The Eukaryotic Translation Initiation Factor 4F Complex Restricts Rotavirus Infection via Regulating the Expression of IRF1 and IRF7
Abstract
1. Introduction
2. Results
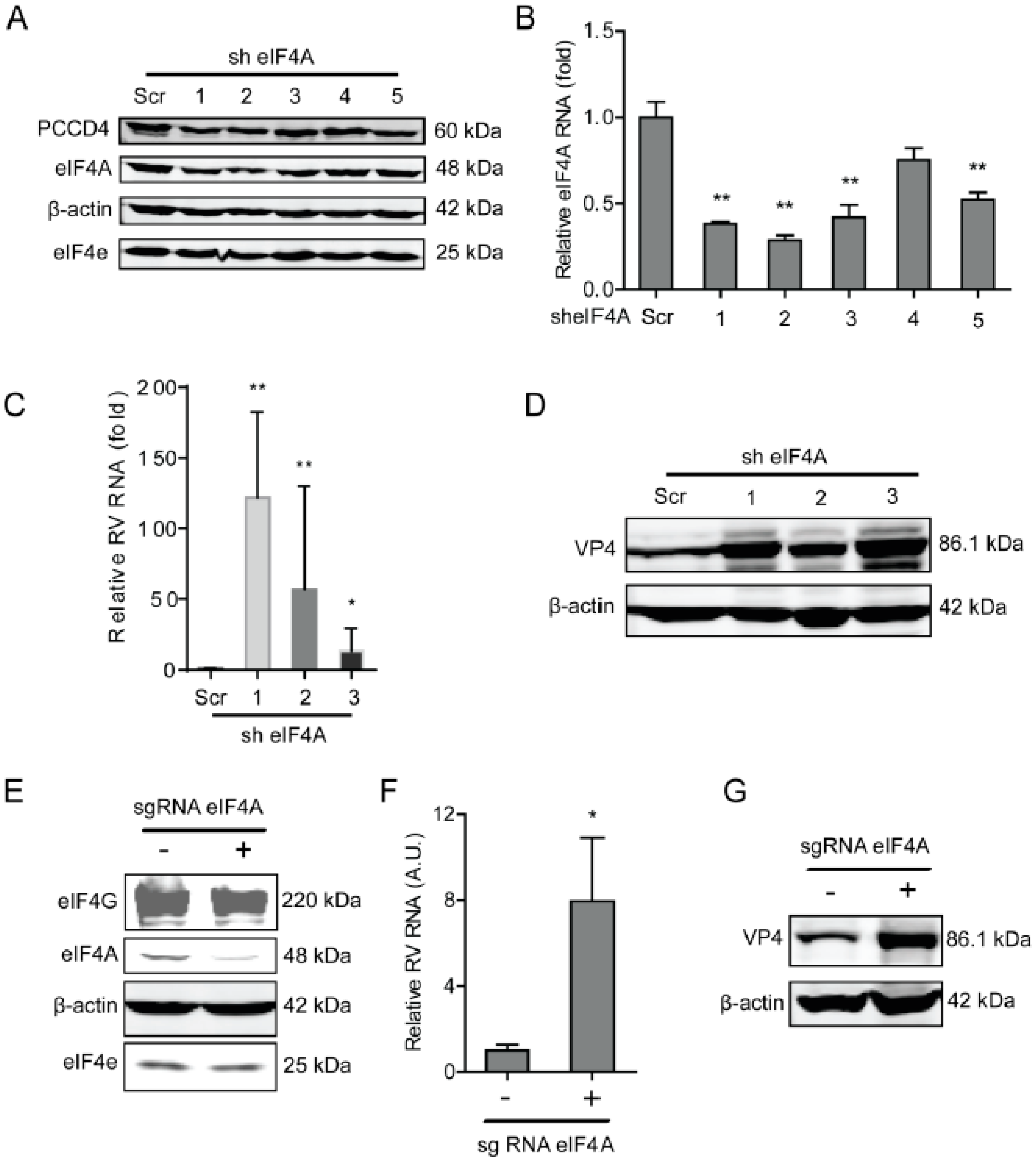
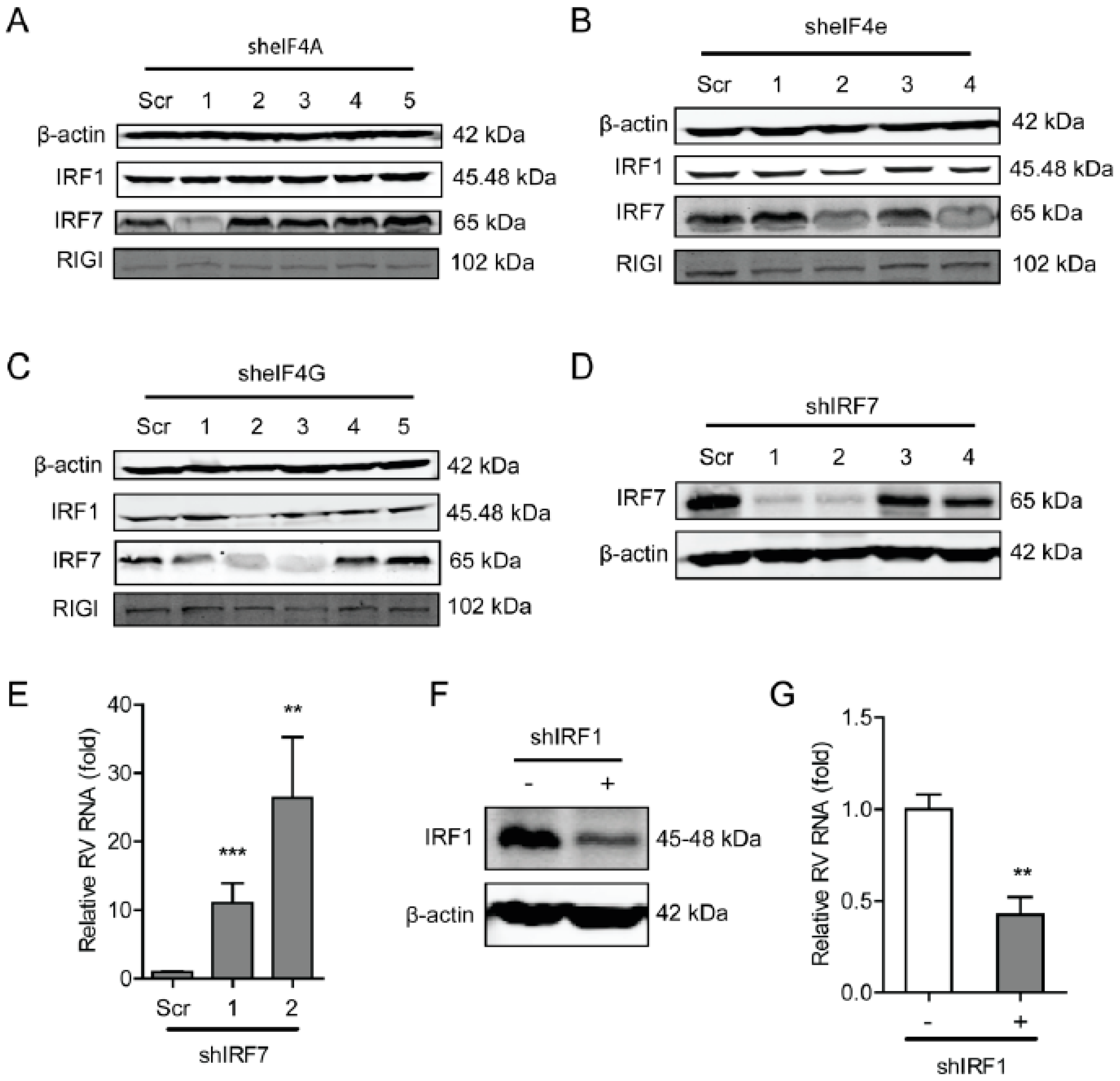
2.1. The eIF4A Inhibits Rotavirus Infection
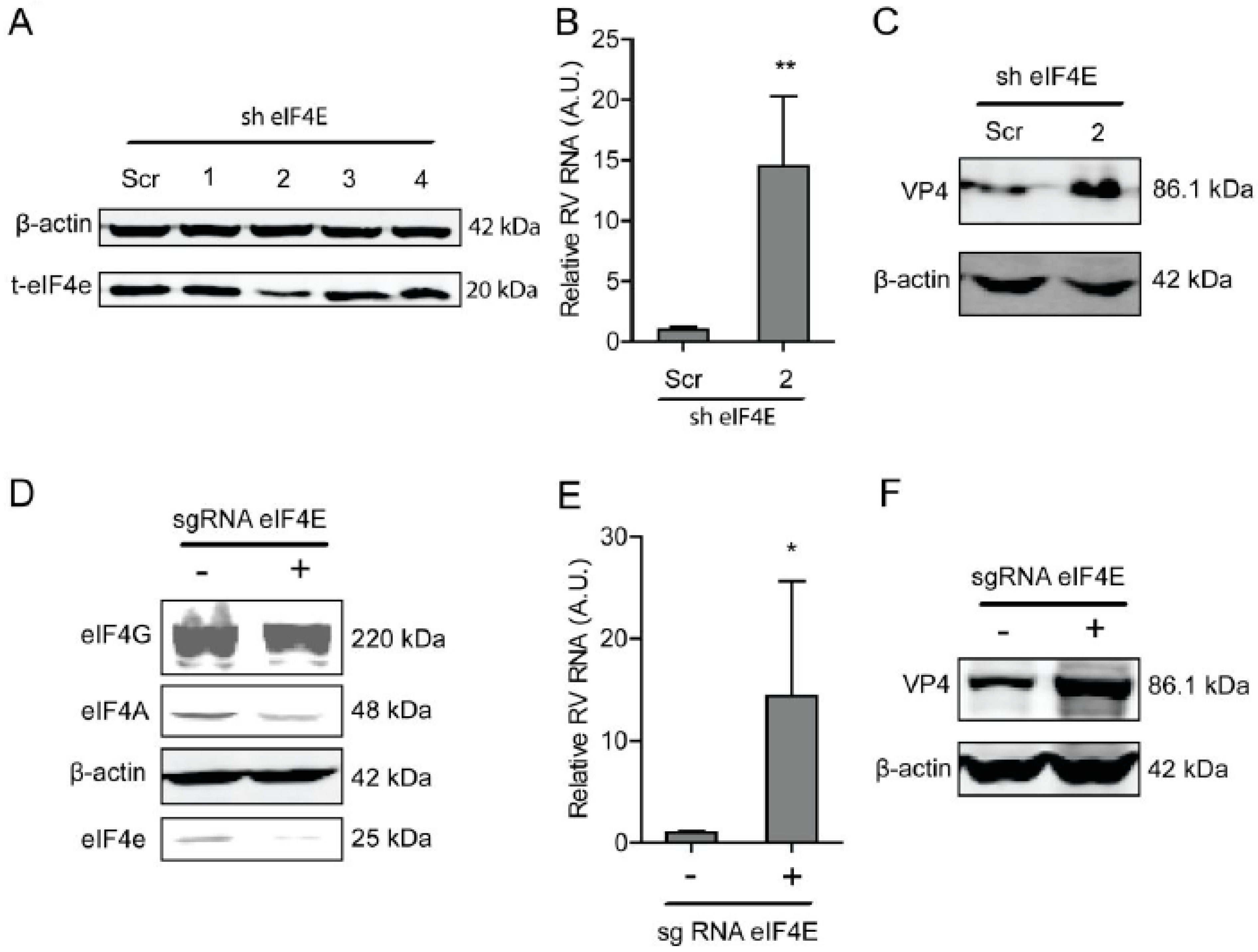
2.2. Blocking eIF4E Promotes Rotavirus Infection
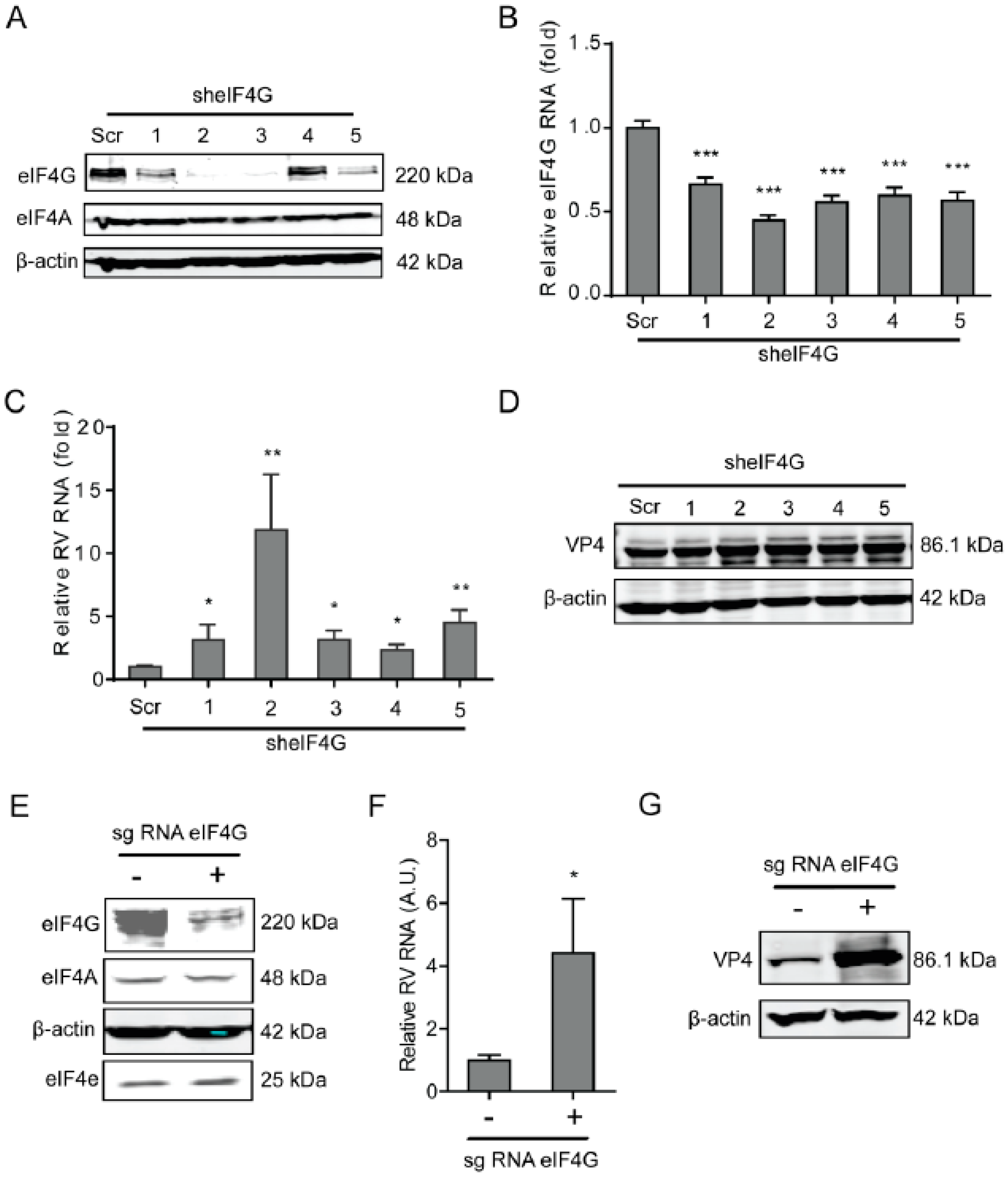
2.3. The eIF4G Suppresses Rotavirus Infection
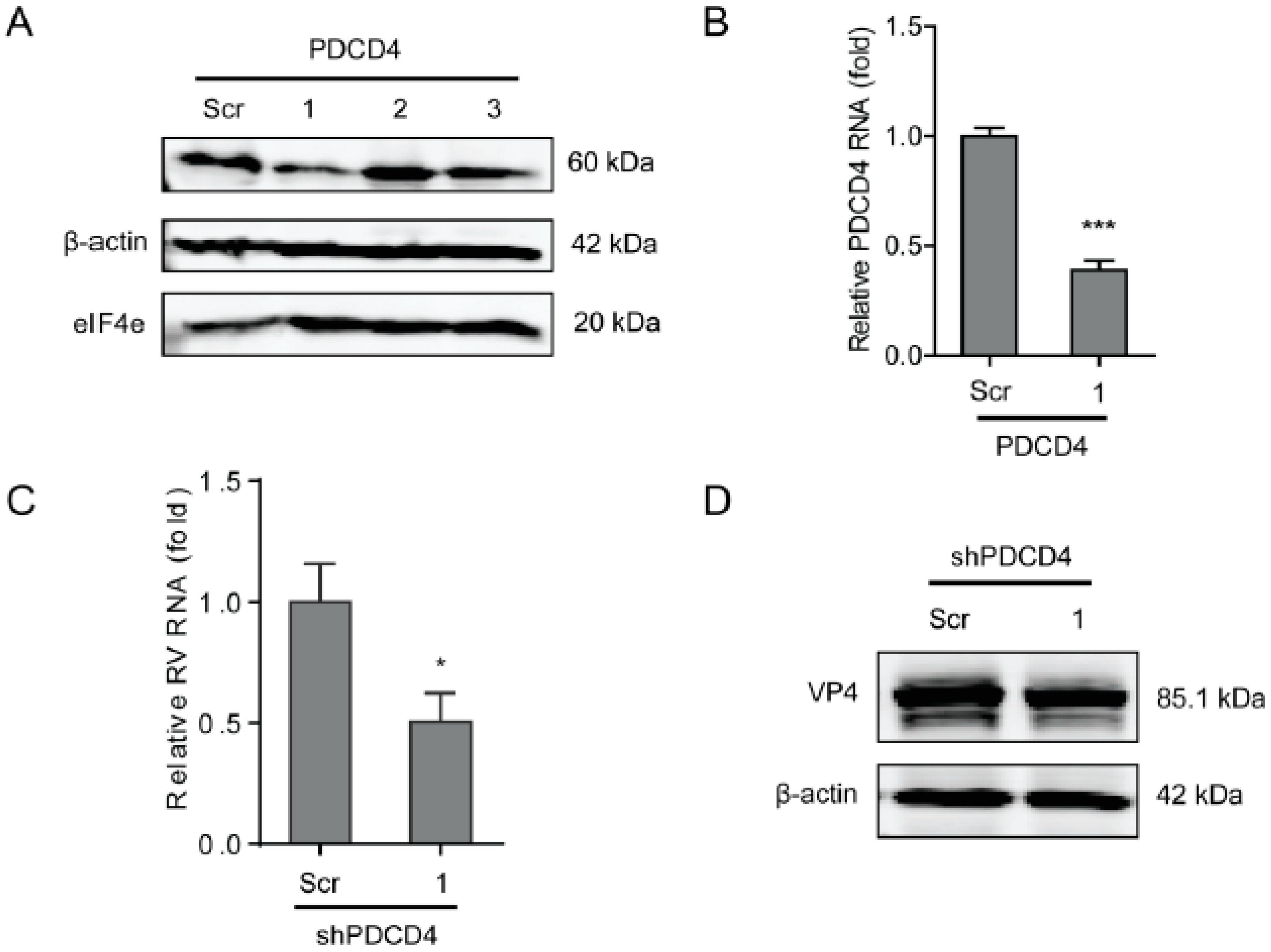
2.4. Programmed Cell Death Protein 4 (PDCD4) Promotes Rotavirus Infection
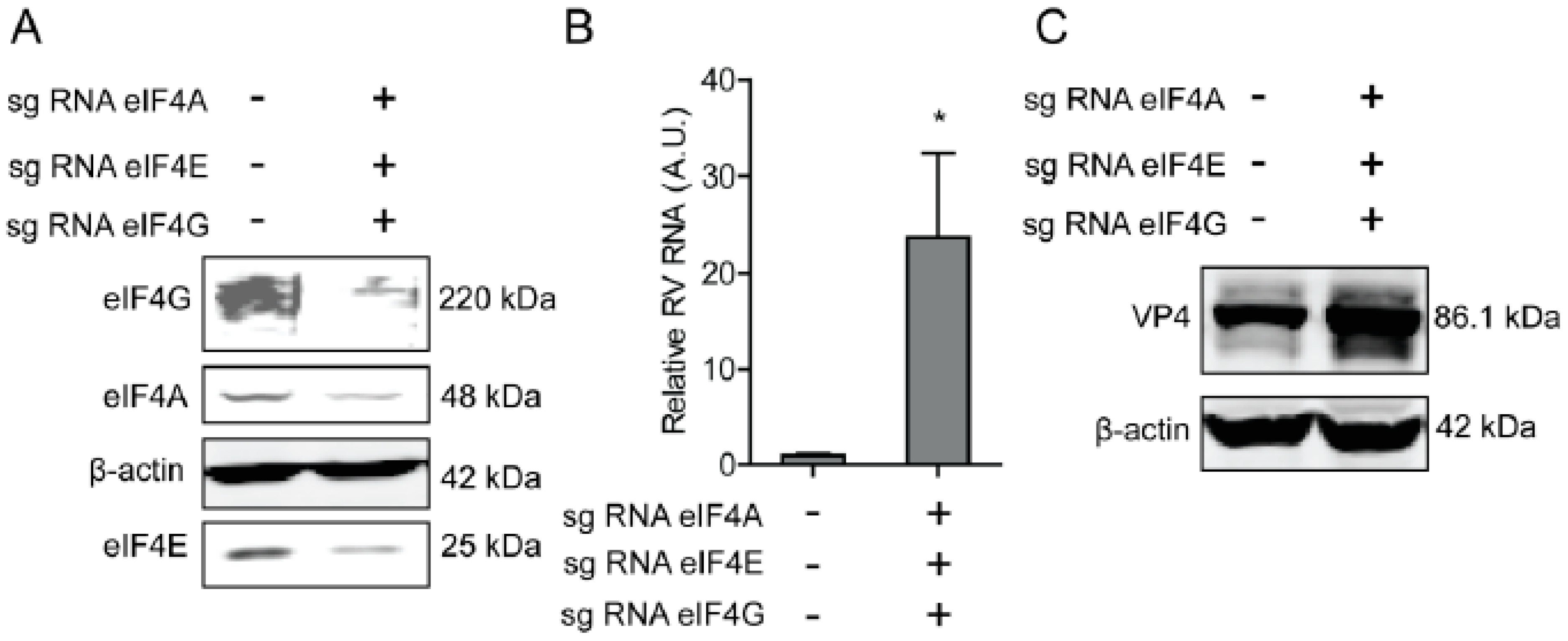
2.5. The eIF4F Complex Inhibits the Level of Antiviral Proteins to Exert Anti-Rotavirus Effect
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Virus
4.2. Inoculation of SA11 Rotavirus in Cells
4.3. Real-Time Quantitative Reverse Transcription PCR (RT-qPCR) Analyses
4.4. Gene Knockdown by shRNA
4.5. Gene Knockout by Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR/Cas9) Assay
4.6. The 3-(4, 5)-Dimethylthiahiazo (-z-y1)-3, 5-di- Phenytetrazoliumromide (MTT) Assay
4.7. Western Blot
4.8. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tate, J.E.; Burton, A.H.; Boschi-Pinto, C.; Steele, A.D.; Duque, J.; Parashar, U.D. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: A systematic review and meta-analysis. Lancet Infect. Dis. 2012, 12, 136–141. [Google Scholar] [CrossRef]
- Tate, J.E.; Burton, A.H.; Boschi-Pinto, C.; Parashar, U.D. Global, Regional, and National Estimates of Rotavirus Mortality in Children <5 Years of Age, 2000–2013. Clin. Infect. Dis. 2016, 62 (Suppl. S2), S96–S105. [Google Scholar]
