Disulfide Bond Engineering of an Endoglucanase from Penicillium verruculosum to Improve Its Thermostability
Abstract
:1. Introduction
2. Results
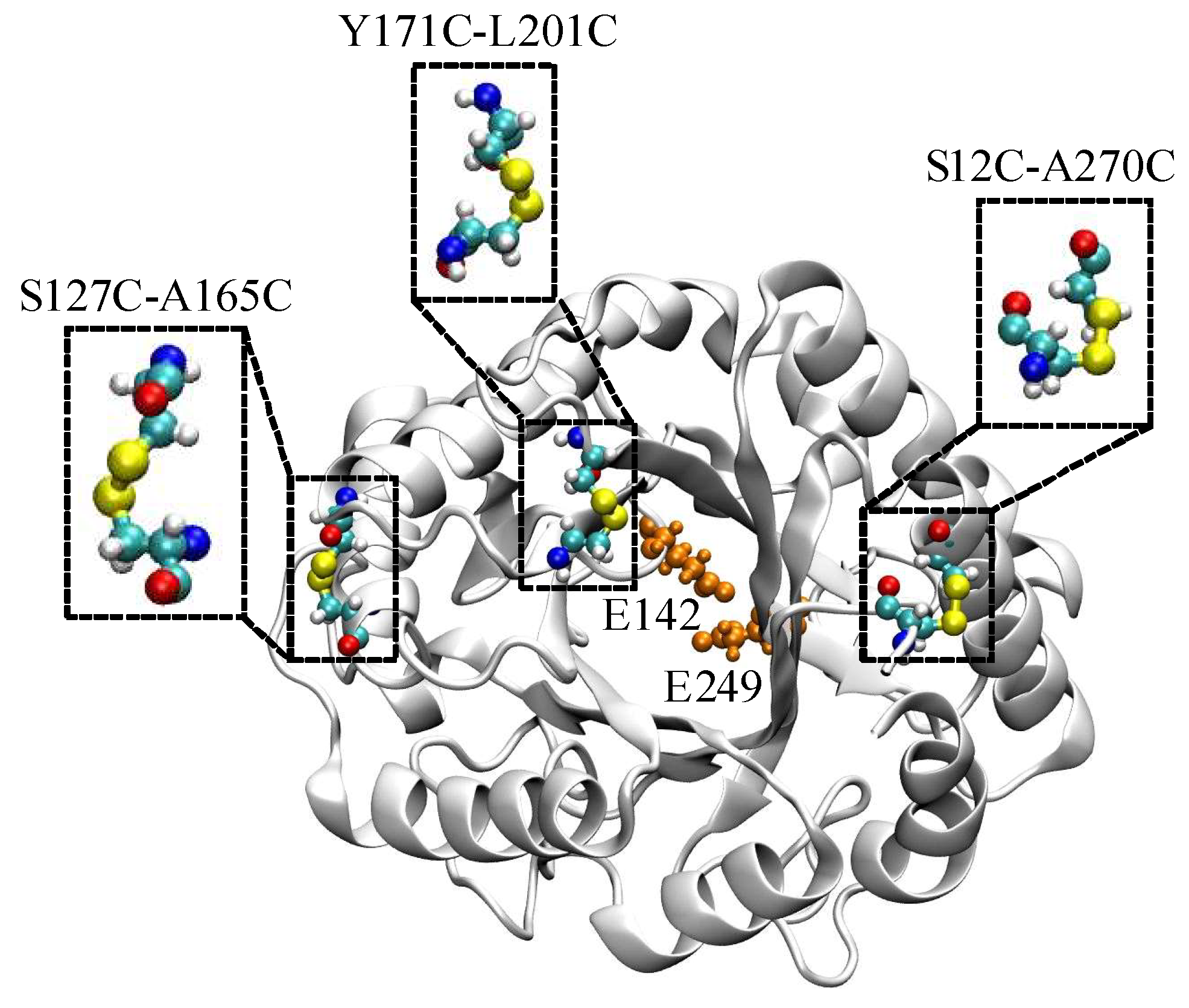
2.1. Computational Design and Screening of Disulfide Bonds
2.2. Production, Purification, and Biochemical Characterization of the EGLII Variants
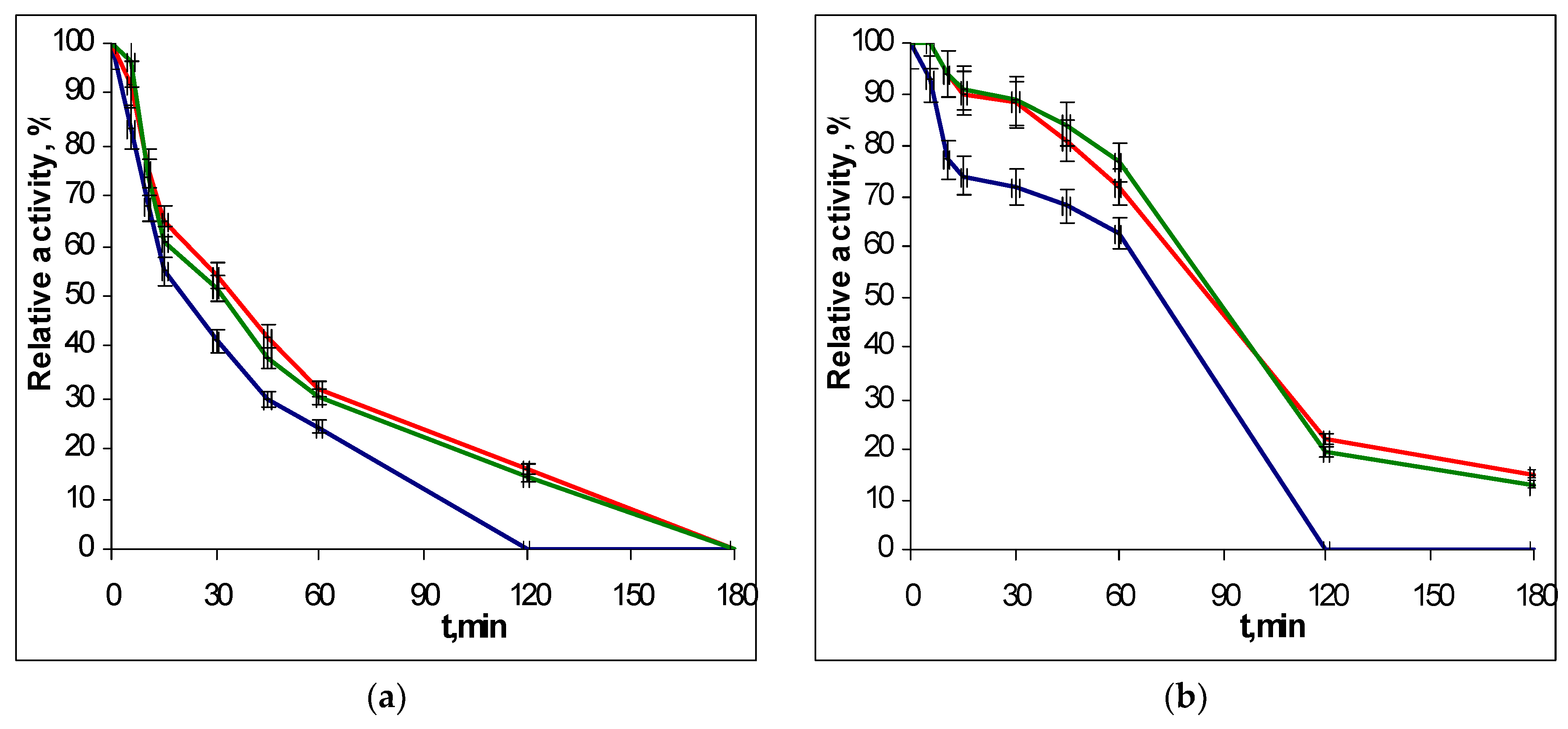
2.3. Thermostability of Recombinant EGLII Forms
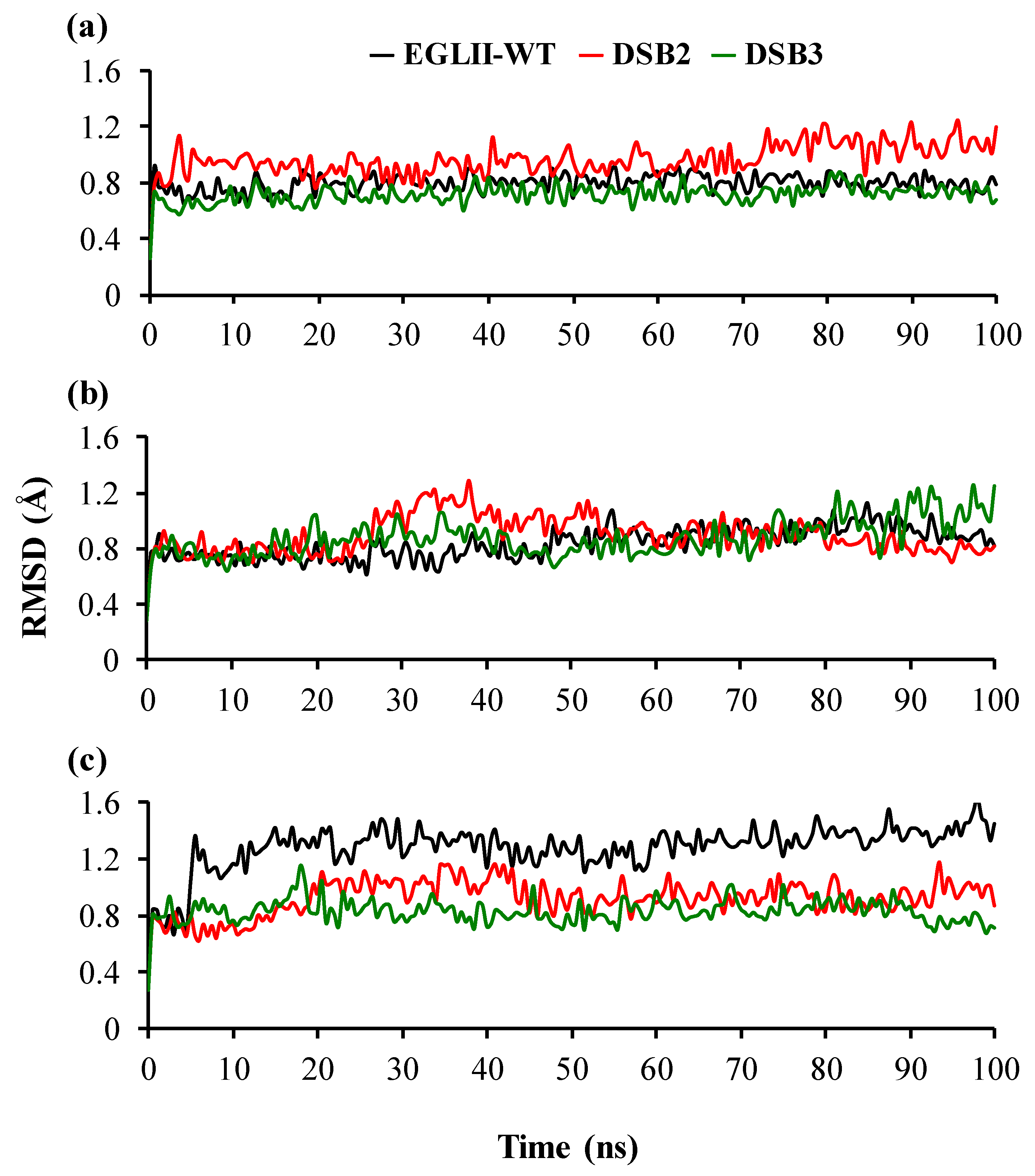
2.4. Analysis of Structural Stability
3. Discussion
4. Materials and Methods
4.1. Disulfide Bond Design
4.2. Microbial Strains
4.3. Enzyme Activity Assays
4.4. Site-Directed Mutagenesis and Protein Expression
4.5. Purification and Charactetization of the Thermostable Variants
4.6. Molecular Modelling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CMC | Carboxymethylcellulose |
| DSB | Disulfide bond |
| EGL | Endoglucanase |
| MD | Molecular dynamics |
References
- Lambertz, C.; Garvey, M.; Klinger, J.; Heesel, D.; Klose, H.; Fischer, R.; Commandeur, U. Challenges and advances in the heterologous expression of cellulolytic enzymes: A review. Biotechnol. Biofuels 2014, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.C.; Mi, L.; Pontrelli, S.; Luo, S. Fuelling the future: Microbial engineering for the production of sustainable biofuels. Nat. Rev. Microbiol. 2016, 14, 288–304. [Google Scholar] [CrossRef] [PubMed]
- Artzi, L.; Bayer, E.A.; Morais, S. Cellulosomes: Bacterial nanomachines for dismantling plant polysaccharides. Nat. Rev. Microbiol. 2017, 15, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Yennamalli, R.M.; Rader, A.J.; Kenny, A.J.; Wolt, J.D.; Sen, T.Z. Endoglucanases: Insights into thermostability for biofuel applications. Biotechnol. Biofuels 2013, 6, 136. [Google Scholar] [CrossRef] [PubMed]
- Scapin, S.M.N.; Souza, F.H.M.; Zanphorlin, L.M.; de Almeida, T.S.; Sade, Y.B.; Cardoso, A.M.; Pinheiro, G.L.; Murakami, M.T. Structure and function of a novel GH8 endoglucanase from the bacterial cellulose synthase complex of Raoultella ornithinolytica. PloS ONE 2017, 12, e0176550. [Google Scholar] [CrossRef]
