Photo-Triggered Reversible Phase Transfer of Azobenzene-Based Ionic Liquid Surfactants between Oil and Water
Abstract
:1. Introduction
2. Results and Discussion
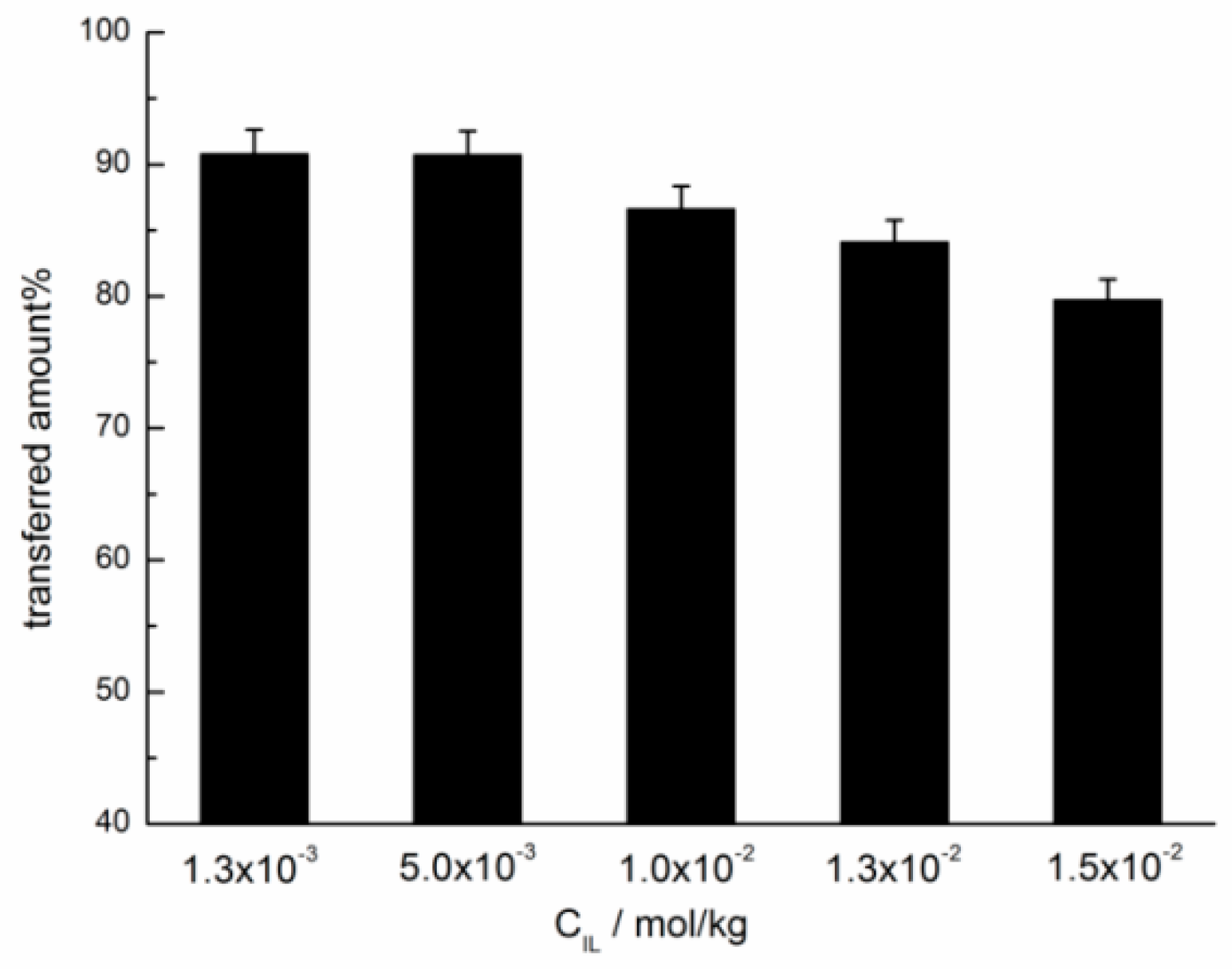
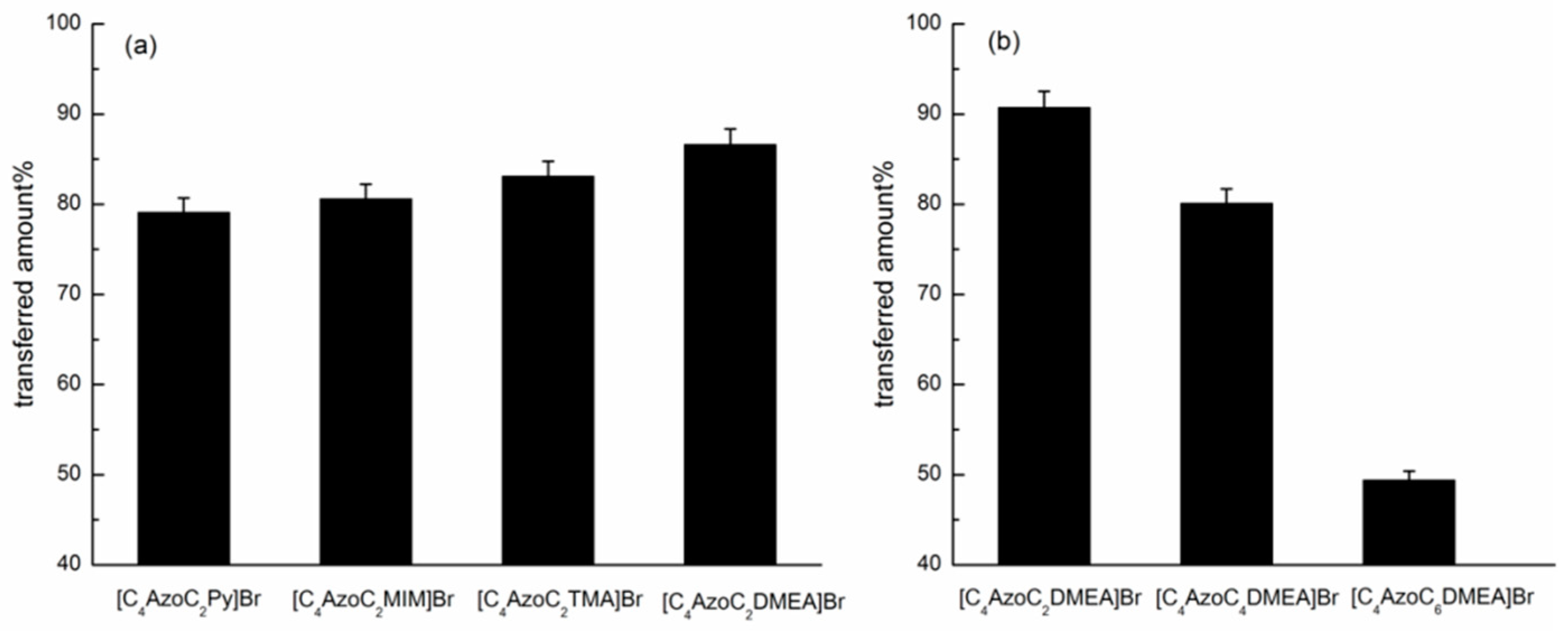
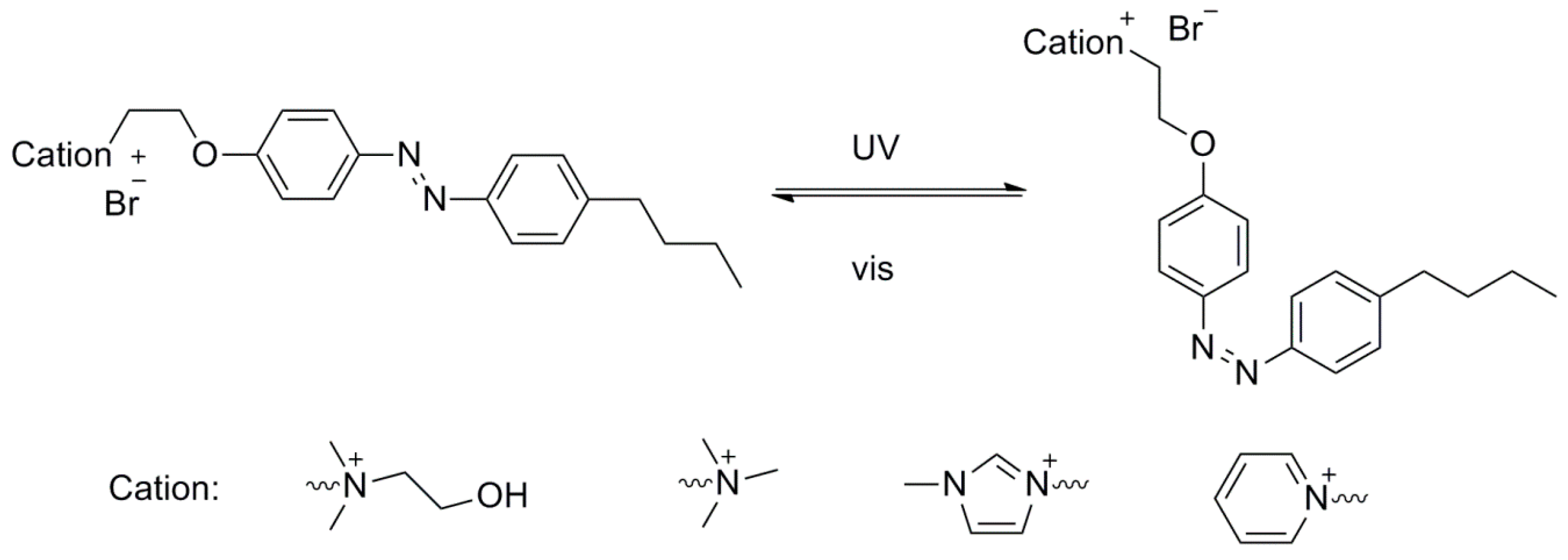
2.1. Photo-Triggered Reversible Phase Transfer of the Azobenzene-Based IL Surfactants
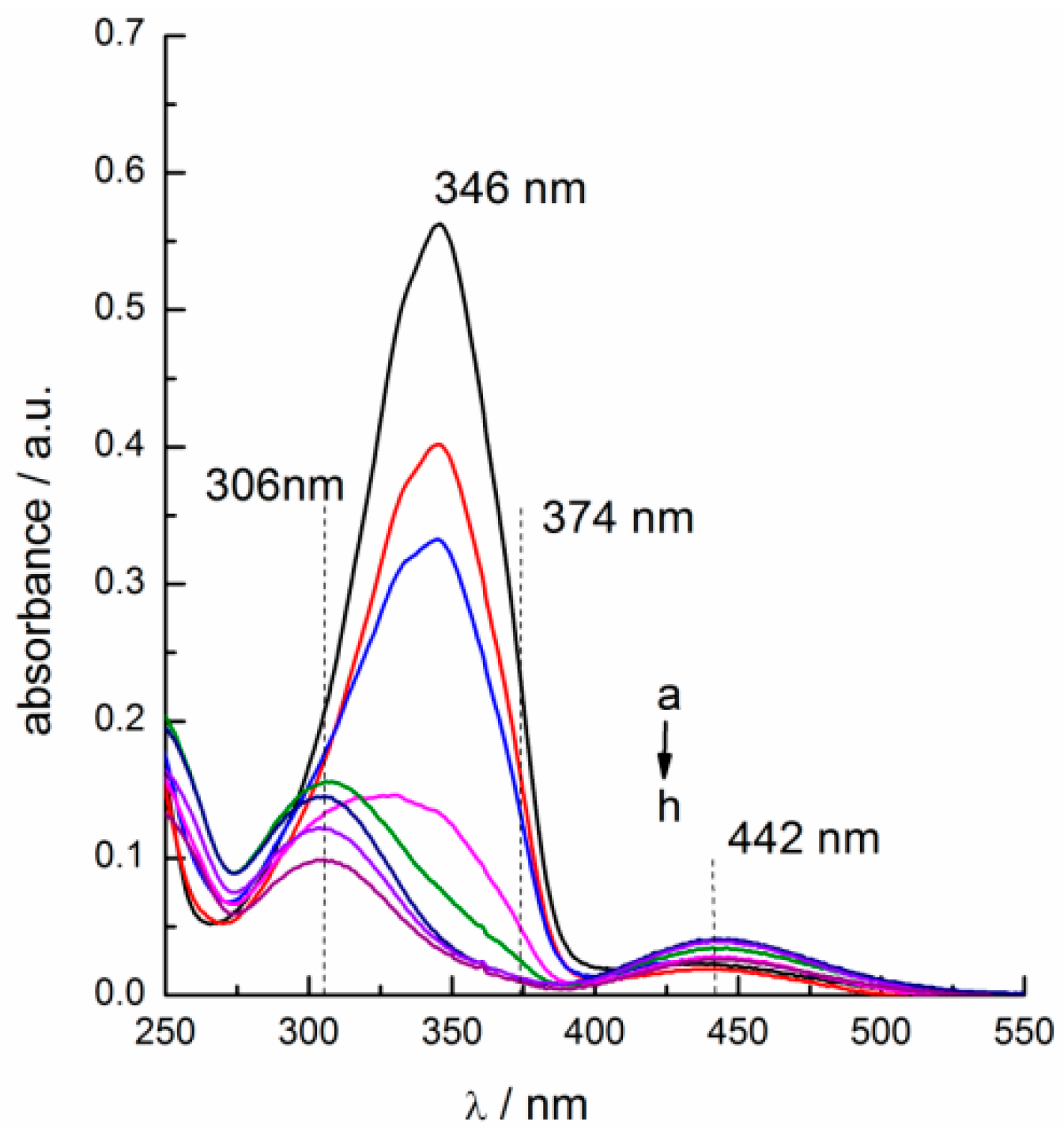
2.2. Photo-Isomerization of the Azobenzene-Based IL Surfactants
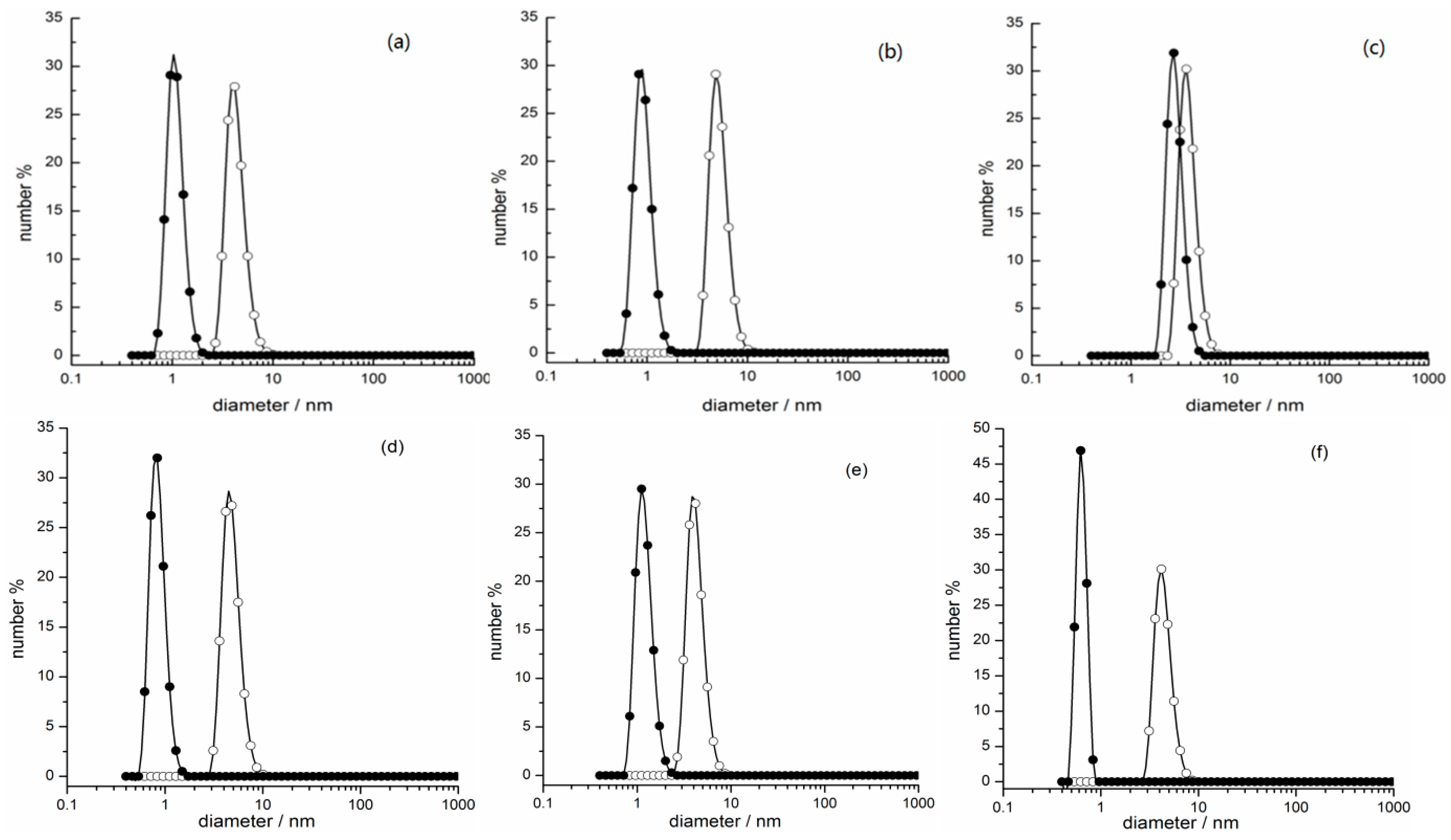
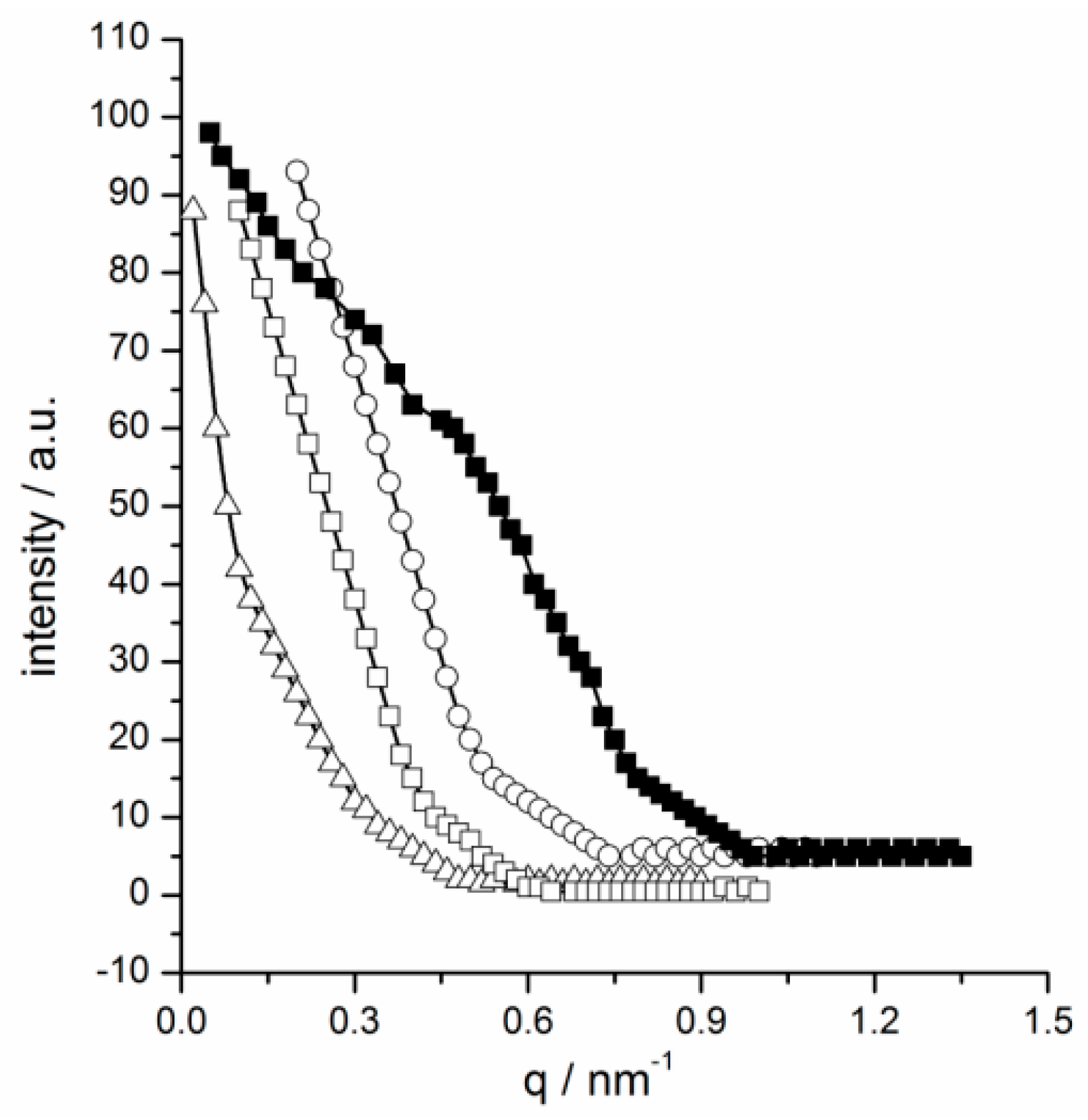
2.3. Photo-Regulated Aggregation of the IL Surfactants
3. Materials and Methods
3.1. Chemicals
3.2. UV-vis Spectrum Measurements
3.3. Partitioning of the Azobenzene-Based IL Surfactants in the Biphasic Oil/Water Systems
3.4. Dynamic Light Scattering Measurements
3.5. Small Angle X-ray Scattering Measurements
3.6. Polarity Measurements of Organic Phase and Water Phase Containing Azobenzene-Based IL Surfactants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
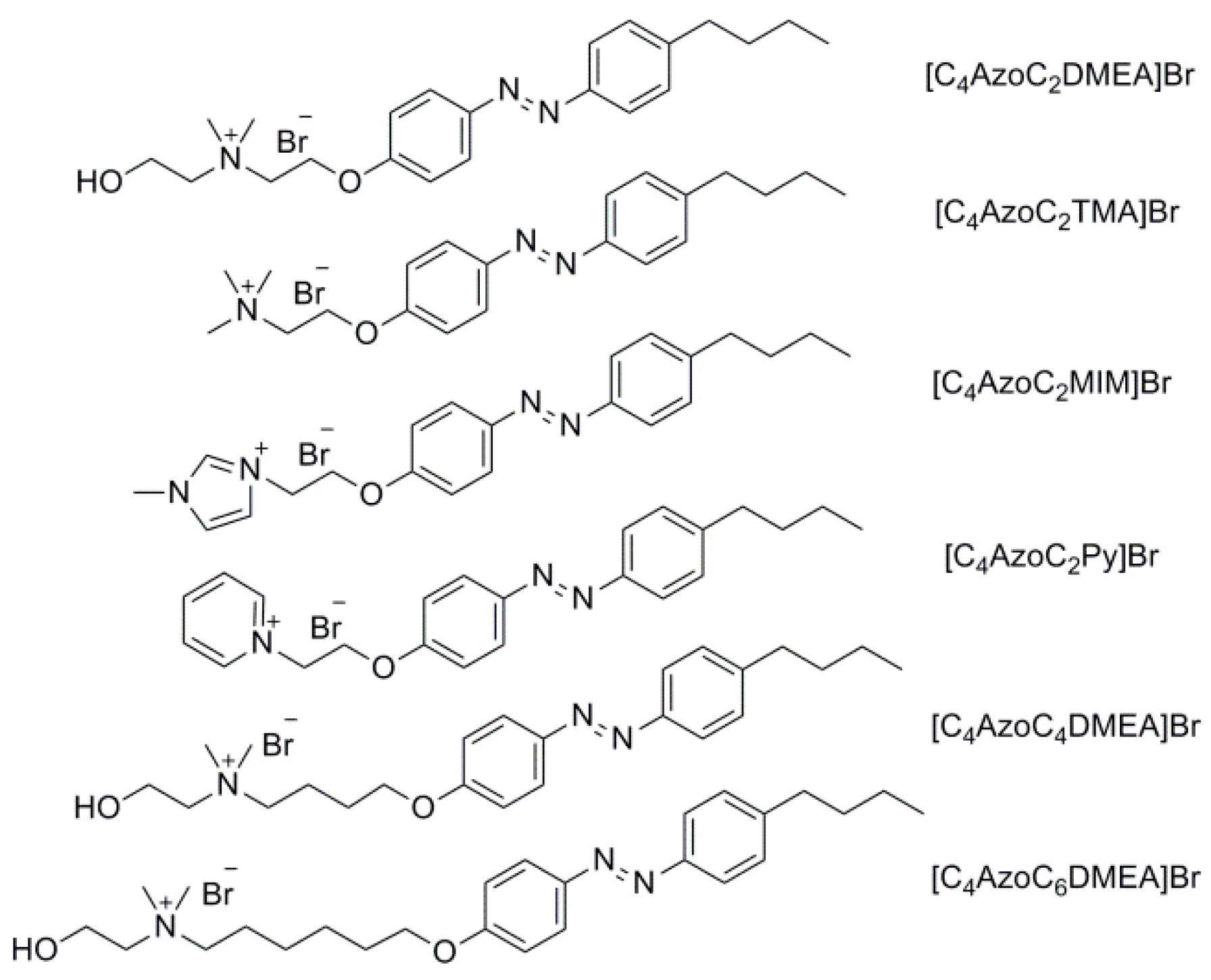
| [C4AzoC2DMEA]Br | 2-(4-((4-butylphenyl)diazenyl)phenoxy)-N-(2-hydroxyethyl)-N,N-dimethyl-ethanaminium bromide |
| [C4AzoC4DMEA]Br | 4-(4-((4-butylphenyl)diazenyl)phenoxy)-N-(2-hydroxyethyl)-N,N-dimethylbutanaminium bromide |
| [C4AzoC6DMEA]Br | 6-(4-((4-butylphenyl)diazenyl)-phenoxy)-N-(2-hydroxyethyl)-N,N-dimethylhexanaminium bromide |
