.; Gladh, H.; Wang, Y.; Moessinger, C.; Jakobsson, L.; Eriksson, U. Neuropilin 1 binds PDGF-D and is a co-receptor in PDGF-D-PDGFRbeta signaling. J. Cell Sci. 2017, 130, 1365–1378. [Google Scholar] [CrossRef] [PubMed]
- Glinka, Y.; Prud’homme, G.J. Neuropilin-1 is a receptor for transforming growth factor beta-1, activates its latent form, and promotes regulatory T cell activity. J. Leukoc. Biol. 2008, 84, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Glinka, Y.; Stoilova, S.; Mohammed, N.; Prud’homme, G.J. Neuropilin-1 exerts co-receptor function for TGF-beta-1 on the membrane of cancer cells and enhances responses to both latent and active TGF-beta. Carcinogenesis 2011, 32, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Vivekanandhan, S.; Mukhopadhyay, D. Genetic status of KRAS influences Transforming Growth Factor-beta (TGF-beta) signaling: An insight into Neuropilin-1 (NRP1) mediated tumorigenesis. Semin. Cancer Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kigel, B.; Rabinowicz, N.; Varshavsky, A.; Kessler, O.; Neufeld, G. Plexin-A4 promotes tumor progression and tumor angiogenesis by enhancement of VEGF and bFGF signaling. Blood 2011, 118, 4285–4296. [Google Scholar] [CrossRef]
- Alto, L.T.; Terman, J.R. Semaphorins and their Signaling Mechanisms. Methods Mol. Biol. 2017, 1493, 1–25. [Google Scholar] [CrossRef]
- Gaur, P.; Bielenberg, D.R.; Samuel, S.; Bose, D.; Zhou, Y.; Gray, M.J.; Dallas, N.A.; Fan, F.; Xia, L.; Lu, J.; et al. Role of class 3 semaphorins and their receptors in tumor growth and angiogenesis. Clin. Cancer. Res. 2009, 15, 6763–6770. [Google Scholar] [CrossRef]
- Maione, F.; Molla, F.; Meda, C.; Latini, R.; Zentilin, L.; Giacca, M.; Seano, G.; Serini, G.; Bussolino, F.; Giraudo, E. Semaphorin 3A is an endogenous angiogenesis inhibitor that blocks tumor growth and normalizes tumor vasculature in transgenic mouse models. J. Clin. Investig. 2009, 119, 3356–3372. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.K.; Shimizu, A.; Kirkpatrick, N.D.; Garkavtsev, I.; Chan, A.W.; di Tomaso, E.; Klagsbrun, M.; Jain, R.K. Merlin/NF2 regulates angiogenesis in schwannomas through a Rac1/semaphorin 3F-dependent mechanism. Neoplasia 2012, 14, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Nasarre, P.; Gemmill, R.M.; Drabkin, H.A. The emerging role of class-3 semaphorins and their neuropilin receptors in oncology. Onco Targets Ther. 2014, 7, 1663–1687. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.C.; Dennis, M.S.; Stawicki, S.; Chanthery, Y.; Pan, Q.; Chen, Y.; Eigenbrot, C.; Yin, J.; Koch, A.W.; Wu, X.; et al. Function blocking antibodies to neuropilin-1 generated from a designed human synthetic antibody phage library. J. Mol. Biol. 2007, 366, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Sawma, P.; Roth, L.; Blanchard, C.; Bagnard, D.; Cremel, G.; Bouveret, E.; Duneau, J.P.; Sturgis, J.N.; Hubert, P. Evidence for new homotypic and heterotypic interactions between transmembrane helices of proteins involved in receptor tyrosine kinase and neuropilin signaling. J. Mol. Biol. 2014, 426, 4099–4111. [Google Scholar] [CrossRef] [PubMed]
- Antipenko, A.; Himanen, J.P.; van Leyen, K.; Nardi-Dei, V.; Lesniak, J.; Barton, W.A.; Rajashankar, K.R.; Lu, M.; Hoemme, C.; Puschel, A.W.; et al. Structure of the semaphorin-3A receptor binding module. Neuron 2003, 39, 589–598. [Google Scholar] [CrossRef]
- Barton, W.A.; Himanen, J.P.; Antipenko, A.; Nikolov, D.B. Structures of axon guidance molecules and their neuronal receptors. Adv. Protein Chem. 2004, 68, 65–106. [Google Scholar] [CrossRef]
- Neufeld, G.; Kessler, O. The semaphorins: Versatile regulators of tumour progression and tumour angiogenesis. Nat. Rev. Cancer 2008, 8, 632–645. [Google Scholar] [CrossRef]
- Valdembri, D.; Caswell, P.T.; Anderson, K.I.; Schwarz, J.P.; Konig, I.; Astanina, E.; Caccavari, F.; Norman, J.C.; Humphries, M.J.; Bussolino, F.; et al. Neuropilin-1/GIPC1 signaling regulates alpha5beta1 integrin traffic and function in endothelial cells. PLoS Biol. 2009, 7, e25. [Google Scholar] [CrossRef]
- Perrot-Applanat, M.; Di Benedetto, M. Autocrine functions of VEGF in breast tumor cells: Adhesion, survival, migration and invasion. Cell Adhes. Migr. 2012, 6, 547–553. [Google Scholar] [CrossRef]
- Yaqoob, U.; Cao, S.; Shergill, U.; Jagavelu, K.; Geng, Z.; Yin, M.; de Assuncao, T.M.; Cao, Y.; Szabolcs, A.; Thorgeirsson, S.; et al. Neuropilin-1 stimulates tumor growth by increasing fibronectin fibril assembly in the tumor microenvironment. Cancer Res. 2012, 72, 4047–4059. [Google Scholar] [CrossRef] [PubMed]
- Goel, H.L.; Pursell, B.; Chang, C.; Shaw, L.M.; Mao, J.; Simin, K.; Kumar, P.; Vander Kooi, C.W.; Shultz, L.D.; Greiner, D.L.; et al. GLI1 regulates a novel neuropilin-2/alpha6beta1 integrin based autocrine pathway that contributes to breast cancer initiation. EMBO Mol. Med. 2013, 5, 488–508. [Google Scholar] [CrossRef]
- Campbell, I.D.; Humphries, M.J. Integrin structure, activation, and interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a004994. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.H.; Carman, C.V.; Springer, T.A. Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 2007, 25, 619–647. [Google Scholar] [CrossRef] [PubMed]
- Humphries, J.D.; Byron, A.; Humphries, M.J. Integrin ligands at a glance. J. Cell Sci. 2006, 119, 3901–3903. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Fleury, C.; Jalalvand, F.; Riesbeck, K. Human pathogens utilize host extracellular matrix proteins laminin and collagen for adhesion and invasion of the host. FEMS Microbiol. Rev. 2012, 36, 1122–1180. [Google Scholar] [CrossRef]
- Humphries, J.D.; Chastney, M.R.; Askari, J.A.; Humphries, M.J. Signal transduction via integrin adhesion complexes. Curr. Opin. Cell Biol. 2018, 56, 14–21. [Google Scholar] [CrossRef]
- Horton, E.R.; Humphries, J.D.; James, J.; Jones, M.C.; Askari, J.A.; Humphries, M.J. The integrin adhesome network at a glance. J. Cell Sci. 2016, 129, 4159–4163. [Google Scholar] [CrossRef]
- Kanchanawong, P.; Waterman, C.M. Advances in light-based imaging of three-dimensional cellular ultrastructure. Curr. Opin. Cell Biol. 2012, 24, 125–133. [Google Scholar] [CrossRef]
- Kanchanawong, P.; Shtengel, G.; Pasapera, A.M.; Ramko, E.B.; Davidson, M.W.; Hess, H.F.; Waterman, C.M. Nanoscale architecture of integrin-based cell adhesions. Nature 2010, 468, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Hoeppner, L.H.; Bach, S.; E, G.; Guo, Y.; Wang, E.; Wu, J.; Cowley, M.J.; Chang, D.K.; Waddell, N.; et al. Neuropilin-2 promotes extravasation and metastasis by interacting with endothelial alpha5 integrin. Cancer Res. 2013, 73, 4579–4590. [Google Scholar] [CrossRef] [PubMed]
- Goel, H.L.; Mercurio, A.M. Enhancing integrin function by VEGF/neuropilin signaling: Implications for tumor biology. Cell Adhes. Migr. 2012, 6, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Wanami, L.S.; Dissanayake, T.R.; Bachelder, R.E. Autocrine semaphorin3A stimulates alpha2 beta1 integrin expression/function in breast tumor cells. Breast Cancer Res. Treat. 2009, 118, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Ellison, T.S.; Atkinson, S.J.; Steri, V.; Kirkup, B.M.; Preedy, M.E.; Johnson, R.T.; Ruhrberg, C.; Edwards, D.R.; Schneider, J.G.; Weilbaecher, K.; et al. Suppression of beta3-integrin in mice triggers a neuropilin-1-dependent change in focal adhesion remodelling that can be targeted to block pathological angiogenesis. Dis. Model. Mech. 2015, 8, 1105–1119. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.D.; Reynolds, L.E.; Kostourou, V.; Reynolds, A.R.; da Silva, R.G.; Tavora, B.; Baker, M.; Marshall, J.F.; Hodivala-Dilke, K.M. Alphav beta3 integrin limits the contribution of neuropilin-1 to vascular endothelial growth factor-induced angiogenesis. J. Biol. Chem. 2009, 284, 33966–33981. [Google Scholar] [CrossRef] [PubMed]
- Castellani, V. The function of neuropilin/L1 complex. Adv. Exp. Med. Biol. 2002, 515, 91–102. [Google Scholar] [PubMed]
- Castellani, V.; Falk, J.; Rougon, G. Semaphorin3A-induced receptor endocytosis during axon guidance responses is mediated by L1 CAM. Mol. Cell. Neurosci. 2004, 26, 89–100. [Google Scholar] [CrossRef]
- Bechara, A.; Nawabi, H.; Moret, F.; Yaron, A.; Weaver, E.; Bozon, M.; Abouzid, K.; Guan, J.L.; Tessier-Lavigne, M.; Lemmon, V.; et al. FAK-MAPK-dependent adhesion disassembly downstream of L1 contributes to semaphorin3A-induced collapse. EMBO J. 2008, 27, 1549–1562. [Google Scholar] [CrossRef]
- Ben-Zvi, A.; Ben-Gigi, L.; Klein, H.; Behar, O. Modulation of semaphorin3A activity by p75 neurotrophin receptor influences peripheral axon patterning. J. Neurosci. 2007, 27, 13000–13011. [Google Scholar] [CrossRef]
- Park, J.E.; Keller, G.A.; Ferrara, N. The vascular endothelial growth factor (VEGF) isoforms: Differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol. Biol. Cell 1993, 4, 1317–1326. [Google Scholar] [CrossRef]
- Jonca, F.; Ortega, N.; Gleizes, P.E.; Bertrand, N.; Plouet, J. Cell release of bioactive fibroblast growth factor 2 by exon 6-encoded sequence of vascular endothelial growth factor. J. Biol. Chem. 1997, 272, 24203–24209. [Google Scholar] [CrossRef] [PubMed]
- Stringer, S.E. The role of heparan sulphate proteoglycans in angiogenesis. Biochem. Soc. Trans. 2006, 34, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Claesson-Welsh, L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb. Perspect. Med. 2012, 2, a006502. [Google Scholar] [CrossRef] [PubMed]
- Vempati, P.; Popel, A.S.; Mac Gabhann, F. Extracellular regulation of VEGF: Isoforms, proteolysis, and vascular patterning. Cytokine Growth Factor Rev. 2014, 25, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Niland, S.; Ditkowski, B.; Parrandier, D.; Roth, L.; Augustin, H.; Eble, J.A. Rhodocetin-alphabeta-induced neuropilin-1-cMet association triggers restructuring of matrix contacts in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Niland, S.; Komljenovic, D.; Macas, J.; Bracht, T.; Bauerle, T.; Liebner, S.; Eble, J.A. Rhodocetin-alphabeta selectively breaks the endothelial barrier of the tumor vasculature in HT1080 fibrosarcoma and A431 epidermoid carcinoma tumor models. Oncotarget 2018, 9, 22406–22422. [Google Scholar] [CrossRef]
