Deletion of Osteopontin Enhances β2-Adrenergic Receptor-Dependent Anti-Fibrotic Signaling in Cardiomyocytes
Abstract
1. Introduction
2. Results
2.1. Regulation of OPN Expression by Aldosterone and the β2AR in H9c2 Cardiomyocytes
2.2. OPN Opposes β2AR cAMP Signaling in H9c2 Cardiomyocytes
2.3. Epac1 Upregulation by OPN CRISPR-Mediated Deletion in H9c2 Cardiomyocytes
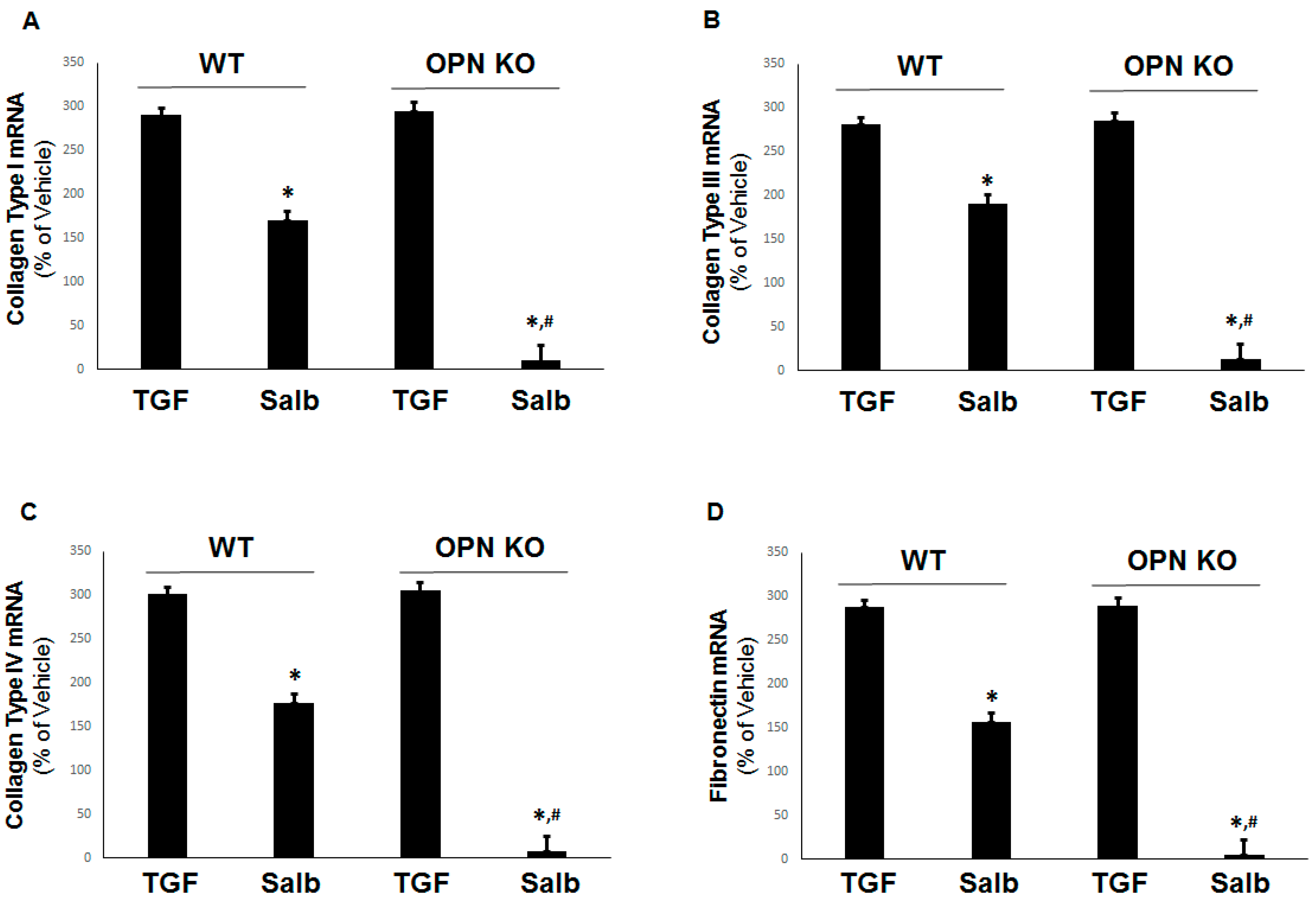
2.4. Prevention of Pro-Fibrotic Gene Expression by OPN Genetic Ablation in H9c2 Cardiomyocytes
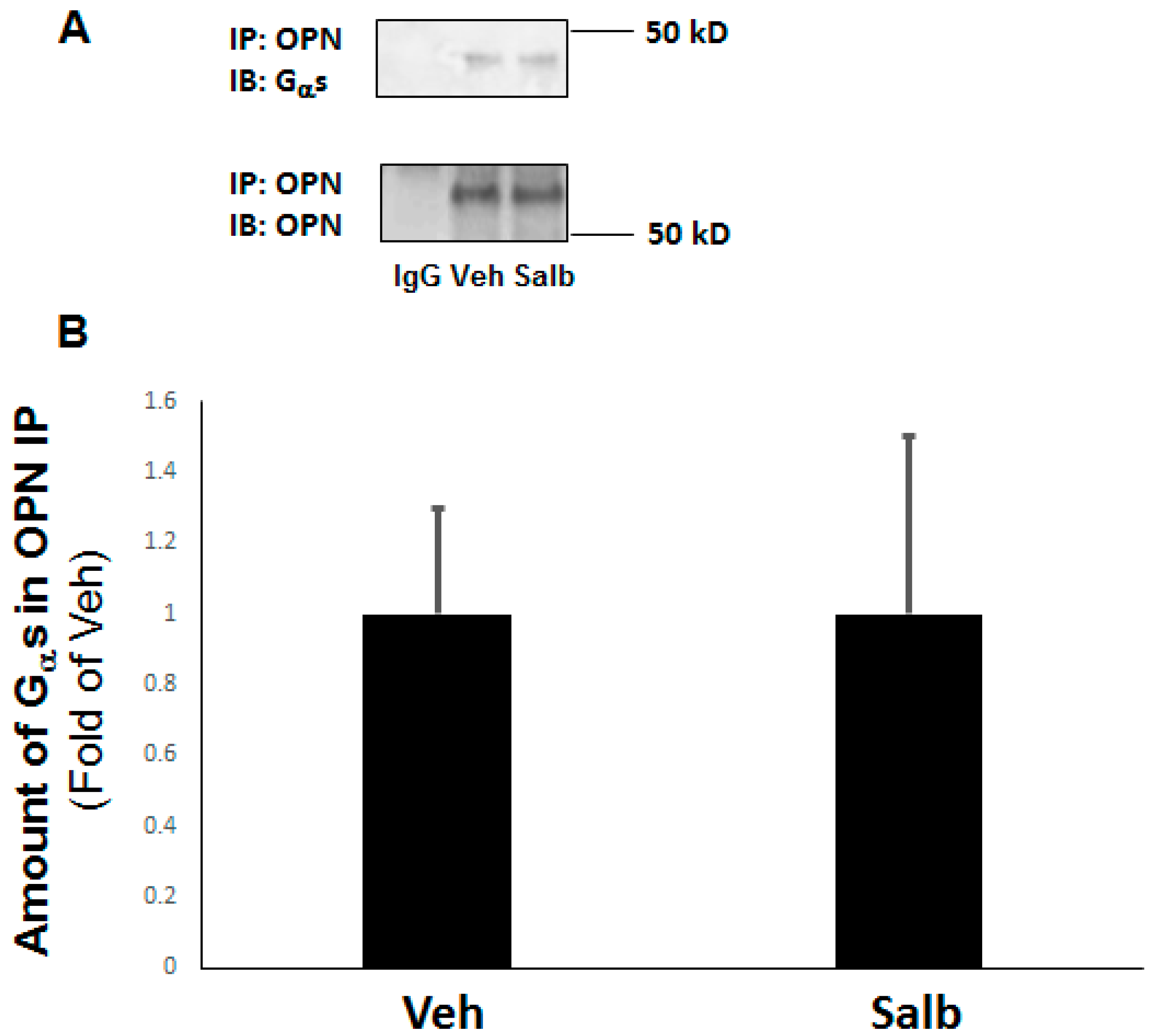
2.5. OPN Inhibits β2AR cAMP Signaling by Directly Interacting with the Gαs/olf Protein Subunit in H9c2 Cardiomyocytes
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and CRISPR-Mediated OPN KO
4.3. Real-Time qPCR
4.4. cAMP Accumulation Determination
4.5. Co-Immunoprecipitation and Western Blotting
4.6. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Berk, B.C.; Fujiwara, K.; Lehoux, S. ECM remodeling in hypertensive heart disease. J. Clin. Investig. 2007, 117, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef] [PubMed]
- Capote, L.A.; Mendez Perez, R.; Lymperopoulos, A. GPCR signaling and cardiac function. Eur. J. Pharmacol. 2015, 763 Pt B, 143–148. [Google Scholar] [CrossRef]
- Lymperopoulos, A.; Rengo, G.; Koch, W.J. Adrenergic nervous system in heart failure: Pathophysiology and therapy. Circ. Res. 2013, 113, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Desimine, V.L.; McCrink, K.A.; Parker, B.M.; Wertz, S.L.; Maning, J.; Lymperopoulos, A. Biased Agonism/Antagonism of Cardiovascular GPCRs for Heart Failure Therapy. Int. Rev. Cell. Mol. Biol. 2018, 339, 41–61. [Google Scholar]
- Salazar, N.C.; Vallejos, X.; Siryk, A.; Rengo, G.; Cannavo, A.; Liccardo, D.; De Lucia, C.; Gao, E.; Leosco, D.; Koch, W.J.; et al. GRK2 blockade with βARKct is essential for cardiac β2-adrenergic receptor signaling towards increased contractility. Cell Commun. Signal. 2013, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Meszaros, J.G.; Gonzalez, A.M.; Endo-Mochizuki, Y.; Villegas, S.; Villarreal, F.; Brunton, L.L. Identification of G protein-coupled signaling pathways in cardiac fibroblasts: Cross talk between G(q) and G(s). Am. J. Physiol. Cell Physiol. 2000, 278, C154–C162. [Google Scholar] [CrossRef]