| [C4AzoC2TMA]Br | 2-(4-((4-butylphenyl)diazenyl)phenoxy)-N,N,N-trimethylethanaminium bromide |
| [C4AzoC2MIM]Br | 3-(2-(4-((4-butylphenyl)diazenyl)phenoxy)ethyl)-1-methyl-imidazolium bromide |
| [C4AzoC2Py]Br | 1-(2-(4-((4-butylphenyl)diazenyl) phenoxy)ethyl)pyridinium bromide |
References
- Liu, G.; Wang, J. Recycling a homogeneous catalyst through a light-controlled phase tag. Angew. Chem. Int. Ed. 2010, 49, 4425–4429. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, B.; Wang, Y.; Yue, L.; Li, W.; Wu, L. A photo-driven polyoxometalate complex shuttle and its homogeneous catalysis and heterogeneous separation. J. Am. Chem. Soc. 2013, 135, 14500–14503. [Google Scholar] [CrossRef]
- Yang, J.; Lee, J.Y.; Ying, J.Y. Phase transfer and its applications in nanotechnology. Chem. Soc. Rev. 2011, 40, 1672–1696. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, J.; Ying, J.Y. Reversible phase transfer of quantum dots and metal nanoparticles. Chem. Commun. 2010, 46, 3179–3181. [Google Scholar] [CrossRef]
- Stocco, A.; Chanana, M.; Su, G.; Cernoch, P.; Binks, B.P.; Wang, D. Bidirectional nanoparticle crossing of oil-water interfaces induced by different stimuli: Insight into phase transfer. Angew. Chem. Int. Ed. 2012, 51, 9647–9651. [Google Scholar] [CrossRef] [PubMed]