- Koch, S.; van Meeteren, L.A.; Morin, E.; Testini, C.; Westrom, S.; Bjorkelund, H.; Le Jan, S.; Adler, J.; Berger, P.; Claesson-Welsh, L. NRP1 presented in trans to the endothelium arrests VEGFR2 endocytosis, preventing angiogenic signaling and tumor initiation. Dev. Cell 2014, 28, 633–646. [Google Scholar] [CrossRef]
- Campos-Mora, M.; Morales, R.A.; Gajardo, T.; Catalan, D.; Pino-Lagos, K. Neuropilin-1 in transplantation tolerance. Front. Immunol. 2013, 4, 405. [Google Scholar] [CrossRef]
- Delgoffe, G.M.; Woo, S.R.; Turnis, M.E.; Gravano, D.M.; Guy, C.; Overacre, A.E.; Bettini, M.L.; Vogel, P.; Finkelstein, D.; Bonnevier, J.; et al. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature 2013, 501, 252–256. [Google Scholar] [CrossRef]
- Bourbie-Vaudaine, S.; Blanchard, N.; Hivroz, C.; Romeo, P.H. Dendritic Cells Can Turn CD4+ T Lymphocytes into Vascular Endothelial Growth Factor-Carrying Cells by Intercellular Neuropilin-1 Transfer. J. Immunol. 2006, 177, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Shimizu, A.; Asano, H.; Kadonosono, T.; Kondoh, S.K.; Geretti, E.; Mammoto, A.; Klagsbrun, M.; Seo, M.K. VEGF-A/NRP1 stimulates GIPC1 and Syx complex formation to promote RhoA activation and proliferation in skin cancer cells. Biol. Open 2015, 4, 1063–1076. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.R.; Baker, D.; James, N.H.; Ratcliffe, K.; Jenkins, M.; Ashton, S.E.; Sproat, G.; Swann, R.; Gray, N.; Ryan, A.; et al. Vascular endothelial growth factor receptors VEGFR-2 and VEGFR-3 are localized primarily to the vasculature in human primary solid cancers. Clin. Cancer. Res. 2010, 16, 3548–3561. [Google Scholar] [CrossRef] [PubMed]
- Prud’homme, G.J.; Glinka, Y. Neuropilins are multifunctional coreceptors involved in tumor initiation, growth, metastasis and immunity. Oncotarget 2012, 3, 921–939. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Fournier, A.; Nakamura, F.; Wang, L.H.; Murakami, Y.; Kalb, R.G.; Fujisawa, H.; Strittmatter, S.M. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell 1999, 99, 59–69. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Li, C.; Zhao, Y.; Wu, L.; Du, X.; Han, Z. VEGF-A/Neuropilin 1 Pathway Confers Cancer Stemness via Activating Wnt/beta-Catenin Axis in Breast Cancer Cells. Cell. Physiol. Biochem. 2017, 44, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Atzori, M.G.; Tentori, L.; Ruffini, F.; Ceci, C.; Lisi, L.; Bonanno, E.; Scimeca, M.; Eskilsson, E.; Daubon, T.; Miletic, H.; et al. The anti-vascular endothelial growth factor receptor-1 monoclonal antibody D16F7 inhibits invasiveness of human glioblastoma and glioblastoma stem cells. J. Exp. Clin. Cancer Res. 2017, 36, 106. [Google Scholar] [CrossRef]
- Shimizu, A.; Zankov, D.P.; Kurokawa-Seo, M.; Ogita, H. Vascular Endothelial Growth Factor-A Exerts Diverse Cellular Effects via Small G Proteins, Rho and Rap. Int. J. Mol. Sci. 2018, 19, 1203. [Google Scholar] [CrossRef]
- Wang, Z.; Ahmad, A.; Li, Y.; Kong, D.; Azmi, A.S.; Banerjee, S.; Sarkar, F.H. Emerging roles of PDGF-D signaling pathway in tumor development and progression. Biochim. Biophys. Acta 2010, 1806, 122–130. [Google Scholar] [CrossRef]
- Hernandez-Garcia, R.; Iruela-Arispe, M.L.; Reyes-Cruz, G.; Vazquez-Prado, J. Endothelial RhoGEFs: A systematic analysis of their expression profiles in VEGF-stimulated and tumor endothelial cells. Vascul. Pharmacol. 2015, 74, 60–72. [Google Scholar] [CrossRef]
- Ghosh, K.; Thodeti, C.K.; Dudley, A.C.; Mammoto, A.; Klagsbrun, M.; Ingber, D.E. Tumor-derived endothelial cells exhibit aberrant Rho-mediated mechanosensing and abnormal angiogenesis in vitro. Proc. Natl. Acad. Sci. USA 2008, 105, 11305–11310. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, X.; Simons, M. Molecular controls of lymphatic VEGFR3 signaling. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Parikh, A.A.; Fan, F.; Liu, W.B.; Ahmad, S.A.; Stoeltzing, O.; Reinmuth, N.; Bielenberg, D.; Bucana, C.D.; Klagsbrun, M.; Ellis, L.M. Neuropilin-1 in human colon cancer: Expression, regulation, and role in induction of angiogenesis. Am. J. Pathol. 2004, 164, 2139–2151. [Google Scholar] [CrossRef]
- Ding, H.; Wu, X.; Roncari, L.; Lau, N.; Shannon, P.; Nagy, A.; Guha, A. Expression and regulation of neuropilin-1 in human astrocytomas. Int. J. Cancer 2000, 88, 584–592. [Google Scholar] [CrossRef]
- Akagi, M.; Kawaguchi, M.; Liu, W.; McCarty, M.F.; Takeda, A.; Fan, F.; Stoeltzing, O.; Parikh, A.A.; Jung, Y.D.; Bucana, C.D.; et al. Induction of neuropilin-1 and vascular endothelial growth factor by epidermal growth factor in human gastric cancer cells. Br. J. Cancer 2003, 88, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Tzahar, E.; Waterman, H.; Chen, X.; Levkowitz, G.; Karunagaran, D.; Lavi, S.; Ratzkin, B.J.; Yarden, Y. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol. Cell. Biol. 1996, 16, 5276–5287. [Google Scholar] [CrossRef] [PubMed]
- Aghajanian, H.; Cho, Y.K.; Manderfield, L.J.; Herling, M.R.; Gupta, M.; Ho, V.C.; Li, L.; Degenhardt, K.; Aharonov, A.; Tzahor, E.; et al. Coronary vasculature patterning requires a novel endothelial ErbB2 holoreceptor. Nat. Commun. 2016, 7, 12038. [Google Scholar] [CrossRef] [PubMed]
- Rizzolio, S.; Battistini, C.; Cagnoni, G.; Apicella, M.; Vella, V.; Giordano, S.; Tamagnone, L. Downregulating neuropilin-2 triggers a novel mechanism enabling EGFR-dependent resistance to oncogene-targeted therapies. Cancer Res. 2018, 78, 1058–1068. [Google Scholar] [CrossRef]
- Ball, S.G.; Bayley, C.; Shuttleworth, C.A.; Kielty, C.M. Neuropilin-1 regulates platelet-derived growth factor receptor signalling in mesenchymal stem cells. Biochem. J. 2010, 427, 29–40. [Google Scholar] [CrossRef]
- Banerjee, S.; Sengupta, K.; Dhar, K.; Mehta, S.; D’Amore, P.A.; Dhar, G.; Banerjee, S.K. Breast cancer cells secreted platelet-derived growth factor-induced motility of vascular smooth muscle cells is mediated through neuropilin-1. Mol. Carcinog. 2006, 45, 871–880. [Google Scholar] [CrossRef]
- Cao, S.; Yaqoob, U.; Das, A.; Shergill, U.; Jagavelu, K.; Huebert, R.C.; Routray, C.; Abdelmoneim, S.; Vasdev, M.; Leof, E.; et al. Neuropilin-1 promotes cirrhosis of the rodent and human liver by enhancing PDGF/TGF-beta signaling in hepatic stellate cells. J. Clin. Investig. 2010, 120, 2379–2394. [Google Scholar] [CrossRef] [PubMed]
- Dhar, K.; Dhar, G.; Majumder, M.; Haque, I.; Mehta, S.; Van Veldhuizen, P.J.; Banerjee, S.K.; Banerjee, S. Tumor cell-derived PDGF-B potentiates mouse mesenchymal stem cells-pericytes transition and recruitment through an interaction with NRP-1. Mol. Cancer 2010, 9, 209. [Google Scholar] [CrossRef] [PubMed]
- Evans, I.M.; Yamaji, M.; Britton, G.; Pellet-Many, C.; Lockie, C.; Zachary, I.C.; Frankel, P. Neuropilin-1 signaling through p130Cas tyrosine phosphorylation is essential for growth factor-dependent migration of glioma and endothelial cells. Mol. Cell. Biol. 2011, 31, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Ponten, A.; Folestad, E.B.; Pietras, K.; Eriksson, U. Platelet-derived growth factor D induces cardiac fibrosis and proliferation of vascular smooth muscle cells in heart-specific transgenic mice. Circ. Res. 2005, 97, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008, 22, 1276–1312. [Google Scholar] [CrossRef] [PubMed]
- Cortez, E.; Gladh, H.; Braun, S.; Bocci, M.; Cordero, E.; Bjorkstrom, N.K.; Miyazaki, H.; Michael, I.P.; Eriksson, U.; Folestad, E.; et al. Functional malignant cell heterogeneity in pancreatic neuroendocrine tumors revealed by targeting of PDGF-DD. Proc. Natl. Acad. Sci. USA 2016, 113, E864–E873. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, P.; Bar-Joseph, I.; Imanishi, Y.; Jarzynka, M.J.; Bogler, O.; Mikkelsen, T.; Hirose, T.; Nishikawa, R.; Cheng, S.Y. Neuropilin-1 promotes human glioma progression through potentiating the activity of the HGF/SF autocrine pathway. Oncogene 2007, 26, 5577–5586. [Google Scholar] [CrossRef]
- Weinstein, I.B. Cancer. Addiction to oncogenes—The Achilles heal of cancer. Science 2002, 297, 63–64. [Google Scholar] [CrossRef]
- Li, L.; Jiang, X.; Zhang, Q.; Dong, X.; Gao, Y.; He, Y.; Qiao, H.; Xie, F.; Xie, X.; Sun, X. Neuropilin-1 is associated with clinicopathology of gastric cancer and contributes to cell proliferation and migration as multifunctional co-receptors. J. Exp. Clin. Cancer Res. 2016, 35, 16. [Google Scholar] [CrossRef]
- Rizzolio, S.; Cagnoni, G.; Battistini, C.; Bonelli, S.; Isella, C.; Van Ginderachter, J.A.; Bernards, R.; Di Nicolantonio, F.; Giordano, S.; Tamagnone, L. Neuropilin-1 upregulation elicits adaptive resistance to oncogene-targeted therapies. J. Clin. Investig. 2018, 128, 3976–3990. [Google Scholar] [CrossRef]
- Cao, Y.; Szabolcs, A.; Dutta, S.K.; Yaqoob, U.; Jagavelu, K.; Wang, L.; Leof, E.B.; Urrutia, R.A.; Shah, V.H.; Mukhopadhyay, D. Neuropilin-1 mediates divergent R-Smad signaling and the myofibroblast phenotype. J. Biol. Chem. 2010, 285, 31840–31848. [Google Scholar] [CrossRef] [PubMed]
- Hirota, S.; Clements, T.P.; Tang, L.K.; Morales, J.E.; Lee, H.S.; Oh, S.P.; Rivera, G.M.; Wagner, D.S.; McCarty, J.H. Neuropilin 1 balances beta8 integrin-activated TGFbeta signaling to control sprouting angiogenesis in the brain. Development 2015, 142, 4363–4373. [Google Scholar] [CrossRef] [PubMed]
- Aspalter, I.M.; Gordon, E.; Dubrac, A.; Ragab, A.; Narloch, J.; Vizan, P.; Geudens, I.; Collins, R.T.; Franco, C.A.; Abrahams, C.L.; et al. Alk1 and Alk5 inhibition by Nrp1 controls vascular sprouting downstream of Notch. Nat. Commun. 2015, 6, 7264. [Google Scholar] [CrossRef] [PubMed]
