Neonicotinoid Insecticides Alter the Transcriptome of Soybean and Decrease Plant Resistance
Abstract
1. Introduction
2. Results
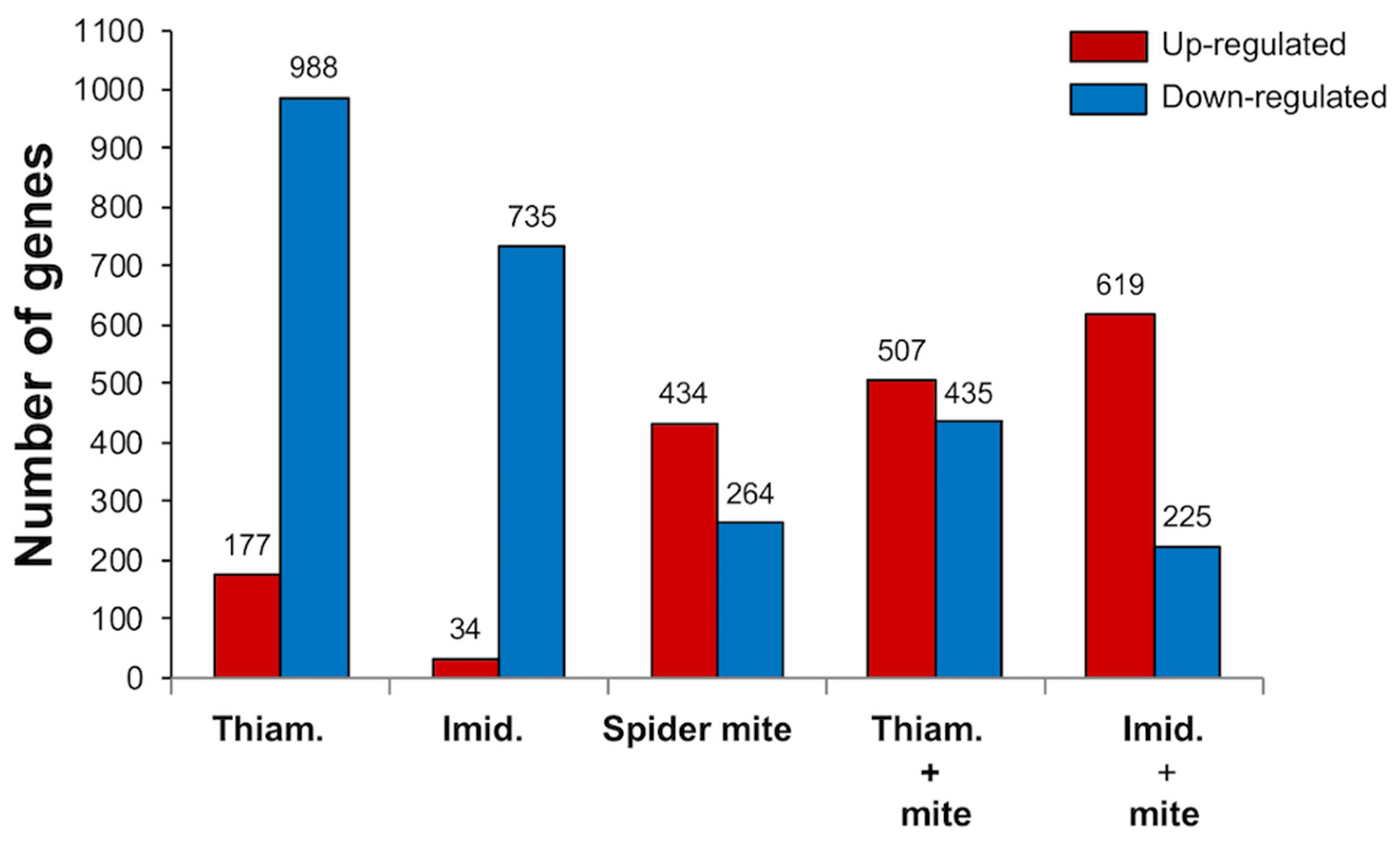
2.1. Neonicotinoid Insecticides Alone and in Combination with Spider Mite Herbivory Altered the Transcriptome of Soybean Plants
2.2. Neonicotinoid Insecticides and Spider Mites Affect Multiple Plant Pathways
2.3. Thiamethoxam Seed Treatments Increase Abundance of Spider Mites
3. Discussion
4. Materials and Methods
4.1. Plant Material, Neonicotinoid Treatments, and Spider Mite Colonies
4.2. Impact of Neonicotinoids and Spider Mite Herbivory on Gene Expression
4.3. Gene Expression Analysis
4.4. Impact of Thiamethoxam on Abundance of Spider Mites
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Laznik, Z.; Znidarcic, D.; Trdan, S. Control of Trialeurodes vaporariorum (Westwood) adults on glasshouse-grown cucumbers in four different growth substrates: An efficacy comparison of foliar application of Steinernema feltiae (Filipjev) and spraying with thiamethoxam. Turk. J. Agric. For. 2011, 35, 631–640. [Google Scholar]
- Laznik, Ž.; Tóth, T.; Lakatos, T.; Vidrih, M.; Trdan, S. Control of the Colorado potato beetle (Leptinotarsa decemlineata [Say]) on potato under field conditions: a comparison of the efficacy of foliar application of two strains of Steinernema feltiae (Filipjev) and spraying with thiamethoxam. J. Plant Dis. Prot. 2010, 117, 129–135. [Google Scholar] [CrossRef]
- Ford, K.A.; Casida, J.E.; Chandran, D.; Gulevich, A.G.; Okrent, R.A.; Durkin, K.A.; Sarpong, R.; Bunnelle, E.M.; Wildermuth, M.C. Neonicotinoid insecticides induce salicylate-associated plant defense responses. Proc. Natl. Acad. Sci. 2010, 107, 17527–17532. [Google Scholar] [CrossRef]
- Szczepaniec, A.; Raupp, M.J.; Parker, R.D.; Kerns, D.; Eubanks, M.D. Neonicotinoid insecticides alter induced defenses and increase susceptibility to spider mites in distantly related crop plants. PLoS ONE 2013, 8, e62620. [Google Scholar] [CrossRef]
- Stamm, M.D.; Enders, L.S.; Donze-Reiner, T.J.; Baxendale, F.P.; Siegfried, B.D.; Heng-Moss, T.M. Transcriptional response of soybean to thiamethoxam seed treatment in the presence and absence of drought stress. BMC Genom. 2014, 15, 1055. [Google Scholar] [CrossRef]
- Douglas, M.R.; Tooker, J.F. Large-scale deployment of seed treatments has driven rapid increase in use of neonicotinoid insecticides and preemptive pest management in US field crops. Environ. Sci. Technol. 2015, 49, 5088–5097. [Google Scholar] [CrossRef]
- Calafiori, M.; Barbieri, A. Effects of seed treatment with insecticide on the germination, nutrients, nodulation, yield and pest control in bean (Phaseolus vulgaris L.) culture. Rev. Ecossistema 2001, 26, 97–104. [Google Scholar]
- Macedo, W.R.; e Castro, P.R.D.C. Thiamethoxam: Molecule moderator of growth, metabolism and production of spring wheat. Pesticide Biochem. Physiol. 2011, 100, 299–304. [Google Scholar] [CrossRef]
- Afifi, M.; Lee, E.; Lukens, L.; Swanton, C. Thiamethoxam as a seed treatment alters the physiological response of maize (Zea mays) seedlings to neighbouring weeds. Pest Manag. Scie. 2015, 71, 505–514. [Google Scholar] [CrossRef]