- Arisaka, A.; Mogaki, R.; Okuro, K.; Aida, T. Caged molecular glues as photoactivatable tags for nuclear translocation of guests in living cells. J. Am. Chem. Soc. 2018, 140, 2687–2692. [Google Scholar] [CrossRef] [PubMed]
- Sakai, N.; Matile, S. Anion-mediated transfer of polyarginine across liquid and bilayer membranes. J. Am. Chem. Soc. 2003, 125, 14348–14356. [Google Scholar] [CrossRef] [PubMed]
- Desset, S.L.; Cole-Hamilton, D.J. Carbon dioxide induced phase switching for homogeneous-catalyst recycling. Angew. Chem. Int. Ed. 2009, 48, 1472–1474. [Google Scholar] [CrossRef]
- Grosseheilmann, J.; Kragl, U. Simple and effective catalyst separation by new CO2-induced switchable organocatalysts. Chemsuschem 2017, 10, 2685–2691. [Google Scholar] [CrossRef] [PubMed]
- Pocovi-Martinez, S.; Frances-Soriano, L.; Zaballos-Garcia, E.; Scaiano, J.C.; Gonzalez-Bejar, M.; Perez-Prieto, J. CO2 switchable nanoparticles: Reversible water/organic-phase exchange of gold nanoparticles by gas bubbling. Rsc Adv. 2013, 3, 4867–4871. [Google Scholar] [CrossRef]
- Pei, X.; Xiong, D.; Wang, H.; Gao, S.; Zhang, X.; Zhang, S.; Wang, J. Reversible phase transfer of carbon dots between an organic phase and aqueous solution triggered by CO2. Angew. Chem. Int. Ed. 2018, 57, 3687–3691. [Google Scholar] [CrossRef]
- Qin, B.; Zhao, Z.; Song, R.; Shanbhag, S.; Tang, Z. A temperature-driven reversible phase transfer of 2-(diethylamino)ethanethiol-stabilized cdte nanoparticles. Angew. Chem. Int. Ed. 2008, 47, 9875–9878. [Google Scholar] [CrossRef]