- Ostrom, R.S.; Naugle, J.E.; Hase, M.; Gregorian, C.; Swaney, J.S.; Insel, P.A.; Brunton, L.L.; Meszaros, J.G. Angiotensin II enhances adenylyl cyclase signaling via Ca2þ/calmodulin. Gq-Gs cross-talk regulates collagen production in cardiac fibroblasts. J. Biol. Chem. 2003, 278, 24461–24468. [Google Scholar] [CrossRef]
- Surinkaew, S.; Aflaki, M.; Takawale, A.; Chen, Y.; Qi, X.Y.; Gillis, M.A.; Shi, Y.F.; Tardif, J.C.; Chattipakorn, N.; Nattel, S. Exchange protein activated by cyclic-adenosine monophosphate (Epac) regulates atrial fibroblast function and controls cardiac remodelling. Cardiovasc. Res. 2019, 115, 94–106. [Google Scholar] [CrossRef]
- Lymperopoulos, A.; Garcia, D.; Walklett, K. Pharmacogenetics of cardiac inotropy. Pharmacogenomics 2014, 15, 1807–1821. [Google Scholar] [CrossRef]
- Bers, D.M. Calcium cycling and signaling in cardiac myocytes. Annu. Rev. Physiol. 2008, 70, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.L. Epac proteins: Multi-purpose cAMP targets. Trends Biochem. Sci. 2006, 31, 680–686. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, J.; Zwartkruis, F.J.; Verheijen, M.H.; Cool, R.H.; Nijman, S.M.; Wittinghofer, A.; Bos, J.L. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 1998, 396, 474–477. [Google Scholar] [CrossRef]
- Yokoyama, U.; Patel, H.H.; Lai, N.C.; Aroonsakool, N.; Roth, D.M.; Insel, P.A. The cyclic AMP effector Epac integrates pro- and anti-fibrotic signals. Proc. Natl. Acad. Sci. USA 2008, 105, 6386–6391. [Google Scholar] [CrossRef] [PubMed]
- Metrich, M.; Lucas, A.; Gastineau, M.; Samuel, J.L.; Heymes, C.; Morel, E.; Lezoualc’h, F. Epac mediates beta-adrenergic receptor-induced cardiomyocyte hypertrophy. Circ. Res. 2008, 102, 959–965. [Google Scholar] [CrossRef]
- Insel, P.A.; Murray, F.; Yokoyama, U.; Romano, S.; Yun, H.; Brown, L.; Snead, A.; Lu, D.; Aroonsakool, N. cAMP and Epac in the regulation of tissue fibrosis. Br. J. Pharmacol. 