The Effect of Conditioned Media of Stem Cells Derived from Lipoma and Adipose Tissue on Macrophages’ Response and Wound Healing in Indirect Co-culture System In Vitro
Abstract
:1. Introduction
2. Results
2.1. Mesenchymal Stem Cell Phenotype and Expression of Stemness-Related Markers
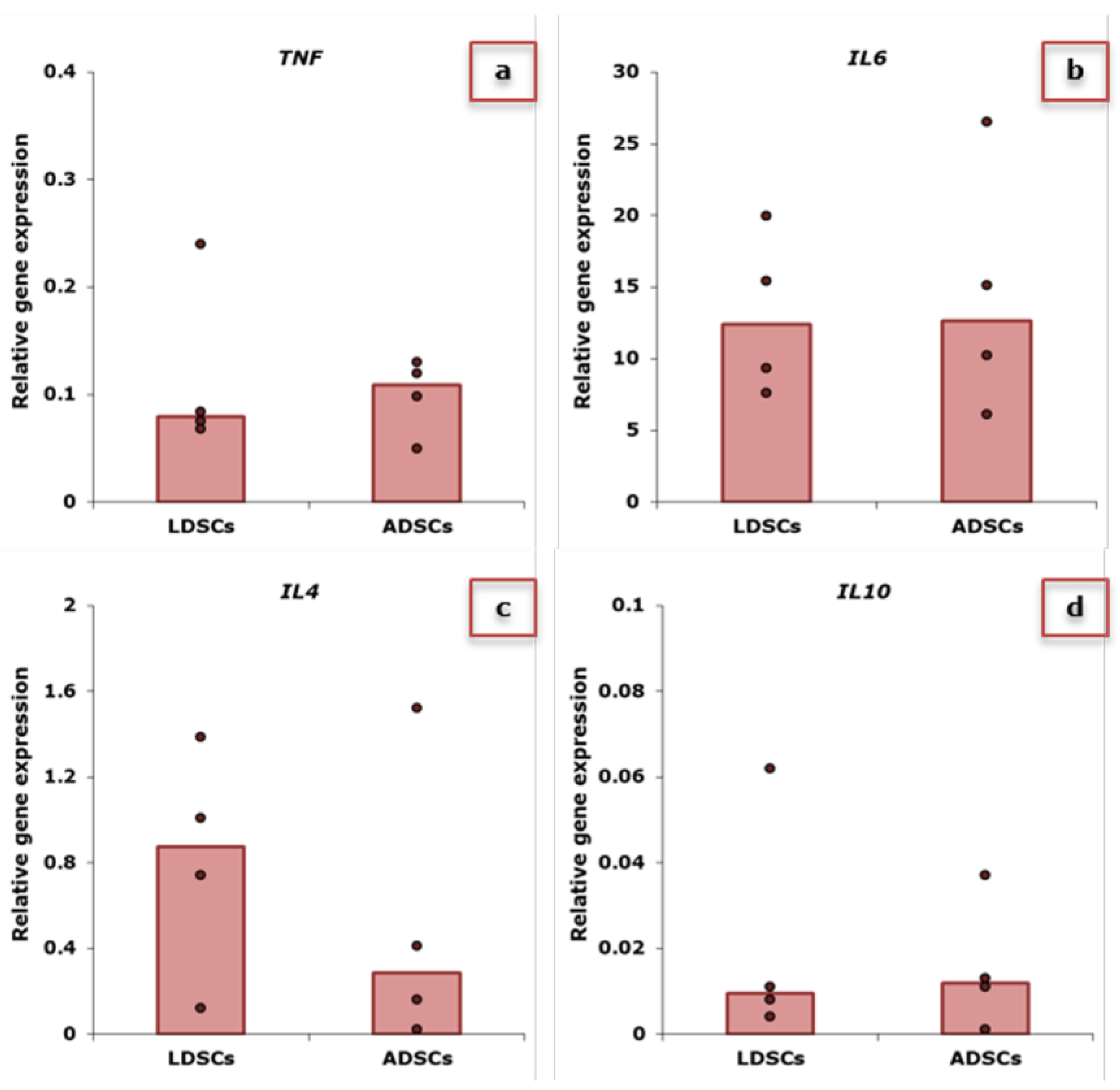
2.2. Expression of Inflammatory-Related Genes in Stem Cells
2.3. Macrophages’ Response to Conditioned Media of Lipoma-Derived Stem Cells (LDSCs) and Adipose-Derived Stem Cells (ADSCs)
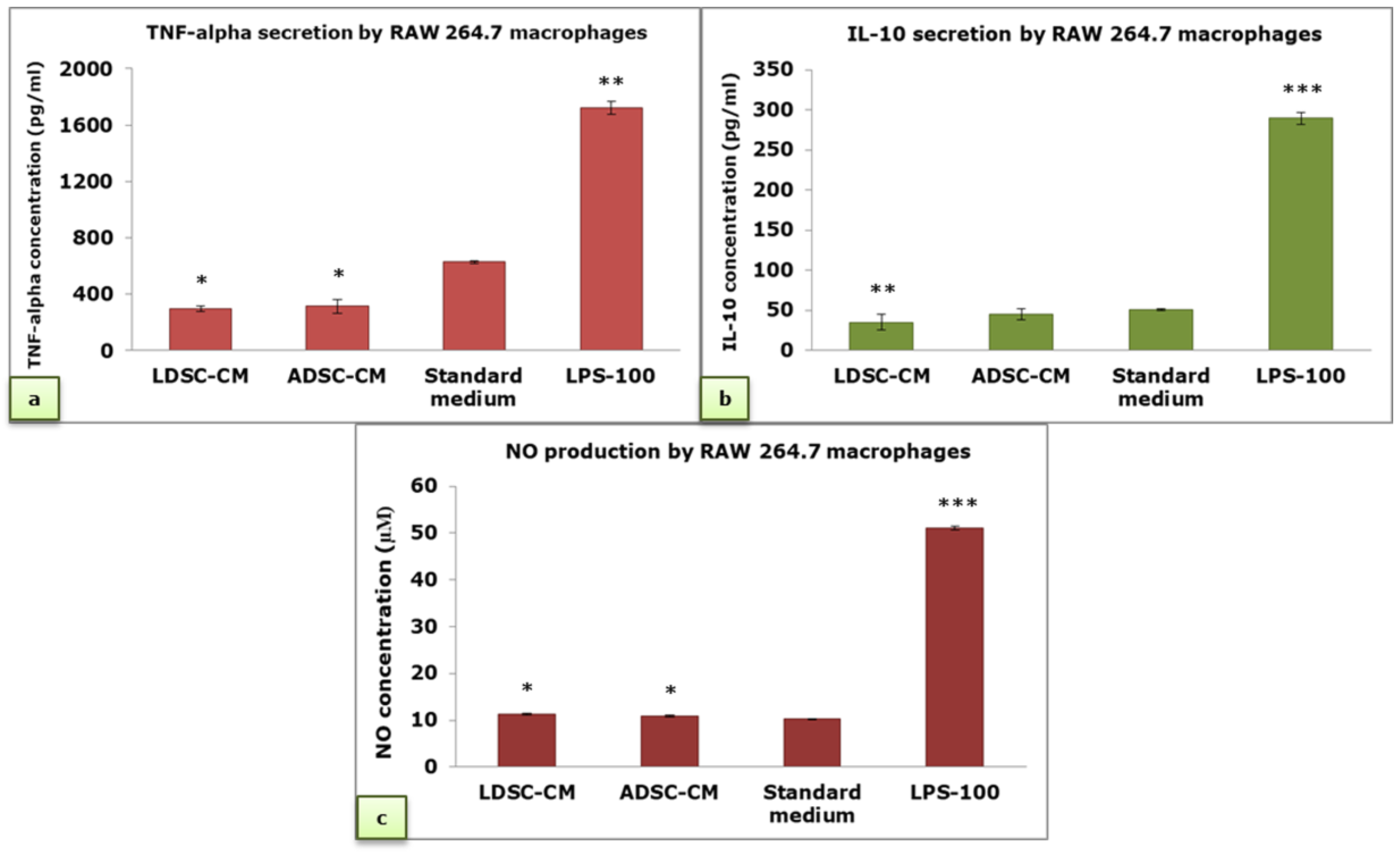
2.4. Immunomodulatory Activity of Conditioned Media of LDSCs and ADSCs
2.5. L929 Bioassay
2.6. In Vitro Wound Healing Analysis
3. Discussion
4. Materials and Methods
4.1. Tissue Sampling
4.2. Isolation and Cultivation of Mesenchymal Stem Cells
4.3. Cell Lines
4.4. Light Microscopy
4.5. Flow Cytometry
4.6. Macrophages’ Response Assays
4.7. Immunomodulatory Assay
4.8. RNA Isolation and Reverse Transcription
4.9. Real-Time PCR
4.10. Enzyme-Linked Immunosorbent Assays (ELISA)
4.11. Nitric Oxide (NO) Measurement
4.12. L929 Bioassay
4.13. In Vitro Wound Healing Assay
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef]
- Baer, P.C.; Geiger, H. Adipose—derived mesenchymal stromal/ stem cells: Tissue localization, characterization, and heterogeneity. Stem Cells Int. 2012, 2012, 812693. [Google Scholar] [CrossRef]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Cvetković, V.J.; Najdanović, J.G.; Vukelić-Nikolić, M.Đ.; Stojanović, S.; Najman, S.J. Osteogenic potential of in vitro osteo-induced adipose-derived mesenchymal stem cells combined with platelet-rich plasma in an ectopic model. Int. Orthop. 2015, 39, 2173–2180. [Google Scholar] [CrossRef] [PubMed]
- Najdanović, J.G.; Cvetković, V.J.; Stojanović, S.; Vukelić-Nikolić, M.Đ.; Stanisavljević, M.N.; Živković, J.M.; Najman, S.J. The influence of adipose-derived stem cells induced into endothelial cells on ectopic vasculogenesis and osteogenesis. Cell. Mol. Bioeng. 2015, 8, 577–590. [Google Scholar] [CrossRef]
- Najman, S.J.; Cvetković, V.J.; Najdanović, J.G.; Stojanović, S.; Vukelić-Nikolić, M.Đ.; Vučković, I.; Petrović, D. Ectopic osteogenic capacity of freshly isolated adipose-derived stromal vascular fraction cells supported with platelet-rich plasma: A simulation of intraoperative procedure. J. Cranio Maxillofac. Surg. 2016, 44, 1750–1760. [Google Scholar] [CrossRef] [PubMed]