- Cataneo, A.; Ferreira, L.; Carvalho, J.; Andréo-Souza, Y.; Corniani, N.; Mischan, M.; Nunes, J. Improved germination of soybean seed treated with thiamethoxam under drought conditions. Seed Sci. Technol. 2010, 38, 248–251. [Google Scholar] [CrossRef]
- Larsen, R.J.; Falk, D.E. Effects of a seed treatment with a neonicotinoid insecticide on germination and freezing tolerance of spring wheat seedlings. Canad. J. Plant Sci. 2013, 93, 535–540. [Google Scholar] [CrossRef]
- Magalhaes, L.C.; Hunt, T.E.; Siegfried, B.D. Efficacy of neonicotinoid seed treatments to reduce soybean aphid populations under field and controlled conditions in Nebraska. J. Econ. Entomol. 2009, 102, 187–195. [Google Scholar] [CrossRef]
- Seagraves, M.P.; Lundgren, J.G. Effects of neonicotinoid seed treatments on soybean aphid and its natural enemies. J. Pest Sci. 2012, 85, 125–132. [Google Scholar] [CrossRef]
- Tomizawa, M.; Casida, J.E. Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Ann. Rev. Entomol. 2003, 48, 339–364. [Google Scholar] [CrossRef]
- Szczepaniec, A.; Creary, S.F.; Laskowski, K.L.; Nyrop, J.P.; Raupp, M.J. Neonicotinoid insecticide imidacloprid causes outbreaks of spider mites on elm trees in urban landscapes. PLoS ONE 2011, 6, e20018. [Google Scholar] [CrossRef]
- Sclar, D.C.; Gerace, D.; Cranshaw, W.S. Observations of population increases and injury by spider mites (Acari: Tetranychidae) on ornamental plants treated with imidacloprid. J. Econ. Entomol. 1998, 91, 250–255. [Google Scholar] [CrossRef]
- Raupp, M.J.; Webb, R.E.; Szczepaniec, A.; Booth, D.; Ahern, R. Incidence, abundance, and severity of mites on hemlocks following applications of imidacloprid. J. Arboric. 2004, 30, 108–113. [Google Scholar]
- Szczepaniec, A.; Raupp, M.J. Direct and indirect effects of imidacloprid on fecundity and abundance of Eurytetranychus buxi (Acari: Tetranychidae) on boxwoods. Exp. Appl. Acarol. 2013, 59, 307–318. [Google Scholar] [CrossRef]
- Gupta, G.; Krischik, V.A. Professional and consumer insecticides for management of adult Japanese beetle on hybrid tea rose. J. Econ. Entomol. 2007, 100, 830–837. [Google Scholar] [CrossRef]
- Ruckert, A.; Allen, L.N.; Ramirez, R.A. Combinations of plant water-stress and neonicotinoids can lead to secondary outbreaks of Banks grass mite (Oligonychus pratensis Banks). PLoS ONE 2018, 13, e0191536. [Google Scholar] [CrossRef]
- Smith, J.F.; Catchot, A.L.; Musser, F.R.; Gore, J. Effects of aldicarb and neonicotinoid seed treatments on twospotted spider mite on cotton. J. Econ. Entomol. 2013, 106, 807–815. [Google Scholar] [CrossRef]
- Zhang, P.-J.; Zheng, S.-J.; van Loon, J.J.A.; Boland, W.; David, A.; Mumm, R.; Dicke, M. Whiteflies interfere with indirect plant defense against spider mites in Lima bean. Proc. Natl. Acad. Sci. USA 2009, 106, 21202–21207. [Google Scholar] [CrossRef]
- Li, C.Y.; Williams, M.M.; Loh, Y.T.; Lee, G.I.; Howe, G.A. Resistance of cultivated tomato to cell content-feeding herbivores is regulated by the octadecanoid-signaling pathway. Plant Physiol. 2002, 130, 494–503. [Google Scholar] [CrossRef]
