Dynamic TMT-Based Quantitative Proteomics Analysis of Critical Initiation Process of Totipotency during Cotton Somatic Embryogenesis Transdifferentiation
Abstract
:1. Introduction
2. Results
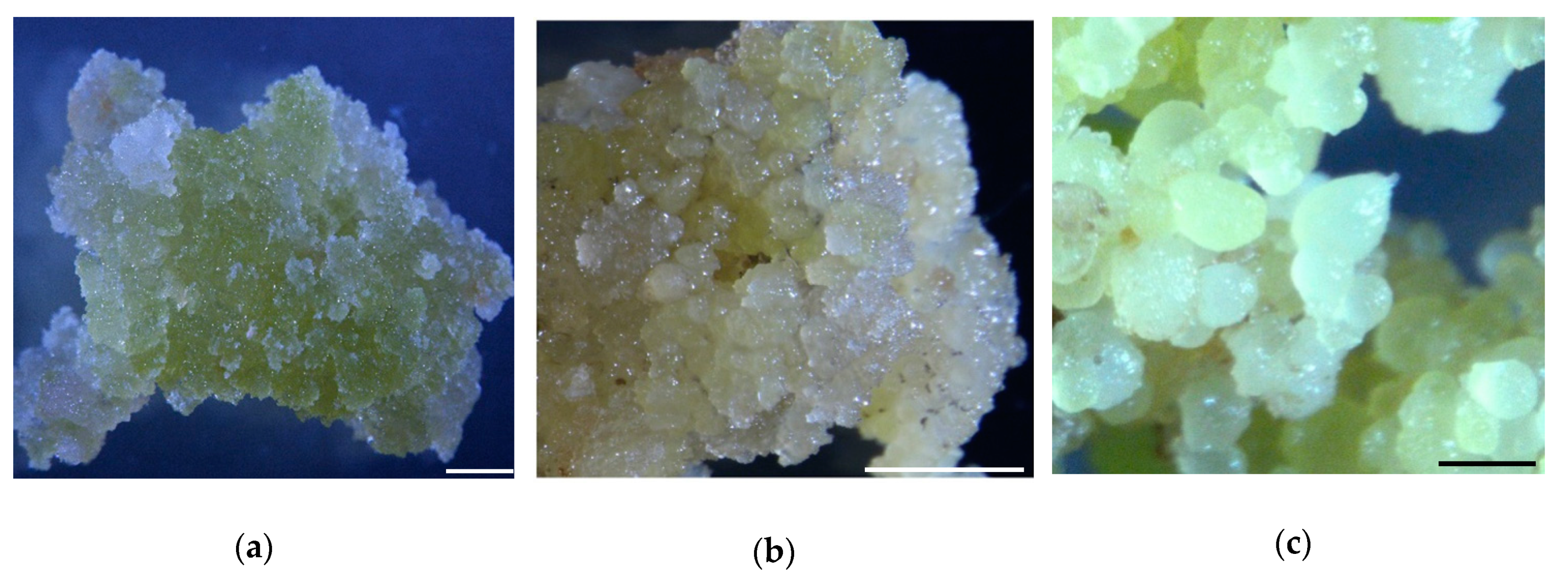
2.1. Somatic Embryogenesis in Cotton
2.2. TMT-Based Quantitative Proteomic Basis Data Analysis and Overall Protein Identification
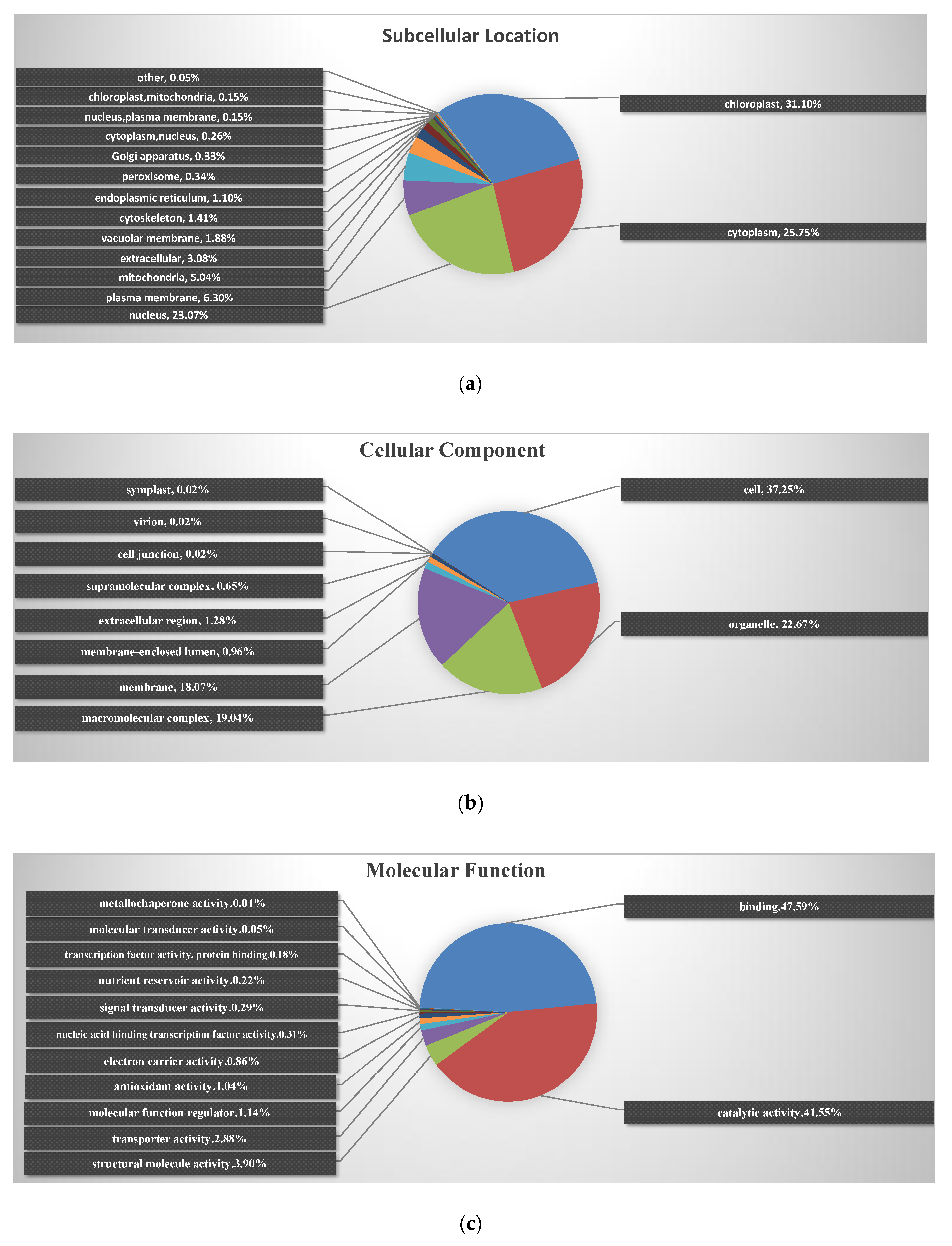
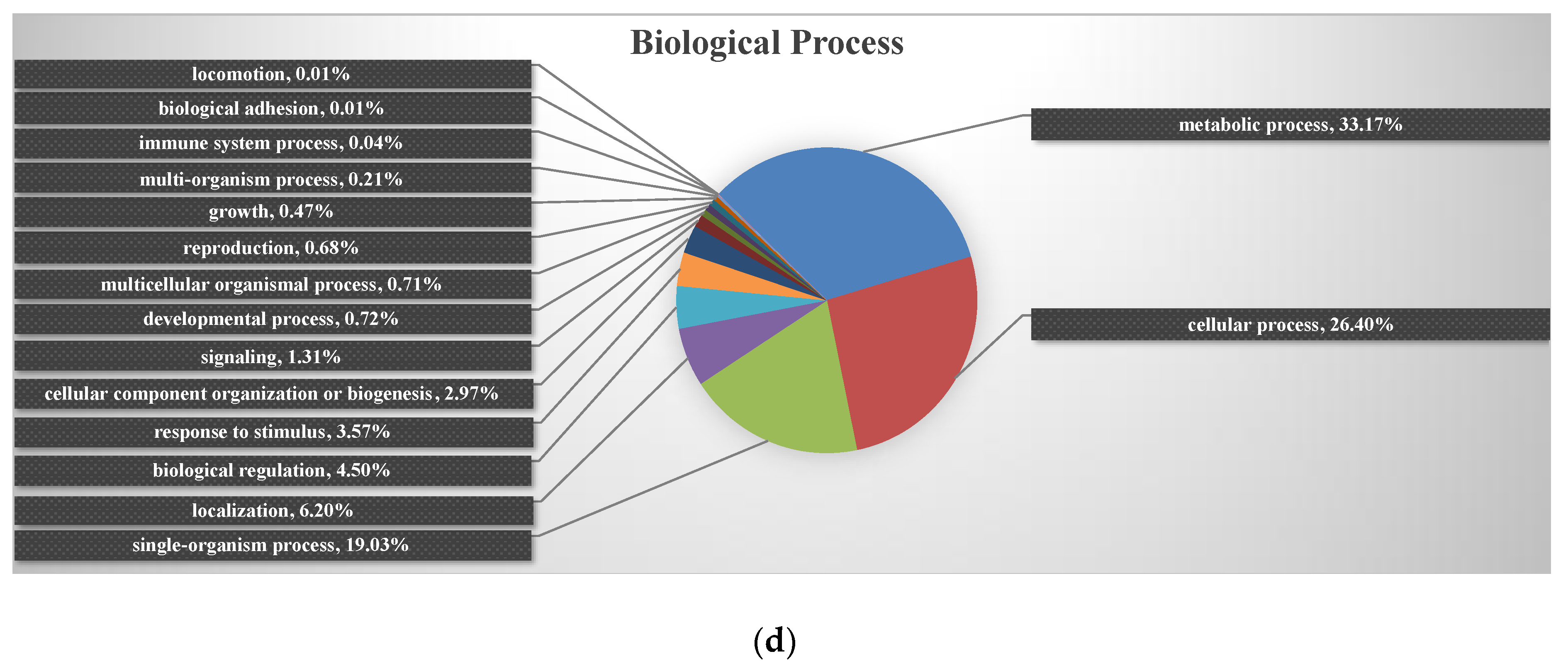
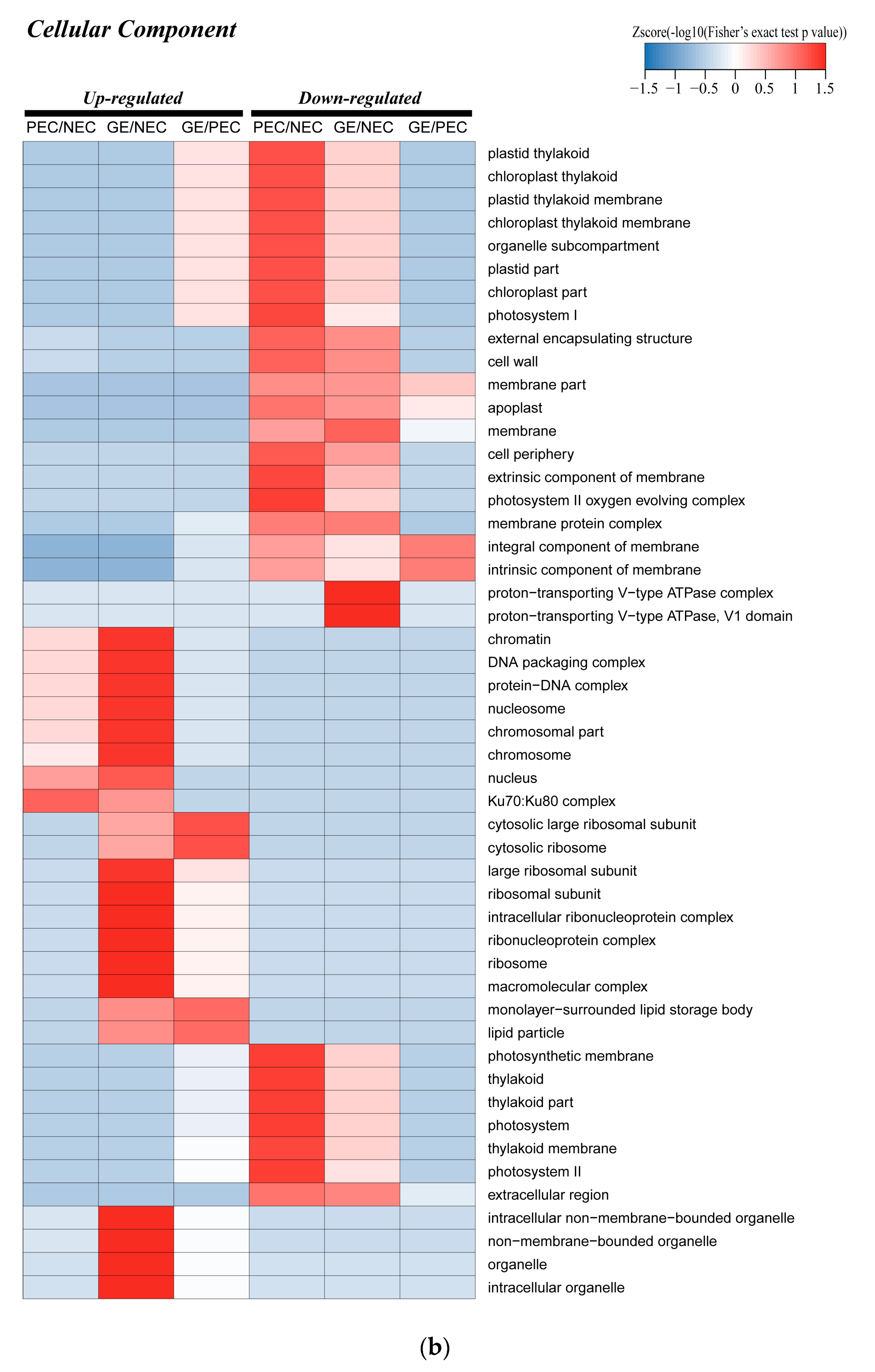
2.3. Enrichment of the Chloroplast Subcellular Location and GO Functional Classification of All Identified Proteins
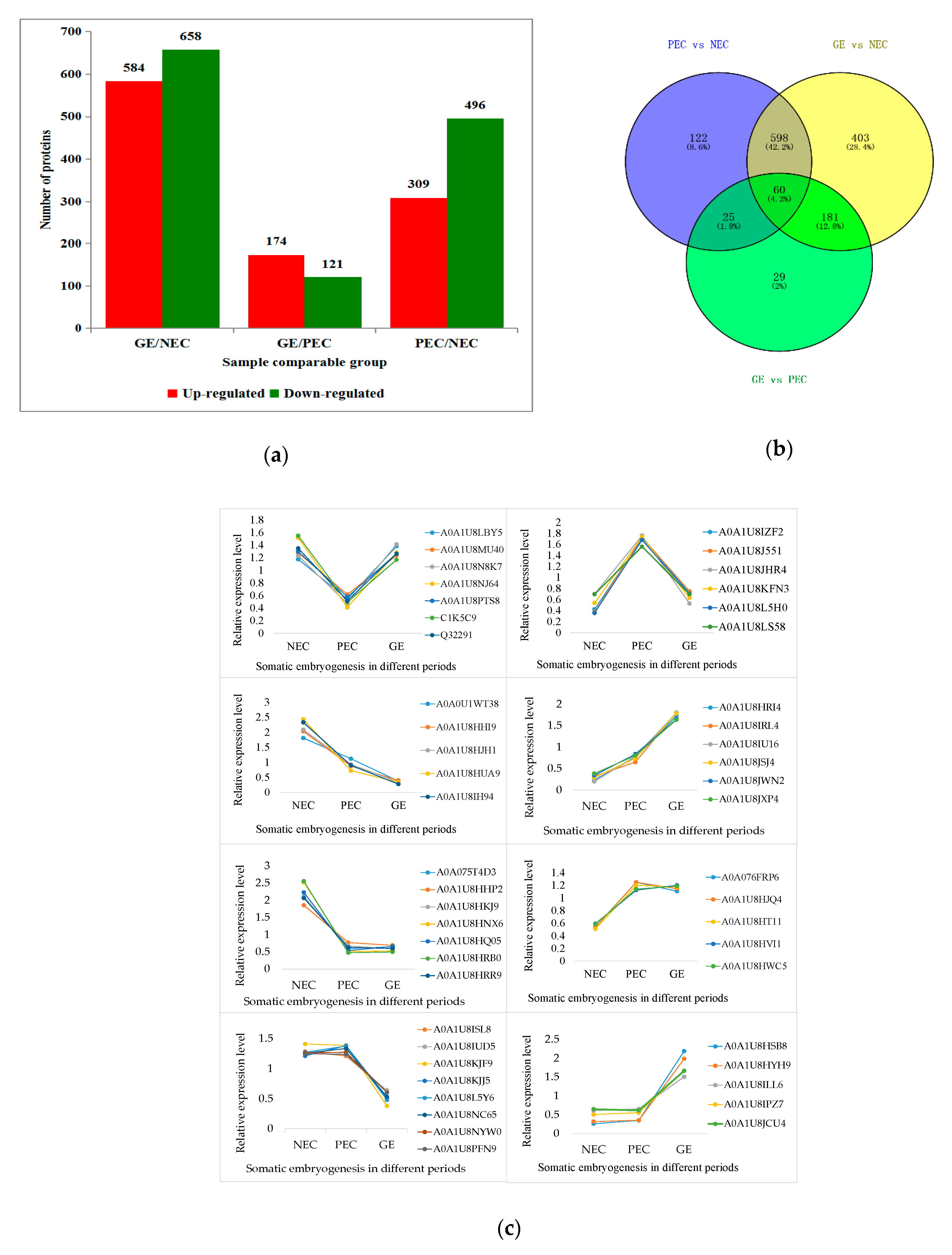
2.4. Identification of Differentially Abundant Proteins
2.5. Enrichment Analysis of DAPs in GO, KEGG and Protein Domain
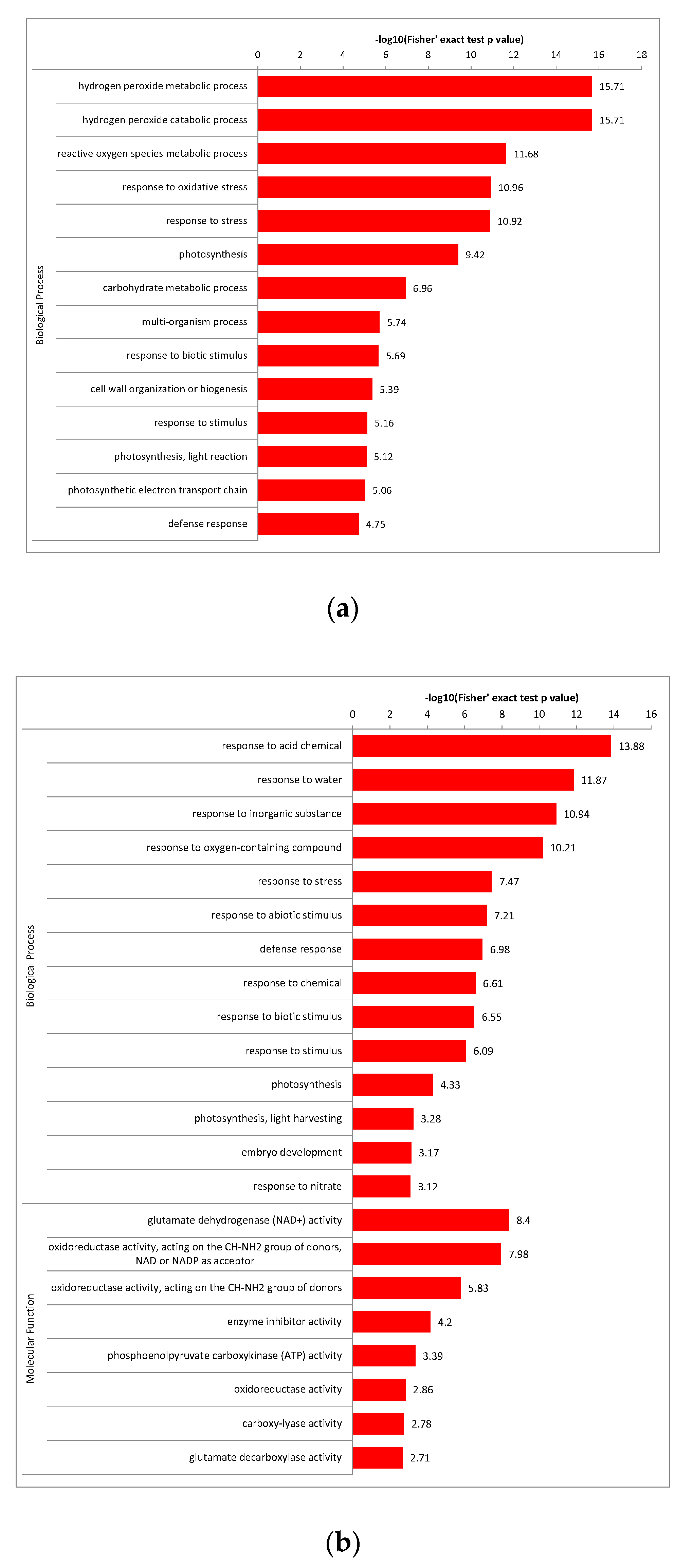
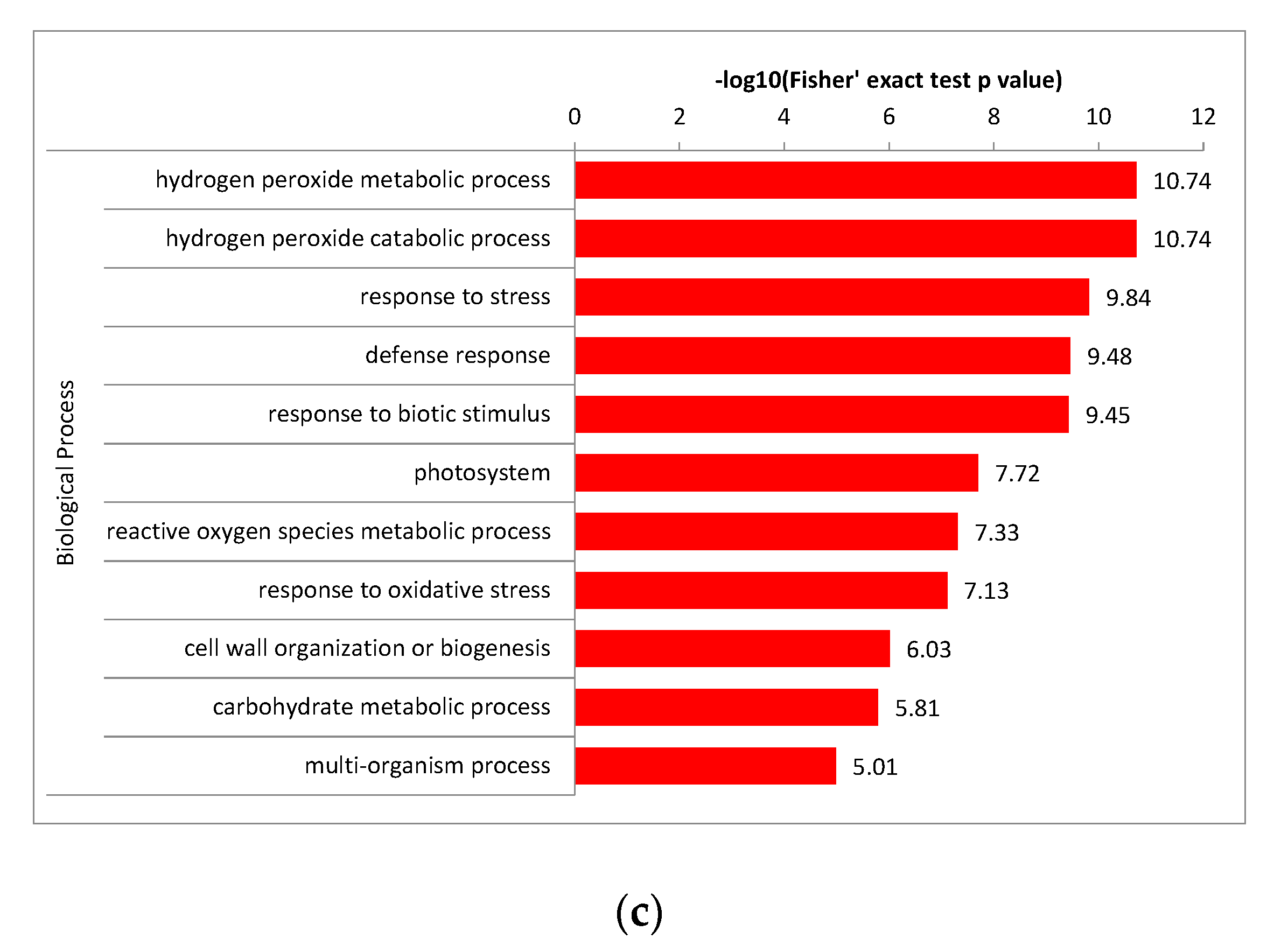
2.5.1. Enrichment Cluster Analysis of DAPs between the Groups in GO Terms
The Enzyme Metabolism Activity of Molecular Function Category in Cotton SE
