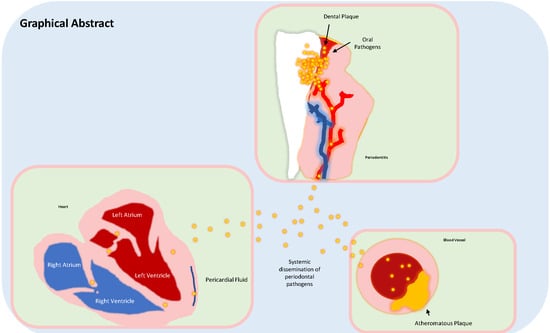
Periodontal Disease: A Risk Factor for Diabetes and Cardiovascular Disease
Abstract
:1. Introduction
2. Pathogenesis of Periodontitis
3. Diabetes and Periodontal Disease
4. Periodontitis as a Risk Factor of Cardiovascular Disease
4.1. Myocardial Infarction
4.2. Endothelial Dysfunction
4.3. Peripheral Artery Disease (PAD)
4.4. Stroke
4.5. Heart Failure
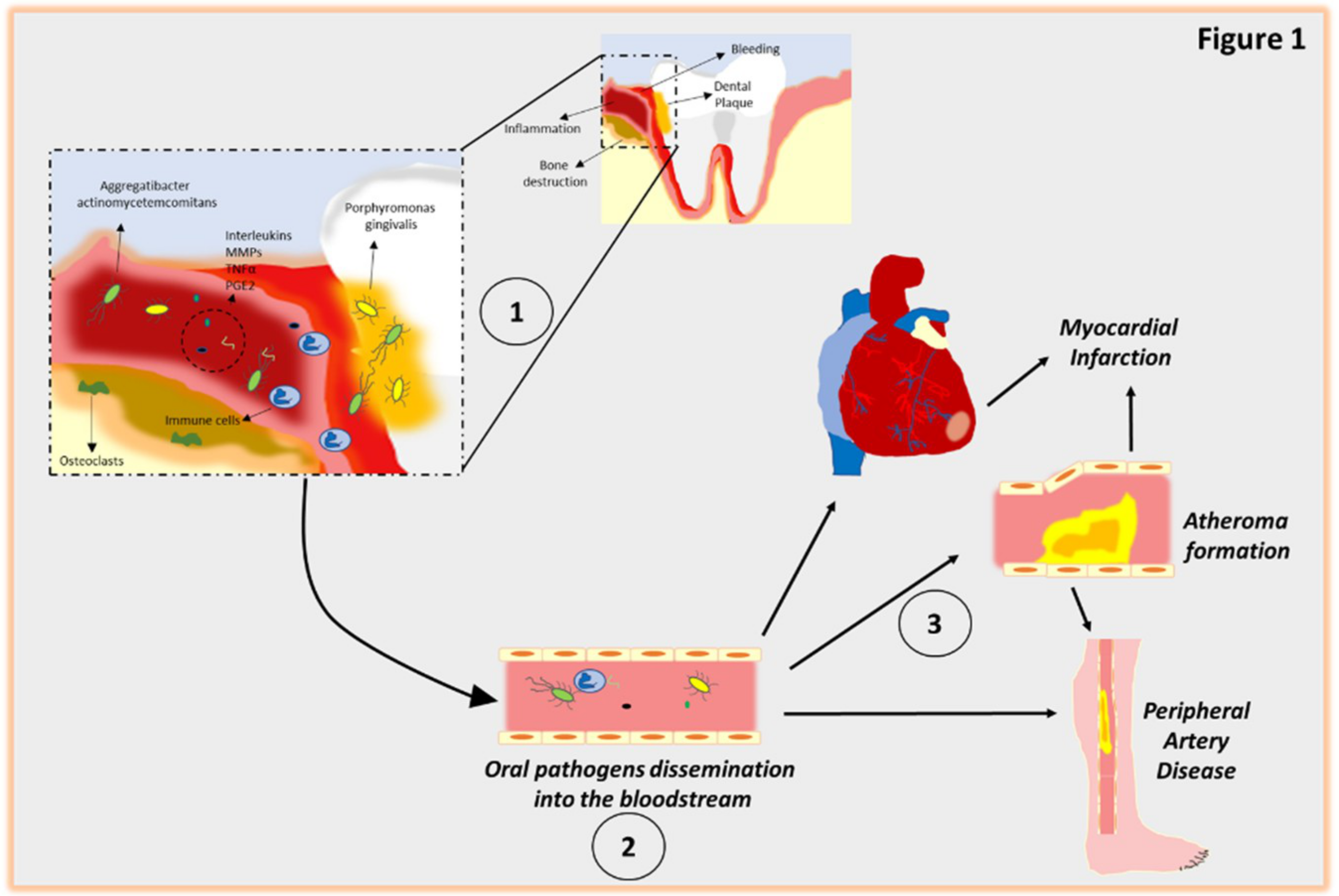
5. Mechanistic Model for the Relationship Between Periodontal Disease and CVD
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CVD | Cardiovascular Disease |
| IL | Interleukin |
| PGE2 | Prostaglandin E2 |
| TNF | Tumor Necrosis Factor |
| MMP | Matrix Metalloproteinase |
| PAD | Peripheral Artery Disease |
| HF | Heart Failure |
| DM | Diabetes Mellitus |
| HSP | Heat shock protein |
| CRP | C-reactive protein |
References
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. (Qassim) 2017, 11, 72–80. [Google Scholar]
- Sudhakara, P.; Gupta, A.; Bhardwaj, A.; Wilson, A. Oral Dysbiotic Communities and Their Implications in Systemic Diseases. Dent. J. (Basel) 2018, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Zhang, X.; Lu, Z.; Li, Y.; Lopes-Virella, M.F.; Yu, H.; Haycraft, C.J.; Li, Q.; Kirkwood, K.L.; Huang, Y. Simvastatin inhibits lipopolysaccharide-induced osteoclastogenesis and reduces alveolar bone loss in experimental periodontal disease. J. Periodontal Res. 2014, 49, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Neely, A.L.; Holford, T.R.; Loe, H.; Anerud, A.; Boysen, H. The natural history of periodontal disease in humans: Risk factors for tooth loss in caries-free subjects receiving no oral health care. J. Clin. Periodontol. 2005, 32, 984–893. [Google Scholar] [CrossRef]
- Monsarrat, P.; Blaizot, A.; Kémoun, P.; Ravaud, P.; Nabet, C.; Sixou, M.; Vergnes, J.N. Clinical research activity in periodontal medicine: A systematic mapping of trial registers. J. Clin. Periodontol. 2016, 43, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Louhelainen, A.M.; Aho, J.; Tuomisto, S.; Aittoniemi, J.; Vuento, R.; Karhunen, P.J.; Pessi, T. Oral bacterial DNA findings in pericardial fluid. J. Oral Microbiol. 2014, 6, 25835. [Google Scholar] [CrossRef]
- Nakano, K.; Inaba, H.; Nomura, R.; Nemoto, H.; Takeda, M.; Yoshioka, H.; Matsue, H.; Takahashi, T.; Taniguchi, K.; Amano, A.; et al. Detection of cariogenic Streptococcus mutans in extirpated heart valve and atheromatous plaque specimens. J. Clin. Microbiol. 2006, 44, 3313–3317. [Google Scholar] [CrossRef]
- Moreno, S.; Parra, B.; Botero, J.E.; Moreno, F.; Vásquez, D.; Fernández, H.; Alba, S.; Gallego, S.; Castillo, G.; Contreras, A. Periodontal microbiota and microorganisms isolated from heart valves in patients undergoing valve replacement surgery in a clinic in Cali, Colombia. Biomedica 2017, 37, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Kozarov, E.; Sweier, D.; Shelburne, C.; Progulske-Fox, A.; Lopatin, D. Detection of bacterial DNA in atheromatous plaques by quantitative PCR. Microbes Infect. 2006, 8, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Cavrini, F.; Sambri, V.; Moter, A.; Servidio, D.; Marangoni, A.; Montebugnoli, L.; Foschi, F.; Prati, C.; Di Bartolomeo, R.; Cevenini, R. Molecular detection of Treponema denticola and Porphyromonas gingivalis in carotid and aortic atheromatous plaques by fish: Report of two cases. J. Med. Microbiol. 2005, 54, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Ishihara, K.; Nakagawa, T.; Hirayama, A.; Inayama, Y. Detection of Treponema denticola in atherosclerotic lesions. J. Clin. Microbiol. 2001, 39, 1114–1117. [Google Scholar] [CrossRef]
- Marcelino, S.L.; Gaetti-Jardim, E.; Nakano, V.; Canônico, L.A.; Nunes, F.D.; Lotufo, R.F.; Pustiglioni, F.E.; Romito, G.A.; Avila-Campos, M.J.; Pessi, T.; et al. Bacterial signatures in thrombus aspirates of patients with myocardial infarction. Circulation 2013, 127, 1219–1228. [Google Scholar]
- Ziebolz, D.; Jahn, C.; Pegel, J.; Semper-Pinnecke, E.; Mausberg, R.F.; Waldmann-Beushausen, R.; Schöndube, F.A.; Danner, B.C. Periodontal bacteria DNA findings in human cardiac tissue—Is there a link of periodontitis to heart valve disease? Int. J. Cardiol. 2018, 251, 74–79. [Google Scholar] [CrossRef]