- Ament, K.; Kant, M.R.; Sabelis, M.W.; Haring, M.A.; Schuurink, R.C. Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiol. 2004, 135, 2025–2037. [Google Scholar] [CrossRef]
- Zheng, S.-J.; van Dijk, J.P.; Bruinsma, M.; Dicke, M. Sensitivity and speed of induced defense of cabbage (Brassica oleracea L.): Dynamics of BoLOX expression patterns during insect and pathogen attack. MPMI 2007, 20, 1332–1345. [Google Scholar] [CrossRef]
- Zhurov, V.; Navarro, M.; Bruinsma, K.A.; Arbona, V.; Santamaria, M.E.; Cazaux, M.; Wybouw, N.; Osborne, E.J.; Ens, C.; Rioja, C.; et al. Reciprocal responses in the interaction between Arabidopsis and the cell-content-feeding chelicerate herbivore spider mite. Plant Physiol. 2014, 164, 384–399. [Google Scholar] [CrossRef]
- Alba, J.M.; Schimmel, B.C.J.; Glas, J.J.; Ataide, L.M.S.; Pappas, M.L.; Villarroel, C.A.; Schuurink, R.C.; Sabelis, M.W.; Kant, M.R. Spider mites suppress tomato defenses downstream of jasmonate and salicylate independently of hormonal crosstalk. New Phytol. 2015, 205, 828–840. [Google Scholar] [CrossRef]
- Thaler, J.S.; Humphrey, P.T.; Whiteman, N.K. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 2012, 17, 260–270. [Google Scholar] [CrossRef]
- Caarls, L.; Pieterse, C.M.J.; Van Wees, S.C.M. How salicylic acid takes transcriptional control over jasmonic acid signaling. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
- Koornneef, A.; Pieterse, C.M. Cross talk in defense signaling. Plant Physiol. 2008, 146, 839–844. [Google Scholar] [CrossRef]
- Kammerhofer, N.; Egger, B.; Dobrev, P.; Vankova, R.; Hofmann, J.; Schausberger, P.; Wieczorek, K. Systemic above- and belowground cross talk: hormone-based responses triggered by Heterodera schachtii and shoot herbivores in Arabidopsis thaliana. J. Exp. Bot. 2015, 66, 7005–7017. [Google Scholar] [CrossRef] [PubMed]
- Cipollini, D.F., Jr. Wind-induced mechanical stimulation increases pest resistance in common bean. Oecologia 1997, 111, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Nachappa, P.; Margolies, D.C.; Nechols, J.R.; Whitfield, A.E.; Rotenberg, D. Tomato spotted wilt virus benefits a non-vector arthropod, Tetranychus urticae, by modulating different plant responses in tomato. PLoS ONE 2013, 8, e75909. [Google Scholar] [CrossRef] [PubMed]
- Santiago, R.; Barros-Rios, J.; Malvar, R.A. Impact of cell wall composition on maize resistance to pests and diseases. Int. J. Mol. Sci. 2013, 14, 6960–6980. [Google Scholar] [CrossRef] [PubMed]
- Elbert, A.; Haas, M.; Springer, B.; Thielert, W.; Nauen, R. Applied aspects of neonicotinoid uses in crop protection. Pest Manag. Sci. 2008, 64, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Stansly, P.A.; Kostyk, B.C.; Reifer, R. Control of tomato pinworm and southern armyworm on staked tomato, 2007. Arthropod Manag. Tests 2008, 33, E50. [Google Scholar] [CrossRef]
- Prager, S.M.; Vindiola, B.; Kund, G.S.; Byrne, F.J.; Trumble, J.T. Considerations for the use of neonicotinoid pesticides in management of Bactericera cockerelli (Šulk)(Hemiptera: Triozidae). Crop Protect. 2013, 54, 84–91. [Google Scholar] [CrossRef]
