The Chemical Transformation of the Cellular Toxin INT (2-(4-Iodophenyl)-3-(4-Nitrophenyl)-5-(Phenyl) Tetrazolium Chloride) as an Indicator of Prior Respiratory Activity in Aquatic Bacteria
Abstract
1. Introduction
2. Results
2.1. The Respiration (R) to In Vivo Formazan Production (F) Relationship.
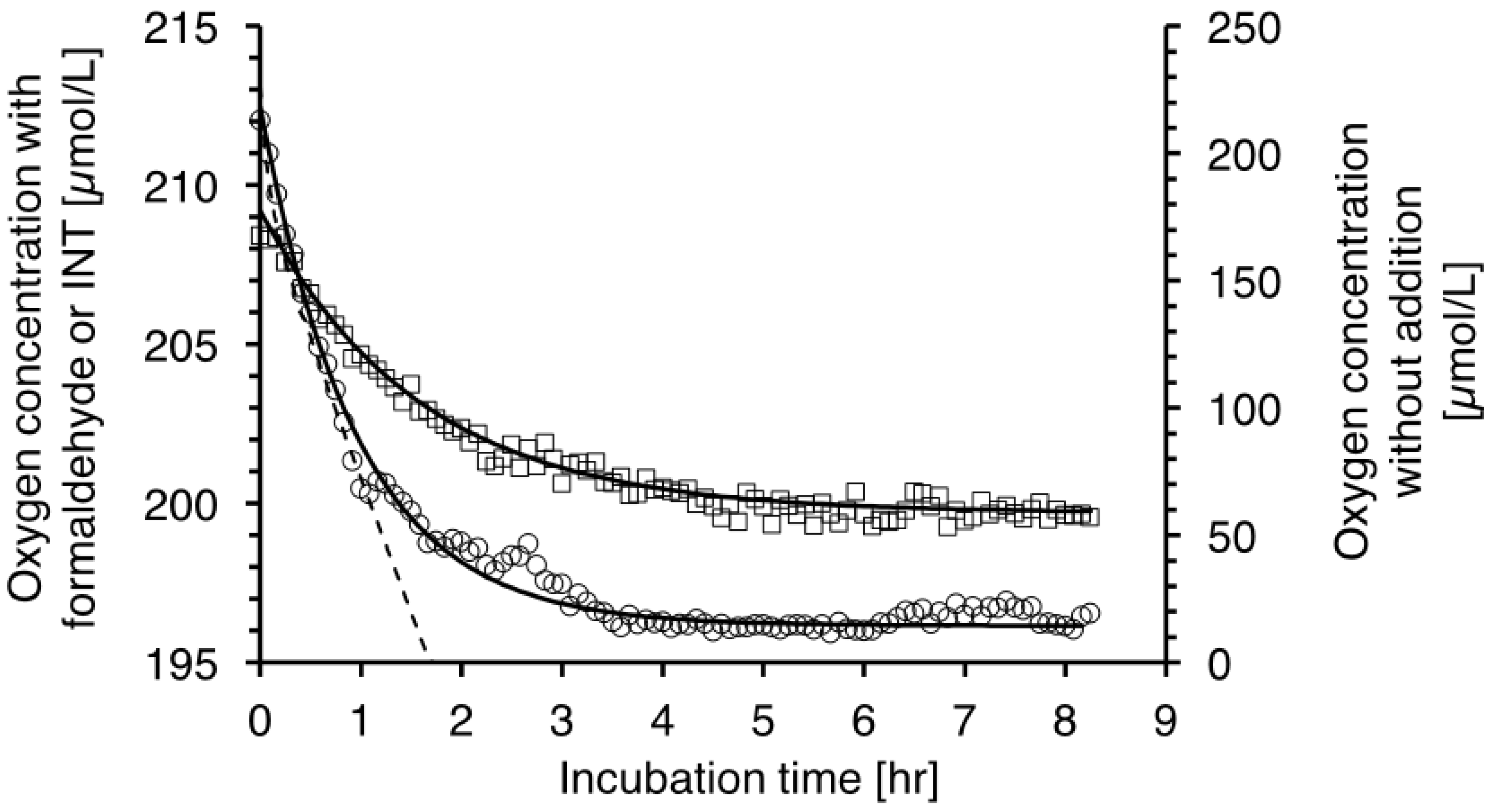
2.2. Oxygen Consumption and Formazan Production Relationship with Specific Growth Rate and Temperature
2.3. Formazan Production and the Rate of Formazan Production in V. Harveyi Batch Cultures.
3. Discussion
3.1. Toxicity of INT
3.2. The ETS In Vitro and In Vivo Methods to Evaluate Respiration Rate
3.3. The INT Reduction Potential Method
4. Materials and Methods
4.1. Sample Collection
4.2. Continuous Culture Preparation
4.3. Batch Cultures of V. harveyi
4.4. Bacterial Abundance
4.5. Oxygen Consumption Determination
4.6. The In Vivo Formazan Formation Measurement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| F | Formazan; μmol/L |
| R | Respiration rates; μmol/L/hr |
| ETS | Electron transport system |
| INT | 2-(4-Iodophenyl)-3-(4-Nitrophenyl)-5-(Phenyl) Tetrazolium Chloride |
References
- Robinson, C. Heterotrophic Bacterial Respiration. In Microbial Ecology of the Oceans; Kirchman, D., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 299–334. [Google Scholar]
- Del Giorgio, P.A.; Williams, P.J.L. Respiration in aquatic ecosystems; Oxford University Press: New York, NY, USA, 2005. [Google Scholar]
- Packard, T.T. The measurement of respiratory electron-transport activity in marine phytoplankton. J. Mar. Syst. 1971, 29, 235–244. [Google Scholar]
- Martínez-García, S.; Fernández, E.; Aranguren-Gassis, M.; Teira, E. In vivo electron transport system activity: A method to estimate respiration in natural marine microbial planktonic communities. Limnol. Oceanogr. Methods 2009, 7, 459–469. [Google Scholar] [CrossRef]
- Ward, B. Photosynthesis and bacterial utilization of phytoplankton exudates - results from pre-incubation and post-incubation size fractionation. Oceanol. Acta 1984, 7, 337–343. [Google Scholar]
- Massana, R.; Pedrόs-Aliό, C.; Casamayor, E.O.; Gasol, J.M. Changes in marine bacterioplankton phylogenetic composition during incubations designed to measure biogeochemically significant parameters. Limnol. Oceanogr. 2001, 46, 1181–1188. [Google Scholar] [CrossRef]