- Horton, J.M.; Bao, C.H.; Bai, Z.F.; Lodge, T.P.; Zhao, B. Temperature- and pH-triggered reversible transfer of doubly responsive hairy particles between water and a hydrophobic ionic liquid. Langmuir 2011, 27, 13324–13334. [Google Scholar] [CrossRef]
- Yao, W.H.; Wang, H.Y.; Cui, G.K.; Li, Z.Y.; Zhu, A.L.; Zhang, S.J.; Wang, J.J. Tuning the hydrophilicity and hydrophobicity of the respective cation and anion: Reversible phase transfer of ionic liquids. Angew. Chem. Int. Ed. 2016, 55, 7934–7938. [Google Scholar] [CrossRef]
- Ueki, T.; Sawamura, S.; Nakamura, Y.; Kitazawa, Y.; Kokubo, H.; Watanabe, M. Thermoreversible nanogel shuttle between ionic liquid and aqueous phases. Langmuir 2013, 29, 13661–13665. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.L.; Wang, T.; Xie, Q.X.; Yu, X.B.; Guo, W.J.; Wu, S.T.; Li, D.F.; Wang, J.H.; Liu, G.Y. A pH-controlled recyclable indolinooxazolidine tagged N-heterocyclic carbene Ru catalyst for olefin metathesis. Dalton Trans. 2017, 46, 5986–5993. [Google Scholar] [CrossRef] [PubMed]
- Richter, T.V.; Bueher, C.; Ludwigs, S. Water- and ionic-liquid-soluble branched polythiophenes bearing anionic and cationic moieties. J. Am. Chem. Soc. 2012, 134, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.X.; Yin, T.X.; Tao, X.Y.; Shen, W.G. Light induced micelle to vesicle transition in an aqueous solution of a surface active ionic liquid. Rsc Adv. 2015, 5, 75806–75809. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, N.; Wang, Z.; Zhang, X. Photocontrolled reversible supramolecular assemblies of an azobenzene-containing surfactant with alpha-cyclodextrin. Angew. Chem. Int. Ed. 2007, 46, 2823–2826. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, S.; Zhang, Q.; Deng, Y. Solvent-dependent photoresponsive conductivity of azobenzene-appended ionic liquids. Chem. Commun. 2011, 47, 6641–6643. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, C.; Qu, X.; Liu, Z.; Yang, Z. Light-triggered reversible phase transfer of composite colloids. Langmuir 2010, 26, 9442–9448. [Google Scholar] [CrossRef]
- Peng, L.; You, M.; Wu, C.; Han, D.; Ocsoy, I.; Chen, T.; Chen, Z.; Tan, W. Reversible phase transfer of nanoparticles based on photoswitchable host-guest chemistry. Acs Nano 2014, 8, 2555–2561. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Ma, W.; Theyssen, N.; Chen, C.; Hou, Z. Temperature-responsive ionic liquids: Fundamental behaviors and catalytic applications. Chem. Rev. 2017, 117, 6881–6928. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, G.; He, H.; Zhang, X.; Wang, J.; Zhang, S. Rodlike micelle structure and formation of ionic liquid in aqueous solution by molecular simulation. Ind. Eng. Chem. Res. 2015, 54, 1681–1688. [Google Scholar] [CrossRef]