- Genco, R.J.; Grossi, S.G.; Ho, A.; Nishimura, F.; Murayama, Y. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J. Periodontol. 2005, 76, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.D.; Offenbacher, S. Systemic effects of periodontitis: Epidemiology of periodontal disease and cardiovascular disease. J. Periodontol. 2005, 76, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Carrizales-Sepúlveda, E.F.; Ordaz-Farías, A.; Vera-Pineda, R.; Flores-Ramírez, R. Periodontal Disease, Systemic Inflammation and the Risk of Cardiovascular Disease. Heart Lung Circ. 2018, 27, 1327–1334. [Google Scholar] [CrossRef]
- Janket, S.-J.; Baird, A.; Chuang, S.; Jones, J.A. Meta-analysis of periodontal disease and risk of coronary heart disease and stroke. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2003, 95, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Khader, Y.S.; Albashaireh, Z.S.M.; Alomari, M.A. Periodontal diseases and the risk of coronary heart and cerebrovascular diseases: A meta-analysis. J. Periodontol. 2004, 75, 1046–1153. [Google Scholar] [CrossRef] [PubMed]
- Seymour, G.J.; Ford, P.J.; Cullinan, M.P.; Leishman, S.; Yamazaki, K. Relationship between periodontal infections and systemic disease. Clin. Microbiol. Infect. 2007, 13 (Suppl. 4), 3–10. [Google Scholar] [CrossRef]
- Santos, C.M.; Lira-Junior, R.; Fischer, R.G.; Santos, A.P.; Oliveira, B.H. Systemic Antibiotics in Periodontal Treatment of Diabetic Patients: A Systematic Review. PLoS ONE 2015, 10, e0145262. [Google Scholar] [CrossRef] [PubMed]
- Guzman, S.; Karima, M.; Wang, H.Y.; van Dyke, T.E. Association between interleukin—1 genotype and periodontal disease in a diabetic population. J. Periodontol. 2003, 74, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.; Hayes, C.; Taylor, G.W. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent. Oral Epidemiol. 2002, 30, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Darveau, R.P. Periodontitis: A polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010, 8, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Slots, J. Periodontology: Past, present, perspectives. Periodontol 2000 2013, 62, 7–19. [Google Scholar] [CrossRef]
- Lourenco, T.G.; Heller, D.; Silva-Boghossian, C.M.; Cotton, S.L.; Paster, B.J.; Colombo, A.P. Microbial signature profiles of periodontally healthy and diseased patients. J. Clin. Periodontol. 2014, 41, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Shaddox, L.M.; Huang, H.; Lin, T.; Hou, W.; Harrison, P.L.; Aukhil, I.; Walker, C.B.; Klepac-Ceraj, V.; Paster, B.J. Microbiological Characterization in Children with Aggressive Periodontitis. J. Dent. Res. 2012, 91, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, H.H.; Nichols, F.C.; Miyasaki, K.T. The role of the cell-mediated immune response to Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in periodontitis. Periodontol 2000 1999, 20, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Hasturk, H.; Kantarci, A. Activation and resolution of periodontal inflammation and its systemic impact. Periodontol 2000. 2015, 69, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Cekici, A.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000 2014, 64, 57–80. [Google Scholar] [CrossRef]
- Hajishengallis, G. Immunomicrobial pathogenesis of periodontitis: Keystones, pathobionts, and host response. Trends Immunol. 2014, 35, 3–11. [Google Scholar] [CrossRef]
- Franco, C.; Patricia, H.R.; Timo, S.; Claudia, B.; Marcela, H. Matrix Metalloproteinases as Regulators of Periodontal Inflammation. Int. J. Mol. Sci. 2017, 18, 440. [Google Scholar] [CrossRef] [PubMed]
- Rengo, G.; Pagano, G.; Paolillo, S.; de Lucia, C.; Femminella, G.D.; Liccardo, D.; Cannavo, A.; Formisano, R.; Petraglia, L.; Komici, K.; et al. Impact of diabetes mellitus on lymphocyte GRK2 protein levels in patients with heart failure. Eur. J. Clin. Investig. 2015, 45, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Belting, S.M.; Hiniker, J.J.; Dummett, C.O. Influence of diabetes mellitus on the severity of periodontal disease. J. Periodontol. 1964, 35, 476–480. [Google Scholar] [CrossRef]