- Bines, J.E.; Danchin, M.; Jackson, P.; Handley, A.; Watts, E.; Lee, K.J.; West, A.; Cowley, D.; Chen, M.Y.; Barnes, G.L.; et al. Safety and immunogenicity of RV3-BB human neonatal rotavirus vaccine administered at birth or in infancy: A randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2015, 15, 1389–1397. [Google Scholar] [CrossRef]
- Vesikari, T.; Karvonen, A.; Prymula, R.; Schuster, V.; Tejedor, J.C.; Cohen, R.; Meurice, F.; Han, H.H.; Damaso, S.; Bouckenooghe, A. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: Randomised, double-blind controlled study. Lancet 2007, 370, 1757–1763. [Google Scholar] [CrossRef]
- Yin, Y.; Metselaar, H.J.; Sprengers, D.; Peppelenbosch, M.P.; Pan, Q. Rotavirus in organ transplantation: Drug-virus-host interactions. Am. J. Transplant. 2015, 15, 585–593. [Google Scholar] [CrossRef]
- Yin, Y.; Bijvelds, M.; Dang, W.; Xu, L.; van der Eijk, A.A.; Knipping, K.; Tuysuz, N.; Dekkers, J.F.; Wang, Y.; de Jonge, J.; et al. Modeling rotavirus infection and antiviral therapy using primary intestinal organoids. Antivir. Res. 2015, 123, 120–131. [Google Scholar] [CrossRef]
- Condemine, W.; Eguether, T.; Courousse, N.; Etchebest, C.; Gardet, A.; Trugnan, G.; Chwetzoff, S. The C-terminus of rotavirus VP4 protein contains an actin binding domain which requires co-operation with the coiled-coil domain for actin remodeling. J. Virol. 2018, 93, e01598-18. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xue, M.; Yu, L.; Luo, G.; Yang, H.; Jia, L.; Zeng, Y.; Li, T.; Ge, S.; Xia, N. Expression and characterization of a novel truncated rotavirus VP4 for the development of a recombinant rotavirus vaccine. Vaccine 2018, 36, 2086–2092. [Google Scholar] [CrossRef] [PubMed]