- Najdanović, J.G.; Cvetković, V.J.; Stojanović, S.; Vukelić-Nikolić, M.Đ.; Čakić-Milošević, M.M.; Živković, J.M.; Najman, S.J. Effects of bone tissue engineering triad components on vascularization process: Comparative gene expression and histological evaluation in an ectopic bone-forming model. Biotechnol. Biotec. Eq. 2016, 30, 1122–1131. [Google Scholar] [CrossRef]
- Leto Barone, A.A.; Khalifian, S.; Lee, W.P.; Brandacher, G. Immunomodulatory effects of adipose-derived stem cells: Fact or fiction? Biomed. Res. Int. 2013, 2013, 383685. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [CrossRef]
- Kyurkchiev, D.; Bochev, I.; Ivanova-Todorova, E.; Mourdjeva, M.; Oreshkova, T.; Belemezova, K.; Kyurkchiev, S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J. Stem Cells 2014, 6, 552–570. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yuan, Q.; Xie, L. Mesenchymal Stem Cell-Based Immunomodulation: Properties and Clinical Application. Stem Cells Int. 2018, 2018, 3057624. [Google Scholar] [CrossRef]
- English, K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol. Cell. Biol. 2013, 91, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, A.; de Rosbo, N.K. The immunomodulatory function of mesenchymal stem cells: Mode of action and pathways. Ann. N. Y. Acad. Sci. 2015, 1351, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Regulski, M.J. Mesenchymal Stem Cells: “Guardians of Inflammation”. Wounds 2017, 29, 20–27. [Google Scholar] [PubMed]
- Shi, Y.; Su, J.; Roberts, A.I.; Shou, P.; Rabson, A.B.; Ren, G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 2012, 33, 136–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.; Xie, N.; Li, W.; Yuan, B.; Shi, Y.; Wang, Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014, 21, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Guillén, M.I.; Platas, J.; Pérez Del Caz, M.D.; Mirabet, V.; Alcaraz, M.J. Paracrine Anti-inflammatory Effects of Adipose Tissue-Derived Mesenchymal Stem Cells in Human Monocytes. Front. Physiol. 2018, 9, 661. [Google Scholar] [CrossRef]
- Madrigal, M.; Rao, K.S.; Riordan, N.H. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J. Transl. Med. 2014, 12, 260. [Google Scholar] [CrossRef] [Green Version]