- Blasco-Baque, V.; Garidou, L.; Pomié, C.; Escoula, Q.; Loubieres, P.; Le Gall-David, S.; Lemaitre, M.; Nicolas, S.; Klopp, P.; Waget, A.; et al. Periodontitis induced by Porphyromonas gingivalis drives periodontal microbiota dysbiosis and insulin resistance via an impaired adaptive immune response. Gut 2017, 66, 872–885. [Google Scholar] [PubMed]
- Liu, Y.; Zhang, Q.J. Periodontitis aggravated pancreatic β-cell dysfunction in diabetic mice through interleukin-12 regulation on Klotho. Diabetes Investig. 2016, 7, 303–311. [Google Scholar] [CrossRef]
- Engebretson, S.; Kocher, T. Evidence that periodontal treatment improves diabetes outcomes: A systematic review and meta-analysis. J. Clin. Periodontol. 2013, 40, S153–S163. [Google Scholar] [CrossRef] [PubMed]
- Teshome, A.; Yitayeh, A. The effect of periodontal therapy on glycemic control and fasting plasma glucose level in type 2 diabetic patients: Systematic review and meta-analysis. BMC Oral Health 2016, 17, 31. [Google Scholar] [CrossRef] [PubMed]
- Faggion, C.M., Jr.; Cullinan, M.P.; Atieh, M. An overview of systematic reviews on the effectiveness of periodontal treatment to improve glycaemic control. J. Periodontal Res. 2016, 51, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Artese, H.P.; Foz, A.M.; Rabelo Mde, S.; Gomes, G.H.; Orlandi, M.; Suvan, J.; D’Aiuto, F.; Romito, G.A. Periodontal therapy and systemic inflammation in type 2 diabetes mellitus: A meta-analysis. PLoS ONE 2015, 10, e0128344. [Google Scholar] [CrossRef]
- De Miguel-Infante, A.; Martinez-Huedo, M.A.; Mora-Zamorano, E.; Hernández-Barrera, V.; Jiménez-Trujillo, I.; de Burgos-Lunar, C.; Cardenas Valladolid, J.; Jiménez-García, R.; Lopez-de-Andrés, A. Periodontal disease in adults with diabetes, prevalence and risk factors. Results of an observational study. Int. J. Clin. Pract. 2018, e13294. [Google Scholar] [CrossRef]
- Salvi, G.E.; Kandylaki, M.; Troendle, A.; Persson, G.R.; Lang, N.P. Experimental gingivitis in type 1 diabetics: A controlled clinical and microbiological study. J. Clin. Periodontol. 2005, 32, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Ardakani, M.R.; Moeintaghavi, A.; Haerian, A.; Ardakani, M.A.; Hashemzadeh, M. Correlation between levels of sulcular and capillary blood glucose. J. Contemp. Dent. Pract. 2009, 10, 10–17. [Google Scholar]
- Sakallioglu, E.E.; Lutfioglu, M.; Sakallioglu, U.; Diraman, E.; Keskiner, I. Fluid dynamics of gingiva in diabetic and systemically healthy periodontitis patients. Arch. Oral Biol. 2008, 53, 646–651. [Google Scholar] [CrossRef]
- Salvi, G.E.; Franco, L.M.; Braun, T.M.; Lee, A.; Persson, G.R.; Lang, N.P.; Giannobile, W.V. Pro-inflammatory biomarkers during experimental gingivitis in patients with type 1 diabetes mellitus: A proof-of-concept study. J. Clin. Periodontol. 2010, 37, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Engebretson, S.; Chertog, R.; Nichols, A.; Hey-Hadavi, J.; Celenti, R.; Grbic, J. Plasma levels of tumour necrosis factor-alpha in patients with chronic periodontitis and type 2 diabetes. J. Clin. Periodontol. 2007, 34, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Noack, B.; Genco, R.J.; Trevisan, M.; Grossi, S.; Zambon, J.J.; de Nardin, E. Periodontal infections contribute to elevated systemic C-reactive protein level. J. Periodontol. 2001, 72, 1221–1227. [Google Scholar] [CrossRef]
- Loos, B.G.; Craandiji, J.; Hoek, F.J.; Wertheim-van Dillen, P.M.E.; van der Velden, U. C-reactive protein and other mark- ers of systemic inflammation in relation to cardiovascular diseases are elevated in periodontitis. J. Periodontol. 2000, 71, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Trevisan, M.; Genco, R.J.; Falkner, K.L.; Dorn, J.P.; Sempos, C.T. Examination of the relation between periodontal health status and cardiovascular risk factors: Serum total and high density lipoprotein cholesterol, C-reactive pro-tein, and plasma fibrinogen. Am. J. Epidemiol. 2000, 151, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wei, B.; Li, J.; Liu, F.; Xuan, D.; Xie, B.; Zhang, J. Association of periodontal parameters with metabolic level and systemic inflammatory markers in patients with type 2 diabetes. J. Periodontol. 2010, 81, 364–371. [Google Scholar] [CrossRef]