2012, 166, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.F.; Seshadri, V.; Huang, K.; Shao, J.S.; Cai, J.; Vattikuti, R.; Schumacher, A.; Loewy, A.P.; Denhardt, D.T.; Rittling, S.R.; et al. An osteopontin-NADPH oxidase signaling cascade promotes pro-matrix metalloproteinase 9 activation in aortic mesenchymal cells. Circ. Res. 2006, 98, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Liaw, L.; Almeida, M.; Hart, C.E.; Schwartz, S.M.; Giachelli, C.M. Osteopontin promotes vascular cell adhesion and spreading and is chemotactic for smooth muscle cells in vitro. Circ. Res. 1994, 74, 214–224. [Google Scholar] [CrossRef]
- Liaw, L.; Birk, D.E.; Ballas, C.B.; Whitsitt, J.S.; Davidson, J.M.; Hogan, B.L. Altered wound healing in mice lacking a functional osteopontin gene (spp1). J. Clin. Investig. 1998, 101, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Irita, J.; Okura, T.; Jotoku, M.; Nagao, T.; Enomoto, D.; Kurata, M.; Desilva, V.R.; Miyoshi, K.; Matsui, Y.; Uede, T.; et al. Osteopontin deficiency protects against aldosterone-induced inflammation, oxidative stress, and interstitial fibrosis in the kidney. Am. J. Physiol. Ren. Physiol. 2011, 301, F833–F834. [Google Scholar] [CrossRef] [PubMed]
- Giachelli, C.M.; Bae, N.; Almeida, M.; Denhardt, D.T.; Alpers, C.E.; Schwartz, S.M. Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. J. Clin. Investig. 1993, 92, 1686–1696. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; McCann, M.; Mangan, S.; Lam, A.; Karan, M. Osteoprotegerin and osteopontin are expressed at high concentrations within symptomatic carotid atherosclerosis. Stroke 2004, 35, 1636–1641. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Jia, N.; Okamoto, H.; Kon, S.; Onozuka, H.; Akino, M.; Liu, L.; Morimoto, J.; Rittling, S.R.; Denhardt, D.; et al. Role of osteopontin in cardiac fibrosis and remodeling in angiotensin II-induced cardiac hypertrophy. Hypertension 2004, 43, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Trueblood, N.A.; Xie, Z.; Communal, C.; Sam, F.; Ngoy, S.; Liaw, L.; Jenkins, A.W.; Wang, J.; Sawyer, D.B.; Bing, O.H.; et al. Exaggerated left ventricular dilation and reduced collagen deposition after myocardial infarction in mice lacking osteopontin. Circ. Res. 2001, 88, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Yano, N.; Suzuki, D.; Endoh, M.; Tseng, A.; Stabila, J.P.; McGonnigal, B.G.; Zhao, T.C.; Padbury, J.F.; Tseng, Y.T. Beta-adrenergic receptor mediated protection against doxorubicin-induced apoptosis in cardiomyocytes: The impact of high ambient glucose. Endocrinology 2008, 149, 6449–6461. [Google Scholar] [CrossRef]