- Jensen, M.S.; Fredriksen, L.; MacKenzie, A.K.; Leiros, I.; Chylenski, P.; Williamson, A.K.; Christopeit, T.; Ostby, H.; Vaaje-Kolstad, G.; Eijsinket, V.G.H. Discovery and characterization of a thermostable two-domain GH6 endoglucanase from a compost metagenome. PloS ONE 2018, 13, e0197862. [Google Scholar] [CrossRef]
- Miotto, L.S.; de Rezende, C.A.; Bernardes, A.; Serpa, V.I.; Tsang, A.; Polikarpovet, I. The characterization of the endoglucanase Cel12A from Gloeophyllum trabeum reveals an enzyme highly active on β-glucan. PLoS ONE 2014, 9, e108393. [Google Scholar] [CrossRef]
- Ghosh, K.; Dill, K. Cellular proteomes have broad distributions of protein stability. Biophys. J. 2010, 99, 3996–4002. [Google Scholar] [CrossRef]
- Park, Y.G.; Jung, M.C.; Song, H.; Jeong, K.W.; Bang, E.; Hwang, G.S.; Kim, Y. Novel structural components contribute to the high thermal stability of acyl carrier protein from Enterococcus faecalis. J. Biol. Chem. 2016, 291, 1692–1702. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.; Mamo, G.; Karlsson, E.N. Potential and utilization of thermophiles and thermostable enzymes in biorefining. Microb. Cell Fact. 2007, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.S.; Liu, D.R. Methods for the directed evolution of proteins. Nat. Rev. Genet. 2015, 16, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Zhu, L.; Schwaneberg, U. Directed evolution 2.0: Improving and deciphering enzyme properties. Chem. Commun. 2015, 51, 9760–9772. [Google Scholar] [CrossRef] [PubMed]
- Muhlmann, M.; Kunze, M.; Ribeiro, J.; Geinitz, B.; Lehmann, C.; Schwaneberg, U.; Commandeur, U.; Buchset, J. Cellulolytic RoboLector—Towards an automated high-throughput screening platform for recombinant cellulase expression. J. Biol. Eng. 2017, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Goedegebuur, F.; Dankmeyer, L.; Gualfetti, P.; Karkehabadi, S.; Hansson, H.; Jana, S.; Huynh, V.; Kelemen, B.R.; Kruithof, P.; Larenas, E.A.; et al. Improving the thermal stability of cellobiohydrolase Cel7A from Hypocrea jecorina by directed evolution. J. Biol. Chem. 2017, 292, 17418–17430. [Google Scholar] [CrossRef] [PubMed]