- Chen, L.; Luo, G.; Xuan, D.; Wei, B.; Liu, F.; Li, J.; Zhang, J. Effects of non-surgical periodontal treatment on clinical response, serum inflammatory parameters, and metabolic control in patients with type 2 diabetes: A randomized study. J. Periodontol. 2012, 83, 435–443. [Google Scholar] [CrossRef]
- Quintero, A.J.; Chaparro, A.; Quirynen, M.; Ramirez, V.; Prieto, D.; Morales, H.; Prada, P.; Hernández, M.; Sanz, A. Effect of two periodontal treatment modalities in patients with uncontrolled type 2 diabetes mellitus: A randomized clinical trial. J. Clin. Periodontol. 2018, 45, 1098–1106. [Google Scholar] [CrossRef]
- Grellmann, A.P.; Sfreddo, C.S.; Maier, J.; Lenzi, T.L.; Zanatta, F.B. Systemic antimicrobials adjuvant to periodontal therapy in diabetic subjects: A meta-analysis. J. Clin. Periodontol. 2016, 43, 250–260. [Google Scholar] [CrossRef]
- Lira Junior, R.; Santos, C.M.M.; Oliveira, B.H.; Fischer, R.G.; Santos, A.P.P. Effects on HbA1c in diabetic patients of adjunctive use of systemic antibiotics in nonsurgical periodontal treatment: A systematic review. J. Dent. 2017, 66, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.S.; Patil, V.P.; Gokhale, N.; Acharya, A.; Kangokar, P. Chronic Periodontitis in Type 2 Diabetes Mellitus: Oxidative Stress as a Common Factor in Periodontal Tissue Injury. J. Clin. Diagn. Res. 2016, 10, BC12–BC16. [Google Scholar] [CrossRef]
- Allen, E.M.; Matthews, J.B.; O’ Halloran, D.J.; Griffiths, H.R.; Chapple, I.L. Oxidative and inflammatory status in Type 2 diabetes patients with periodontitis. J. Clin. Periodontol. 2011, 38, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Polak, D.; Shapira, L. An update on the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J. Clin. Periodontol. 2018, 45, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Kjellström, B.; Gustafsson, A.; Nordendal, E.; Norhammar, A.; Nygren, Å.; Näsman, P.; Rydén, L.; Åsberg, M. PAROKRANK steering committee. Symptoms of depression and their relation to myocardial infarction and periodontitis. Eur. J. Cardiovasc. Nurs. 2017, 16, 468–474. [Google Scholar] [CrossRef] [PubMed]
- De Nardin, E. The role of inflammatory and immunological mediators in periodontitis and cardiovascular disease. Ann. Periodontol. 2001, 6, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Shrihari, T.G. Potential correlation between periodontitis and coronary heart disease--an overview. Gen. Dent. 2012, 60, 20–24. [Google Scholar]
- Wożakowska-Kapłon, B.; Włosowicz, M.; Gorczyca-Michta, I.; Górska, R. Oral health status and the occurrence and clinical course of myocardial infarction in hospital phase: A case-control study. Cardiol. J. 2013, 20, 370–377. [Google Scholar] [CrossRef]
- Kodovazenitis, G.; Pitsavos, C.; Papadimitriou, L.; Vrotsos, I.A.; Stefanadis, C.; Madianos, P.N. Association between periodontitis and acute myocardial infarction: A case-control study of a nondiabetic population. J. Periodontal Res. 2014, 49, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, S.; Toyokawa, S.; Miyoshi, Y.; Suyama, Y.; Inoue, K.; Kobayashi, Y. Five-year follow-up study of the association between periodontal disease and myocardial infarction among Japanese male workers: MY Health Up Study. J. Public Health (Oxf.) 2015, 37, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Rydén, L.; Buhlin, K.; Ekstrand, E.; de Faire, U.; Gustafsson, A.; Holmer, J.; Kjellström, B.; Lindahl, B.; Norhammar, A.; Nygren, Å.; et al. Periodontitis Increases the Risk of a First Myocardial Infarction: A Report From the PAROKRANK Study. Circulation 2016, 133, 576–578. [Google Scholar] [PubMed]
- Mattila, K.J.; Nieminen, M.S.; Valtonen, V.V.; Rasi, V.P.; Kesäniemi, Y.A.; Syrjälä, S.L.; Jungell, P.S.; Isoluoma, M.; Hietaniemi, K.; Jokinen, M.J. Association between dental health and acute myocardial infarction. BMJ 1989, 298, 779–781. [Google Scholar] [CrossRef]
- Willershausen, B.; Kasaj, A.; Willershausen, I.; Zahorka, D.; Briseño, B.; Blettner, M.; Genth-Zotz, S.; Münzel, T. Association between chronic dental infection and acute myocardial infarction. J. Endod. 2009, 35, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Jansson, L.; Lavstedt, S.; Frithiof, L.; Theobald, H. Relationship between oral health and mortality in cardiovascular diseases. J. Clin. Periodontol. 2001, 28, 762–768. [Google Scholar] [CrossRef]