- Vivekanandhan, S.; Yang, L.; Cao, Y.; Wang, E.; Dutta, S.K.; Sharma, A.K.; Mukhopadhyay, D. Genetic status of KRAS modulates the role of Neuropilin-1 in tumorigenesis. Sci. Rep. 2017, 7, 12877. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Yin, W.; Wang, Y.; Zhou, L.; Liu, Y.; Yang, G.; Wang, J.; Lu, J. MiR-206 suppresses epithelial mesenchymal transition by targeting TGF-beta signaling in estrogen receptor positive breast cancer cells. Oncotarget 2016, 7, 24537–24548. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, S.; Wu, B.; Fang, J.; Zhu, M.; Sun, L.; Zhang, L.; Zhang, Y.; Sun, M.; Guo, L.; et al. Transforming growth factor-beta1 promotes breast cancer metastasis by downregulating miR-196a-3p expression. Oncotarget 2017, 8, 49110–49122. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, G.; Sabag, A.D.; Rabinovicz, N.; Kessler, O. Semaphorins in angiogenesis and tumor progression. Cold Spring Harb. Perspect. Med. 2012, 2, a006718. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, A.; Doci, C.L.; Gutkind, J.S. Semaphorin signaling in angiogenesis, lymphangiogenesis and cancer. Cell Res. 2012, 22, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Hu, J.; Uemura, A.; Tetzlaff, F.; Augustin, H.G.; Fischer, A. Semaphorin-3C signals through Neuropilin-1 and PlexinD1 receptors to inhibit pathological angiogenesis. EMBO Mol. Med. 2015, 7, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Yu, J.S. Semaphorin 3C and its receptors in cancer and cancer stem-like cells. Biomedicines 2018, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Bielenberg, D.R.; Hida, Y.; Shimizu, A.; Kaipainen, A.; Kreuter, M.; Kim, C.C.; Klagsbrun, M. Semaphorin 3F, a chemorepulsant for endothelial cells, induces a poorly vascularized, encapsulated, nonmetastatic tumor phenotype. J. Clin. Investig. 2004, 114, 1260–1271. [Google Scholar] [CrossRef] [PubMed]
- Kessler, O.; Shraga-Heled, N.; Lange, T.; Gutmann-Raviv, N.; Sabo, E.; Baruch, L.; Machluf, M.; Neufeld, G. Semaphorin-3F is an inhibitor of tumor angiogenesis. Cancer Res. 2004, 64, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Li, S.; Chepeha, D.B.; Giordano, T.J.; Li, J.; Zhang, H.; Polverini, P.J.; Nor, J.; Kitajewski, J.; Wang, C.Y. Crosstalk between tumor and endothelial cells promotes tumor angiogenesis by MAPK activation of Notch signaling. Cancer Cell 2005, 8, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Benedito, R.; Roca, C.; Sörensen, I.; Adams, S.; Gossler, A.; Fruttiger, M.; Adams, R.H. The Notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell 2009, 137, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Abramsson, A.; Betsholtz, C. Endothelial/pericyte interactions. Circ. Res. 2005, 97, 512–523. [Google Scholar] [CrossRef] [PubMed]
- von Tell, D.; Armulik, A.; Betsholtz, C. Pericytes and vascular stability. Exp. Cell Res. 2006, 312, 623–629. [Google Scholar] [CrossRef]
- Gaengel, K.; Genove, G.; Armulik, A.; Betsholtz, C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 630–638. [Google Scholar] [CrossRef]
- Gu, C.; Giraudo, E. The role of semaphorins and their receptors in vascular development and cancer. Exp. Cell Res. 2013, 319, 1306–1316. [Google Scholar] [CrossRef]
- Aguilera, K.Y.; Brekken, R.A. Recruitment and retention: Factors that affect pericyte migration. Cell. Mol. Life Sci. 2014, 71, 299–309. [Google Scholar] [CrossRef]
- Xian, X.; Hakansson, J.; Stahlberg, A.; Lindblom, P.; Betsholtz, C.; Gerhardt, H.; Semb, H. Pericytes limit tumor cell metastasis. J. Clin. Investig. 2006, 116, 642–651. [Google Scholar] [CrossRef]
- Chakraborty, G.; Kumar, S.; Mishra, R.; Patil, T.V.; Kundu, G.C. Semaphorin 3A suppresses tumor growth and metastasis in mice melanoma model. PLoS ONE 2012, 7, e33633. [Google Scholar] [CrossRef]
- Fukasawa, M.; Matsushita, A.; Korc, M. Neuropilin-1 interacts with integrin beta1 and modulates pancreatic cancer cell growth, survival and invasion. Cancer Biol. Ther. 2007, 6, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Goel, H.L.; Mercurio, A.M. VEGF targets the tumour cell. Nat. Rev. Cancer 2013, 13, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Byzova, T.V.; Goldman, C.K.; Pampori, N.; Thomas, K.A.; Bett, A.; Shattil, S.J.; Plow, E.F. A mechanism for modulation of cellular responses to VEGF: Activation of the integrins. Mol. Cell 2000, 6, 851–860. [Google Scholar] [CrossRef]
- Serini, G.; Valdembri, D.; Zanivan, S.; Morterra, G.; Burkhardt, C.; Caccavari, F.; Zammataro, L.; Primo, L.; Tamagnone, L.; Logan, M.; et al. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature 2003, 424, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Valdembri, D.; Regano, D.; Maione, F.; Giraudo, E.; Serini, G. Class 3 semaphorins in cardiovascular development. Cell Adhes. Migr. 2016, 10, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Goel, H.L.; Pursell, B.; Standley, C.; Fogarty, K.; Mercurio, A.M. Neuropilin-2 regulates alpha6beta1 integrin in the formation of focal adhesions and signaling. J. Cell Sci. 2012, 125, 497–506. [Google Scholar] [CrossRef]
- Muders, M.H.; Zhang, H.; Wang, E.; Tindall, D.J.; Datta, K. Vascular endothelial growth factor-C protects prostate cancer cells from oxidative stress by the activation of mammalian target of rapamycin complex-2 and AKT-1. Cancer Res. 2009, 69, 6042–6048. [Google Scholar] [CrossRef]
- Facchinetti, V.; Ouyang, W.; Wei, H.; Soto, N.; Lazorchak, A.; Gould, C.; Lowry, C.; Newton, A.C.; Mao, Y.; Miao, R.Q.; et al. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J. 2008, 27, 1932–1943. [Google Scholar] [CrossRef]
- D’Haene, N.; Sauvage, S.; Maris, C.; Adanja, I.; Le Mercier, M.; Decaestecker, C.; Baum, L.; Salmon, I. VEGFR1 and VEGFR2 involvement in extracellular galectin-1- and galectin-3-induced angiogenesis. PLoS ONE 2013, 8, e67029. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Wang, Y.; Wu, X. The Role of Galectins in Cervical Cancer Biology and Progression. BioMed Res. Int. 2018, 2018, 2175927. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.H.; Ying, N.W.; Wu, M.H.; Chiang, W.F.; Hsu, C.L.; Wong, T.Y.; Jin, Y.T.; Hong, T.M.; Chen, Y.L. Galectin-1, a novel ligand of neuropilin-1, activates VEGFR-2 signaling and modulates the migration of vascular endothelial cells. Oncogene 2008, 27, 3746–3753. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; E, G.; Wang, E.; Pal, K.; Dutta, S.K.; Bar-Sagi, D.; Mukhopadhyay, D. VEGF exerts an angiogenesis-independent function in cancer cells to promote their malignant progression. Cancer Res. 2012, 72, 3912–3918. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, K.; Shirakihara, T.; Nakano, A.; Imamura, T.; Miyazono, K.; Saitoh, M. Role of Ras signaling in the induction of snail by transforming growth factor-beta. J. Biol. Chem. 2009, 284, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, A.; Seerapu, H.R. Regulation of VEGF signaling by membrane traffic. Cell. Signal. 2012, 24, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Lanahan, A.A.; Hermans, K.; Claes, F.; Kerley-Hamilton, J.S.; Zhuang, Z.W.; Giordano, F.J.; Carmeliet, P.; Simons, M. VEGF receptor 2 endocytic trafficking regulates arterial morphogenesis. Dev. Cell 2010, 18, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Lanahan, A.; Zhang, X.; Fantin, A.; Zhuang, Z.; Rivera-Molina, F.; Speichinger, K.; Prahst, C.; Zhang, J.; Wang, Y.; Davis, G.; et al. The neuropilin 1 cytoplasmic domain is required for VEGF-A-dependent arteriogenesis. Dev. Cell 2013, 25, 156–168. [Google Scholar] [CrossRef]
- Kawamura, H.; Li, X.; Goishi, K.; van Meeteren, L.A.; Jakobsson, L.; Cebe-Suarez, S.; Shimizu, A.; Edholm, D.; Ballmer-Hofer, K.; Kjellen, L.; et al. Neuropilin-1 in regulation of VEGF-induced activation of p38MAPK and endothelial cell organization. Blood 2008, 112, 3638–3649. [Google Scholar] [CrossRef]
- Cai, H.; Reed, R.R. Cloning and characterization of neuropilin-1-interacting protein: A PSD-95/Dlg/ZO-1 domain-containing protein that interacts with the cytoplasmic domain of neuropilin-1. J. Neurosci. 1999, 19, 6519–6527. [Google Scholar] [CrossRef]
- Prahst, C.; Heroult, M.; Lanahan, A.A.; Uziel, N.; Kessler, O.; Shraga-Heled, N.; Simons, M.; Neufeld, G.; Augustin, H.G. Neuropilin-1-VEGFR-2 complexing requires the PDZ-binding domain of neuropilin-1. J. Biol. Chem. 2008, 283, 25110–25114. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, L.; Sun, K.; Khan, A.A.; Yan, J.; Liu, H.; Lu, A.; Gu, N. Neuropilin-1 (NRP-1)/GIPC1 pathway mediates glioma progression. Tumour Biol. 2016, 37, 13777–13788. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.B.; Braun, G.B.; Friman, T.; Aza-Blanc, P.; Ruidiaz, M.E.; Sugahara, K.N.; Teesalu, T.; Ruoslahti, E. An endocytosis pathway initiated through neuropilin-1 and regulated by nutrient availability. Nat. Commun. 2014, 5, 4904. [Google Scholar] [CrossRef]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Greenwald, D.R.; Ruoslahti, E. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science 2010, 328, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, L.; Nandy, D.; Zhang, Y.; Basu, A.; Radisky, D.; Mukhopadhyay, D. Neuropilin-1 upholds dedifferentiation and propagation phenotypes of renal cell carcinoma cells by activating Akt and sonic hedgehog axes. Cancer Res. 2008, 68, 8667–8672. [Google Scholar] [CrossRef] [PubMed]
- Hillman, R.T.; Feng, B.Y.; Ni, J.; Woo, W.M.; Milenkovic, L.; Hayden Gephart, M.G.; Teruel, M.N.; Oro, A.E.; Chen, J.K.; Scott, M.P. Neuropilins are positive regulators of Hedgehog signal transduction. Genes Dev. 2011, 25, 2333–2346. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Milenkovic, L.; Suyama, K.; Hartl, T.; Purzner, T.; Winans, A.; Meyer, T.; Scott, M.P. Phosphodiesterase 4D acts downstream of Neuropilin to control Hedgehog signal transduction and the growth of medulloblastoma. eLife 2015, 4, e07068. [Google Scholar] [CrossRef] [PubMed]
- Po, A.; Silvano, M.; Miele, E.; Capalbo, C.; Eramo, A.; Salvati, V.; Todaro, M.; Besharat, Z.M.; Catanzaro, G.; Cucchi, D.; et al. Noncanonical GLI1 signaling promotes stemness features and in vivo growth in lung adenocarcinoma. Oncogene 2017, 36, 4641–4652. [Google Scholar] [CrossRef]
- Snuderl, M.; Batista, A.; Kirkpatrick, N.D.; Ruiz de Almodovar, C.; Riedemann, L.; Walsh, E.C.; Anolik, R.; Huang, Y.; Martin, J.D.; Kamoun, W.; et al. Targeting placental growth factor/neuropilin 1 pathway inhibits growth and spread of medulloblastoma. Cell 2013, 152, 1065–1076. [Google Scholar] [CrossRef]
- Yogi, K.; Sridhar, E.; Goel, N.; Jalali, R.; Goel, A.; Moiyadi, A.; Thorat, R.; Panwalkar, P.; Khire, A.; Dasgupta, A.; et al. MiR-148a, a microRNA upregulated in the WNT subgroup tumors, inhibits invasion and tumorigenic potential of medulloblastoma cells by targeting Neuropilin 1. Oncoscience 2015, 2, 334–348. [Google Scholar] [CrossRef]
- Liu, W.; Wu, T.; Dong, X.; Zeng, Y.A. Neuropilin-1 is upregulated by Wnt/beta-catenin signaling and is important for mammary stem cells. Sci. Rep. 2017, 7, 10941. [Google Scholar] [CrossRef]