- Shivange, A.V.; Roccatano, D.; Schwaneberg, U. Iterative key-residues interrogation of a phytase with thermostability increasing substitutions identified in directed evolution. Appl. Microbiol. Biotechnol. 2016, 100, 227–242. [Google Scholar] [CrossRef]
- Wang, X.; Ma, R.; Xie, X.; Liu, W.; Tu, T.; Zheng, F.; You, S.; Ge, J.; Xie, H.; Yao, B.; et al. Thermostability improvement of a Talaromyces leycettanus xylanase by rational protein engineering. Sci. Rep. 2017, 7, 15287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Yang, G.; Xie, Y.; Xu, L.; An, J.; Cui, L.; Feng, Y. Modulation of the thermostability and substrate specificity of Candida rugosa lipase1 by altering the acyl-binding residue Gly414 at the α-helix-connecting bend. Enzyme Microb. Technol. 2016, 82, 34–41. [Google Scholar] [CrossRef]
- Modarres, H.P.; Mofrad, M.; Sanati-Nezhad, A. Protein thermostability engineering. RSC Adv. 2016, 6, 115252–115270. [Google Scholar] [CrossRef]
- Kruger, D.M.; Rathi, P.C.; Pfleger, C.; Gohlke, H. CNA web server: Rigidity theory-based thermal unfolding simulations of proteins for linking structure, thermostability, and function. Nucleic Acids Res. 2013, 41, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.H.; Yu, T.H.; Wong, K.B. Stabilizing salt-bridge enhances protein thermostability by reducing the heat capacity change of unfolding. PLoS ONE 2011, 6, e21624. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.B.; Dombkowski, A.A. Disulfide by Design 2.0: A web-based tool for disulfide engineering in proteins. BMC Bioinf. 2013, 14, 346. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Zhu, L.; Xu, X.; Li, Q. Rational design of disulfide bonds increases thermostability of a mesophilic 1,3-1,4-β-glucanase from Bacillus terquilensis. PLoS ONE 2016, 11, e0154036. [Google Scholar] [CrossRef]
- Yang, H.; Liu, L.; Li, J.; Chen, J.; Du, G. Rational design to improve protein thermostability: Recent advances and prospects. ChemBioEng. Rev. 2015, 2, 87–94. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.; Luo, X.; Li, J.; Reed, S.A.; Xiao, H.; Young, T.S.; Schultz, P.G. Enhancing protein stability with extended disulfide bonds. Proc. Natl. Acad. Sci. USA 2016, 113, 5910–5915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogg, P.J. Disulfide bonds as switches for protein function. Trends Biochem. Sci. 2003, 28, 210–214. [Google Scholar] [CrossRef]