The Photosynthesis-Related Proteins of the Cellular Component Category in Cotton SE
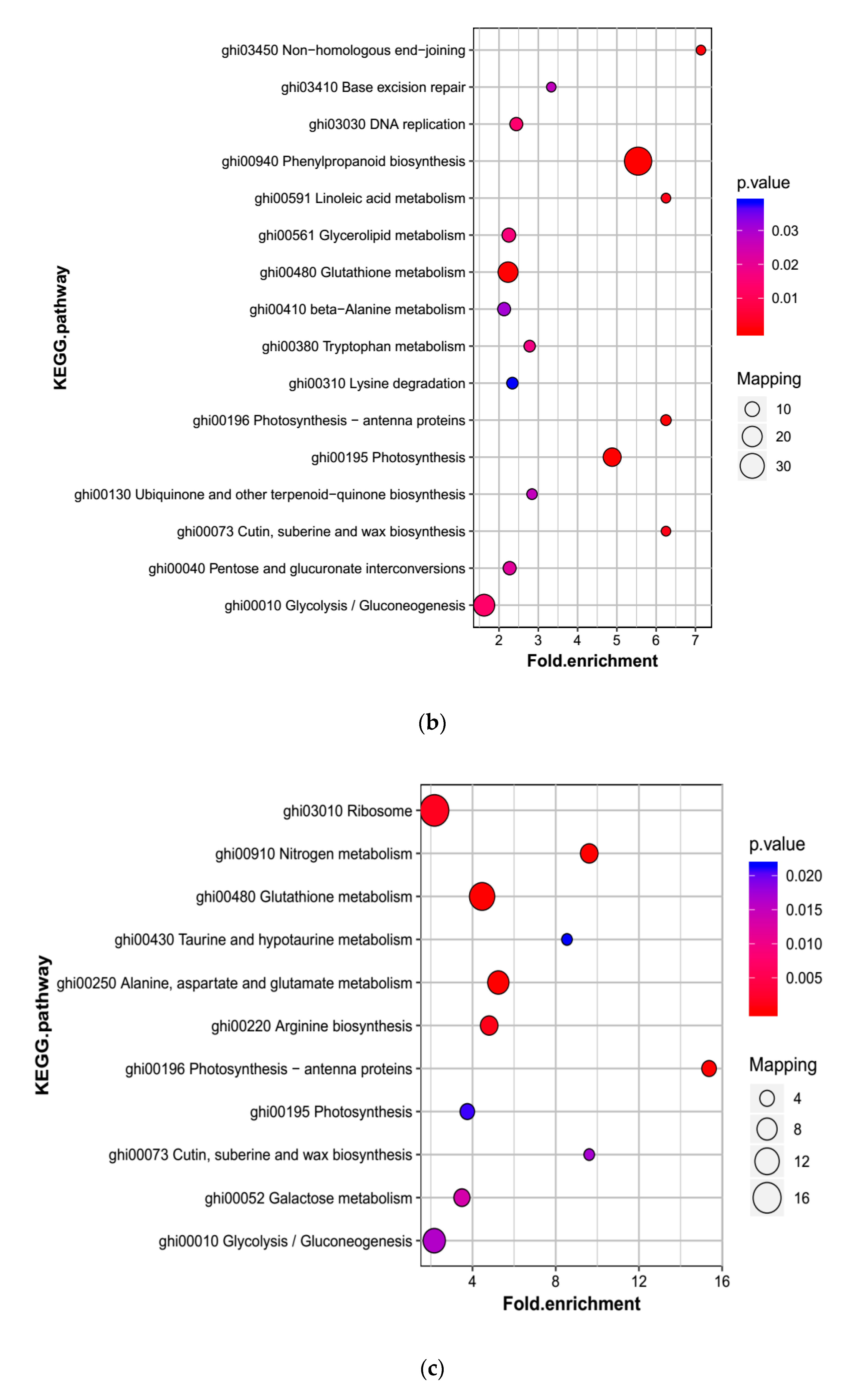
2.5.2. Enrichment Analysis in KEGG of the DAPs Involved in Phenylpropanoid Biosynthesis, Nitrogen Metabolism, Photosynthesis and Other Related Biological Processes
Enrichment Analysis in KEGG Clusters of Related Biological Processes among Groups
KEGG Pathway Enrichment Analysis of Related Biological Processes within the Sample Groups
2.5.3. Enrichment Cluster Analysis of Differential Proteins Functional Domain
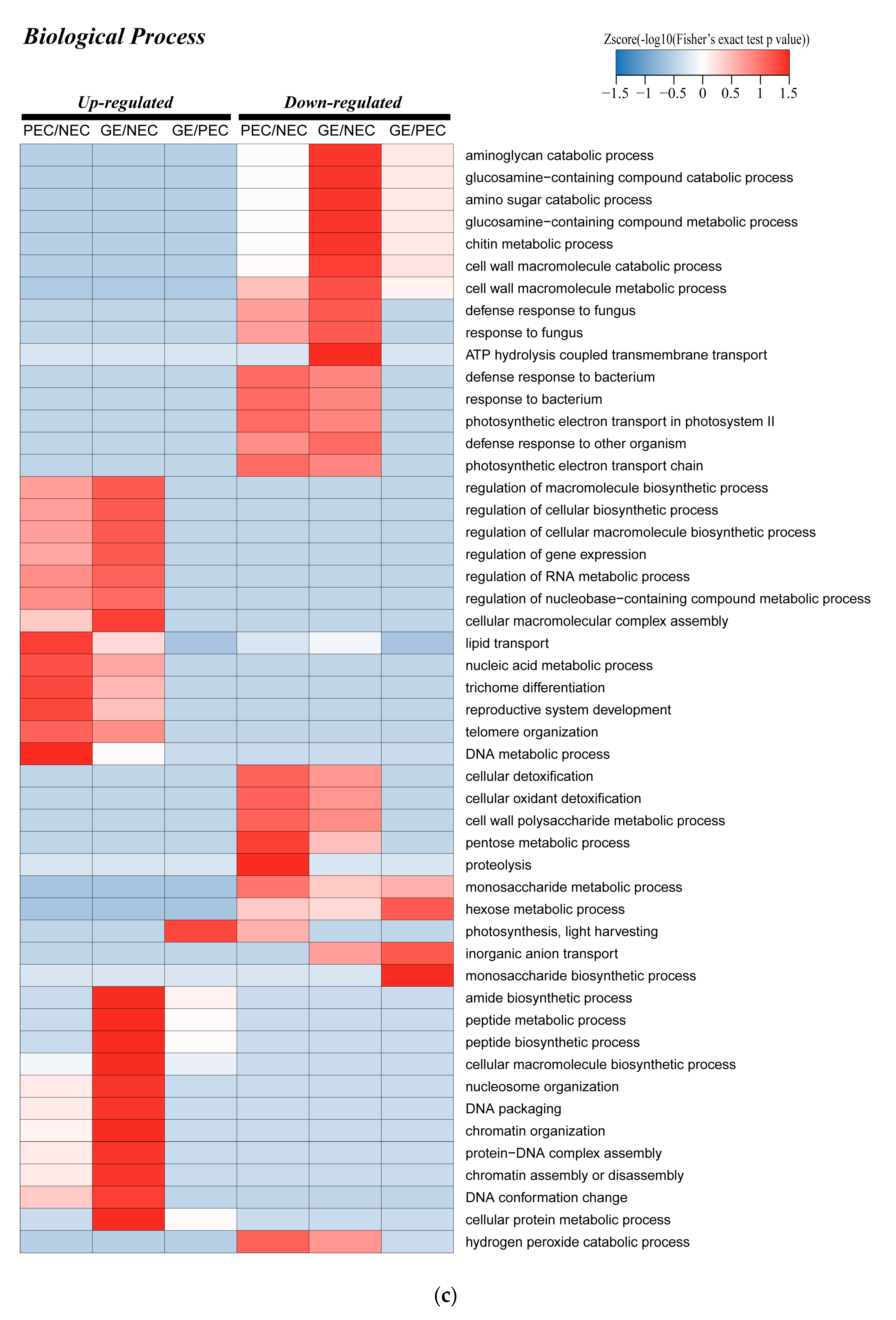
2.6. Enrichment Analysis of the Major Biological Process between Different Comparison Groups
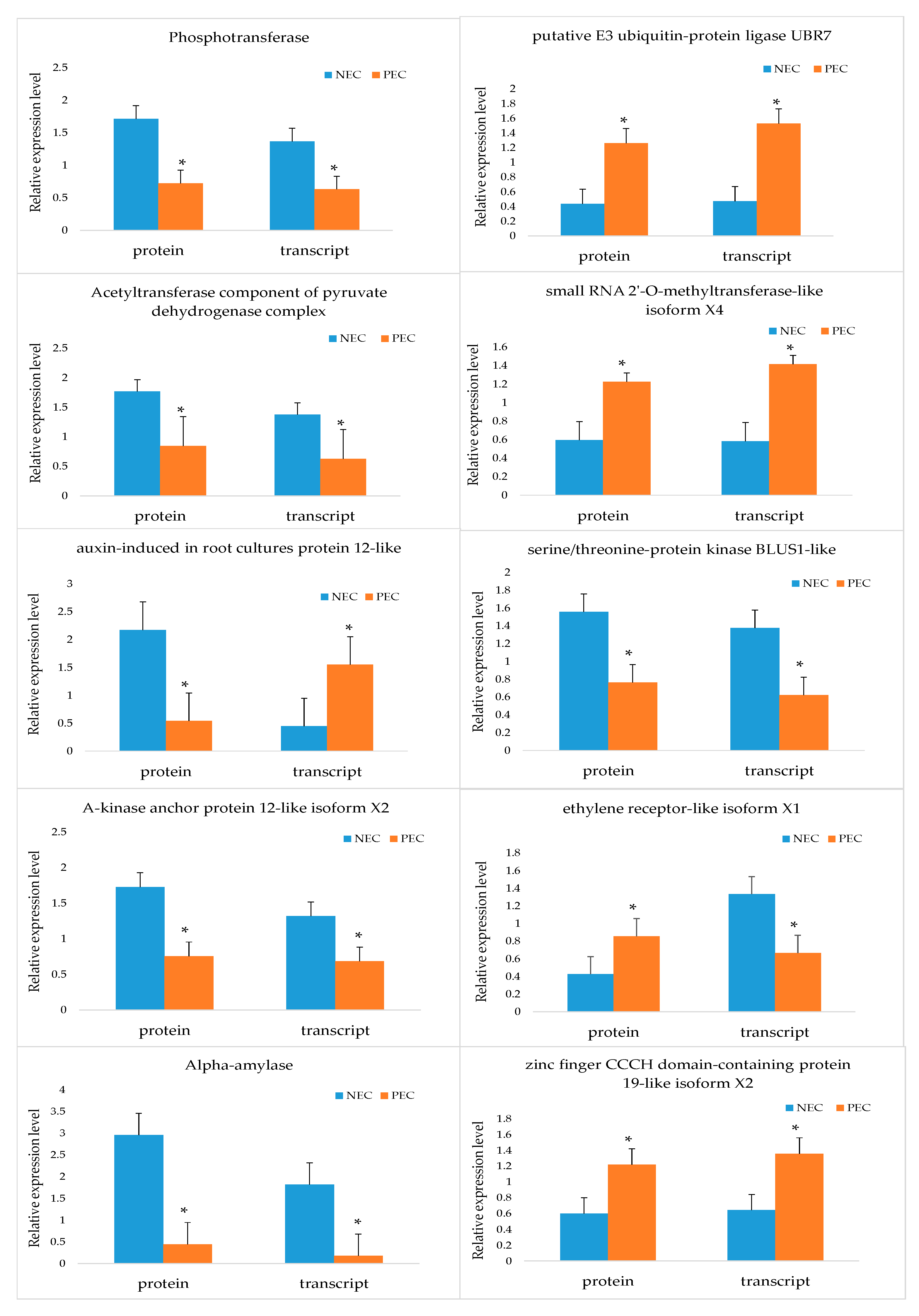
2.7. Several Major DAPs are Associated with SE Regulation and Modification
2.8. Comparative and Complementary Proteome of the Candidate DAPs
3. Discussion
3.1. DAPs Enriched in Crucial Biological Processes Associated with Cotton SE
3.1.1. Peroxidase Proteins Involved in Phenylpropanoid Biosynthesis Affect SE
3.1.2. Photosynthesis in Cotton SE