- Mlih, M.; Abdulrahman, N.; Gadeau, A.P.; Mohamed, I.A.; Jaballah, M.; Mraiche, F. Na(+)/H (+) exchanger isoform 1 induced osteopontin expression in cardiomyocytes involves NFAT3/Gata4. Mol. Cell. Biochem. 2015, 404, 211–220. [Google Scholar] [CrossRef]
- Parker, B.M.; Wertz, S.L.; Pollard, C.M.; Desimine, V.L.; Maning, J.; McCrink, K.A.; Lymperopoulos, A. Novel Insights into the Crosstalk between Mineralocorticoid Receptor and G Protein-Coupled Receptors in Heart Adverse Remodeling and Disease. Int. J. Mol. Sci. 2018, 19, 3764. [Google Scholar] [CrossRef]
- Kiyosue, A.; Nagata, D.; Myojo, M.; Sato, T.; Takahashi, M.; Satonaka, H.; Nagai, R.; Hirata, Y. Aldosterone-induced osteopontin gene transcription in vascular smooth muscle cells involves glucocorticoid response element. Hypertens. Res. 2011, 34, 1283–1287. [Google Scholar] [CrossRef]
- Nagao, M.; Feinstein, T.N.; Ezura, Y.; Hayata, T.; Notomi, T.; Saita, Y.; Hanyu, R.; Hemmi, H.; Izu, Y.; Takeda, S.; et al. Sympathetic control of bone mass regulated by osteopontin. Proc. Natl. Acad. Sci. USA 2011, 108, 17767–17772. [Google Scholar] [CrossRef]
- Lymperopoulos, A.; Rengo, G.; Zincarelli, C.; Kim, J.; Koch, W.J. Adrenal beta-arrestin 1 inhibition in vivo attenuates post-myocardial infarction progression to heart failure and adverse remodeling via reduction of circulating aldosterone levels. J. Am. Coll. Cardiol. 2011, 57, 356–365. [Google Scholar] [CrossRef]
- Cao, C.; Luo, X.; Ji, X.; Wang, Y.; Zhang, Y.; Zhang, P.; Zhong, L. Osteopontin regulates the proliferation of rat aortic smooth muscle cells in response to gingipains treatment. Mol. Cell. Probes 2017, 33, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Yoshimoto, T.; Hirono, Y.; Suzuki, N.; Sakurada, M.; Tsuchiya, K.; Minami, I.; Iwashima, F.; Sakai, H.; Tateno, T.; et al. Aldosterone increases osteopontin gene expression in rat endothelial cells. Biochem. Biophys. Res. Commun. 