- Gattuso, J.-P.; Peduzzi, S.; Pizay, M.-D.; Tonolla, M. Changes in freshwater bacterial community composition during measurements of microbial and community respiration. J. Plankton Res. 2002, 24, 1197–1206. [Google Scholar] [CrossRef]
- Aranguren-Gassis, M.; Teira, E.; Serret, P.; Martínez-García, S.; Fernández, E. Potential overestimation of bacterial respiration rates in oligotrophic plankton communities. Mar. Ecol. Prog. Ser. 2012, 453, 1–10. [Google Scholar] [CrossRef]
- Packard, T.T.; Williams, P.J.L. Rates of respiratory oxygen consumption and electron transport in surface seawater from the northwest Atlantic. Ocean. Acta 1981, 4, 351–358. [Google Scholar]
- Christensen, J.P.; Owens, T.G.; Devol, A.H.; Packard, T.T. Respiration and physiological state in marine bacteria. Mar. Biol. 1980, 55, 267–276. [Google Scholar] [CrossRef]
- Packard, T.T.; Berdalet, E.; Blasco, D.; Roy, S.O.; St-Amand, L.; Lagacé, B.; Lee, K.; Gagnó, J.-P. Oxygen consumption in the marine bacterium Pseudomonas nautica predicted from ETS activity and bisubstrate enzyme kinetics. J. Plankton Res. 1996, 18, 1819–1835. [Google Scholar] [CrossRef]
- Villegas-Mendoza, J.; Cajal-Medrano, R.; Maske, H. INT (2-(4-Iodophenyl)-3-(4-Nitrophenyl)-5-(Phenyl) Tetrazolium Chloride) Is Toxic to Prokaryote Cells Precluding Its Use with Whole Cells as a Proxy for In Vivo Respiration. Microb. Ecol. 2015, 70, 1004–1011. [Google Scholar] [CrossRef]
- Richardson, D.J. Structural and Functional Flexibility of Bacterial Respiromes. In Bacterial Physiology; El-Sharoud, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 97–128. [Google Scholar]
- Madigan, M.T.; Madigan, M.T.; Brock, T.D. Brock Biology of Microorganisms; Pearson/Benjamin Cummings: San Francisco, CA, USA, 2009. [Google Scholar]
- Matsushita, K.; Toyama, H.; Adachi, O. Respiratory Chains in Acetic Acid Bacteria: Membranebound Periplasmic Sugar and Alcohol Respirations; Springer: Dordrecht, The Netherlands, 2004; pp. 81–99. [Google Scholar]
- Sone, N.; Hägerhäll, C.; Sakamoto, J. Aerobic Respiration in the Gram-Positive Bacteria. In Respiration in Archaea and Bacteria: Diversity of Prokaryotic Systems.; Zannoni, D., Ed.; Springer: Dordrecht, The Netherlands, 2004; pp. 35–62. [Google Scholar]
- Trevors, J.T.; Mayfield, C.I.; Inniss, W.E. Measurement of Electron Transport System (ETS) activity in soil. Microb. Ecol. 1982, 8, 163–168. [Google Scholar] [CrossRef]