- Kim, D.; Lee, V.; Dorsey, T.B.; Niklason, L.E.; Gui, L.; Dai, G. Neuropilin-1 Mediated Arterial Differentiation of Murine Pluripotent Stem Cells. Stem Cells Dev. 2018, 27, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, K.; Murray, S.; Manousou, K.; Tikas, I.; Dervenis, C.; Sgouros, J.; Rontogianni, D.; Lakis, S.; Bobos, M.; Poulios, C.; et al. Genotyping and mRNA profiling reveal actionable molecular targets in biliary tract cancers. Am. J. Cancer Res. 2018, 8, 2–15. [Google Scholar] [PubMed]
- Takakura, N. Formation and regulation of the cancer stem cell niche. Cancer Sci. 2012, 103, 1177–1181. [Google Scholar] [CrossRef] [PubMed]
- Samuel, S.; Gaur, P.; Fan, F.; Xia, L.; Gray, M.J.; Dallas, N.A.; Bose, D.; Rodriguez-Aguayo, C.; Lopez-Berestein, G.; Plowman, G.; et al. Neuropilin-2 mediated beta-catenin signaling and survival in human gastro-intestinal cancer cell lines. PLoS ONE 2011, 6, e23208. [Google Scholar] [CrossRef]
- Grandclement, C.; Pallandre, J.R.; Valmary Degano, S.; Viel, E.; Bouard, A.; Balland, J.; Remy-Martin, J.P.; Simon, B.; Rouleau, A.; Boireau, W.; et al. Neuropilin-2 expression promotes TGF-beta1-mediated epithelial to mesenchymal transition in colorectal cancer cells. PLoS ONE 2011, 6, e20444. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Guo, Y.; Kim, K.; McQueen, P.; Ghaffar, S.; Christ, A.; Lin, C.; Eskander, R.; Zi, X.; Hoang, B.H. Neuropilin-2 expression is inhibited by secreted Wnt antagonists and its down-regulation is associated with reduced tumor growth and metastasis in osteosarcoma. Mol. Cancer 2015, 14, 86. [Google Scholar] [CrossRef]
- Parker, M.W.; Linkugel, A.D.; Goel, H.L.; Wu, T.; Mercurio, A.M.; Vander Kooi, C.W. Structural basis for VEGF-C binding to neuropilin-2 and sequestration by a soluble splice form. Structure 2015, 23, 677–687. [Google Scholar] [CrossRef]
- Lichtenberger, B.M.; Tan, P.K.; Niederleithner, H.; Ferrara, N.; Petzelbauer, P.; Sibilia, M. Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell 2010, 140, 268–279. [Google Scholar] [CrossRef]
- Sun, C.; Wang, L.; Huang, S.; Heynen, G.J.; Prahallad, A.; Robert, C.; Haanen, J.; Blank, C.; Wesseling, J.; Willems, S.M.; et al. Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature 2014, 508, 118–122. [Google Scholar] [CrossRef]
- Wang, J.; Huang, S.K.; Marzese, D.M.; Hsu, S.C.; Kawas, N.P.; Chong, K.K.; Long, G.V.; Menzies, A.M.; Scolyer, R.A.; Izraely, S.; et al. Epigenetic changes of EGFR have an important role in BRAF inhibitor-resistant cutaneous melanomas. J. Investig. Dermatol. 2015, 135, 532–541. [Google Scholar] [CrossRef]
- Prud’homme, G.J.; Glinka, Y.; Lichner, Z.; Yousef, G.M. Neuropilin-1 is a receptor for extracellular miRNA and AGO2/miRNA complexes and mediates the internalization of miRNAs that modulate cell function. Oncotarget 2016, 7, 68057–68071. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Egranov, S.D.; Yang, L.; Lin, C. Molecular Mechanisms of Long Noncoding RNAs-mediated Cancer Metastasis. Genes Chromosomes Cancer 2018. [Google Scholar] [CrossRef]
- Chen, Z.P.; Wei, J.C.; Wang, Q.; Yang, P.; Li, W.L.; He, F.; Chen, H.C.; Hu, H.; Zhong, J.B.; Cao, J. Long noncoding RNA 00152 functions as a competing endogenous RNA to regulate NRP1 expression by sponging with miRNA206 in colorectal cancer. Int. J. Oncol. 2018, 53, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, M.; Huang, Y.; Feng, L.; Chen, H.; Hu, Y.; Chen, H.; Zhang, K.; Zheng, L.; Zheng, S. MicroRNA-320b promotes colorectal cancer proliferation and invasion by competing with its homologous microRNA-320a. Cancer Lett. 2015, 356, 669–675. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, Y.M.; Li, L.C.; Wang, L.L.; Wu, X.L. MicroRNA-338 inhibits growth, invasion and metastasis of gastric cancer by targeting NRP1 expression. PLoS ONE 2014, 9, e94422. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, Z.; Wang, Y.; Gu, W. MiR-338 suppresses the growth and metastasis of OSCC cells by targeting NRP1. Mol. Cell. Biochem. 2015, 398, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Liu, X.C.; Du, J.; Zhang, Y.J. MiR-152 regulates metastases of non-small cell lung cancer cells by targeting neuropilin-1. Int. J. Clin. Exp. Pathol. 2015, 8, 14235–14240. [Google Scholar]
- Zhang, L.; Chen, Y.; Wang, H.; Zheng, X.; Li, C.; Han, Z. miR-376a inhibits breast cancer cell progression by targeting neuropilin-1 NR. Onco Targets Ther. 2018, 11, 5293–5302. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, L.; Khan, A.A.; Li, B.; Gu, B.; Lin, F.; Su, X.; Yan, J. miRNA-124-3p/neuropilin-1(NRP-1) axis plays an important role in mediating glioblastoma growth and angiogenesis. Int. J. Cancer 2018, 143, 635–644. [Google Scholar] [CrossRef]
- Epis, M.R.; Giles, K.M.; Candy, P.A.; Webster, R.J.; Leedman, P.J. miR-331-3p regulates expression of neuropilin-2 in glioblastoma. J. Neurooncol. 2014, 116, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Chopp, M.; Lu, Y.; Buller, B.; Jiang, F. MiR-15b and miR-152 reduce glioma cell invasion and angiogenesis via NRP-2 and MMP-3. Cancer Lett. 2013, 329, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, M.; Hu, Y.; Shi, N.; Yu, H.; Liu, H.; Lian, H. miR-486-5p attenuates tumor growth and lymphangiogenesis by targeting neuropilin-2 in colorectal carcinoma. Onco Targets Ther. 2016, 9, 2865–2871. [Google Scholar] [CrossRef] [PubMed]
- Pagani, E.; Ruffini, F.; Antonini Cappellini, G.C.; Scoppola, A.; Fortes, C.; Marchetti, P.; Graziani, G.; D’Atri, S.; Lacal, P.M. Placenta growth factor and neuropilin-1 collaborate in promoting melanoma aggressiveness. Int. J. Oncol. 2016, 48, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Pellet-Many, C.; Frankel, P.; Jia, H.; Zachary, I. Neuropilins: Structure, function and role in disease. Biochem. J. 2008, 411, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Janssen, B.J.; Malinauskas, T.; Weir, G.A.; Cader, M.Z.; Siebold, C.; Jones, E.Y. Neuropilins lock secreted semaphorins onto plexins in a ternary signaling complex. Nat. Struct. Mol. Biol. 2012, 19, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Palodetto, B.; da Silva Santos Duarte, A.; Rodrigues Lopes, M.; Adolfo Corrocher, F.; Marconi Roversi, F.; Soares Niemann, F.; Priscila Vieira Ferro, K.; Leda Figueiredo Longhini, A.; Melo Campos, P.; Favaro, P.; et al. SEMA3A partially reverses VEGF effects through binding to neuropilin-1. Stem Cell Res. 2017, 22, 70–78. [Google Scholar] [CrossRef]
- Chu, W.; Song, X.; Yang, X.; Ma, L.; Zhu, J.; He, M.; Wang, Z.; Wu, Y. Neuropilin-1 promotes epithelial-to-mesenchymal transition by stimulating nuclear factor-kappa B and is associated with poor prognosis in human oral squamous cell carcinoma. PLoS ONE 2014, 9, e101931. [Google Scholar] [CrossRef]
- Nasarre, P.; Gemmill, R.M.; Potiron, V.A.; Roche, J.; Lu, X.; Baron, A.E.; Korch, C.; Garrett-Mayer, E.; Lagana, A.; Howe, P.H.; et al. Neuropilin-2 Is upregulated in lung cancer cells during TGF-beta1-induced epithelial-mesenchymal transition. Cancer Res. 2013, 73, 7111–7121. [Google Scholar] [CrossRef]
- Hirata, E.; Sahai, E. Tumor Microenvironment and Differential Responses to Therapy. Cold Spring Harb. Perspect. Med. 2017, 7, a026781. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Otranto, M.; Sarrazy, V.; Bonte, F.; Hinz, B.; Gabbiani, G.; Desmouliere, A. The role of the myofibroblast in tumor stroma remodeling. Cell Adhes. Migr. 2012, 6, 203–219. [Google Scholar] [CrossRef] [PubMed]
- von Ahrens, D.; Bhagat, T.D.; Nagrath, D.; Maitra, A.; Verma, A. The role of stromal cancer-associated fibroblasts in pancreatic cancer. J. Hematol. Oncol. 2017, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.M.; Fusenig, N.E. Friends or foes—Bipolar effects of the tumour stroma in cancer. Nat. Rev. Cancer 2004, 4, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Polanska, U.M.; Orimo, A. Carcinoma-associated fibroblasts: Non-neoplastic tumour-promoting mesenchymal cells. J. Cell. Physiol. 2013, 228, 1651–1657. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, B.; Webb, D.J. Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem. Soc. Trans. 2017, 45, 229–236. [Google Scholar] [CrossRef]
- Kuzet, S.E.; Gaggioli, C. Fibroblast activation in cancer: When seed fertilizes soil. Cell Tissue Res. 2016, 365, 607–619. [Google Scholar] [CrossRef]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef]
- Ramamonjisoa, N.; Ackerstaff, E. Characterization of the Tumor Microenvironment and Tumor-Stroma Interaction by Non-invasive Preclinical Imaging. Front. Oncol. 2017, 7, 3. [Google Scholar] [CrossRef]
- Hinz, B.; Phan, S.H.; Thannickal, V.J.; Prunotto, M.; Desmouliere, A.; Varga, J.; De Wever, O.; Mareel, M.; Gabbiani, G. Recent developments in myofibroblast biology: Paradigms for connective tissue remodeling. Am. J. Pathol. 2012, 180, 1340–1355. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, H.F. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 1986, 315, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xiao, C.H.; Tan, L.D.; Wang, Q.S.; Li, X.Q.; Feng, Y.M. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-b signalling. Br. J. Cancer 2014, 110, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Marshall, J.F. The role of integrins in TGFb activation in the tumour stroma. Cell Tissue Res. 2016, 365, 657–673. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Sahai, E. Mechanisms and impact of altered tumour mechanics. Nat. Cell Biol. 2018, 20, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Zhao, S.; Zhou, B.P.; Mi, J. Metabolic reprogramming of the tumour microenvironment. FEBS J. 2015, 282, 3892–3898. [Google Scholar] [CrossRef] [PubMed]
- Marchiq, I.; Pouyssegur, J. Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H(+) symporters. J. Mol. Med. 2016, 94, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Gaggioli, C.; Hooper, S.; Hidalgo-Carcedo, C.; Grosse, R.; Marshall, J.F.; Harrington, K.; Sahai, E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol. 2007, 9, 1392–1400. [Google Scholar] [CrossRef]
- Roy, S.; Bag, A.K.; Dutta, S.; Polavaram, N.S.; Islam, R.; Schellenburg, S.; Banwait, J.; Guda, C.; Ran, S.; Hollingsworth, M.A.; et al. Macrophage-Derived Neuropilin-2 Exhibits Novel Tumor-Promoting Functions. Cancer Res. 2018, 78, 5600–5617. [Google Scholar] [CrossRef]