- Jiao, T.; Zhuang, X.; He, H.; Zhao, L.; Li, C.; Chen, H.; Zhang, S. An ionic liquid extraction process for the separation of indole from wash oil. Green Chem. 2015, 1, 3783–3790. [Google Scholar] [CrossRef]
- Fihey, A.; Perrier, A.; Browne, W.R.; Jacquemin, D. Multiphotochromic molecular systems. Chem. Soc. Rev. 2015, 44, 3719–3759. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, H.; Chu, M.; Guan, P.; Zhao, Y.; Zhao, Y.; Wang, J. Photo-isomerization and light-modulated aggregation behavior of azobenzene-based ionic liquids in aqueous solutions. Rsc Adv. 2017, 7, 44688–44695. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.; Lu, F.; Sun, P.; Gao, X.; Shi, L.; Zheng, L. Photoresponsive self-assembly of surface active ionic liquid. Langmuir 2016, 32, 8163–8170. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, L.; Bing, W.; Zhang, Z.; Li, Z.; Ren, J.; Qu, X. Light controlled reversible inversion of nanophosphor-stabilized pickering emulsions for biphasic enantioselective biocatalysis. J. Am. Chem. Soc. 2014, 136, 7498–7504. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, X.; Feng, Y.; Chen, Y.; Zhao, Y.; Wang, H.; Xu, Q.; Wang, J. A reversible conductivity modulation of azobenzene-based ionic liquids in aqueous solutions using UV/vis light. Phys. Chem. Chem. Phys. 2018, 20, 12808–12816. [Google Scholar] [CrossRef] [PubMed]