- Kadokura, H.; Katzen, F.; Beckwith, J. Protein disulfide bond formation in prokaryotes. Annu. Rev. Biochem. 2003, 72, 111–135. [Google Scholar] [CrossRef]
- Liu, L.; Deng, Z.; Yang, H.; Li, J.; Shin, H.D.; Chen, R.R.; Du, G.; Chen, J. In silico rational design and systems engineering of disulfide bridges in the catalytic domain of an alkaline α-amylase from Alkalimonas amylolytica to improve thermostability. Appl. Environ. Microbiol. 2013, 80, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Badieyan, S.; Bevan, D.R.; Zhang, C. Study and design of stability in GH5 cellulases. Biotechnol. Bioeng. 2012, 109, 31–44. [Google Scholar] [CrossRef]
- Rigoldi, F.; Donini, S.; Giacomina, f.; Sorana, F.; Redaelli, A.; Bandiera, T.; Parisini, E.; Gautieri, A. Thermal stabilization of the deglycating enzyme Amadoriase I by rational design. Sci. Rep. 2018, 8, 3042. [Google Scholar] [CrossRef] [PubMed]
- Morozova, V.V.; Gusakov, A.V.; Andrianov, R.M.; Pravilnikov, A.G.; Osipov, D.O.; Sinitsyn, A.P. Cellulases of Penicillium verruculosum. Biotechnol. J. 2010, 5, 871–880. [Google Scholar] [CrossRef]
- Dotsenko, A.S.; Rozhkova, A.M.; Gusakov, A.V. Properties and N-glycosylation of recombinant endoglucanase II from Penicillium verruculosum. Moscow Univ. Chem. Bull. 2015, 70, 283–286. [Google Scholar] [CrossRef]
- Salam, N.K.; Adzhigirey, M.; Sherman, W.; Pearlman, D.A. Structure-based approach to the prediction of disulfide bonds in proteins. J. Protein Eng. Des. Sel. 2014, 27, 365–374. [Google Scholar] [CrossRef] [Green Version]
- Dombkowski, A.A.; Sultana, K.Z.; Craig, D.B. Protein disulfide engineering. J. FEBS Lett. 2014, 588, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Feige, M.J.; Hendershot, L.M. Disulfide bonds in ER protein folding and homeostasis. Curr. Opin. Cell Biol. 2011, 23, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Fass, D. Disulfide bonding in protein biophysics. Annu. Rev. Biophys. 2012, 41, 63–79. [Google Scholar] [CrossRef]