- Huseth, A.S.; Lindholm, J.; Groves, C.L.; Groves, R.L. Variable concentration of soil-applied insecticides in potato over time: Implications for management of Leptinotarsa decemlineata. Pest Manag. Sci. 2014, 70, 1863–1871. [Google Scholar] [CrossRef]
- Nicholas, A.H.; Spooner-Hart, R.N.; Vickers, R.A. Control of woolly aphid, Eriosoma lanigerum (Hausmann) (Hemiptera: Pemphigidae) on mature apple trees using insecticide soil-root drenches. Aust. J. Entomol. 2003, 42, 6–11. [Google Scholar] [CrossRef]
- Dombrecht, B.; Xue, G.P.; Sprague, S.J.; Kirkegaard, J.A.; Ross, J.J.; Reid, J.B.; Fitt, G.P.; Sewelam, N.; Schenk, P.M.; Manners, J.M.; et al. MYC2 dfferentially modulates diverse jasmonate-dependent functions in Arabidopsis. Plant Cell 2007, 19, 2225–2245. [Google Scholar] [CrossRef]
- Fernández-Calvo, P.; Chini, A.; Fernández-Barbero, G.; Chico, J.-M.; Gimenez-Ibanez, S.; Geerinck, J.; Eeckhout, D.; Schweizer, F.; Godoy, M.; Franco-Zorrilla, J.M.; et al. The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. Plant Cell 2011, 23, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K.; Manners, J.M. MYC2: The master in action. Mol. Plant 2013, 6, 686–703. [Google Scholar] [CrossRef] [PubMed]
- Breen, S.; Williams, S.J.; Outram, M.; Kobe, B.; Solomon, P.S. Emerging insights into the functions of pathogenesis-related protein 1. Trends Plant Sci. 2017, 22, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Grones, P.; Friml, J. Auxin transporters and binding proteins at a glance. J. Cell Sci. 2015, 128, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Naseem, M.; Kaltdorf, M.; Dandekar, T. The nexus between growth and defence signalling: Auxin and cytokinin modulate plant immune response pathways. J. Exp. Bot. 2015, 66, 4885–4896. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Gray, W.M. SAUR proteins as effectors of hormonal and environmental signals in plant growth. Mol. Plant 2015, 8, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xia, X.; Yu, G.; Wang, J.; Wu, J.; Wang, M.; Yang, Y.; Shi, K.; Yu, Y.; Chen, Z.; et al. Brassinosteroids play a critical role in the regulation of pesticide metabolism in crop plants. Sci. Rep. 2015, 5, 9018. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Kumar, R.; Shahzad, B.; Thukral, A.K.; Bhardwaj, R. Brassinosteroid-mediated pesticide detoxification in plants: A mini-review. Cogent Food Agric. 2018, 4, 1436212. [Google Scholar] [CrossRef]
- Yokoyama, R.; Nishitani, K. A comprehensive expression analysis of all members of a gene family encoding cell-wall enzymes allowed us to predict cis-regulatory regions involved in cell-wall construction in specific organs of Arabidopsis. Plant Cell Physiol. 2001, 42, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.A.; Rehman, H.M.; Imtiaz, M.; Baloch, F.S.; Lee, J.D.; Yang, S.H.; Lee, S.I.; Chung, G. Systems identification and characterization of cell wall reassembly and degradation related genes in Glycine max (L.) Merill, a bioenergy legume. Sci. Rep. 2017, 7, 10862. [Google Scholar] [CrossRef] [PubMed]