- Zakrevskyy, Y.; Richter, M.; Zakrevska, S.; Lomadze, N.; von Klitzing, R.; Santer, S. Light-controlled reversible manipulation of microgel particle size using azobenzene-containing surfactant. Adv. Fun. Mater. 2012, 22, 5000–5009. [Google Scholar] [CrossRef]
- Lu, T.; Huang, J.; Liang, D. Salt effect on microstructures in cationic gemini surfactant solutions as studied by dynamic light scattering. Langmuir 2008, 24, 1740–1744. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, C. Polarity of ionic liquids determined empirically by means of solvatochromic pyridinium N-phenolate betaine dyes. Green Chem. 2005, 7, 339–351. [Google Scholar] [CrossRef]

| Organic Phase | R | IL Transfer Efficiency % |
|---|---|---|
| n-octanol | 1:2 | 70.8 |
| Mixed organic solvent 1 | 1:1 | 66.0 |
| Mixed organic solvent 1 | 1:1.5 | 81.1 |
| Mixed organic solvent 1 | 1:2 | 86.6 |
| Mixed organic solvent 1 | 1:2.5 | 89.5 |
| Compound | Isomerization Efficiency % |
|---|---|
| [C4AzoC2Py]Br 1 | 92.9 |
| [C4AzoC2MIM]Br 1 | 93.6 |
| [C4AzoC2TMA]Br 1 | 94.2 |
| [C4AzoC2DMEA]Br 1 | 95.2 |
| [C4AzoC2DMEA]Br 2 | 94.9 |
| [C4AzoC4DMEA]Br 2 | 93.1 |
| [C4AzoC6DMEA]Br 2 | 91.5 |
| Compound | Organic Phase | Water Phase | ||||
|---|---|---|---|---|---|---|
| Before UV Irradiation | After UV Irradiation | δET(30) | Before UV Irradiation | After UV Irradiation | δET(30) | |