- Imai, M.; Akatani, K.; Ikegami, N.; Furuichi, Y. Capped and conserved terminal structures in human rotavirus genome double-stranded RNA segments. J. Virol. 1983, 47, 125–136. [Google Scholar]
- Vende, P.; Piron, M.; Castagne, N.; Poncet, D. Efficient translation of rotavirus mRNA requires simultaneous interaction of NSP3 with the eukaryotic translation initiation factor eIF4G and the mRNA 3’ end. J. Virol. 2000, 74, 7064–7071. [Google Scholar] [CrossRef] [PubMed]
- Gratia, M.; Sarot, E.; Vende, P.; Charpilienne, A.; Baron, C.H.; Duarte, M.; Pyronnet, S.; Poncet, D. Rotavirus NSP3 Is a Translational Surrogate of the Poly(A) Binding Protein-Poly(A) Complex. J. Virol. 2015, 89, 8773–8782. [Google Scholar] [CrossRef]
- Mamane, Y.; Petroulakis, E.; LeBacquer, O.; Sonenberg, N. mTOR, translation initiation and cancer. Oncogene 2006, 25, 6416–6422. [Google Scholar] [CrossRef] [PubMed]
- Gingras, A.C.; Raught, B.; Sonenberg, N. eIF4 initiation factors: Effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 1999, 68, 913–963. [Google Scholar] [CrossRef] [PubMed]
- Zan-Kowalczewska, M.; Bretner, M.; Sierakowska, H.; Szczesna, E.; Filipowicz, W.; Shatkin, A.J. Removal of 5’-terminal m7G from eukaryotic mRNAs by potato nucleotide pyrophosphatase and its effect on translation. Nucleic Acids Res. 1977, 4, 3065–3081. [Google Scholar] [CrossRef]
- Montero, H.; Garcia-Roman, R.; Mora, S.I. eIF4E as a control target for viruses. Viruses 2015, 7, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Malka-Mahieu, H.; Newman, M.; Desaubry, L.; Robert, C.; Vagner, S. Molecular Pathways: The eIF4F Translation Initiation Complex-New Opportunities for Cancer Treatment. Clin. Cancer Res. 2017, 23, 21–25. [Google Scholar] [CrossRef]