- Akamatsu, Y.; Yamamoto, T.; Yamamoto, K.; Oseko, F.; Kanamura, N.; Imanishi, J.; Kita, M. Porphyromonas gingivalis induces myocarditis and/or myocardial infarction in mice and IL-17A is involved in pathogenesis of these diseases. Arch. Oral Biol. 2011, 56, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Hujoel, P.P.; Drangsholt, M.; Spiekerman, C.; Derouen, T.A. Examining the link between coronary heart disease and the elimination of chronic dental infections. J. Am. Dent. Assoc. 2001, 132, 883–889. [Google Scholar] [CrossRef]
- Howell, T.H.; Ridker, P.M.; Ajani, U.A.; Hennekens, C.H.; Christen, W.G. Periodontal disease and risk of subsequent cardiovascular disease in U.S. male physicians. J. Am. Coll. Cardiol. 2001, 37, 445–450. [Google Scholar] [CrossRef]
- Sidhu, R.K. Association between Acute Myocardial Infarction and Periodontitis: A Review of the Literature. J. Int. Acad. Periodontol. 2016, 18, 23–33. [Google Scholar]
- Lockhart, P.B.; Bolger, A.F.; Papapanou, P.N.; Osinbowale, O.; Trevisan, M.; Levison, M.E.; Taubert, K.A.; Newburger, J.W.; Gornik, H.L.; Gewitz, M.H.; et al. American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, Council on Epidemiology and Prevention, Council on Peripheral Vascular Disease, and Council on Clinical Cardiology. Periodontal disease and atherosclerotic vascular disease: Does the evidence support an independent association? A scientific statement from the American Heart Association. Circulation 2012, 125, 2520–2544. [Google Scholar] [PubMed]
- Park, K.H.; Park, W.J. Endothelial Dysfunction: Clinical Implications in Cardiovascular Disease and Therapeutic Approaches. J. Korean Med. Sci. 2015, 30, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Radomski, M.W.; Palmer, R.M.; Moncada, S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br. J. Pharmacol. 1987, 92, 181. [Google Scholar] [CrossRef] [PubMed]
- Garg, U.C.; Hassid, A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J. Clin. Investig. 1989, 83, 1774. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Goto, C.; Jitsuiki, D.; Umemura, T.; Nishioka, K.; Hidaka, T.; Takemoto, H.; Nakamura, S.; Soga, J.; Chayama, K.; et al. Periodontal infection is associated with endothelial dysfunction in healthy subjects and hypertensive patients. Hypertension 2008, 51, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Moura, M.F.; Navarro, T.P.; Silva, T.A.; Cota, L.O.M.; Soares Dutra Oliveira, A.M.; Costa, F.O. Periodontitis and Endothelial Dysfunction: Periodontal Clinical Parameters and Levels of Salivary Markers Interleukin-1β, Tumor Necrosis Factor-α, Matrix Metalloproteinase-2, Tissue Inhibitor of Metalloproteinases-2 Complex, and Nitric Oxide. J. Periodontol. 2017, 88, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.C.; DalBó, S.; Striechen, T.M.; Farias, J.M.; Olchanheski, L.R., Jr.; Mendes, R.T.; Vellosa, J.C.; Fávero, G.M.; Sordi, R.; Assreuy, J.; et al. Experimental periodontitis promotes transient vascular inflammation and endothelial dysfunction. Arch. Oral Biol. 2013, 58, 1187–1198. [Google Scholar] [CrossRef] [PubMed]
- Campi, P.; Herrera, B.S.; de Jesus, F.N.; Napolitano, M.; Teixeira, S.A.; Maia-Dantas, A.; Spolidorio, L.C.; Akamine, E.H.; Mayer, M.P.A.; de Carvalho, M.H.C.; et al. Endothelial dysfunction in rats with ligature-induced periodontitis: Participation of nitric oxide and cycloxygenase-2-derived products. Arch. Oral Biol. 2016, 63, 66–74. [Google Scholar] [CrossRef]
- Maekawa, T.; Takahashi, N.; Honda, T.; Yonezawa, D.; Miyashita, H.; Okui, T.; Tabeta, K.; Yamazaki, K. Porphyromonas gingivalis antigens and interleukin-6 stimulate the production of monocyte chemoattractant protein-1 via the upregulation of early growth response-1 transcription in human coronary artery endothelial cells. J. Vasc. Res. 2010, 47, 346–354. [Google Scholar] [CrossRef]
- Bhagat, K.; Moss, R.; Collier, J.; Vallance, P. Endothelial “stunning” following a brief exposure to endotoxin: A mechanism to link infection and infarction? Cardiovasc. Res. 1996, 32, 822–829. [Google Scholar]