2005, 336, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Miura, R.; Nakamura, K.; Miura, D.; Miura, A.; Hisamatsu, K.; Kajiya, M.; Hashimoto, K.; Nagase, S.; Morita, H.; Fukushima Kusano, K.; et al. Aldosterone synthesis and cytokine production in human peripheral blood mononuclear cells. J. Pharmacol. Sci. 2006, 102, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yousefi, K.; Ding, W.; Singh, J.; Shehadeh, L.A. Osteopontin RNA aptamer can prevent and reverse pressure overload-induced heart failure. Cardiovasc. Res. 2017, 113, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Yousefi, K.; Goncalves, S.; Goldstein, B.J.; Sabater, A.L.; Kloosterboer, A.; Ritter, P.; Lambert, G.; Mendez, A.J.; Shehadeh, L.A. Osteopontin deficiency ameliorates Alport pathology by preventing tubular metabolic deficits. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Coculescu, B.I.; Manole, G.; Dincă, G.V.; Coculescu, E.C.; Berteanu, C.; Stocheci, C.M. Osteopontin—A biomarker of disease, but also of stage stratification of the functional myocardial contractile deficit by chronic ischaemic heart disease. J. Enzyme Inhib. Med. Chem. 2019, 34, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Poggio, P.; Grau, J.B.; Field, B.C.; Sainger, R.; Seefried, W.F.; Rizzolio, F.; Ferrari, G. Osteopontin controls endothelial cell migration in vitro and in excised human valvular tissue from patients with calcific aortic stenosis and controls. J. Cell. Physiol. 2011, 226, 2139–2149. [Google Scholar] [CrossRef]
- Dai, J.; Peng, L.; Fan, K.; Wang, H.; Wei, R.; Ji, G.; Cai, J.; Lu, B.; Li, B.; Zhang, D.; et al. Osteopontin induces angiogenesis through activation of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene 2009, 28, 3412–3422. [Google Scholar] [CrossRef]
- Xiang, Y.; Kobilka, B.K. Myocyte adrenoceptor signaling pathways. Science 2003, 300, 1530–1532. [Google Scholar] [CrossRef]
- Santulli, G. Sympathetic Nervous System Signaling in Heart Failure and Cardiac Aging. In Pathophysiology and Pharmacotherapy of Cardiovascular Disease; Jagadeesh, G., Balakumar, P., Maung, U.K., Eds.; Adis: Cham, Switzerland, 2015; pp. 83–105. [Google Scholar]
- Lymperopoulos, A.; Rengo, G.; Koch, W.J. GRK2 inhibition in heart failure: Something old, something new. Curr. Pharm. Des. 2012, 18, 186–191. [Google Scholar] [CrossRef]