3.1.3. Response to Environment Stresses during SE of Cotton
3.1.4. Effects of Nitrogen Metabolism Related to SE
3.2. Other DAPs of Regulatory Factors Associated with Cotton SE
3.2.1. Phytohormone Response Related Proteins
3.2.2. Signal Transduction Related Proteins
3.2.3. SE Associated Proteins of Aquaporins and Fatty Acid Metabolism
3.2.4. Regulation of SE-related Proteins, Transcription, Posttranscription and Modification
4. Materials and Methods
4.1. Plant Materials and Culture Conditions
4.2. Protein Extraction and Identification
4.2.1. Protein Samples Preparation
4.2.2. Trypsin Digestion
4.2.3. TMT Labeling
4.2.4. HPLC Fractionation and LC-MS/MS Analysis
4.2.5. Database Search
4.3. Bioinformatics
4.3.1. Annotation Methods
GO Annotation
Domain Annotation
KEGG Pathway Annotation
Subcellular Localization Prediction
4.3.2. Functional Enrichment
Enrichment of Gene Ontology Analysis
Enrichment of Pathway Analysis
Enrichment of Protein Domain Analysis
Enrichment-Based Clustering
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| DAPs | Differentially accumulated proteins |
| EC | Embryogenic calli |
| GA | Gibberellin |
| GEs | Globular embryos |
| GO | Gene Ontology |
| iTRAQ | Isobaric tags for relative and absolute quantitation |
| KEGG | Kyoto encyclopedia of genes and genomes |
| MS | Murashige and Skoog |
| MSB | Vitamins medium |
| NEC | Nonembryogenic calli |
| PEC | Primary embryogenic calli |
| PEM | Proembryogenic cell mass |
| SE | Somatic embryogenesis |
| SEM | Somatic embryogenesis masses |
| SSH | Suppression subtractive hybridization |
| TMT | The isobaric labels tandem mass tags |
References
- Wilkins, T.A.; Rajasekaran, K.; Anderson, D.M. Cotton biotechnology. Crit. Rev. Plant Sci. 2000, 19, 511–550. [Google Scholar] [CrossRef]
- Brookes, G.; Barfoot, P. GM Crops: Global Socio-Economic and Environmental Impacts 1996–2010; PG Economics, Ltd.: Dorchester, UK, 2012; Available online: http://www.pgeconomics.co.uk/page/33/global-impact-2012 (accessed on 13 January 2013).