- Zhai, Y.; Brun, N.R.; Bundschuh, M.; Schrama, M.; Hin, E.; Vijver, M.G.; Hunting, E.R. Microbially-mediated indirect effects of silver nanoparticles on aquatic invertebrates. Aquat. Sci. 2018, 80, 44. [Google Scholar] [CrossRef]
- Hunting, E.R.; de Goeij, J.M.; Asselman, M.; van Soest, R.W.M.; van der Geest, H.G. Degradation of Mangrove-Derived Organic Matter in Mangrove Associated Sponges. Bull. Mar. Sci. 2010, 86, 871–877. [Google Scholar] [CrossRef]
- Maldonado, F.; Packard, T.T.; Gómez, M. Understanding tetrazolium reduction and the importance of substrates in measuring respiratory electron transport activity. J. Exp. Mar. Bio. Ecol. 2012, 434–435, 110–118. [Google Scholar] [CrossRef]
- Smith, J.J.; McFeters, G.A. Mechanisms of INT (2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyl tetrazolium chloride), and CTC (5-cyano-2,3-ditolyl tetrazolium chloride) reduction in Escherichia coli K-12. J. Microbiol. Methods 1997, 29, 161–175. [Google Scholar] [CrossRef]
- May, P.S.; Winter, J.W.; Fried, G.H.; Antopol, W. Effect of Tetrazolium Salts on Selected Bacterial Species. Exp. Biol. Med. 1960, 105, 364–366. [Google Scholar] [CrossRef]
- Ping, L.; Mavridou, D.A.I.; Emberly, E.; Westermann, M.; Ferguson, S.J. Vital dye reaction and granule localization in periplasm of escherichia coli. PLoS ONE 2012. [Google Scholar] [CrossRef]
- Packard, T.T.; Christensen, J.P. Respiration and vertical carbon flux in the Gulf of Maine water column. J. Mar. Res. 2004, 62, 93–115. [Google Scholar] [CrossRef]
- Packard, T.T.; Codispoti, L.A. Respiration, mineralization, and biochemical properties of the particulate matter in the southern Nansen Basin water column in April 1981. Deep Sea Res. Part I Oceanogr. Res. Pap. 2007, 54, 403–414. [Google Scholar] [CrossRef]
- Martínez-García, S.; Fernández, E.; del Valle, D.; Karl, D.; Teira, E. Experimental assessment of marine bacterial respiration. Aquat. Microb. Ecol. 2013, 70, 189–205. [Google Scholar] [CrossRef]
- García, F.C.; García-Martín, E.E.; Taboada, F.G.; Sal, S.; Serret, P.; López-Urrutia, Á. The allometry of the smallest: Superlinear scaling of microbial metabolic rates in the Atlantic Ocean. ISME J. 2016, 10, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Cajal-Medrano, R.; Maske, H. Growth efficiency and respiration at different growth rates in glucose-limited chemostats with natural marine bacteria populations. Aquat. Microb. Ecol. 2005, 38, 125–133. [Google Scholar] [CrossRef]