- Pisera, A.; Campo, A.; Campo, S. Structure and functions of the translation initiation factor eIF4E and its role in cancer development and treatment. J. Genet. Genom. 2018, 45, 13–24. [Google Scholar] [CrossRef]
- Chang, Y.; Huh, W.K. Ksp1-dependent phosphorylation of eIF4G modulates post-transcriptional regulation of specific mRNAs under glucose deprivation conditions. Nucleic Acids Res. 2018, 46, 3047–3060. [Google Scholar] [CrossRef] [PubMed]
- Biyanee, A.; Ohnheiser, J.; Singh, P.; Klempnauer, K.H. A novel mechanism for the control of translation of specific mRNAs by tumor suppressor protein Pdcd4: Inhibition of translation elongation. Oncogene 2015, 34, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Dennis, M.D.; Jefferson, L.S.; Kimball, S.R. Role of p70S6K1-mediated phosphorylation of eIF4B and PDCD4 proteins in the regulation of protein synthesis. J. Biol. Chem. 2012, 287, 42890–42899. [Google Scholar] [CrossRef]
- Gale, M., Jr.; Tan, S.L.; Katze, M.G. Translational control of viral gene expression in eukaryotes. Microbiol. Mol. Biol. Rev. 2000, 64, 239–280. [Google Scholar] [CrossRef]
- Walsh, D. Manipulation of the host translation initiation complex eIF4F by DNA viruses. Biochem. Soc. Trans. 2010, 38, 1511–1516. [Google Scholar] [CrossRef]
- Chaudhry, Y.; Nayak, A.; Bordeleau, M.E.; Tanaka, J.; Pelletier, J.; Belsham, G.J.; Roberts, L.O.; Goodfellow, I.G. Caliciviruses differ in their functional requirements for eIF4F components. J. Biol. Chem. 2006, 281, 25315–25325. [Google Scholar] [CrossRef] [PubMed]
- Cencic, R.; Desforges, M.; Hall, D.R.; Kozakov, D.; Du, Y.; Min, J.; Dingledine, R.; Fu, H.; Vajda, S.; Talbot, P.J.; et al. Blocking eIF4E-eIF4G interaction as a strategy to impair coronavirus replication. J. Virol. 2011, 85, 6381–6389. [Google Scholar] [CrossRef]