- Ranty, B.; Aldon, D.; Cotelle, V.; Galaud, J.-P.; Thuleau, P.; Mazars, C. Calcium sensors as key hubs in plant responses to biotic and abiotic stresses. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Scholz, S.S.; Heyer, M. A role for calmodulin-like proteins in herbivore defense path- ways in plants. J. Int. Soc. Endocytobiol. 2016, 27, 12. [Google Scholar]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, L.; Kumar, D.; Mukhopadhyay, K. WRKY transcription factors: Involvement in plant–pathogen interactions. In Recent Advances in Applied Microbiology; Shukla, P., Ed.; Springer Singapore: Singapore, 2017; pp. 229–246. ISBN 978-981-10-5275-0. [Google Scholar]
- Banerjee, A.; Roychoudhury, A. WRKY proteins: Signaling and regulation of expression during abiotic stress responses. Available online: https://www.hindawi.com/journals/tswj/2015/807560/abs/ (accessed on 11 January 2019).
- Higashi, K.; Ishiga, Y.; Inagaki, Y.; Toyoda, K.; Shiraishi, T.; Ichinose, Y. Modulation of defense signal transduction by flagellin-induced WRKY41 transcription factor in Arabidopsis thaliana. Mol. Genet. Genom. 2008, 279, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Achnine, L.; Kota, P.; Liu, C.-J.; Reddy, M.S.S.; Wang, L. The phenylpropanoid pathway and plant defence—A genomics perspective. Mol. Plant Pathol. 2002, 3, 371–390. [Google Scholar] [CrossRef]
- Camera, S.L.; Gouzerh, G.; Dhondt, S.; Hoffmann, L.; Fritig, B.; Legrand, M.; Heitz, T. Metabolic reprogramming in plant innate immunity: The contributions of phenylpropanoid and oxylipin pathways. Immunol. Rev. 2004, 198, 267–284. [Google Scholar] [CrossRef]
- Lv, M.; Kong, H.; Liu, H.; Lu, Y.; Zhang, C.; Liu, J.; Ji, C.; Zhu, J.; Su, J.; Gao, X. Induction of phenylalanine ammonia-lyase (PAL) in insect damaged and neighboring undamaged cotton and maize seedlings. Int. J. Pest Manag. 2017, 63, 166–171. [Google Scholar] [CrossRef]
- Goujon, T.; Sibout, R.; Eudes, A.; MacKay, J.; Jouanin, L. Genes involved in the biosynthesis of lignin precursors in Arabidopsis thaliana. Plant Physiol. Biochem. 2003, 41, 677–687. [Google Scholar] [CrossRef]
- Zhao, Q.; Nakashima, J.; Chen, F.; Yin, Y.; Fu, C.; Yun, J.; Shao, H.; Wang, X.; Wang, Z.-Y.; Dixon, R.A. Laccase is necessary and nonredundant with peroxidase for lignin polymerization during vascular development in Arabidopsis. Plant Cell 2013. [Google Scholar] [CrossRef]
- Le Gall, H.; Philippe, F.; Domon, J.-M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell wall metabolism in response to abiotic stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef]
- Barakat, A.; Bagniewska-Zadworna, A.; Frost, C.J.; Carlson, J.E. Phylogeny and expression profiling of CAD and CAD-like genes in hybrid Populus (P. deltoides × P. nigra): evidence from herbivore damage for subfunctionalization and functional divergence. BMC Plant Biol. 2010, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Choi, H.; An, G. Roles of lignin biosynthesis and regulatory genes in plant development. J. Integr. Plant Biol. 2015, 57, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Martel, C.; Zhurov, V.; Navarro, M.; Martinez, M.; Cazaux, M.; Auger, P.; Migeon, A.; Estrella Santamaria, M.; Wybouw, N.; Diaz, I.; et al. Tomato whole genome transcriptional response to Tetranychus urticae identifies divergence of spider mite-induced responses between tomato and Arabidopsis. Mol. Plant-Microbe Interact. 2015, 28, 343–361. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C.M. Hormonal modulation of plant immunity. Ann. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed]