- Bulleid, N.J. Disulfide bond formation in the mammalian endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 2012, 11, a013219. [Google Scholar] [CrossRef]
- Chang, M.; Chu, X.; Lv, J.; Li, Q.; Tian, J.; Wu, N. Improving the thermostability of acidic pullulanase from Bacillus naganoensis by rational design. PloS ONE 2016, 11, e0165006. [Google Scholar] [CrossRef]
- Wedemeyer, W.J.; Welker, E.; Naravan, M.; Scheraga, H.A. Disulfide bonds and protein folding. Biochemistry 2000, 39, 4207–4216. [Google Scholar] [CrossRef]
- Eijsink, V.; Vriend, G.; Van den Burg, B. Engineering a hyperstable enzyme by manipulation of early steps in the unfolding process. Biocatal. Biotransf. 2001, 19, 443–458. [Google Scholar] [CrossRef]
- Ribeiro, T.; Lordelo, M.M.S.; Ponte, P.I.P.; Maçãs, B.; Prates, J.A.M.; Fontes, M.A.; Falcão, L.; Freire, J.P.B.; Ferreira, L.M.A.; Fontes, C.M.G.A. Levels of endogenous -glucanase activity in barley affect the efficacy of exogenous enzymes used to supplement barley-based diets for poultry. Poultry Sci. 2011, 90, 1245–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Luo, H.; Shi, P.; Huang, H.; Bai, Y.; Yao, B. A highly-active endo-1,3-1,4-β-glucanase from thermophilic Talaromyces emersonii CBS394.64 with application potential in the brewing and feed industries. Process Biochem. 2014, 49, 1448–1456. [Google Scholar] [CrossRef]
- Singh, R. A review of algorithmic techniques for disulfide-bond determination. Brief. Funct. Genomics 2008, 7, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Sinitsyn, A.P.; Rozhkova, A.M. Penicillium canescens host as the platform for development of a new recombinant strain producers of carbohydrases. In Microorganisms in Biorefineries; Kamm, B., Ed.; Springer: Berlin, Germany, 2015; pp. 1–19. [Google Scholar]
- Nelson, N. A photometric adaptation of the Somogyi method for the determination of sugars. J. Biol. Chem. 1944, 153, 375–379. [Google Scholar]
- Sinitsyna, O.A.; Bukhtoyarov, F.E.; Gusakov, A.V.; Okunev, O.N.; Bekkarevitch, A.O.; Vinetsky, Y.P.; Sinitsyn, A.P. Isolation and properties of major components of Penicillium canescens extracellular enzyme complex. Biochemistry (Moscow) 2003, 68, 1200–1209. [Google Scholar] [CrossRef]