- Boisnard, A.; Albar, L.; Thiemele, D.; Rondeau, M.; Ghesquiere, A. Evaluation of genes from eIF4E and eIF4G multigenic families as potential candidates for partial resistance QTLs to Rice yellow mottle virus in rice. Theor. Appl. Genet. 2007, 116, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Colina, R.; Costa-Mattioli, M.; Dowling, R.J.; Jaramillo, M.; Tai, L.H.; Breitbach, C.J.; Martineau, Y.; Larsson, O.; Rong, L.; Svitkin, Y.V.; et al. Translational control of the innate immune response through IRF-7. Nature 2008, 452, 323–328. [Google Scholar] [CrossRef]
- Herdy, B.; Jaramillo, M.; Svitkin, Y.V.; Rosenfeld, A.B.; Kobayashi, M.; Walsh, D.; Alain, T.; Sean, P.; Robichaud, N.; Topisirovic, I.; et al. Translational control of the activation of transcription factor NF-kappaB and production of type I interferon by phosphorylation of the translation factor eIF4E. Nat. Immunol. 2012, 13, 543–550. [Google Scholar] [CrossRef]
- Cerezo, M.; Guemiri, R.; Druillennec, S.; Girault, I.; Malka-Mahieu, H.; Shen, S.; Allard, D.; Martineau, S.; Welsch, C.; Agoussi, S.; et al. Translational control of tumor immune escape via the eIF4F-STAT1-PD-L1 axis in melanoma. Nat. Med. 2018, 24, 1877–1886. [Google Scholar] [CrossRef]
- Burgui, I.; Yanguez, E.; Sonenberg, N.; Nieto, A. Influenza virus mRNA translation revisited: Is the eIF4E cap-binding factor required for viral mRNA translation? J. Virol. 2007, 81, 12427–12438. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.; Arias, C.; Perez, C.; Halladin, D.; Escandon, M.; Ueda, T.; Watanabe-Fukunaga, R.; Fukunaga, R.; Mohr, I. Eukaryotic translation initiation factor 4F architectural alterations accompany translation initiation factor redistribution in poxvirus-infected cells. Mol. Cell. Biol. 2008, 28, 2648–2658. [Google Scholar] [CrossRef]