- Baglio, S.R.; Pegtel, D.M.; Baldini, N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front. Physiol. 2012, 3, 359. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef]
- Shingyochi, Y.; Orbay, H.; Mizuno, H. Adipose-derived stem cells for wound repair and regeneration. Expert Opin. Biol. Ther. 2015, 15, 1285–1292. [Google Scholar] [CrossRef]
- Lin, T.M.; Chang, H.W.; Wang, K.H.; Kao, A.P.; Chang, C.C.; Wen, C.H.; Lai, C.S.; Lin, S.D. Isolation and identification of mesenchymal stem cells from human lipoma tissue. Biochem. Biophys. Res. Commun. 2007, 361, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Eto, H.; Inoue, K.; Aoi, N.; Kato, H.; Araki, J.; Higashino, T.; Yoshimura, K. Cellular and molecular features of lipoma tissue: Comparison with normal adipose tissue. Br. J. Dermatol. 2009, 161, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Tremp, M.; Menzi, N.; Tchang, L.; di Summa, P.G.; Schaefer, D.J.; Kalbermatten, D.F. Adipose-Derived Stromal Cells from Lipomas: Isolation, Characterisation and Review of the Literature. Pathobiology 2016, 83, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Makiguchi, T.; Terashi, H.; Hashikawa, K.; Yokoo, S.; Kusaka, J. Osteolipoma in the glabella: Pathogenesis associated with mesenchymal lipoma-derived stem cells. J. Craniofac. Surg. 2013, 24, 1310–1313. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, S.; Najman, S.; Korać, A. Stem Cells Derived from Lipoma and Adipose Tissue-Similar Mesenchymal Phenotype but Different Differentiation Capacity Governed by Distinct Molecular Signature. Cells 2018, 7, 260. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.W.; Gao, J.H.; Lu, F.; Zheng, X.D. The differences between adipose tissue derived stem cells and lipoma mesenchymal stem cells in characteristics (In Chinese). Zhonghua Zheng Xing Wai Ke Za Zhi 2010, 26, 125–132. [Google Scholar]
- Mafi, P.; Hindocha, S.; Mafi, R.; Griffin, M.; Khan, W.S. Adult mesenchymal stem cells and cell surface characterization—A systematic review of the literature. Open Orthop. J. 2011, 5, 253–260. [Google Scholar] [CrossRef] [PubMed]