- Singhmar, P.; Huo, X.; Eijkelkamp, N.; Berciano, S.R.; Baameur, F.; Mei, F.C.; Zhu, Y.; Cheng, X.; Hawke, D.; Mayor, F., Jr.; et al. Critical role for Epac1 in inflammatory pain controlled by GRK2-mediated phosphorylation of Epac1. Proc. Natl. Acad. Sci. USA 2016, 113, 3036–3041. [Google Scholar] [CrossRef] [PubMed]
- Belden, Z.; Deiuliis, J.A.; Dobre, M.; Rajagopalan, S. The Role of the Mineralocorticoid Receptor in Inflammation: Focus on Kidney and Vasculature. Am. J. Nephrol. 2017, 46, 298–314. [Google Scholar] [CrossRef]
- Brown, N.J. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat. Rev. Nephrol. 2013, 9, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Fuller, P.J.; Yang, J.; Young, M.J. 30 years of the mineralocorticoid receptor: Coregulators as mediators of mineralocorticoid receptor signaling diversity. J. Endocrinol. 2017, 234, T23–T34. [Google Scholar] [CrossRef] [PubMed]
- Faresse, N. Post-translational modifications of the mineralocorticoid receptor: How to dress the receptor according to the circumstances? J. Steroid. Biochem. Mol. Biol. 2014, 143, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Walther, R.F.; Atlas, E.; Carrigan, A.; Rouleau, Y.; Edgecombe, A.; Visentin, L.; Lamprecht, C.; Addicks, G.C.; Haché, R.J.; Lefebvre, Y.A. A serine/threonine-rich motif is one of three nuclear localization signals that determine unidirectional transport of the mineralocorticoid receptor to the nucleus. J. Biol. Chem. 2005, 280, 17549–17561. [Google Scholar] [CrossRef] [PubMed]
- Komolov, K.E.; Du, Y.; Duc, N.M.; Betz, R.M.; Rodrigues, J.P.G.L.M.; Leib, R.D.; Patra, D.; Skiniotis, G.; Adams, C.M.; Dror, R.O.; et al. Structural and Functional Analysis of a β2-Adrenergic Receptor Complex with GRK5. Cell 2017, 169, 407–421. [Google Scholar] [CrossRef] [PubMed]
- McCrink, K.A.; Maning, J.; Vu, A.; Jafferjee, M.; Marrero, C.; Brill, A.; Bathgate-Siryk, A.; Dabul, S.; Koch, W.J.; Lymperopoulos, A. β-Arrestin2 Improves Post-Myocardial Infarction Heart Failure via Sarco(endo)plasmic Reticulum Ca2+-ATPase-Dependent Positive Inotropy in Cardiomyocytes. Hypertension 2017, 70, 972–981. [Google Scholar] [CrossRef]