- Vos, I.A.; Pieterse, C.M.J.; Wees, S.C.M. van Costs and benefits of hormone-regulated plant defences. Plant Pathol. 2013, 62, 43–55. [Google Scholar] [CrossRef]
- Schweiger, R.; Heise, A.-M.; Persicke, M.; Müller, C. Interactions between the jasmonic and salicylic acid pathway modulate the plant metabolome and affect herbivores of different feeding types. Plant Cell Environ. 2014, 37, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E.; Mithöfer, A.; Boland, W. Before gene expression: early events in plant–insect interaction. Trends Plant Sci. 2007, 12, 310–316. [Google Scholar] [CrossRef]
- Li, Q.; Xie, Q.-G.; Smith-Becker, J.; Navarre, D.A.; Kaloshian, I. Mi-1-mediated aphid resistance involves salicylic acid and mitogen-activated protein kinase signaling cascades. MPMI 2006, 19, 655–664. [Google Scholar] [CrossRef]
- Bostock, R.M.; Karban, R.; Thaler, J.S.; Weyman, P.D.; Gilchrist, D. Signal interactions in induced resistance to pathogens and insect herbivores. Eur. J. Plant Pathol. 2001, 107, 103–111. [Google Scholar] [CrossRef]
- Mozoruk, J.; Hunnicutt, L.E.; Cave, R.D.; Hunter, W.B.; Bausher, M.G. Profiling transcriptional changes in Citrus sinensis (L.) Osbeck challenged by herbivory from the xylem-feeding leafhopper Homalodisca coagulata (Say) by cDNA macroarray analysis. Plant Sci. 2006, 170, 1068–1080. [Google Scholar] [CrossRef]
- Barros-Rios, J.; Malvar, R.A.; Jung, H.-J.G.; Santiago, R. Cell wall composition as a maize defense mechanism against corn borers. Phytochemistry 2011, 72, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Villada, E.S.; González, E.G.; López-Sesé, A.I.; Castiel, A.F.; Gómez-Guillamón, M.L. Hypersensitive response to Aphis gossypii Glover in melon genotypes carrying the VAT gene. J. Exp. Bot. 2009, 60, 3269–3277. [Google Scholar] [CrossRef] [PubMed]
- Bensoussan, N.; Santamaria, M.E.; Zhurov, V.; Diaz, I.; Grbić, M.; Grbić, V. Plant-herbivore interaction: dissection of the cellular pattern of Tetranychus urticae feeding on the host plant. Front. Plant Sci. 2016, 7, 1105. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Riquelme, J.; Zhurov, V.; Rioja, C.; Pérez-Moreno, I.; Torres-Pérez, R.; Grimplet, J.; Carbonell-Bejerano, P.; Bajda, S.; Van Leeuwen, T.; Martínez-Zapater, J.M. Comparative genome-wide transcriptome analysis of Vitis vinifera responses to adapted and non-adapted strains of two-spotted spider mite, Tetranyhus urticae. BMC Genom. 2016, 17, 74. [Google Scholar] [CrossRef] [PubMed]
- Boissot, N.; Schoeny, A.; Vanlerberghe-Masutti, F. VAT, an amazing gene conferring resistance to aphids and viruses they carry: from molecular structure to field effects. Front. Plant Sci. 2016, 7, 1420. [Google Scholar] [CrossRef]