- De Ugarte, D.A.; Alfonso, Z.; Zuk, P.A.; Elbarbary, A.; Zhu, M.; Ashjian, P.; Benhaim, P.; Hedrick, M.H.; Fraser, J.K. Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunol. Lett. 2003, 89, 267–270. [Google Scholar] [CrossRef]
- Alipour, R.; Sadeghi, F.; Hashemi-Beni, B.; Zarkesh-Esfahani, S.H.; Heydari, F.; Mousavi, S.B.; Adib, M.; Narimani, M.; Esmaeili, N. Phenotypic characterizations and comparison of adult dental stem cells with adipose-derived stem cells. Int. J. Prev. Med. 2010, 1, 164–171. [Google Scholar]
- Zavan, B.; De Francesco, F.; D’Andrea, F.; Ferroni, L.; Gardin, C.; Salzillo, R.; Nicoletti, G.; Ferraro, G.A. Persistence of CD34 Stem Marker in Human Lipoma: Searching for Cancer Stem Cells. Int. J. Biol. Sci. 2015, 11, 1127–1139. [Google Scholar] [CrossRef] [Green Version]
- Gronthos, S.; Franklin, D.M.; Leddy, H.A.; Robey, P.G.; Storms, R.W.; Gimble, J.M. Surface protein characterization of human adipose tissue-derived stromal cells. J. Cell Physiol. 2001, 189, 54–63. [Google Scholar] [CrossRef]
- Meliga, E.; Strem, B.M.; Duckers, H.J.; Serruys, P.W. Adipose-derived cells. Cell Transplant. 2007, 16, 963–970. [Google Scholar] [CrossRef]
- Tang, B.; Li, X.; Liu, Y.; Chen, X.; Li, X.; Chu, Y.; Zhu, H.; Liu, W.; Xu, F.; Zhou, F.; et al. The Therapeutic Effect of ICAM-1-Overexpressing Mesenchymal Stem Cells on Acute Graft-Versus-Host Disease. Cell Physiol. Biochem. 2018, 46, 2624–2635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, G.; Roberts, A.I.; Shi, Y. Adhesion molecules: Key players in Mesenchymal stem cell-mediated immunosuppression. Cell Adh. Migr. 2011, 5, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Rubtsov, Y.; Goryunov, K.; Romanov, A.; Suzdaltseva, Y.; Sharonov, G.; Tkachuk, V. Molecular Mechanisms of Immunomodulation Properties of Mesenchymal Stromal Cells: A New Insight into the Role of ICAM-1. Stem Cells Int. 2017, 2017, 6516854. [Google Scholar] [CrossRef] [PubMed]
- Park, C.W.; Kim, K.S.; Bae, S.; Son, H.K.; Myung, P.K.; Hong, H.J.; Kim, H. Cytokine secretion profiling of human mesenchymal stem cells by antibody array. Int. J. Stem Cells 2009, 2, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Shang, Q.; Pan, Z.; Bai, Y.; Li, Z.; Zhang, H.; Zhang, Q.; Guo, C.; Zhang, L.; Wang, Q. Exosomes From Adipose-Derived Stem Cells Attenuate Adipose Inflammation and Obesity Through Polarizing M2 Macrophages