- Zhao, X.Y.; Su, Y.H.; Cheng, Z.J.; Zang, X.S. Cell fate switch during in vitro plant organogenesis. J. Integr. Plant Biol. 2008, 50, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Mujib, A. Somatic Embryogenesis in Ornamentals and Its Applications; Springer: New Delhi, India, 2016; pp. 67–86. [Google Scholar]
- Fehér, A. The initiation phase of somatic embryogenesis: What we know and what we don’t. Acta Biol. Szeged. 2008, 52, 53–56. [Google Scholar]
- Fehér, A. Somatic embryogenesis—stress-induced remodeling of plant cell fate. BBA-Gene Regul. Mech. 2015, 1849, 385–402. [Google Scholar]
- Stewards, F.C.; Mapbs, M.O.; Mears, K. Growth and organized development of cultured cells I. Organization in cultures grown from freely suspended cells. Am. J. Bot. 1958, 45, 705–708. [Google Scholar] [CrossRef]
- Rao, A.Q.; Hussain, S.S.; Shahzad, M.S.; Bokhari, S.Y.; Raza, M.H.; Rakha, A.; Majeed, A.; Shahid, A.A.; Saleem, Z.; Husnain, T.; Riazuddin, S. Somatic embryogenesis in wild relatives of cotton (Gossypium Spp.). J. Zhejiang Univ. Sci. B 2006, 7, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.A.; Sharp, W.R.; Flick, C.E. Growth and Behavior of Cell Cultures: Embryogenesis and Organogenesis. Plant Tissue Cultures; Trevor, A.T., Ed.; Academic Press: New York, NY, USA, 1981; pp. 45–113. [Google Scholar]
- Scowcroft, W.R. Genetic Variability in Tissue Culture: Impact on Germplasm Conservation and Utilization; IBPGR: Roma, Italy, 1984; p. 41. Available online: http://www.sidalc.net/cgi-bin/wxis.exe/?IsisScript=bac.xis&method=post&formato=2&cantidad=1&expresion=mfn=048729 (accessed on 3 April 2019).
- Ge, X.; Zhang, C.; Wang, Q.; Yang, Z.; Wang, Y.; Zhang, X.; Wu, Z.; Hou, Y.; Wu, J.; Li, F. iTRAQ protein profile differential analysis between somatic globular and cotyledonary embryos reveals stress, hormone, and respiration involved in increasing plantlet regeneration of Gossypium hirsutum L. J. Proteome Res. 2014, 14, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Sakhanokho, H.F.; Rajasekaran, K. Cotton regeneration in vitro. In Fiber Plants; Ramawat, K.G., Ed.; Springer: Cham, Switzerland, 2016; pp. 87–110. [Google Scholar]
- Fehér, A. Why somatic plant cells start to form embryos? In Somatic Embryogenesis; Mujib, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 85–101. [Google Scholar]
- Karami, O.; Aghavaisi, B.; Pour, A.M. Molecular aspects of somatic-to-embryogenic transition in plants. J. Chem. Biol. 2009, 2, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Elhiti, M.; Stasolla, C.; Wang, A. Molecular regulation of plant somatic embryogenesis. In Vitro Cell. Dev. Biol. Plant 2013, 49, 631–642. [Google Scholar] [CrossRef]
- Fehér, A.; Pasternak, T.P.; Dudits, D. Transition of somatic plant cells to an embryogenic state. Plant Cell Tissue Org. 2003, 74, 201–228. [Google Scholar] [CrossRef]
- Ikeda-Iwai, M.; Umehara, M.; Satoh, S.; Kamada, H. Stress-induced somatic embryogenesis in vegetative tissues of Arabidopsis thaliana. Plant J. 2003, 34, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.J.; Nolan, K.E. Invited review: Genetic regulation of somatic embryogenesis with particular reference to Arabidopsis thaliana and Medicago truncatula. In Vitro Cell. Dev. Biol. Plant 2006, 42, 473–481. [Google Scholar] [CrossRef]
- Reinert, J. Über die Kontrolle der Morphogenese und die Induktion von Adventivembryonen an Gewebekulturen aus Karotten. Planta 1959, 53, 318–333. [Google Scholar] [CrossRef]
- Quiroz-Figueroa, F.R.; Rojas-Herrera, R.; Galaz-Avalos, R.M.; Loyola-Vargas, V.M. Embryo production through somatic embryogenesis can be used to study cell differentiation in plants. Plant Cell Tissue Org. 2006, 86, 285. [Google Scholar] [CrossRef]
- Mathieu, M.; Lelu-Walter, M.A.; Blervacq, A.S.; David, H.; Hawkins, S.; Neutelings, G. Germin-like genes are expressed during somatic embryogenesis and early development of conifers. Plant Mol. Biol. 2006, 61, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, T.A. In Vitro Embryogenesis in Plants. Springer Science & Business Media: Dordrecht, The Netherlands, 1995; pp. 267–308. [Google Scholar]
- Lakshmanan, P.; Taji, A. Somatic embryogenesis in leguminous plants. Plant Biol. 2000, 2, 136–148. [Google Scholar] [CrossRef]
- Elhiti, M.; Tahir, M.; Gulden, R.H.; Khamiss, K.; Stasolla, C. Modulation of embryo-forming capacity in culture through the expression of Brassica genes involved in the regulation of the shoot apical meristem. J. Exp. Bot. 2010, 61, 4069–4085. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Niu, Q.W.; Frugis, G.; Chua, N. The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J. 2002, 30, 349–359. [Google Scholar] [CrossRef]
- Weijers, D.; Sauer, M.; Meurette, O.; Friml, J.; Ljung, K.; Sandberg, G.; Hooykaas, P.; Offringa, R. Maintenance of embryonic auxin distribution for apical–basal patterning by PIN-FORMED–dependent auxin transport in Arabidopsis. Plant Cell 2005, 17, 2517–2526. [Google Scholar] [CrossRef]
- Su, Y.H.; Zhao, X.Y.; Liu, Y.B.; Zhang, C.L.; O’Neill, S.D.; Zhang, X.S. Auxin-induced WUS expression is essential for embryonic stem cell renewal during somatic embryogenesis in Arabidopsis. Plant J. 2009, 59, 448–460. [Google Scholar] [CrossRef]
- Vogler, H.; Kuhlemeier, C. Simple hormones but complex signalling. Curr. Opin. Plant Biol. 2003, 6, 51–56. [Google Scholar] [CrossRef]
- Braybrook, S.A.; Harada, J.J. LECs go crazy in embryo development. Trends Plant Sci. 2008, 13, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Giraudat, J.; Parcy, F.; Bertauche, N.; Gosti, F.; Leung, J.; Morris, P.C.; Bouvier-Durand, M.; Vartanian, N. Current advances in abscisic acid action and signalling. In Signals and Signal Transduction Pathways in Plants; Palme, K., Ed.; Springer: Dordrecht, The Netherlands, 1994; pp. 321–341. [Google Scholar]
- Ton, J.; Mauch-Mani, B. β-amino-butyric acid-induced resistance against necrotrophic pathogens is based on ABA-dependent priming for callose. Plant J. 2004, 38, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Letarte, J.; Simion, E.; Miner, M.; Kasha, K.J. Arabinogalactans and arabinogalactan-proteins induce embryogenesis in wheat (Triticum aestivum L.) microspore culture. Plant Cell 2006, 24, 691. [Google Scholar] [CrossRef] [PubMed]
- Singla, B.; Tyagi, A.K.; Khurana, J.P.; Khurana, P. Analysis of expression profile of selected genes expressed during auxin-induced somatic embryogenesis in leaf base system of wheat (Triticum aestivum) and their possible interactions. Plant Mol. Biol. 2007, 65, 677–692. [Google Scholar] [CrossRef] [PubMed]
- Mantiri, F.R.; Kurdyukov, S.; Lohar, D.P.; Sharopova, N.; Saeed, N.A.; Wang, X.D.; Vandenbosch, K.A.; Rose, R.J. The transcription factor MtSERF1 of the ERF subfamily identified by transcriptional profiling is required for somatic embryogenesis induced by auxin plus cytokinin in Medicago truncatula. Plant Physiol. 2008, 146, 1622–1636. [Google Scholar] [CrossRef] [PubMed]
- Kulinska-Lukaszek, K.; Tobojka, M.; Adamiok, A.; Kurczynska, E.U. Expression of the BBM gene during somatic embryogenesis of Arabidopsis thaliana. Biol. Plant. 2012, 56, 389–394. [Google Scholar] [CrossRef]