- Connor, J.H.; Lyles, D.S. Vesicular stomatitis virus infection alters the eIF4F translation initiation complex and causes dephosphorylation of the eIF4E binding protein 4E-BP1. J. Virol. 2002, 76, 10177–10187. [Google Scholar] [CrossRef]
- Walsh, D.; Perez, C.; Notary, J.; Mohr, I. Regulation of the translation initiation factor eIF4F by multiple mechanisms in human cytomegalovirus-infected cells. J. Virol. 2005, 79, 8057–8064. [Google Scholar] [CrossRef]
- Strong, R.; Belsham, G.J. Sequential modification of translation initiation factor eIF4GI by two different foot-and-mouth disease virus proteases within infected baby hamster kidney cells: Identification of the 3Cpro cleavage site. J. Gen. Virol. 2004, 85, 2953–2962. [Google Scholar] [CrossRef] [PubMed]
- Yanguez, E.; Castello, A.; Welnowska, E.; Carrasco, L.; Goodfellow, I.; Nieto, A. Functional impairment of eIF4A and eIF4G factors correlates with inhibition of influenza virus mRNA translation. Virology 2011, 413, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xu, L.; Wang, Y.; Wang, W.; Sprengers, D.; Metselaar, H.J.; Peppelenbosch, M.P.; Pan, Q. Requirement of the eukaryotic translation initiation factor 4F complex in hepatitis E virus replication. Antivir. Res. 2015, 124, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Linero, F.; Welnowska, E.; Carrasco, L.; Scolaro, L. Participation of eIF4F complex in Junin virus infection: Blockage of eIF4E does not impair virus replication. Cell. Microbiol. 2013, 15, 1766–1782. [Google Scholar] [CrossRef]