and Beiging in White Adipose Tissue. Diabetes 2018, 67, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Domenis, R.; Cifù, A.; Quaglia, S.; Pistis, C.; Moretti, M.; Vicario, A.; Parodi, P.C.; Fabris, M.; Niazi, K.R.; Soon-Shiong, P.; et al. Pro inflammatory stimuli enhance the immunosuppressive functions of adipose mesenchymal stem cells-derived exosomes. Sci. Rep. 2018, 8, 13325. [Google Scholar] [CrossRef]
- Shang, Q.; Bai, Y.; Wang, G.; Song, Q.; Guo, C.; Zhang, L.; Wang, Q. Delivery of Adipose-Derived Stem Cells Attenuates Adipose Tissue Inflammation and Insulin Resistance in Obese Mice Through Remodeling Macrophage Phenotypes. Stem Cells Dev. 2015, 24, 2052–2064. [Google Scholar] [CrossRef] [PubMed]
- Ghahremani Piraghaj, M.; Soudi, S.; Ghanbarian, H.; Bolandi, Z.; Namaki, S.; Hashemi, S.M. Effect of efferocytosis of apoptotic mesenchymal stem cells (MSCs) on C57BL/6 peritoneal macrophages function. Life Sci. 2018, 212, 203–212. [Google Scholar] [CrossRef]
- Chae, H.K.; Song, W.J.; Ahn, J.O.; Li, Q.; Lee, B.Y.; Kweon, K.; Park, S.C.; Youn, H.Y. Immunomodulatory effects of soluble factors secreted by feline adipose tissue-derived mesenchymal stem cells. Vet. Immunol. Immunopathol. 2017, 191, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Han, S.M.; Song, W.J.; Park, S.C.; Ryu, M.O.; Youn, H.Y. Anti-inflammatory Effects of Oct4/Sox2-overexpressing Human Adipose Tissue-derived Mesenchymal Stem Cells. In Vivo 2017, 31, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Gao, X.; Wang, J. Human adipose-derived mesenchymal stem cell-conditioned media suppresses inflammatory bone loss in a lipopolysaccharide-induced murine model. Exp. Ther. Med. 2018, 15, 1839–1846. [Google Scholar] [CrossRef]
- Cho, D.I.; Kim, M.R.; Jeong, H.Y.; Jeong, H.C.; Jeong, M.H.; Yoon, S.H.; Kim, Y.S.; Ahn, Y. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp. Mol. Med. 2014, 46, e70. [Google Scholar] [CrossRef] [PubMed]
- Takafuji, Y.; Hori, M.; Mizuno, T.; Harada-Shiba, M. Humoral factors secreted from adipose tissue-derived mesenchymal stem cells ameliorate atherosclerosis in Ldlr-/- mice. Cardiovasc. Res. 2018, cvy271. [Google Scholar] [CrossRef]