- Yang, Z.; Li, C.; Wang, Y.; Zhang, C.; Wu, Z.; Zhang, X.; Liu, C.; Li, F. GhAGL15s, preferentially expressed during somatic embryogenesis, promote embryogenic callus formation in cotton (Gossypium hirsutum L.). Mol. Genet. Genom. 2014, 289, 873–883. [Google Scholar] [CrossRef]
- Zheng, Q.; Zheng, Y.; Perry, S.E. AGAMOUS-Like15 Promotes Somatic Embryogenesis in Arabidopsis thaliana and Glycine max in Part by Control of Ethylene Biosynthesis and Response. Plant Physiol. 2013, 161, 2113–2127. [Google Scholar] [CrossRef]
- Schmidt, E.D.; Guzzo, F.; Toonen, M.A.; de Vries, S.C. A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development 1997, 124, 2049–2062. [Google Scholar]
- Zeng, F.; Zhang, X.; Zhu, L.; Tu, L.; Guo, X.; Nie, Y. Isolation and characterization of genes associated to cotton somatic embryogenesis by suppression subtractive hybridization and macroarray. Plant Mol. Biol. 2006, 60, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Schäfer, J.; Kuhn, K.; Kienle, S.; Schwarz, J.; Schmidt, G.; Neumann, T.; Johnstone, R.; Mohammed, A.K.; Hamon, C. Tandem mass tags: A novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Anal. Chem. 2003, 75, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Pagel, O.; Loroch, S.; Sickmann, A.; Zahedi, R.P. Current strategies and findings in clinically relevant post-translational modification-specific proteomics. Expert Rev. Proteom. 2015, 12, 235–253. [Google Scholar] [CrossRef] [PubMed]
- Klubicová, K.; Uvácková, L.; Danchenko, M.; Nemecek, P.; Skultéty, L.; Salaj, J.; Salaj, T. Insights into the early stage of Pinus nigra Arn. somatic embryogenesis using discovery proteomics. J. Proteom. 2017, 169, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Sghaier-Hammami, B.; Drira, N.; Jorrín-Novo, J.V. Comparative 2-DE proteomic analysis of date palm (Phoenix dactylifera L.) somatic and zygotic embryos. J. Proteom. 2009, 73, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.G.; Cheng, W.H.; Tian, W.G.; Li, Y.J.; Liu, F.; Xue, F.; Zhu, Q.H.; Sun, Y.Q.; Sun, J. iTRAQ-based comparative proteomic analysis provides insights into somatic embryogenesis in Gossypium hirsutum L. Plant Mol. Biol. 2018, 96, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.M.; Parreira, J.R.; Santos, R.; Duque, A.S.; Francisco, R.; Tomé, D.F.; Ricardo, C.P.; Coelho, A.V.; Fevereiro, P. A proteomics study of the induction of somatic embryogenesis in Medicago truncatula using 2DE and MALDI-TOF/TOF. Physiol. Plant. 2012, 146, 236–249. [Google Scholar] [CrossRef]
- Passardi, F.; Theiler, G.; Zamocky, M.; Cosioa, C.; Rouhier, N.; Teixera, F.; Margis-Pinheiroe, M.; Ioannidis, V.; Penel, C.; Falquet, L.; Dunand, C. PeroxiBase: The peroxidase database. Phytochemistry 2007, 68, 1605–1611. [Google Scholar] [CrossRef]
- Baaziz, M.; Saaidi, M. Preliminary identification of date palm cultivars by esterase isoenzymes and peroxidase activities. Can. J. Bot. 1988, 66, 89–93. [Google Scholar] [CrossRef]
- Baaziz, M. The activity and preliminary characterization of peroxidases in leaves of cultivars of date palm, Phoenix dactylifera L. New Phytol. 1989, 111, 403–411. [Google Scholar] [CrossRef]
- Abohatem, M.; Zouine, J.; El Hadrami, I. Low concentrations of BAP and high rate of subcultures improve the establishment and multiplication of somatic embryos in date palm suspension cultures by limiting oxidative browning associated with high levels of total phenols and peroxidase activities. Sci. Hortic. Amst. 2011, 130, 344–348. [Google Scholar] [CrossRef]
- Afreen, F.; Zobayed, S.M.A.; Kozai, T. Photoautotrophic culture of Coffea arabusta somatic embryos: Photosynthetic ability and growth of different stage embryos. Ann. Bot. 2002, 90, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Rival, A.; Beulé, T.; Lavergne, D.; Nato, A.; Havaux, M.; Puard, M. Development of photosynthetic characteristics in oil palm during in vitro micropropagation. J. Plant Physiol. 1997, 150, 520–527. [Google Scholar] [CrossRef]
- Karami, O.; Saidi, A. The molecular basis for stress-induced acquisition of somatic embryogenesis. Mol. Biol. Rep. 2010, 37, 2493–2507. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Xing, G.; Zhou, G.; Liu, X.; Wang, Y. The induced and regulatory effects of plant hormones in somatic embryogenesis. Hereditas 2000, 22, 349–354. [Google Scholar]
- Jiménez, V.M. Regulation of in vitro somatic embryogenesis with emphasis on to the role of endogenous hormones. Rev. Bras. Fisiol. Veg. 2001, 13, 196–223. [Google Scholar] [CrossRef]
- Kumria, R.; Sunnichan, V.G.; Das, D.K.; Gupta, S.K.; Reddy, V.S.; Bhatnagar, R.K.; Leelavathi, S. High-frequency somatic embryo production and maturation into normal plants in cotton (Gossypium hirsutum) through metabolic stress. Plant Cell Rep. 2003, 21, 635–639. [Google Scholar] [PubMed]
- Patnaik, D.; Mahalakshmi, A.; Khurana, P. Effect of water stress and heavy metals on induction of somatic embryogenesis in wheat leaf base cultures. Indian J. Exp. Biol. 2005, 43, 740–745. [Google Scholar] [PubMed]
- Cheong, Y.H.; Chang, H.S.; Gupta, R.; Wang, X.; Zhu, T.; Luan, S. Transcriptional profiling reveals novel interactions between wounding, pathogen, abiotic stress, and hormonal responses in Arabidopsis. Plant Physiol. 2002, 129, 661–677. [Google Scholar] [CrossRef]
- Karami, O.; Deljou, A.; Esna-Ashari, M.; Ostad-Ahmadi, P. Effect of sucrose concentrations on somatic embryogenesis in carnation (Dianthus caryophyllus L.). Sci. Hortic. 2006, 110, 340–344. [Google Scholar] [CrossRef]
- Karami, O.; Deljou, A.; Kordestani, G.K. Secondary somatic embryogenesis of carnation (Dianthus caryophyllus L.). Plant Cell 2008, 92, 273–280. [Google Scholar] [CrossRef]
- Klimaszewska, K.; Cyr, D.R.; Sutton, B.C.S. Influence of gelling agents on culture medium gel strength, water availability, tissue water potential, and maturation response in embryogenic cultures of Pinus strobus L. In Vitro Cell Dev. Biol. Plant 2000, 36, 279–286. [Google Scholar] [CrossRef]
- Kiyosue, T. Somatic embryogenesis in higher plants. J. Plant Res. 1993, 3, 75–82. [Google Scholar]
- Lehmann, T.; Polcyn, W.; Ratajczak, L. Glutamate dehydrogenase isoenzymes in yellow lupine root nodules. III. Affinity for ammonia. Acta Physiol. Plant 1990, 12, 259–263. [Google Scholar]
- Yamaya, T.; Oaks, A. Synthesis of glutamate by mitochondria–an anaplerotic function for glutamate dehydrogenase. Physiol. Plant. 1987, 70, 749–756. [Google Scholar] [CrossRef]
- Gancedo, J.M. Yeast carbon catabolite repression. Microbiol. Mol. Biol. R. 1998, 62, 334–361. [Google Scholar]
- Saier, M.H. Protein phosphorylation and allosteric control of inducer exclusion and catabolite repression by the bacterial phosphoenolpyruvate: Sugar phosphotransferase system. Microbiol. Mol. Biol. Rev. 1989, 53, 109–120. [Google Scholar]
- Nishiwaki, M.; Fujino, K.; Koda, Y.; Masuda, K.; Kikuta, Y. Somatic embryogenesis induced by the simple application of abscisic acid to carrot (Daucus carota L.) seedlings in culture. Planta 2000, 211, 756–759. [Google Scholar] [CrossRef]