- Hajishengallis, G.; Wang, M.; Harokopakis, E.; Triantafilou, M.; Triantafilou, K. Porphyromonas gingivalis fimbriae proactively modulate beta2 integrin adhesive activity and promote binding to and internalization by macrophages. Infect. Immun. 2006, 74, 5658–5666. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Moser, B.; Roth-Walter, F.; Giacona, M.B.; Harja, E.; Papapanou, P.N.; Schmidt, A.M.; Lalla, E. Infection with a periodontal pathogen increases mononuclear cell adhesion to human aortic endothelial cells. Atherosclerosis 2007, 190, 271–281. [Google Scholar] [CrossRef]
- Hashizume, T.; Kurita-Ochiai, T.; Yamamoto, M. Porphyromonas gingivalis stimulates monocyte adhesion to human umbilical vein endothelial cells. FEMS Immunol. Med. Microbiol. 2011, 62, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Kolattukudy, P.E. Role of MCP-1 in cardiovascular disease: Molecular mechanisms and clinical implications. Clin. Sci. (Lond.) 2009, 117, 95–109. [Google Scholar] [CrossRef]
- Nakamura, N.; Yoshida, M.; Umeda, M.; Huang, Y.; Kitajima, S.; Inoue, Y.; Ishikawa, I.; Iwai, T. Extended exposure of lipopolysaccharide fraction from Porphyromonas gingivalis facilitates mononuclear cell adhesion to vascular endothelium via Toll-like receptor-2 dependent mechanism. Atherosclerosis 2008, 196, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Ansai, T.; Yamamoto, E.; Awano, S.; Yu, W.; Turner, A.J.; Takehara, T. Effects of periodontopathic bacteria on the expression of endothelin-1 in gingival epithelial cells in adult periodontitis. Clin. Sci. (Lond.) 2002, 103, 327S–331S. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H. Endothelial dysfunction and vascular disease—a 30th anniversary update. Acta Physiol. (Oxf.) 2017, 219, 22–96. [Google Scholar] [CrossRef] [PubMed]
- Seinost, G.; Wimmer, G.; Skerget, M.; Thaller, E.; Brodmann, M.; Gasser, R.; Bratschko, R.O.; Pilger, E. Periodontal treatment improves endothelial dysfunction in patients with severe periodontitis. Am. Heart J. 2005, 149, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Elter, J.R.; Hinderliter, A.L.; Offenbacher, S.; Beck, J.D.; Caughey, M.; Brodala, N.; Madianos, P.N. The effects of periodontal therapy on vascular endothelial function: A pilot trial. Am. Heart J. 2006, 151, 47. [Google Scholar] [CrossRef]
- Tonetti, M.S.; D’Aiuto, F.; Nibali, L.; Donald, A.; Storry, C.; Parkar, M.; Suvan, J.; Hingorani, A.D.; Vallance, P.; Deanfield, J. Treatment of periodontitis and endothelial function. N. Engl. J. Med. 2007, 356, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, M.; Suvan, J.; Petrie, A.; Donos, N.; Masi, S.; Hingorani, A.; Deanfield, J.; D’Aiuto, F. Association between periodontal disease and its treatment, flow-mediated dilatation and carotid intima-media thickness: A systematic review and meta-analysis. Atherosclerosis 2014, 236, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Houcken, W.; Teeuw, W.J.; Bizzarro, S.; Alvarez Rodriguez, E.; Mulders, T.A.; van den Born, B.J.; Loos, B.G. Arterial stiffness in periodontitis patients and controls. A case–control and pilot intervention study. J. Hum. Hypertens. 2016, 30, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Vidal, F.; Cordovil, I.; Figueredo, C.M.; Fischer, R.G. Non-surgical periodontal treatment reduces cardiovascular risk in refractory hypertensive patients: A pilot study. J. Clin. Periodontol. 2013, 40, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Cannavo, A.; Liccardo, D.; Lymperopoulos, A.; Gambino, G.; D’Amico, M.L.; Rengo, F.; Koch, W.J.; Leosco, D.; Ferrara, N.; Rengo, G. β Adrenergic Receptor Kinase C-Terminal Peptide Gene-Therapy Improves β2-Adrenergic Receptor-Dependent Neoangiogenesis after Hindlimb Ischemia. J. Pharmacol. Exp. Ther. 2016, 356, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhao, L.S.; Cai, C.; Shi, Q.; Wen, N.; Xu, J. Association between periodontitis and peripheral artery disease: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2018, 18, 141. [Google Scholar] [CrossRef]