- Cheng, Y.; Shi, Z.-P.; Jiang, L.-B.; Ge, L.-Q.; Wu, J.-C.; Jahn, G.C. Possible connection between imidacloprid-induced changes in rice gene transcription profiles and susceptibility to the brown plant hopper Nilaparvata lugens Stål (Hemiptera: Delphacidae). Pesticide Biochem. Physiol. 2012, 102, 213–219. [Google Scholar] [CrossRef]
- Kawano, T. Roles of the reactive oxygen species-generating peroxidase reactions in plant defense and growth induction. Plant Cell Rep. 2003, 21, 829–837. [Google Scholar]
- Gulsen, O.; Eickhoff, T.; Heng-Moss, T.; Shearman, R.; Baxendale, F.; Sarath, G.; Lee, D. Characterization of peroxidase changes in resistant and susceptible warm-season turfgrasses challenged by Blissus occiduus. Arthropod-Plant Interact. 2010, 4, 45–55. [Google Scholar] [CrossRef]
- Mai, V.C.; Bednarski, W.; Borowiak-Sobkowiak, B.; Wilkaniec, B.; Samardakiewicz, S.; Morkunas, I. Oxidative stress in pea seedling leaves in response to Acyrthosiphon pisum infestation. Phytochemistry 2013, 93, 49–62. [Google Scholar] [CrossRef]
- Regan, K.; Ordosch, D.; Glover, K.D.; Tilmon, K.J.; Szczepaniec, A. Effects of a pyrethroid and two neonicotinoid insecticides on population dynamics of key pests of soybean and abundance of their natural enemies. Crop Protect. 2017, 98, 24–32. [Google Scholar] [CrossRef]
- Schmutz, J.; Cannon, S.B.; Schlueter, J.; Ma, J.; Mitros, T.; Nelson, W.; Hyten, D.L.; Song, Q.; Thelen, J.J.; Cheng, J.; et al. Genome sequence of the palaeopolyploid soybean. Nature 2010, 463, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Zhou, X.; Ling, Y.; Zhang, Z.; Su, Z. agriGO: A GO analysis toolkit for the agricultural community. Nucleic Acids Res. 2010, 38, W64–W70. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.-Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef] [PubMed]
- R Core Team R: A language and environment for statistical computing. Available online: http://www.R-project.org (accessed on 2 December 2017).






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wulff, J.A.; Kiani, M.; Regan, K.; Eubanks, M.D.; Szczepaniec, A. Neonicotinoid Insecticides Alter the Transcriptome of Soybean and Decrease Plant Resistance. Int. J. Mol. Sci. 2019, 20, 783. https://doi.org/10.3390/ijms20030783
Wulff JA, Kiani M, Regan K, Eubanks MD, Szczepaniec A. Neonicotinoid Insecticides Alter the Transcriptome of Soybean and Decrease Plant Resistance. International Journal of Molecular Sciences. 2019; 20(3):783. https://doi.org/10.3390/ijms20030783
Chicago/Turabian StyleWulff, Jason A., Mahnaz Kiani, Karly Regan, Micky D. Eubanks, and Adrianna Szczepaniec. 2019. "Neonicotinoid Insecticides Alter the Transcriptome of Soybean and Decrease Plant Resistance" International Journal of Molecular Sciences 20, no. 3: 783. https://doi.org/10.3390/ijms20030783
APA StyleWulff, J. A., Kiani, M., Regan, K., Eubanks, M. D., & Szczepaniec, A. (2019). Neonicotinoid Insecticides Alter the Transcriptome of Soybean and Decrease Plant Resistance. International Journal of Molecular Sciences, 20(3), 783. https://doi.org/10.3390/ijms20030783