- Lenarcic, E.M.; Ziehr, B.; De Leon, G.; Mitchell, D.; Moorman, N.J. Differential role for host translation factors in host and viral protein synthesis during human cytomegalovirus infection. J. Virol. 2014, 88, 1473–1483. [Google Scholar] [CrossRef]
- Montero, H.; Arias, C.F.; Lopez, S. Rotavirus Nonstructural Protein NSP3 is not required for viral protein synthesis. J. Virol. 2006, 80, 9031–9038. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Dang, W.; Zhou, X.; Xu, L.; Wang, W.; Cao, W.; Chen, S.; Su, J.; Cai, X.; Xiao, S.; et al. PI3K-Akt-mTOR axis sustains rotavirus infection via the 4E-BP1 mediated autophagy pathway and represents an antiviral target. Virulence 2018, 9, 83–98. [Google Scholar] [CrossRef]
- Ruoff, R.; Katsara, O.; Kolupaeva, V. Cell type-specific control of protein synthesis and proliferation by FGF-dependent signaling to the translation repressor 4E-BP. Proc. Natl. Acad. Sci. USA 2016, 113, 7545–7550. [Google Scholar] [CrossRef]
- Wei, N.A.; Liu, S.S.; Leung, T.H.; Tam, K.F.; Liao, X.Y.; Cheung, A.N.; Chan, K.K.; Ngan, H.Y. Loss of Programmed cell death 4 (Pdcd4) associates with the progression of ovarian cancer. Mol. Cancer 2009, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Petersson, J.; Ageberg, M.; Sanden, C.; Olofsson, T.; Gullberg, U.; Drott, K. The p53 target gene TRIM22 directly or indirectly interacts with the translation initiation factor eIF4E and inhibits the binding of eIF4E to eIF4G. Biol. Cell 2012, 104, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Chen, S.; Hakim, M.S.; Wang, W.; Xu, L.; Dang, W.; Qu, C.; Verhaar, A.P.; Su, J.; Fuhler, G.M.; et al. 6-Thioguanine inhibits rotavirus replication through suppression of Rac1 GDP/GTP cycling. Antivir. Res. 2018, 156, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Wang, Y.; Dang, W.; Xu, L.; Su, J.; Zhou, X.; Wang, W.; Felczak, K.; van der Laan, L.J.; Pankiewicz, K.W.; et al. Mycophenolic acid potently inhibits rotavirus infection with a high barrier to resistance development. Antivir. Res. 2016, 133, 41–49. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Feng, C.; Fang, Y.; Zhou, X.; Xu, L.; Wang, W.; Kong, X.; P. Peppelenbosch, M.; Pan, Q.; Yin, Y. The Eukaryotic Translation Initiation Factor 4F Complex Restricts Rotavirus Infection via Regulating the Expression of IRF1 and IRF7. Int. J. Mol. Sci. 2019, 20, 1580. https://doi.org/10.3390/ijms20071580
Chen S, Feng C, Fang Y, Zhou X, Xu L, Wang W, Kong X, P. Peppelenbosch M, Pan Q, Yin Y. The Eukaryotic Translation Initiation Factor 4F Complex Restricts Rotavirus Infection via Regulating the Expression of IRF1 and IRF7. International Journal of Molecular Sciences. 2019; 20(7):1580. https://doi.org/10.3390/ijms20071580
Chicago/Turabian StyleChen, Sunrui, Cui Feng, Yan Fang, Xinying Zhou, Lei Xu, Wenshi Wang, Xiangdong Kong, Maikel P. Peppelenbosch, Qiuwei Pan, and Yuebang Yin. 2019. "The Eukaryotic Translation Initiation Factor 4F Complex Restricts Rotavirus Infection via Regulating the Expression of IRF1 and IRF7" International Journal of Molecular Sciences 20, no. 7: 1580. https://doi.org/10.3390/ijms20071580
APA StyleChen, S., Feng, C., Fang, Y., Zhou, X., Xu, L., Wang, W., Kong, X., P. Peppelenbosch, M., Pan, Q., & Yin, Y. (2019). The Eukaryotic Translation Initiation Factor 4F Complex Restricts Rotavirus Infection via Regulating the Expression of IRF1 and IRF7. International Journal of Molecular Sciences, 20(7), 1580. https://doi.org/10.3390/ijms20071580