- Stojanović, S.; Najman, S. Macrophages—The Key Actors in Adipose Tissue Remodeling and Dysfunction. In Cell Biology—New Insights; Najman, S., Ed.; IntechOpen: London, UK, 2016; pp. 187–196. ISBN 978-953-51-2242-5. [Google Scholar]
- Cherubino, M.; Rubin, J.P.; Miljkovic, N.; Kelmendi-Doko, A.; Marra, K.G. Adipose-derived stem cells for wound healing applications. Ann. Plast. Surg. 2011, 66, 210–215. [Google Scholar] [CrossRef]
- Maxson, S.; Lopez, E.A.; Yoo, D.; Danilkovitch-Miagkova, A.; Leroux, M.A. Concise review: Role of mesenchymal stem cells in wound repair. Stem Cells Transl. Med. 2012, 1, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Ridiandries, A.; Tan, J.T.M.; Bursill, C.A. The Role of Chemokines in Wound Healing. Int. J. Mol. Sci. 2018, 19, 3217. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jin, S.Y.; Song, J.S.; Seo, K.K.; Cho, K.H. Paracrine effects of adipose-derived stem cells on keratinocytes and dermal fibroblasts. Ann. Dermatol. 2012, 24, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.T.; Redmond, S.L.; Marano, R.J.; Atlas, M.D.; von Unge, M.; Aabel, P.; Dilley, R.J. Paracrine Activity from Adipose-Derived Stem Cells on In Vitro Wound Healing in Human Tympanic Membrane Keratinocytes. Stem Cells Dev. 2017, 26, 405–418. [Google Scholar] [CrossRef]
- Guo, J.; Hu, H.; Gorecka, J.; Bai, H.; He, H.; Assi, R.; Isaji, T.; Wang, T.; Setia, O.; Lopes, L.; et al. Adipose-derived mesenchymal stem cells accelerate diabetic wound healing in a similar fashion as bone marrow-derived cells. Am. J. Physiol. Cell. Physiol. 2018, 315, C885–C896. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Yang, D.; Xu, J.; Si, Z.; Jin, X.; Zhang, J. Locally administered adipose-derived stem cells accelerate wound healing through differentiation and vasculogenesis. Cell Transplant. 2011, 20, 205–216. [Google Scholar] [CrossRef]
- Osugi, M.; Katagiri, W.; Yoshimi, R.; Inukai, T.; Hibi, H.; Ueda, M. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng. Part A 2012, 18, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, W.; Osugi, M.; Kawai, T.; Ueda, M. Novel cell-free regeneration of bone using stem cell-derived growth factors. Int. J. Oral Maxillofac. Implants 2013, 28, 1009–1016. [Google Scholar] [CrossRef]