- Chen, Y.W.; Umeda, M.; Nagasawa, T.; Takeuchi, Y.; Huang, Y.; Inoue, Y.; Iwai, T.; Izumi, Y.; Ishikawa, I. Periodontitis may increase the risk of peripheral arterial disease. Eur. J. Vasc. Endovasc. Surg. 2008, 35, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.B.; Shin, M.S.; Han, D.H.; Sukhbaatar, M.; Kim, M.S.; Shin, H.S.; Kim, H.D. Periodontitis is associated with the risk of subclinical atherosclerosis and peripheral arterial disease in Korean adults. Atherosclerosis 2016, 251, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Çalapkorur, M.U.; Alkan, B.A.; Tasdemir, Z.; Akcali, Y.; Saatçi, E. Association of peripheral arterial disease with periodontal disease: Analysis of inflammatory cytokines and an acute phase protein in gingival crevicular fluid and serum. J. Periodontal Res. 2017, 52, 532–539. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e66. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.M.; Pangilinan, P.H., Jr.; Rodriguez, G.M. The stroke rehabilitation paradigm. Phys. Med. Rehabil. Clin. N. Am. 2007, 18, 631–650. [Google Scholar] [CrossRef] [PubMed]
- Leira, Y.; Seoane, J.; Blanco, M.; Rodríguez-Yáñez, M.; Takkouche, B.; Blanco, J.; Castillo, J. Association between periodontitis and ischemic stroke: A systematic review and meta-analysis. Eur. J. Epidemiol. 2017, 32, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Sfyroeras, G.S.; Roussas, N.; Saleptsis, V.G.; Argyriou, C.; Giannoukas, A.D. Association between periodontal disease and stroke. J. Vasc. Surg. 2012, 55, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Lafon, A.; Pereira, B.; Dufour, T.; Rigouby, V.; Giroud, M.; Béjot, Y.; Tubert-Jeannin, S. Periodontal disease and stroke: A meta-analysis of cohort studies. Eur. J. Neurol. 2014, 21, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Pussinen, P.J.; Alfthan, G.; Rissanen, H.; Reunanen, A.; Asikainen, S.; Knekt, P. Antibodies to periodontal pathogens and stroke risk. Stroke 2004, 35, 2020–2023. [Google Scholar] [CrossRef] [PubMed]
- Ghizoni, J.S.; Taveira, L.A.; Garlet, G.P.; Ghizoni, M.F.; Pereira, J.R.; Dionísio, T.J.; Brozoski, D.T.; Santos, C.F.; Sant’Ana, A.C. Increased levels of Porphyromonas gingivalis are associated with ischemic and hemorrhagic cerebrovascular disease in humans: An in vivo study. J. Appl. Oral Sci. 2012, 20, 104–112. [Google Scholar] [CrossRef]
- Hosomi, N.; Aoki, S.; Matsuo, K.; Deguchi, K.; Masugata, H.; Murao, K.; Ichihara, N.; Ohyama, H.; Dobashi, H.; Nezu, T.; et al. Association of serum anti-periodontal pathogen antibody with ischemic stroke. Cerebrovasc. Dis. 2012, 34, 385–392. [Google Scholar] [CrossRef]
- Ponikowski, P.; Anker, S.D.; AlHabib, K.F.; Cowie, M.R.; Force, T.L.; Hu, S.; Jaarsma, T.; Krum, H.; Rastogi, V.; Rohde, L.E.; et al. Heart failure: Preventing disease and death worldwide. ESC Heart Fail. 2014, 1, 4–25. [Google Scholar] [CrossRef] [PubMed]
- Cannavo, A.; Komici, K.; Bencivenga, L.; D’amico, M.L.; Gambino, G.; Liccardo, D.; Ferrara, N.; Rengo, G. GRK2 as a therapeutic target for heart failure. Expert Opin. Ther. Targets 2018, 22, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, H.; Herrmann, K.; Franke, J.; Karimi, A.; Täger, T.; Cebola, R.; Katus, H.A.; Zugck, C.; Frankenstein, L. Periodontitis in Chronic Heart Failure. Tex. Heart Inst. J. 2016, 43, 297–304. [Google Scholar] [CrossRef]
- Wood, N.; Johnson, R.B. The relationship between tomato intake and congestive heart failure risk in periodontitis subjects. J. Clin. Periodontol. 2004, 31, 574–580. [Google Scholar] [CrossRef]
- Schulze-Späte, U.; Mizani, I.; Salaverry, K.R.; Chang, J.; Wu, C.; Jones, M.; Kennel, P.J.; Brunjes, D.L.; Choo, T.H.; Kato, T.S.; et al. Periodontitis and bone metabolism in patients with advanced heart failure and after heart transplantation. ESC Heart Fail. 2017, 4, 169–177. [Google Scholar] [CrossRef]
- Carinci, F.; Martinelli, M.; Contaldo, M.; Santoro, R.; Pezzetti, F.; Lauritano, D.; Candotto, V.; Mucchi, D.; Palmieri, A.; Tagliabue, A.; et al. Focus on periodontal disease and development of endocarditis. J. Biol. Regul. Homeost. Agents 2018, 32, 143–147. [Google Scholar]
- Oliveira, F.A.; Forte, C.P.; Silva, P.G.; Lopes, C.B.; Montenegro, R.C.; Santos, Â.K.; Sobrinho, C.R.; Mota, M.R.; Sousa, F.B.; Alves, A.P. Molecular Analysis of Oral Bacteria in Heart Valve of Patients With Cardiovascular Disease by Real-Time Polymerase Chain Reaction. Medicine (Baltimore) 2015, 94, e2067. [Google Scholar] [CrossRef]