- Dotsenko, A.S.; Gusakov, A.V.; Volkov, P.V.; Rozhkova, A.M.; Sinitsyn, A.P. N-linked glycosylation of recombinant cellobiohydrolase I (Cel7A) from Penicillium verruculosum and its effect on the enzyme activity. Biotechnol. Bioeng. 2016, 113, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Sanger, F.; Nicklen, S.; Chase, A.R. DNA Sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef] [PubMed]
- Aleksenko, A.; Makarova, N.; Nikolaev, I.; Clutterbuck, A. Integrative and replicative transformation of Penicillium canescens with a heterologous nitrate-reductase gene. Curr. Genet. 1995, 28, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Peterson, G.L. Review of the Folin phenol protein quantitation method of Lowry, Rosebrough, Farr and Randall. Anal. Biochem. 1979, 100, 201–220. [Google Scholar] [CrossRef]
- Van Durme, J.; Delgado, J.; Stricher, F.; Serrano, L.; Schymkowitz, J.; Rousseau, F. A graphical interface for the FoldX forcefield. Bioinformatics 2011, 27, 1711–1712. [Google Scholar] [CrossRef] [Green Version]
- Schymkowitz, J.; Borg, J.; Stricher, F.; Nys, R.; Rousseau, F.; Serrano, L. The FoldX web server: An online force field. Nucleic Acids Res. 2005, 33, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Krieger, E.; Joo, K.; Lee, J.; Lee, J.; Raman, S.; Thompson, J.; Tyka, M.; Baker, D.; Karplus, K. Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: Four approaches that performed well in CASP8. Proteins Struct. Funct. Bioinf. 2009, 77, 114–122. [Google Scholar] [CrossRef]
- Hess, B.; Kutzner, C.; van der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Pall, S.; Abraham, M.J.; Kutzner, C.; Hess, B.; Lindahl, E. Tackling exascale software challenges in molecular dynamics simulations with GROMACS. In Proceedings of the International Conference on Exascale Applications and Software (EASC), Stockholm, Sweden, 2–3 April 2014; Markidis, S., Laure, E., Eds.; Springer: Basel, Switzerland, 2015; pp. 3–27. [Google Scholar]
- Lindahl, E.; Hess, B.; van der Spoel, D. GROMACS 3.0: A package for molecular simulation and trajectory analysis. J. Mol. Model. 2001, 7, 306–317. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Kusalik, P.G.; Svishchev, I.M. The spatial structure in liquid water. Science 1994, 265, 1219–1221. [Google Scholar] [CrossRef]