| [C4AzoC2DMEA]Br | 82.3 | 83.8 | 1.5 | 83.2 | 84.7 | 1.5 |
| [C4AzoC4DMEA]Br | 82.3 | 83.5 | 1.2 | 82.9 | 84.3 | 1.4 |
| [C4AzoC6DMEA]Br | 82.9 | 83.5 | 0.6 | 83.5 | 84.3 | 0.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Feng, Y.; Yuan, X.; Wang, H.; Zhao, Y.; Wang, J. Photo-Triggered Reversible Phase Transfer of Azobenzene-Based Ionic Liquid Surfactants between Oil and Water. Int. J. Mol. Sci. 2019, 20, 1685. https://doi.org/10.3390/ijms20071685
Li Z, Feng Y, Yuan X, Wang H, Zhao Y, Wang J. Photo-Triggered Reversible Phase Transfer of Azobenzene-Based Ionic Liquid Surfactants between Oil and Water. International Journal of Molecular Sciences. 2019; 20(7):1685. https://doi.org/10.3390/ijms20071685
Chicago/Turabian StyleLi, Zhiyong, Ying Feng, Xiaoqing Yuan, Huiyong Wang, Yuling Zhao, and Jianji Wang. 2019. "Photo-Triggered Reversible Phase Transfer of Azobenzene-Based Ionic Liquid Surfactants between Oil and Water" International Journal of Molecular Sciences 20, no. 7: 1685. https://doi.org/10.3390/ijms20071685
APA StyleLi, Z., Feng, Y., Yuan, X., Wang, H., Zhao, Y., & Wang, J. (2019). Photo-Triggered Reversible Phase Transfer of Azobenzene-Based Ionic Liquid Surfactants between Oil and Water. International Journal of Molecular Sciences, 20(7), 1685. https://doi.org/10.3390/ijms20071685