- Sekinishi, A.; Suzuki, J.; Aoyama, N.; Ogawa, M.; Watanabe, R.; Kobayashi, N.; Hanatani, T.; Ashigaki, N.; Hirata, Y.; Nagai, R.; et al. Periodontal pathogen Aggregatibacter actinomycetemcomitans deteriorates pressure overload-induced myocardial hypertrophy in mice. Int. Heart J. 2012, 53, 324–330. [Google Scholar] [CrossRef]
- Kozarov, E.V.; Dorn, B.R.; Shelburne, C.E.; Dunn, W.A., Jr.; Progulske-Fox, A. Human atherosclerotic plaque contains viable invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, e17–e18. [Google Scholar] [CrossRef] [PubMed]
- Lanter, B.B.; Sauer, K.; Davies, D.G. Bacteria present in carotid arterial plaques are found as biofilm deposits which may contribute to enhanced risk of plaque rupture. MBio 2014, 5, e01206–e01214. [Google Scholar] [CrossRef]
- Lanter, B.B.; Davies, D.G. Propionibacterium acnes Recovered from Atherosclerotic Human Carotid Arteries Undergoes Biofilm Dispersion and Releases Lipolytic and Proteolytic Enzymes in Response to Norepinephrine Challenge In Vitro. Infect. Immun. 2015, 83, 3960–3971. [Google Scholar] [CrossRef] [PubMed]
- Snow, D.E.; Everett, J.; Mayer, G.; Cox, S.B.; Miller, B.; Rumbaugh, K.; Wolcott, R.A.; Wolcott, R.D. The presence of biofilm structures in atherosclerotic plaques of arteries from legs amputated as a complication of diabetic foot ulcers. J. Wound Care 2016, 25, S16–S22. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kolltveit, K.M.; Tronstad, L.; Olsen, I. Systemic Diseases Caused by Oral Infection. Clin. Microbiol. Rev. 2000, 13, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Filho, I.S.; Freitas Coelho, J.M.; da Cruz, S.S.; Passos, J.S.; Teixeirade Freitas, C.O.; Aragao Farias, N.S.; Amorim da Silva, R.; Silva Pereira, M.N.; Lima, T.L.; Barreto, M.L. Chronic periodontitis and C-reactive protein levels. J. Periodontol. 2011, 82, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: Moving an inflammatory hypothesis toward consensus. J. Am. Coll. Cardiol. 2007, 49, 2129–2138. [Google Scholar] [CrossRef]
- Kleindienst, R.; Xu, Q.; Willeit, J.; Waldenberger, F.R.; Weimann, S.; Wick, G. Immunology of atherosclerosis: Demonstration of heat shock protein 60 expression and T lymphocytes bearing alpha/beta or gamma/delta receptor in human atherosclerotic lesions. Am. J. Pathol. 1993, 142, 1927–1937. [Google Scholar] [PubMed]
- Loesche, W.J.; Lopatin, D.E. Interactions between periodontal disease, medical diseases and immunity in the older individual. Periodontology 2000 1998, 16, 80–105. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Chiang, C.H.; Huang, C.C.; Chung, C.M.; Chan, W.L.; Huang, P.H.; Lin, S.J.; Chen, J.W.; Leu, H.B. The association of tooth scaling and decreased cardiovascular disease: A nationwide population-based study. Am. J. Med. 2012, 125, 568–575. [Google Scholar] [CrossRef]
- Chou, S.H.; Tung, Y.C.; Lin, Y.S.; Wu, L.S.; Lin, C.P.; Liou, E.J.; Chang, C.J.; Kung, S.; Chu, P.H. Major Adverse Cardiovascular Events in Treated Periodontitis: A Population-Based Follow-Up Study from Taiwan. PLoS ONE 2015, 10, e0130807. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liccardo, D.; Cannavo, A.; Spagnuolo, G.; Ferrara, N.; Cittadini, A.; Rengo, C.; Rengo, G. Periodontal Disease: A Risk Factor for Diabetes and Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 1414. https://doi.org/10.3390/ijms20061414
Liccardo D, Cannavo A, Spagnuolo G, Ferrara N, Cittadini A, Rengo C, Rengo G. Periodontal Disease: A Risk Factor for Diabetes and Cardiovascular Disease. International Journal of Molecular Sciences. 2019; 20(6):1414. https://doi.org/10.3390/ijms20061414
Chicago/Turabian StyleLiccardo, Daniela, Alessandro Cannavo, Gianrico Spagnuolo, Nicola Ferrara, Antonio Cittadini, Carlo Rengo, and Giuseppe Rengo. 2019. "Periodontal Disease: A Risk Factor for Diabetes and Cardiovascular Disease" International Journal of Molecular Sciences 20, no. 6: 1414. https://doi.org/10.3390/ijms20061414
APA StyleLiccardo, D., Cannavo, A., Spagnuolo, G., Ferrara, N., Cittadini, A., Rengo, C., & Rengo, G. (2019). Periodontal Disease: A Risk Factor for Diabetes and Cardiovascular Disease. International Journal of Molecular Sciences, 20(6), 1414. https://doi.org/10.3390/ijms20061414