- Dejda, A.; Mawambo, G.; Daudelin, J.F.; Miloudi, K.; Akla, N.; Patel, C.; Andriessen, E.M.; Labrecque, N.; Sennlaub, F.; Sapieha, P. Neuropilin-1-Expressing Microglia Are Associated with Nascent Retinal Vasculature Yet Dispensable for Developmental Angiogenesis. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1530–1536. [Google Scholar] [CrossRef]
- Karkkainen, M.J.; Alitalo, K. Lymphatic endothelial regulation, lymphoedema, and lymph node metastasis. Semin. Cell Dev. Biol. 2002, 13, 9–18. [Google Scholar] [CrossRef]
- Shevach, E.M. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 2009, 30, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Cui, J. Present and future of cancer immunotherapy: A tumor microenvironmental perspective. Oncol. Lett. 2018, 16, 4105–4113. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.H.; Alitalo, K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007, 8, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Billioux, A.; Modlich, U.; Bicknell, R. Angiogenesis. In The Cancer Handbook, 2nd ed.; Alison, M., Ed.; John Wiley & Sons: New York, NY, USA, 2007; Volume 1, pp. 144–154. [Google Scholar]
- Hanahan, D.; Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef]
- Blouw, B.; Song, H.; Tihan, T.; Bosze, J.; Ferrara, N.; Gerber, H.-P.; Johnson, R.S.; Bergers, G. The hypoxic response of tumors is dependent on their microenvironment. Cancer Cell 2003, 4, 133–146. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Cavaco, A.; Rezaei, M.; Niland, S.; Eble, J.A. Collateral damage intended—Cancer-associated fibroblasts and vasculature are potential targets in cancer therapy. Int. J. Mol. Sci. 2017, 18, 2355. [Google Scholar] [CrossRef] [PubMed]
- Nagy, J.A.; Chang, S.H.; Dvorak, A.M.; Dvorak, H.F. Why are tumour blood vessels abnormal and why is it important to know? Br. J. Cancer 2009, 100, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Holash, J.; Maisonpierre, P.C.; Compton, D.; Boland, P.; Alexander, C.R.; Zagzag, D.; Yancopoulos, G.D.; Wiegand, S.J. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 1999, 284, 1994–1998. [Google Scholar] [CrossRef] [PubMed]
- Dome, B.; Hendrix, M.J.; Paku, S.; Tovari, J.; Timar, J. Alternative vascularization mechanisms in cancer: Pathology and therapeutic implications. Am. J. Pathol. 2007, 170, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Donnem, T.; Hu, J.; Ferguson, M.; Adighibe, O.; Snell, C.; Harris, A.L.; Gatter, K.C.; Pezzella, F. Vessel co-option in primary human tumors and metastases: An obstacle to effective anti-angiogenic treatment? Cancer Med. 2013, 2, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Djonov, V.; Schmid, M.; Tschanz, S.A.; Burri, P.H. Intussusceptive angiogenesis: Its role in embryonic vascular network formation. Circul. Res. 2000, 86, 286–292. [Google Scholar] [CrossRef]
- Kurz, H.; Burri, P.H.; Djonov, V.G. Angiogenesis and vascular remodeling by intussusception: From form to function. News Physiol. Sci. 2003, 18, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Gianni-Barrera, R.; Trani, M.; Reginato, S.; Banfi, A. To sprout or to split? VEGF, Notch and vascular morphogenesis. Biochem. Soc. Trans. 2011, 39, 1644–1648. [Google Scholar] [CrossRef] [PubMed]
- Lyden, D.; Hattori, K.; Dias, S.; Costa, C.; Blaikie, P.; Butros, L.; Chadburn, A.; Heissig, B.; Marks, W.; Witte, L.; et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat. Med. 2001, 7, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Reyes, M.; Dudek, A.; Jahagirdar, B.; Koodie, L.; Marker, P.H.; Verfaillie, C.M.C.I.N. Origin of endothelial progenitors in human postnatal bone marrow. J. Clin. Investi. 2002, 109, 337–346. [Google Scholar] [CrossRef]
- Ribatti, D. The involvement of endothelial progenitor cells in tumor angiogenesis. J. Cell. Mol. Med. 2004, 8, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Dudley, A.C. Tumor endothelial cells. Cold Spring Harb. Perspect. Med. 2012, 2, a006536. [Google Scholar] [CrossRef]
- Delgado-Bellido, D.; Serrano-Saenz, S.; Fernandez-Cortes, M.; Oliver, F.J. Vasculogenic mimicry signaling revisited: Focus on non-vascular VE-cadherin. Mol. Cancer 2017, 16, 65. [Google Scholar] [CrossRef]
- Dong, J.; Zhao, Y.; Huang, Q.; Fei, X.; Diao, Y.; Shen, Y.; Xiao, H.; Zhang, T.; Lan, Q.; Gu, X. Glioma stem/progenitor cells contribute to neovascularization via transdifferentiation. Stem Cell Rev. 2011, 7, 141–152. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Q.; Li, X.Y.; Yang, Q.Y.; Xu, W.W.; Liu, G.L. Short-term anti-vascular endothelial growth factor treatment elicits vasculogenic mimicry formation of tumors to accelerate metastasis. J. Exp. Clin. Cancer Res. 2012, 31, 16. [Google Scholar] [CrossRef] [PubMed]
- Knopik-Skrocka, A.; Kręplewska, P.; Jarmołowska-Jurczyszyn, D. Tumor blood vessels and vasculogenic mimicry—Current knowledge and searching for new cellular/molecular targets of anti-angiogenic therapy. Adv. Cell Biol. 2017, 5, 50–71. [Google Scholar] [CrossRef]
- Forster, J.C.; Harriss-Phillips, W.M.; Douglass, M.J.; Bezak, E. A review of the development of tumor vasculature and its effects on the tumor microenvironment. Hypoxia 2017, 5, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Fantin, A.; Vieira, J.M.; Plein, A.; Denti, L.; Fruttiger, M.; Pollard, J.W.; Ruhrberg, C. NRP1 acts cell autonomously in endothelium to promote tip cell function during sprouting angiogenesis. Blood 2013, 121, 2352–2362. [Google Scholar] [CrossRef] [PubMed]
- Larrivee, B.; Prahst, C.; Gordon, E.; del Toro, R.; Mathivet, T.; Duarte, A.; Simons, M.; Eichmann, A. ALK1 signaling inhibits angiogenesis by cooperating with the Notch pathway. Dev. Cell 2012, 22, 489–500. [Google Scholar] [CrossRef]
- Guttmann-Raviv, N.; Kessler, O.; Shraga-Heled, N.; Lange, T.; Herzog, Y.; Neufeld, G. The neuropilins and their role in tumorigenesis and tumor progression. Cancer Lett. 2006, 231, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Migliozzi, M.T.; Mucka, P.; Bielenberg, D.R. Lymphangiogenesis and metastasis--a closer look at the neuropilin/semaphorin3 axis. Microvasc. Res. 2014, 96, 68–76. [Google Scholar] [CrossRef]
- Jurisic, G.; Maby-El Hajjami, H.; Karaman, S.; Ochsenbein, A.M.; Alitalo, A.; Siddiqui, S.S.; Ochoa Pereira, C.; Petrova, T.V.; Detmar, M. An unexpected role of semaphorin3a-neuropilin-1 signaling in lymphatic vessel maturation and valve formation. Circ. Res. 2012, 111, 426–436. [Google Scholar] [CrossRef]
- Serini, G.; Tamagnone, L. Bad vessels beware! Semaphorins will sort you out! EMBO Mol. Med. 2015, 7, 1251–1253. [Google Scholar] [CrossRef]
- Mumblat, Y.; Kessler, O.; Ilan, N.; Neufeld, G. Full-Length Semaphorin-3C Is an Inhibitor of Tumor Lymphangiogenesis and Metastasis. Cancer Res. 2015, 75, 2177–2186. [Google Scholar] [CrossRef]
- Miyato, H.; Tsuno, N.H.; Kitayama, J. Semaphorin 3C is involved in the progression of gastric cancer. Cancer Sci. 2012, 103, 1961–1966. [Google Scholar] [CrossRef]
- Herman, J.G.; Meadows, G.G. Increased class 3 semaphorin expression modulates the invasive and adhesive properties of prostate cancer cells. Int. J. Oncol. 2007, 30, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Man, J.; Shoemake, J.; Zhou, W.; Fang, X.; Wu, Q.; Rizzo, A.; Prayson, R.; Bao, S.; Rich, J.N.; Yu, J.S. Sema3C promotes the survival and tumorigenicity of glioma stem cells through Rac1 activation. Cell Rep. 2014, 9, 1812–1826. [Google Scholar] [CrossRef]
- Bassi, D.E.; Fu, J.; Lopez de Cicco, R.; Klein-Szanto, A.J. Proprotein convertases: “master switches” in the regulation of tumor growth and progression. Mol. Carcinog. 2005, 44, 151–161. [Google Scholar] [CrossRef]
- Riabov, V.; Gudima, A.; Wang, N.; Mickley, A.; Orekhov, A.; Kzhyshkowska, J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front. Physiol. 2014, 5, 75. [Google Scholar] [CrossRef] [PubMed]
- Esselens, C.; Malapeira, J.; Colome, N.; Casal, C.; Rodriguez-Manzaneque, J.C.; Canals, F.; Arribas, J. The cleavage of semaphorin 3C induced by ADAMTS1 promotes cell migration. J. Biol. Chem. 2010, 285, 2463–2473. [Google Scholar] [CrossRef]
- Jain, R.K. Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell 2014, 26, 605–622. [Google Scholar] [CrossRef] [PubMed]
- Maione, F.; Capano, S.; Regano, D.; Zentilin, L.; Giacca, M.; Casanovas, O.; Bussolino, F.; Serini, G.; Giraudo, E. Semaphorin 3A overcomes cancer hypoxia and metastatic dissemination induced by antiangiogenic treatment in mice. J. Clin. Investig. 2012, 122, 1832–1848. [Google Scholar] [CrossRef]
- Acevedo, L.M.; Barillas, S.; Weis, S.M.; Gothert, J.R.; Cheresh, D.A. Semaphorin 3A suppresses VEGF-mediated angiogenesis yet acts as a vascular permeability factor. Blood 2008, 111, 2674–2680. [Google Scholar] [CrossRef] [PubMed]
- Cerani, A.; Tetreault, N.; Menard, C.; Lapalme, E.; Patel, C.; Sitaras, N.; Beaudoin, F.; Leboeuf, D.; De Guire, V.; Binet, F.; et al. Neuron-derived semaphorin 3A is an early inducer of vascular permeability in diabetic retinopathy via neuropilin-1. Cell Metab. 2013, 18, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Gioelli, N.; Maione, F.; Camillo, C.; Ghitti, M.; Valdembri, D.; Morello, N.; Darche, M.; Zentilin, L.; Cagnoni, G.; Qiu, Y.; et al. A rationally designed NRP1-independent superagonist SEMA3A mutant is an effective anticancer agent. Sci. Transl. Med. 2018, 10, eaah4807. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Tosetti, F.; Li, V.W.; Noonan, D.M.; Li, W.W. Cancer prevention by targeting angiogenesis. Nat. Rev. Clin. Oncol. 2012, 9, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Ruffini, F.; D’Atri, S.; Lacal, P.M. Neuropilin-1 expression promotes invasiveness of melanoma cells through vascular endothelial growth factor receptor-2-dependent and -independent mechanisms. Int. J. Oncol. 2013, 43, 297–306. [Google Scholar] [CrossRef]
- Torti, D.; Trusolino, L. Oncogene addiction as a foundational rationale for targeted anti-cancer therapy: Promises and perils. EMBO Mol. Med. 2011, 3, 623–636. [Google Scholar] [CrossRef]
- Fallahi-Sichani, M.; Becker, V.; Izar, B.; Baker, G.J.; Lin, J.R.; Boswell, S.A.; Shah, P.; Rotem, A.; Garraway, L.A.; Sorger, P.K. Adaptive resistance of melanoma cells to RAF inhibition via reversible induction of a slowly dividing de-differentiated state. Mol. Syst. Biol. 2017, 13, 905. [Google Scholar] [CrossRef]