- Fyta, M.; Netz, R.R. Ionic force field optimization based on single-ion and ion-pair solvation properties: Going beyond standard mixing rules. J. Chem. Phys. 2012, 136, 124103. [Google Scholar] [CrossRef]
- Dolinsky, T.J.; Nielsen, J.E.; McCammon, J.A.; Baker, N.A. PDB2PQR: An automated pipeline for the setup of Poisson–Boltzmann electrostatics calculations. Nucleic Acids Res. 2004, 32, 665–667. [Google Scholar] [CrossRef]
- Dolinsky, T.J.; Czodrowski, P.; Li, H.; Nielsen, J.E.; Jensen, J.H.; Klebe, G.; Baker, N.A. PDB2PQR: Expanding and upgrading automated preparation of biomolecular structures for molecular simulations. Nucleic Acids Res. 2007, 35, 522–525. [Google Scholar] [CrossRef]
- Onufriev, A.V.; Alexov, E. Protonation and pK changes in protein-ligand binding. Q. Rev. Biophys. 2013, 46, 181–209. [Google Scholar] [CrossRef]
- Wang, J.; Cieplak, P.; Kollman, P.A. How well does a restrained electrostatic potential (RESP) model perform in calculating conformational energies of organic and biological molecules? J. Comput. Chem. 2000, 21, 1049–1074. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Norberg, J.; Nilsson, L. On the truncation of long-range electrostatic interactions in DNA. Biophys. J. 2000, 79, 1537–1553. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef] [Green Version]
- Hess, B. P-LINCS: A parallel linear constraint solver for molecular simulation. J. Chem. Theory Comput. 2008, 4, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Maiorov, V.N.; Crippen, G.M. Size-independent comparison of protein three-dimensional structures. Proteins Struct. Funct. Bioinf. 1995, 22, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Lobanov, M.Y.; Bogatyreva, N.S.; Galzitskaya, O.V. Radius of gyration as an indicator of protein structure compactness. Mol. Biol. (Moscow) 2008, 42, 623–628. [Google Scholar] [CrossRef]


| Substrate | EGLII-wt | EGLII-DSB2 | EGLII-DSB3 |
|---|---|---|---|
| β-Glucan | 57 ± 4 | 68 ± 6 | 66 ± 5 |
| CMC | 53 ± 3 | 61 ± 4 | 64 ± 4 |
| Mutation | Primer Name | Sequence |