- Borovikova, A.A.; Ziegler, M.E.; Banyard, D.A.; Wirth, G.A.; Paydar, K.Z.; Evans, G.R.D.; Widgerow, A.D. Adipose-Derived Tissue in the Treatment of Dermal Fibrosis: Antifibrotic Effects of Adipose-Derived Stem Cells. Ann. Plast. Surg. 2018, 80, 297–307. [Google Scholar] [CrossRef]
- Živković, J.M.; Najman, S.J.; Vukelić, M.Đ.; Stojanović, S.; Aleksić, M.V.; Stanisavljević, M.N.; Najdanović, J.G. Osteogenic effect of inflammatory macrophages loaded onto mineral bone substitute in subcutaneous implants. Arch. Biol. Sci. 2015, 67, 173–186. [Google Scholar] [CrossRef]
- Shiau, M.Y.; Chiou, H.L.; Lee, Y.L.; Kuo, T.M.; Chang, Y.H. Establishment of a consistent L929 bioassay system for TNF-alpha quantitation to evaluate the effect of lipopolysaccharide, phytomitogens and cytodifferentiation agents on cytotoxicity of TNF-alpha secreted by adherent human mononuclear cells. Mediators Inflamm. 2001, 10, 199–208. [Google Scholar] [CrossRef]
- Weissgerber, T.L.; Milic, N.M.; Winham, S.J.; Garovic, V.D. Beyond bar and line graphs: Time for a new data presentation paradigm. PLoS Biol. 2015, 13, e1002128. [Google Scholar] [CrossRef]






| Type of Conditioned Media (CM) | Ratio between NR Assay and CV Test | Ratio between NBT Test and CV Test | Ratio between MTT Test and CV Test | |||
|---|---|---|---|---|---|---|
| LDSC-CM | 1.25 ± 0.11 | p = 0.1 | 1.33 ± 0.18 | p = 0.06 | 1.04 ± 0.16 | p = 0.23 |
| ADSC-CM | 1.13 ± 0.10 | 1.12 ± 0.14 | 0.92 ± 0.14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojanović, S.; Najman, S. The Effect of Conditioned Media of Stem Cells Derived from Lipoma and Adipose Tissue on Macrophages’ Response and Wound Healing in Indirect Co-culture System In Vitro. Int. J. Mol. Sci. 2019, 20, 1671. https://doi.org/10.3390/ijms20071671
Stojanović S, Najman S. The Effect of Conditioned Media of Stem Cells Derived from Lipoma and Adipose Tissue on Macrophages’ Response and Wound Healing in Indirect Co-culture System In Vitro. International Journal of Molecular Sciences. 2019; 20(7):1671. https://doi.org/10.3390/ijms20071671
Chicago/Turabian StyleStojanović, Sanja, and Stevo Najman. 2019. "The Effect of Conditioned Media of Stem Cells Derived from Lipoma and Adipose Tissue on Macrophages’ Response and Wound Healing in Indirect Co-culture System In Vitro" International Journal of Molecular Sciences 20, no. 7: 1671. https://doi.org/10.3390/ijms20071671