- Wilson, T.R.; Fridlyand, J.; Yan, Y.; Penuel, E.; Burton, L.; Chan, E.; Peng, J.; Lin, E.; Wang, Y.; Sosman, J.; et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature 2012, 487, 505–509. [Google Scholar] [CrossRef]
- Graziani, G.; Lacal, P.M. Neuropilin-1 as therapeutic target for malignant melanoma. Front. Oncol. 2015, 5, 125. [Google Scholar] [CrossRef]
- Lambrechts, D.; Lenz, H.J.; de Haas, S.; Carmeliet, P.; Scherer, S.J. Markers of response for the antiangiogenic agent bevacizumab. J. Clin. Oncol. 2013, 31, 1219–1230. [Google Scholar] [CrossRef]
- Baumgarten, P.; Blank, A.E.; Franz, K.; Hattingen, E.; Dunst, M.; Zeiner, P.; Hoffmann, K.; Bahr, O.; Mader, L.; Goeppert, B.; et al. Differential expression of vascular endothelial growth factor A, its receptors VEGFR-1, -2, and -3 and co-receptors neuropilin-1 and -2 does not predict bevacizumab response in human astrocytomas. Neuro Oncol. 2016, 18, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Bais, C.; Mueller, B.; Brady, M.F.; Mannel, R.S.; Burger, R.A.; Wei, W.; Marien, K.M.; Kockx, M.M.; Husain, A.; Birrer, M.J.; et al. Tumor Microvessel Density as a Potential Predictive Marker for Bevacizumab Benefit: GOG-0218 Biomarker Analyses. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef] [PubMed]
- Jamil, M.O.; Hathaway, A.; Mehta, A. Tivozanib: Status of development. Curr. Oncol. Rep. 2015, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.J.; Wey, J.S.; Belcheva, A.; McCarty, M.F.; Trevino, J.G.; Evans, D.B.; Ellis, L.M.; Gallick, G.E. Neuropilin-1 suppresses tumorigenic properties in a human pancreatic adenocarcinoma cell line lacking neuropilin-1 coreceptors. Cancer Res. 2005, 65, 3664–3670. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T.; Kawakami, T.; Abe, Y.; Nishi, M.; Onoda, N.; Miyazaki, N.; Oida, Y.; Yamazaki, H.; Ueyama, Y.; Nakamura, M. The preserved expression of neuropilin (NRP) 1 contributes to a better prognosis in colon cancer. Oncol. Rep. 2006, 15, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, G.; Shi, Y.; Qiu, R.; Khan, A.A. Neuropilin-1 (NRP-1) and Magnetic Nanoparticles, a Potential Combination for Diagnosis and Therapy of Gliomas. Curr. Pharm. Des. 2015, 21, 5434–5449. [Google Scholar] [CrossRef]
- Schuch, G.; Machluf, M.; Bartsch, G., Jr.; Nomi, M.; Richard, H.; Atala, A.; Soker, S. In vivo administration of vascular endothelial growth factor (VEGF) and its antagonist, soluble neuropilin-1, predicts a role of VEGF in the progression of acute myeloid leukemia in vivo. Blood 2002, 100, 4622–4628. [Google Scholar] [CrossRef]
- Sugahara, K.N.; Scodeller, P.; Braun, G.B.; de Mendoza, T.H.; Yamazaki, C.M.; Kluger, M.D.; Kitayama, J.; Alvarez, E.; Howell, S.B.; Teesalu, T.; et al. A tumor-penetrating peptide enhances circulation-independent targeting of peritoneal carcinomatosis. J. Control. Release 2015, 212, 59–69. [Google Scholar] [CrossRef]
- Simon-Gracia, L.; Hunt, H.; Scodeller, P.; Gaitzsch, J.; Kotamraju, V.R.; Sugahara, K.N.; Tammik, O.; Ruoslahti, E.; Battaglia, G.; Teesalu, T. iRGD peptide conjugation potentiates intraperitoneal tumor delivery of paclitaxel with polymersomes. Biomaterials 2016, 104, 247–257. [Google Scholar] [CrossRef]
- Zhang, H.; Tam, S.; Ingham, E.S.; Mahakian, L.M.; Lai, C.Y.; Tumbale, S.K.; Teesalu, T.; Hubbard, N.E.; Borowsky, A.D.; Ferrara, K.W. Ultrasound molecular imaging of tumor angiogenesis with a neuropilin-1-targeted microbubble. Biomaterials 2015, 56, 104–113. [Google Scholar] [CrossRef]
- Bumbaca, D.; Xiang, H.; Boswell, C.A.; Port, R.E.; Stainton, S.L.; Mundo, E.E.; Ulufatu, S.; Bagri, A.; Theil, F.P.; Fielder, P.J.; et al. Maximizing tumour exposure to anti-neuropilin-1 antibody requires saturation of non-tumour tissue antigenic sinks in mice. Br. J. Pharmacol. 2012, 166, 368–377. [Google Scholar] [CrossRef]
- Feldman, D.R.; Baum, M.S.; Ginsberg, M.S.; Hassoun, H.; Flombaum, C.D.; Velasco, S.; Fischer, P.; Ronnen, E.; Ishill, N.; Patil, S.; et al. Phase I trial of bevacizumab plus escalated doses of sunitinib in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 2009, 27, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Weekes, C.D.; Beeram, M.; Tolcher, A.W.; Papadopoulos, K.P.; Gore, L.; Hegde, P.; Xin, Y.; Yu, R.; Shih, L.M.; Xiang, H.; et al. A phase I study of the human monoclonal anti-NRP1 antibody MNRP1685A in patients with advanced solid tumors. Investig. New Drugs 2014, 32, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, A.; LoRusso, P.M.; Messersmith, W.A.; Papadopoulos, K.P.; Gore, L.; Beeram, M.; Ramakrishnan, V.; Kim, A.H.; Beyer, J.C.; Mason Shih, L.; et al. A Phase Ib study evaluating MNRP1685A, a fully human anti-NRP1 monoclonal antibody, in combination with bevacizumab and paclitaxel in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2014, 73, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, K.M.; Rennert, P.D.; Freeman, G.J. Combination cancer immunotherapy and new immunomodulatory targets. Nat. Rev. Drug Discov. 2015, 14, 561–584. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, A.; Allerston, C.K.; Jia, H.; Herzog, B.; Garza-Garcia, A.; Winfield, N.; Ellard, K.; Aqil, R.; Lynch, R.; Chapman, C.; et al. Small molecule inhibitors of the neuropilin-1 vascular endothelial growth factor A (VEGF-A) interaction. J. Med. Chem. 2010, 53, 2215–2226. [Google Scholar] [CrossRef] [PubMed]
- Binetruy-Tournaire, R.; Demangel, C.; Malavaud, B.; Vassy, R.; Rouyre, S.; Kraemer, M.; Plouet, J.; Derbin, C.; Perret, G.; Mazie, J.C. Identification of a peptide blocking vascular endothelial growth factor (VEGF)-mediated angiogenesis. EMBO J. 2000, 19, 1525–1533. [Google Scholar] [CrossRef]
- Barr, M.P.; Byrne, A.M.; Duffy, A.M.; Condron, C.M.; Devocelle, M.; Harriott, P.; Bouchier-Hayes, D.J.; Harmey, J.H. A peptide corresponding to the neuropilin-1-binding site on VEGF(165) induces apoptosis of neuropilin-1-expressing breast tumour cells. Br. J. Cancer 2005, 92, 328–333. [Google Scholar] [CrossRef]
- Sidman, R.L.; Li, J.; Lawrence, M.; Hu, W.; Musso, G.F.; Giordano, R.J.; Cardo-Vila, M.; Pasqualini, R.; Arap, W. The peptidomimetic Vasotide targets two retinal VEGF receptors and reduces pathological angiogenesis in murine and nonhuman primate models of retinal disease. Sci. Transl. Med. 2015, 7, 309ra165. [Google Scholar] [CrossRef]
- Hong, T.M.; Chen, Y.L.; Wu, Y.Y.; Yuan, A.; Chao, Y.C.; Chung, Y.C.; Wu, M.H.; Yang, S.C.; Pan, S.H.; Shih, J.Y.; et al. Targeting neuropilin 1 as an antitumor strategy in lung cancer. Clin. Cancer. Res. 2007, 13, 4759–4768. [Google Scholar] [CrossRef]
- Jia, H.; Aqil, R.; Cheng, L.; Chapman, C.; Shaikh, S.; Jarvis, A.; Chan, A.W.; Hartzoulakis, B.; Evans, I.M.; Frolov, A.; et al. N-terminal modification of VEGF-A C terminus-derived peptides delineates structural features involved in neuropilin-1 binding and functional activity. ChemBioChem 2014, 15, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.; Mota, F.; Steadman, D.; Soudy, C.; Miyauchi, J.T.; Crosby, S.; Jarvis, A.; Reisinger, T.; Winfield, N.; Evans, G.; et al. Small Molecule Neuropilin-1 Antagonists Combine Antiangiogenic and Antitumor Activity with Immune Modulation through Reduction of Transforming Growth Factor Beta (TGFbeta) Production in Regulatory T-Cells. J. Med. Chem. 2018, 61, 4135–4154. [Google Scholar] [CrossRef] [PubMed]
- Tymecka, D.; Puszko, A.K.; Lipinski, P.F.J.; Fedorczyk, B.; Wilenska, B.; Sura, K.; Perret, G.Y.; Misicka, A. Branched pentapeptides as potent inhibitors of the vascular endothelial growth factor 165 binding to Neuropilin-1: Design, synthesis and biological activity. Eur. J. Med. Chem. 2018, 158, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Nasarre, C.; Roth, M.; Jacob, L.; Roth, L.; Koncina, E.; Thien, A.; Labourdette, G.; Poulet, P.; Hubert, P.; Cremel, G.; et al. Peptide-based interference of the transmembrane domain of neuropilin-1 inhibits glioma growth in vivo. Oncogene 2010, 29, 2381–2392. [Google Scholar] [CrossRef] [PubMed]
- Arpel, A.; Gamper, C.; Spenle, C.; Fernandez, A.; Jacob, L.; Baumlin, N.; Laquerriere, P.; Orend, G.; Cremel, G.; Bagnard, D. Inhibition of primary breast tumor growth and metastasis using a neuropilin-1 transmembrane domain interfering peptide. Oncotarget 2016, 7, 54723–54732. [Google Scholar] [CrossRef] [PubMed]
- Leng, Q.; Woodle, M.C.; Mixson, A.J. NRP1 transport of cancer therapeutics mediated by tumor-penetrating peptides. Drugs Future 2017, 42, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Zanuy, D.; Kotla, R.; Nussinov, R.; Teesalu, T.; Sugahara, K.N.; Aleman, C.; Haspel, N. Sequence dependence of C-end rule peptides in binding and activation of neuropilin-1 receptor. J. Struct. Biol. 2013, 182, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Simon-Gracia, L.; Hunt, H.; Teesalu, T. Peritoneal carcinomatosis targeting with tumor homing peptides. Molecules 2018, 23, 1190. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Zhang, H.; Zhang, J.P.; Li, Y.; Zhao, B.; Feng, G.K.; Du, Y.; Xiong, D.; Zhong, Q.; Liu, W.L.; et al. Neuropilin 1 is an entry factor that promotes EBV infection of nasopharyngeal epithelial cells. Nat. Commun. 2015, 6, 6240. [Google Scholar] [CrossRef]
- Liu, X.; Lin, P.; Perrett, I.; Lin, J.; Liao, Y.P.; Chang, C.H.; Jiang, J.; Wu, N.; Donahue, T.; Wainberg, Z.; et al. Tumor-penetrating peptide enhances transcytosis of silicasome-based chemotherapy for pancreatic cancer. J. Clin. Investig. 2017, 127, 2007–2018. [Google Scholar] [CrossRef]
- Ruoslahti, E. Tumor penetrating peptides for improved drug delivery. Adv. Drug Deliv. Rev. 2017, 110–111, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Thoreau, F.; Vanwonterghem, L.; Henry, M.; Coll, J.L.; Boturyn, D. Design of RGD-ATWLPPR peptide conjugates for the dual targeting of alphaVbeta3 integrin and neuropilin-1. Org. Biomol. Chem. 2018, 16, 4101–4107. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov [Internet]. National Library of Medicine (US): Bethesda, MD, USA. Identifier: NCT03517176, CEND-1 in combination with nanoparticle albumin bound-paclitaxel (abraxane) and gemcitabine in metastatic pancreatic cancer; 7 May 2018; [1 page]. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03517176?term=Cend-1&cond=cancer&rank=1 (accessed on 23 January 2019).