- Kirchman, D. Statistical analysis of direct counts of microbial abundance. In Handbook of Methods in Aquatic Microbial Ecology; Kemp, P.F., Sherr, B.F., Sherr, E.B., Cole, J.J., Eds.; Lewis Publishing: Boca Raton, FL, USA, 1993; pp. 117–119. [Google Scholar]
- Roland, F.; Caraco, N.F.; Cole, J.J.; del Giorgio, P. Rapid and precise determination of dissolved oxygen by spectrophotometry: Evaluation of interference from color and turbidity. Limnol. Oceanogr. 1999, 44, 1148–1154. [Google Scholar] [CrossRef]
- Protocols for the Joint Global Ocean Flux Study (JGOFS) Core Measurements. JGOFS Rep. Number 19 1996, 170.
- Marchand, D.; Prairie, Y.T.; Del Giorgio, P.A. Linking forest fires to lake metabolism and carbon dioxide emissions in the boreal region of Northern Québec. Glob. Chang. Biol. 2009, 15, 2861–2873. [Google Scholar] [CrossRef]
- Berggren, M.; Lapierre, J.-F.; del Giorgio, P.A. Magnitude and regulation of bacterioplankton respiratory quotient across freshwater environmental gradients. ISME J. 2012, 6, 984–993. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villegas-Mendoza, J.; Cajal-Medrano, R.; Maske, H. The Chemical Transformation of the Cellular Toxin INT (2-(4-Iodophenyl)-3-(4-Nitrophenyl)-5-(Phenyl) Tetrazolium Chloride) as an Indicator of Prior Respiratory Activity in Aquatic Bacteria. Int. J. Mol. Sci. 2019, 20, 782. https://doi.org/10.3390/ijms20030782
Villegas-Mendoza J, Cajal-Medrano R, Maske H. The Chemical Transformation of the Cellular Toxin INT (2-(4-Iodophenyl)-3-(4-Nitrophenyl)-5-(Phenyl) Tetrazolium Chloride) as an Indicator of Prior Respiratory Activity in Aquatic Bacteria. International Journal of Molecular Sciences. 2019; 20(3):782. https://doi.org/10.3390/ijms20030782
Chicago/Turabian StyleVillegas-Mendoza, Josué, Ramón Cajal-Medrano, and Helmut Maske. 2019. "The Chemical Transformation of the Cellular Toxin INT (2-(4-Iodophenyl)-3-(4-Nitrophenyl)-5-(Phenyl) Tetrazolium Chloride) as an Indicator of Prior Respiratory Activity in Aquatic Bacteria" International Journal of Molecular Sciences 20, no. 3: 782. https://doi.org/10.3390/ijms20030782
APA StyleVillegas-Mendoza, J., Cajal-Medrano, R., & Maske, H. (2019). The Chemical Transformation of the Cellular Toxin INT (2-(4-Iodophenyl)-3-(4-Nitrophenyl)-5-(Phenyl) Tetrazolium Chloride) as an Indicator of Prior Respiratory Activity in Aquatic Bacteria. International Journal of Molecular Sciences, 20(3), 782. https://doi.org/10.3390/ijms20030782