- Jin, L.G.; Li, H.; Liu, J.Y. Molecular characterization of three ethylene responsive element binding factor genes from cotton. J. Integr. Plant Biol. 2010, 52, 485–495. [Google Scholar] [CrossRef]
- Ho, S.L.; Huang, L.F.; Lu, C.A.; He, S.L.; Wang, C.C.; Yu, S.P.; Chen, J.; Yu, S.M. Sugar starvation-and GA-inducible calcium-dependent protein kinase 1 feedback regulates GA biosynthesis and activates a 14-3-3 protein to confer drought tolerance in rice seedlings. Plant Mol. Biol. 2013, 81, 347–361. [Google Scholar] [CrossRef]
- Friml, J.; Benková, E.; Blilou, I.; Wisniewska, J.; Hamann, T.; Ljung, K.; Woody, S.; Sandberg, G.; Scheres, B.; Jürgens, G.; Palme, K. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell 2002, 108, 661–673. [Google Scholar] [CrossRef]
- Blilou, I.; Xu, J.; Wildwater, M.; Willemsen, V.; Paponov, I.; Friml, J.; Heidstra, R.; Aida, M.; Palme, K.; Scheres, B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 2005, 433, 39. [Google Scholar] [CrossRef] [PubMed]
- Hagen, G.; Kleinschmidt, A.; Guilfoyle, T. Auxin-regulated gene expression in intact soybean hypocotyl and excised hypocotyl sections. Planta 1984, 162, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Hagen, G.; Guilfoyle, T.J. Rapid induction of selective transcription by auxins. Plant Mol. Biol. 1985, 5, 1197–1203. [Google Scholar] [CrossRef]
- Yang, X.Y.; Zhang, X.L. Regulation of somatic embryogenesis in higher plants. Crit. Rev. Plant Sci. 2010, 29, 36–57. [Google Scholar] [CrossRef]
- Gallie, D.R.; Young, T.E. The ethylene biosynthetic and perception machinery is differentially expressed during endosperm and embryo development in maize. Mol. Genet. Genom. 2004, 271, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Langhansova, L.; Konradova, H.; Vaněk, T. Polyethylene glycol and abscisic acid improve maturation and regeneration of Panax ginseng somatic embryos. Plant Cell Rep. 2004, 22, 725–730. [Google Scholar] [CrossRef]
- Nieves, N.; Martinez, M.E.; Castillo, R.; Blanco, M.A.; González-Olmedo, J.L. Effect of abscisic acid and jasmonic acid on partial desiccation of encapsulated somatic embryos of sugarcane. Plant Cell Tissue Org. 2001, 65, 15–21. [Google Scholar] [CrossRef]
- Von, A.S.; Hakman, I. Regulation of somatic embryo development in Picea abies by abscisic acid (ABA). J. Plant Physiol. 1988, 132, 164–169. [Google Scholar]
- Jürgens, G. Apical–basal pattern formation in Arabidopsis embryogenesis. EMBO J. 2001, 20, 3609–3616. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Anil, V.S.; Rao, K.S. Calcium-mediated signaling during sandalwood somatic embryogenesis. Role for exogenous calcium as second messenger. Plant Physiol. 2000, 123, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Noah, A.M.; Niemenak, N.; Sunderhaus, S.; Haase, C.; Omokolo, D.N.; Winkelmann, T.; Braun, H.P. Comparative proteomic analysis of early somatic and zygotic embryogenesis in Theobroma cacao L. J. Proteom. 2013, 78, 123–133. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, B.; Park, J.; Plaxton, W.C. The remarkable diversity of plant PEPC (phosphoenolpyruvate carboxylase): Recent insights into the physiological functions and post-translational controls of non-photosynthetic PEPCs. Biochem. J. 2011, 436, 15–34. [Google Scholar] [CrossRef]
- Aivalakis, G.; Dimou, M.; Flemetakis, E.; Plati, F.; Katinakis, P.; Drossopoulos, J.B. Immunolocalization of carbonic anhydrase and phosphoenolpyruvate carboxylase in developing seeds of Medicago sativa. Plant Physiol. Biochem. 2004, 42, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Rolletschek, H.; Borisjuk, L.; Radchuk, R.; Miranda, M.; Heim, U.; Wobus, U.; Weber, H. Seed-specific expression of a bacterial phosphoenolpyruvate carboxylase in Vicia narbonensis increases protein content and improves carbon economy. Plant Biotechnol. J. 2004, 2, 211–219. [Google Scholar] [CrossRef]
- Jing, D.; Zhang, J.; Xia, Y.; Kong, L.; OuYang, F.; Zhang, S.; Zhang, H.; Wang, J. Proteomic analysis of stress-related proteins and metabolic pathways in Picea asperata somatic embryos during partial desiccation. Plant Biotechnol. J. 2017, 15, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Boursiac, Y.; Chen, S.; Luu, D.T.; Sorieul, M.; van den Dries, N.; Maurel, C. Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiol. 2005, 139, 790–805. [Google Scholar] [CrossRef] [PubMed]
- Loqué, D.; Ludewig, U.; Yuan, L.; von Wirén, N. Tonoplast intrinsic proteins AtTIP2; 1 and AtTIP2; 3 facilitate NH3 transport into the vacuole. Plant Physiol. 2005, 137, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Leitão, L.; Prista, C.; Moura, T.F.; Loureiro-Dias, M.C.; Soveral, G. Grapevine aquaporins: Gating of a tonoplast intrinsic protein (TIP2; 1) by cytosolic pH. PLoS ONE 2012, 7, e33219. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.J.; Saxena, P.K. Modulation of mineral and free fatty acid profiles during thidiazuron mediated somatic embryogenesis in peanuts (Arachis hypogeae L.). J. Plant Physiol. 1997, 151, 358–361. [Google Scholar] [CrossRef]
- Hundertmark, M.; Hincha, D.K. LEA (late embryogenesis abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genom. 2008, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Palovaara, J.; Hakman, I. Conifer WOX-related homeodomain transcription factors, developmental consideration and expression dynamic of WOX2 during Picea abies somatic embryogenesis. Plant Mol. Biol. 2008, 66, 533–549. [Google Scholar] [CrossRef] [PubMed]
- Höck, J.; Meister, G. The Argonaute protein family. Genome Biol. 2008, 9, 210. [Google Scholar] [CrossRef] [PubMed]
- Szyrajew, K.; Bielewicz, D.; Dolata, J.; Wójcik, A.M.; Nowak, K.; Szczygieł-Sommer, A.; Szweykowska-Kulinska, Z.; Jarmolowski, A.; Gaj, M.D. MicroRNAs are intensively regulated during induction of somatic embryogenesis in Arabidopsis. Front. Plant Sci. 2017, 8, 18. [Google Scholar] [CrossRef]
- Takahata, K. Isolation of carrot Argonaute1 from subtractive somatic embryogenesis cDNA library. Biosci. Biotechnol. Biochem. 2008, 72, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.; Law, D.A.; Stasolla, C. Molecular characterization of PgAGO, a novel conifer gene of the ARGONAUTE family expressed in apical cells and required for somatic embryo development in spruce. Tree Physiol. 2006, 26, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Munksgaard, D.; Mattsson, O.; Okkels, F.T. Somatic embryo development in carrot is associated with an increase in levels of S-adenosylmethionine, S-adenosylhomocysteine and DNA methylation. Physiol. Plant. 1995, 93, 5–10. [Google Scholar] [CrossRef]
- Jarillo, J.A.; Piñeiro, M.; Cubas, P.; Martínez-Zapater, J.M. Chromatin remodeling in plant development. Int. J. Dev. Biol. 2009, 53, 1581–1596. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.H.; Zhu, H.G.; Tian, W.G.; Zhu, S.H.; Xiong, X.P.; Sun, Y.Q.; Zhu, Q.H.; Sun, J. De novo transcriptome analysis reveals insights into dynamic homeostasis regulation of somatic embryogenesis in upland cotton (G. hirsutum L.). Plant Mol. Biol. 2016, 92, 279–292. [Google Scholar] [CrossRef]