- Zhang, L.; Parry, G.C.; Levin, E.G. Inhibition of tumor cell migration by LD22-4, an N-terminal fragment of 24-kDa FGF2, is mediated by Neuropilin 1. Cancer Res. 2013, 73, 3316–3325. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Donate, F.; Parry, G.C.; Guan, X.; Maher, P.; Levin, E.G. Inhibition of cell migration and angiogenesis by the amino-terminal fragment of 24kD basic fibroblast growth factor. J. Biol. Chem. 2002, 277, 31056–31061. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.G.; Sikora, L.; Ding, L.; Rao, S.P.; Sriramarao, P. Suppression of tumor growth and angiogenesis in vivo by a truncated form of 24-kd fibroblast growth factor (FGF)-2. Am. J. Pathol. 2004, 164, 1183–1190. [Google Scholar] [CrossRef]
- Kim, Y.J.; Bae, J.; Shin, T.H.; Kang, S.H.; Jeong, M.; Han, Y.; Park, J.H.; Kim, S.K.; Kim, Y.S. Immunoglobulin Fc-fused, neuropilin-1-specific peptide shows efficient tumor tissue penetration and inhibits tumor growth via anti-angiogenesis. J. Control. Release 2015, 216, 56–68. [Google Scholar] [CrossRef]
- Paasonen, L.; Sharma, S.; Braun, G.B.; Kotamraju, V.R.; Chung, T.D.; She, Z.G.; Sugahara, K.N.; Yliperttula, M.; Wu, B.; Pellecchia, M.; et al. New p32/gC1qR Ligands for Targeted Tumor Drug Delivery. ChemBioChem 2016, 17, 570–575. [Google Scholar] [CrossRef]
- Sharma, S.; Kotamraju, V.R.; Molder, T.; Tobi, A.; Teesalu, T.; Ruoslahti, E. Tumor-Penetrating Nanosystem Strongly Suppresses Breast Tumor Growth. Nano Lett. 2017, 17, 1356–1364. [Google Scholar] [CrossRef]
- Hunt, H.; Simon-Gracia, L.; Tobi, A.; Kotamraju, V.R.; Sharma, S.; Nigul, M.; Sugahara, K.N.; Ruoslahti, E.; Teesalu, T. Targeting of p32 in peritoneal carcinomatosis with intraperitoneal linTT1 peptide-guided pro-apoptotic nanoparticles. J. Control. Release 2017, 260, 142–153. [Google Scholar] [CrossRef]
- Liang, D.S.; Zhang, W.J.; Wang, A.T.; Su, H.T.; Zhong, H.J.; Qi, X.R. Treating metastatic triple negative breast cancer with CD44/neuropilin dual molecular targets of multifunctional nanoparticles. Biomaterials 2017, 137, 23–36. [Google Scholar] [CrossRef]
- Thomas, E.; Colombeau, L.; Gries, M.; Peterlini, T.; Mathieu, C.; Thomas, N.; Boura, C.; Frochot, C.; Vanderesse, R.; Lux, F.; et al. Ultrasmall AGuIX theranostic nanoparticles for vascular-targeted interstitial photodynamic therapy of glioblastoma. Int. J. Nanomed. 2017, 12, 7075–7088. [Google Scholar] [CrossRef] [PubMed]