|---|---|---|
| S12C | S12C-fwd | 5′-AACGTGCTTCTTGTTTCGAATGGTTCGGT-3′ |
| S12C-rev | 5′-ACCGAACCATTCGAAACAAGAAGCACGTT-3′ | |
| A270C | A270C-fwd | 5′-TGCTGGATTATTTGTGTGAAAACTCAGACGT-3′ |
| A270C-rev | 5′-ACGTCTGAGTTTTCACACAAATAATCCAGC-3′ | |
| S127C | S127C-fwd | 5′-TGGTCCACACTGGCCTGTCAATTCAAATCA-3′ |
| S127C-rev | 5′-TGATTTGAATTGACAGGCCAGTGTGGACCA-3′ | |
| A165C | A165C-fwd | 5′-ATGGCATCCGCGACTGTGGTGCAACAA-3′ |
| A165C-rev | 5′-TTGTTGCACCACAGTCGCGGATGCCA-3′ | |
| Y171C | Y171C-fwd | 5′-TGGCGCAACAACTCAATGTATCTTCGTTGA-3′ |
| Y171C-rev | 5′-TCAACGAAGATACATTGAGTTGTTGCGCCA-3′ | |
| L201C | L201C-fwd | 5′-ACTGACCCTTCTGATTGTATCGTCTACGAGAT-3′ |
| L201C-rev | 5′-ATCTCGTAGACGATACAATCAGAAGGGTCAGT-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashirova, A.; Pramanik, S.; Volkov, P.; Rozhkova, A.; Nemashkalov, V.; Zorov, I.; Gusakov, A.; Sinitsyn, A.; Schwaneberg, U.; Davari, M.D. Disulfide Bond Engineering of an Endoglucanase from Penicillium verruculosum to Improve Its Thermostability. Int. J. Mol. Sci. 2019, 20, 1602. https://doi.org/10.3390/ijms20071602
Bashirova A, Pramanik S, Volkov P, Rozhkova A, Nemashkalov V, Zorov I, Gusakov A, Sinitsyn A, Schwaneberg U, Davari MD. Disulfide Bond Engineering of an Endoglucanase from Penicillium verruculosum to Improve Its Thermostability. International Journal of Molecular Sciences. 2019; 20(7):1602. https://doi.org/10.3390/ijms20071602
Chicago/Turabian StyleBashirova, Anna, Subrata Pramanik, Pavel Volkov, Aleksandra Rozhkova, Vitaly Nemashkalov, Ivan Zorov, Alexander Gusakov, Arkady Sinitsyn, Ulrich Schwaneberg, and Mehdi D. Davari. 2019. "Disulfide Bond Engineering of an Endoglucanase from Penicillium verruculosum to Improve Its Thermostability" International Journal of Molecular Sciences 20, no. 7: 1602. https://doi.org/10.3390/ijms20071602
APA StyleBashirova, A., Pramanik, S., Volkov, P., Rozhkova, A., Nemashkalov, V., Zorov, I., Gusakov, A., Sinitsyn, A., Schwaneberg, U., & Davari, M. D. (2019). Disulfide Bond Engineering of an Endoglucanase from Penicillium verruculosum to Improve Its Thermostability. International Journal of Molecular Sciences, 20(7), 1602. https://doi.org/10.3390/ijms20071602