- Bathgate-Siryk, A.; Dabul, S.; Pandya, K.; Walklett, K.; Rengo, G.; Cannavo, A.; De Lucia, C.; Liccardo, D.; Gao, E.; Leosco, D.; et al. Negative impact of β-arrestin-1 on post-myocardial infarction heart failure via cardiac and adrenal-dependent neurohormonal mechanisms. Hypertension 2014, 63, 404–412. [Google Scholar] [CrossRef]
- Jafferjee, M.; Reyes Valero, T.; Marrero, C.; McCrink, K.A.; Brill, A.; Lymperopoulos, A. GRK2 Up-Regulation Creates a Positive Feedback Loop for Catecholamine Production in Chromaffin Cells. Mol. Endocrinol. 2016, 30, 372–381. [Google Scholar] [CrossRef]
- Lymperopoulos, A.; Rengo, G.; Funakoshi, H.; Eckhart, A.D.; Koch, W.J. Adrenal GRK2 upregulation mediates sympathetic overdrive in heart failure. Nat. Med. 2007, 13, 315–323. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pollard, C.M.; Desimine, V.L.; Wertz, S.L.; Perez, A.; Parker, B.M.; Maning, J.; McCrink, K.A.; Shehadeh, L.A.; Lymperopoulos, A. Deletion of Osteopontin Enhances β2-Adrenergic Receptor-Dependent Anti-Fibrotic Signaling in Cardiomyocytes. Int. J. Mol. Sci. 2019, 20, 1396. https://doi.org/10.3390/ijms20061396
Pollard CM, Desimine VL, Wertz SL, Perez A, Parker BM, Maning J, McCrink KA, Shehadeh LA, Lymperopoulos A. Deletion of Osteopontin Enhances β2-Adrenergic Receptor-Dependent Anti-Fibrotic Signaling in Cardiomyocytes. International Journal of Molecular Sciences. 2019; 20(6):1396. https://doi.org/10.3390/ijms20061396
Chicago/Turabian StylePollard, Celina M., Victoria L. Desimine, Shelby L. Wertz, Arianna Perez, Barbara M. Parker, Jennifer Maning, Katie A. McCrink, Lina A. Shehadeh, and Anastasios Lymperopoulos. 2019. "Deletion of Osteopontin Enhances β2-Adrenergic Receptor-Dependent Anti-Fibrotic Signaling in Cardiomyocytes" International Journal of Molecular Sciences 20, no. 6: 1396. https://doi.org/10.3390/ijms20061396
APA StylePollard, C. M., Desimine, V. L., Wertz, S. L., Perez, A., Parker, B. M., Maning, J., McCrink, K. A., Shehadeh, L. A., & Lymperopoulos, A. (2019). Deletion of Osteopontin Enhances β2-Adrenergic Receptor-Dependent Anti-Fibrotic Signaling in Cardiomyocytes. International Journal of Molecular Sciences, 20(6), 1396. https://doi.org/10.3390/ijms20061396