- Roth, L.; Agemy, L.; Kotamraju, V.R.; Braun, G.; Teesalu, T.; Sugahara, K.N.; Hamzah, J.; Ruoslahti, E. Transtumoral targeting enabled by a novel neuropilin-binding peptide. Oncogene 2012, 31, 3754–3763. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.N.; Braun, G.B.; de Mendoza, T.H.; Kotamraju, V.R.; French, R.P.; Lowy, A.M.; Teesalu, T.; Ruoslahti, E. Tumor-penetrating iRGD peptide inhibits metastasis. Mol. Cancer Ther. 2015, 14, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.M.; Aidoudi-Ahmed, S.; Sharma, S.; Kotamraju, V.R.; Foster, P.J.; Sugahara, K.N.; Ruoslahti, E.; Rutt, B.K. Nanoparticles coated with the tumor-penetrating peptide iRGD reduce experimental breast cancer metastasis in the brain. J. Mol. Med. 2015, 93, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Zakraoui, O.; Marcinkiewicz, C.; Aloui, Z.; Othman, H.; Grepin, R.; Haoues, M.; Essafi, M.; Srairi-Abid, N.; Gasmi, A.; Karoui, H.; et al. Lebein, a snake venom disintegrin, suppresses human colon cancer cells proliferation and tumor-induced angiogenesis through cell cycle arrest, apoptosis induction and inhibition of VEGF expression. Mol. Carcinog. 2017, 56, 18–35. [Google Scholar] [CrossRef] [PubMed]
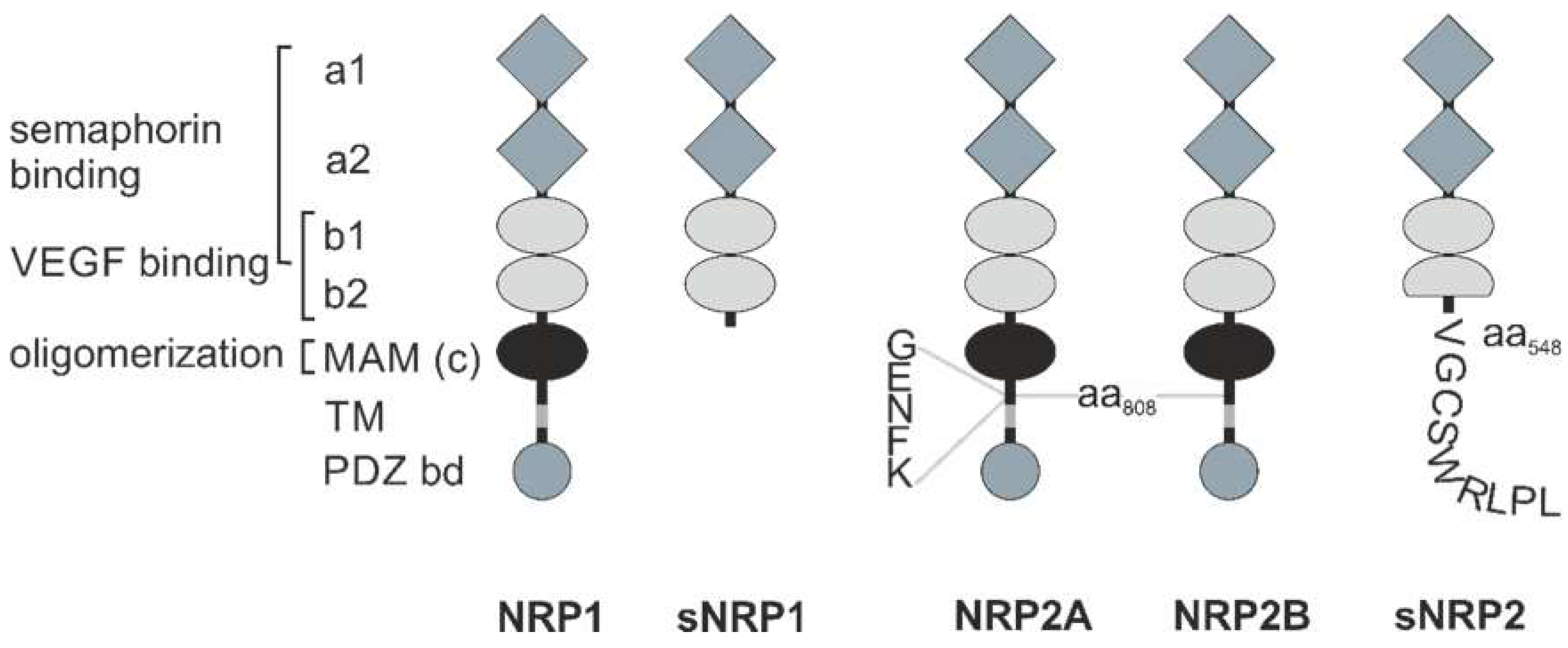

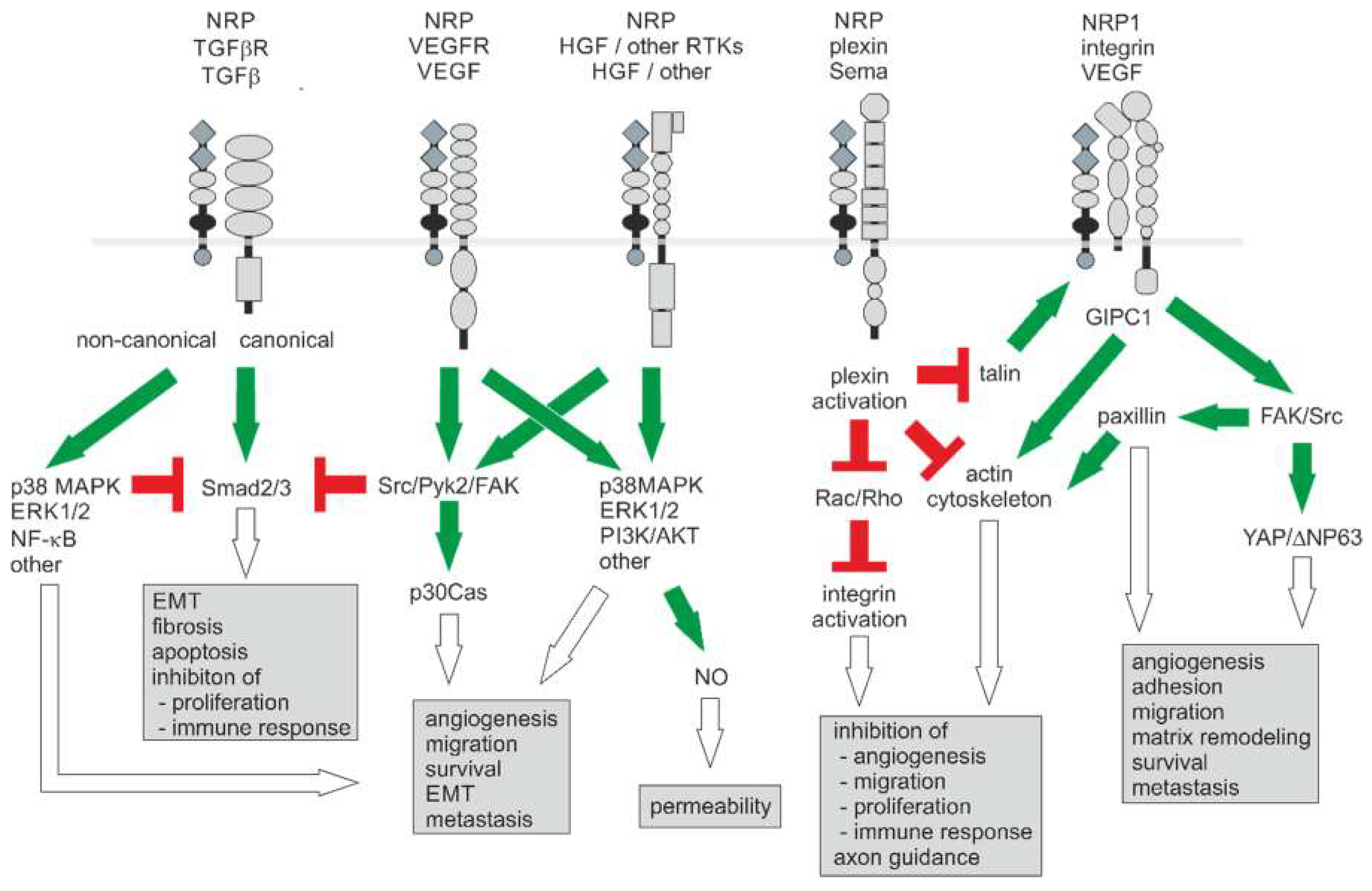
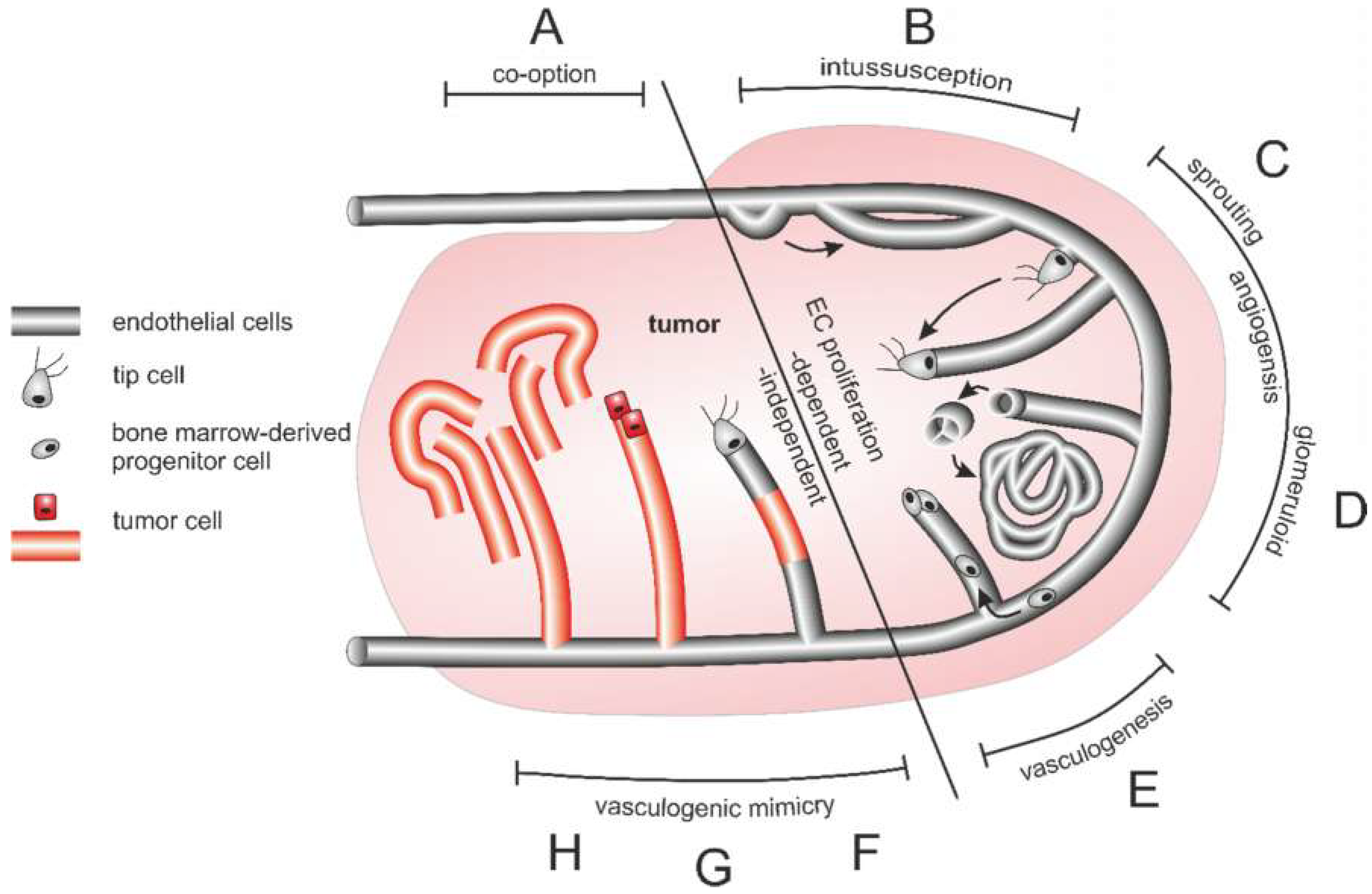
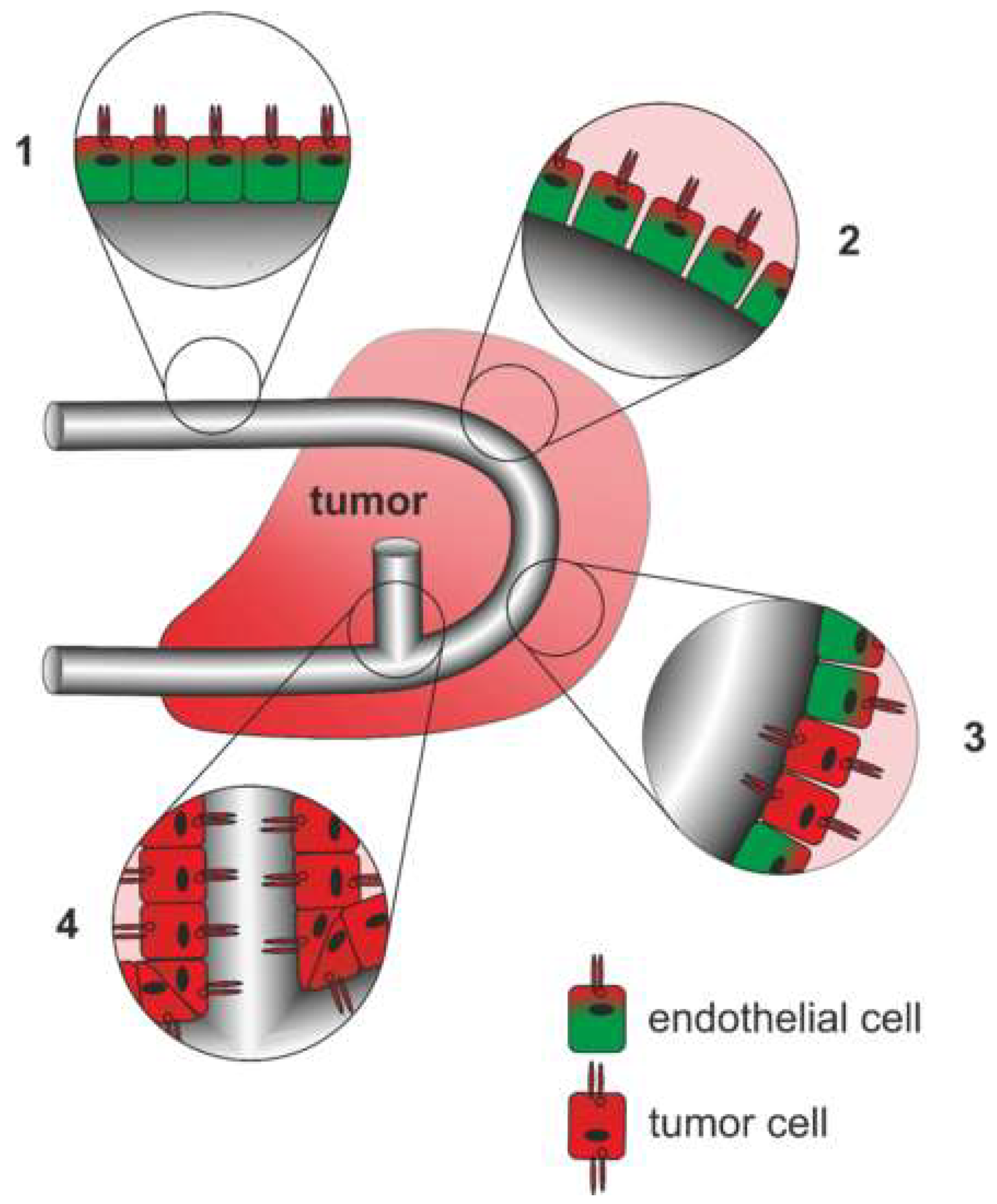
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niland, S.; Eble, J.A. Neuropilins in the Context of Tumor Vasculature. Int. J. Mol. Sci. 2019, 20, 639. https://doi.org/10.3390/ijms20030639
Niland S, Eble JA. Neuropilins in the Context of Tumor Vasculature. International Journal of Molecular Sciences. 2019; 20(3):639. https://doi.org/10.3390/ijms20030639
Chicago/Turabian StyleNiland, Stephan, and Johannes A. Eble. 2019. "Neuropilins in the Context of Tumor Vasculature" International Journal of Molecular Sciences 20, no. 3: 639. https://doi.org/10.3390/ijms20030639
APA StyleNiland, S., & Eble, J. A. (2019). Neuropilins in the Context of Tumor Vasculature. International Journal of Molecular Sciences, 20(3), 639. https://doi.org/10.3390/ijms20030639