- Cao, A.; Zheng, Y.; Yu, Y.; Wang, X.; Shao, D.; Sun, J.; Cui, B. Comparative Transcriptome Analysis of SE initial dedifferentiation in cotton of different SE capability. Sci. Rep. 2017, 7, 8583. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chen, Y.; Ding, Y.; Wu, J.; Wang, P.; Yu, Y.; Wei, X.; Wang, Y.; Zhang, C.; Li, F.; Ge, X. Effects of GhWUS from upland cotton (Gossypium hirsutum L.) on somatic embryogenesis and shoot regeneration. Plant Sci. 2018, 270, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, M.; Li, Y.; Zhang, Q.; Lindsey, K.; Daniell, H.; Jin, S.; Zhang, X. Multi-omics analyses reveal epigenomics basis for cotton somatic embryogenesis through successive regeneration acclimation process. Plant Biotechnol. J. 2019, 17, 435–450. [Google Scholar] [CrossRef] [PubMed]
- da Cunha Soares, T.; da Silva, C.R.C.; Chagas Carvalho, J.M.F.; Cavalcanti, J.J.V.; de Lima, L.M.; de Albuquerque Melo Filho, P.; Severino, L.S.; Dos Santos, R.C. Validating a probe from GhSERK1 gene for selection of cotton genotypes with somatic embryogenic capacity. J. Biotechnol. 2018, 270, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, X.; Li, B.; Chen, L.; Min, L.; Zhang, X. GhL1L1 affects cell fate specification by regulating GhPIN1-mediated auxin distribution. Plant Biotechnol. J. 2019, 17, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, X.; Nie, Y.; Jin, S.X.; Liang, S.G. Factors affecting somatic embryogenesis and plant regeneration from a range of recalcitrant genotypes of Chinese cottons (Gossypium hirsutum L.). In Vitro Cell. Dev. Biol. Plant 2004, 40, 371–375. [Google Scholar] [CrossRef]
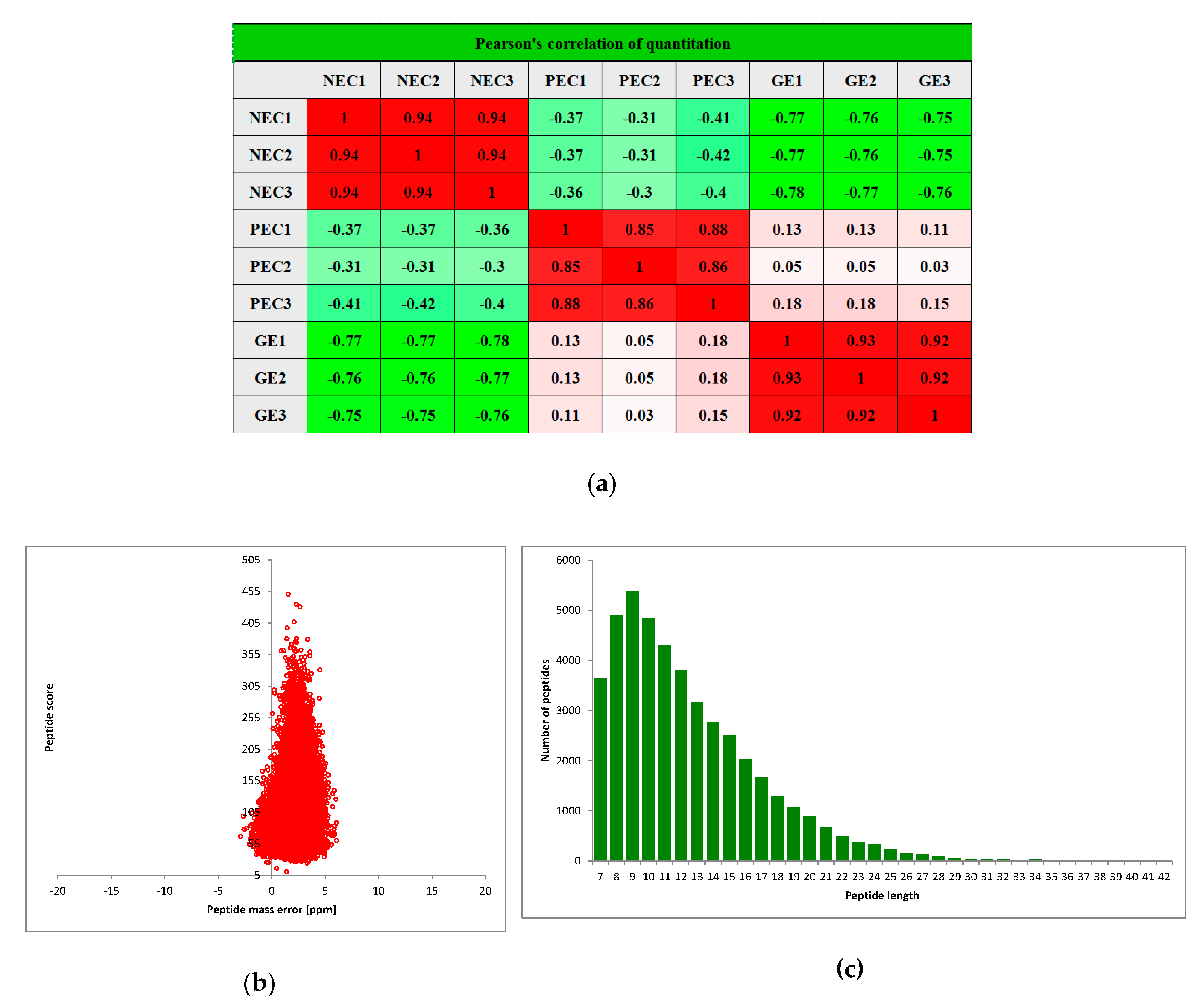




| Total Spectrum | Matched Spectrum | Peptides | Unique Peptides | Identified Proteins | Quantifiable Proteins |
|---|---|---|---|---|---|
| 360,720 | 74,579 (20.7%) | 45,062 | 27,673 | 9369 | 6730 |
| Gene ID | Gene Name | Protein ID | Protein Description | Pathway Annotation | PEC/NEC Ratio | GE/PEC Ratio | GE/NEC Ratio |
|---|---|---|---|---|---|---|---|
| LOC107907377 | PIN2 | A0A120KAE0 | Auxin efflux carrier component | Auxin signal | 2.36 | — | — |
| LOC107909506 | GH3.17 | A0A1U8JQJ4 | Indole-3-acetic acid-amido synthetase GH3.17-like isoform X2 | Auxin signal | 2.14 | — | — |
| LOC107948437 | ETR1 | A0A1U8NHA4 | ethylene receptor-like isoform X1 | Ethylene signal | 2.02 | — | 3.705 |
| LOC107938108 | GASL1 | M1GN42 | GA-stimulated transcript-like protein 1 | GA signal | 0.25 | 0.27 | 0.068 |
| LOC107955576 | GASL4 | M1GMV2 | GA-stimulated transcript-like protein 4 | GA signal | 3.00 | — | |
| LOC107950128 | PYR1 | A0A1U8NR07 | Abscisic acid receptor PYR1-like | ABA signal | — | 2.31 | 2.361 |
| LOC107893363 | At5g01020 | A0A1U8I6G4 | serine/threonine-protein kinase At5g01020-like | Signal transduction | 0.50 | — | 0.482 |
| LOC107897915 | — | A0A1U8IRF6 | A-kinase anchor protein 12-like isoform X2 | Signal transduction | 0.44 | — | 0.459 |
| LOC107909143 | — | A0A1U8JP78 | leucine-rich repeat receptor-like protein kinase PXC2 | Signal transduction | 0.45 | — | 0.36 |
| LOC107945188 | BAM3 | A0A1U8N9I0 | leucine-rich repeat receptor-like Ser/Thr -protein kinase BAM3 | Signal transduction | 2.03 | — | — |
| LOC107935259 | PCKA | A0A1U8M980 | phosphoenolpyruvate carboxykinase [ATP]-like | Signal transduction | 2.08 | 0.38 | — |
| LOC107943515 | At1g56140 | A0A1U8N4H5 | probable LRR receptor-like serine/threonine-protein kinase At1g56140 | Signal transduction | 0.29 | — | 0.215 |
| LOC107931208 | TPK1 | A0A1U8LYM7 | thiamine pyrophosphokinase 1-like isoform X1 | Signal transduction | 0.46 | — | 0.469 |
| LOC107905700 | PFK | A0A1U8JGW8 | ATP-dependent 6-phosphofructokinase | Signal transduction | — | 2.55 | 2.504 |
| LOC107943957 | PV42A | A0A1U8N623 | SNF1-related protein kinase regulatory subunit gamma-like PV42a | Signal transduction | — | 2.83 | 2.757 |
| LOC107937641 | CPK11 | A0A1U8MGW7 | calcium-dependent protein kinase 11-like | Signal transduction | 2.25 | — | 3.84 |
| LOC107930954 | CML27 | A0A1U8LUL1 | probable calcium-binding protein CML27 | Signal transduction | 4.16 | — | 2.88 |
| LOC107916423 | RHN1 | A0A1U8KFK5 | ras-related protein RHN1-like | Signal transduction | — | 0.47 | 0.333 |
| LOC107889787 | — | A0A1U8HV05 | Embryonic protein DC-8-like | Somatic embryogenesis related proteins | — | 3.58 | 4.522 |
| LOC107937048 | Lea2A-A | Q03791 | Embryogenesis abundant protein | Somatic embryogenesis related proteins | — | 4.42 | 4.746 |
| LOC107941722 | WOX9 | A0A1U8MVD7 | WUSCHEL-related homeobox 9-like | Transcription factor | 2.58 | — | — |
| LOC107905698 | NFYB6 | A0A1U8JC47 | Nuclear transcription factor Y subunit B-6 | Transcription factor | 3.22 | 2.07 | 6.661 |
| bHLH4 | W5XUY9 | BHLH4 transcription factor | Transcription factor | 2.16 | — | — | |
| LOC107920272 | NFYB9 | A0A1U8KSD1 | nuclear transcription factor Y subunit B-9-like | Transcription factor | 4.07 | — | 2.509 |
| LOC107931333 | A0A1U8LVZ2 | transcription factor HBP-1b (C38)-like | Transcription factor | 2.40 | — | — | |
| LOC107924015 | PHL1 | A0A1U8L8P3 | Protein PHR1-LIKE 1-like | Transcription factor | 0.15 | — | 0.166 |
| LOC107891610 | At1g07170 | A0A1U8I119 | PHD finger-like domain-containing protein 5B | Zinc finger | 3.49 | — | 4.21 |
| LOC107909066 | NERD | A0A1U8JUI6 | zinc finger CCCH domain-containing protein 19-like isoform X2 | Zinc finger | 2.03 | — | — |
| LOC107927097 | TAF15B | A0A1U8LG36 | transcription TFIID subunit 15b-like | Zinc finger | 0.44 | — | — |
| LOC107962890 | ZHD5 | A0A1U8PUW9 | zinc-finger homeodomain protein 5-like | Zinc finger | — | 2.93 | 5.554 |
| LOC107890886 | AGO1 | A0A1U8HY77 | protein argonaute 1-like isoform X2 | Posttranscriptional regulation | 8.44 | — | 6.734 |
| LOC107906203 | AGO4 | A0A1U8JEA7 | protein argonaute 4-like | Posttranscriptional regulation | 2.38 | — | 2.426 |
| LOC107962954 | HEN1 | A0A1U8PXD5 | small RNA 2’-O-methyltransferase-like isoform X4 | Posttranscriptional regulation | 2.06 | — | — |
| LOC107891032 | IDM1 | A0A1U8HYR9 | increased DNA methylation 1-like isoform X4 | Modification-related protein | 2.62 | 0.49 | — |
| LOC107906306 | MMT1 | A0A1U8JEK1 | Methionine S-methyltransferase | Modification-related protein | 0.46 | — | 0.458 |
| LOC107948568 | SUVH4 | A0A1U8NHS0 | histone-lysine N-methyltransferase, H3 lysine-9 specific SUVH4-like isoform X2 | Modification-related protein | 0.28 | — | 0.196 |
| LOC107943854 | CCOAOMT | A0A1U8N5R4 | caffeoyl-CoA O-methyltransferase -like | Modification-related protein | 0.35 | — | 0.363 |
| LOC107953938 | EMB1691 | A0A1U8P3T9 | methyltransferase-like protein 1 | Modification-related protein | 2.33 | — | 2.774 |
| LOC107916882 | IAMT1 | A0A1U8KGS5 | indole-3-acetate O-methyltransferase 1 | Modification-related protein | 2.32 | — | — |
| LOC107958653 | — | A0A1U8PI31 | chromatin modification-related protein MEAF6-like isoform X3 | Modification-related protein | — | 3.48 | 5.641 |
| LOC107926365 | — | A0A1U8LDK7 | RNA cytidine acetyltransferase | Modification-related protein | 2.17 | — | 2.333 |
| LOC107960303 | — | A0A1U8PLN7 | Acetyltransferase component of pyruvate dehydrogenase complex | Modification-related protein | 0.48 | — | 0.404 |
| LOC107947121 | UBR7 | A0A1U8NDR8 | putative E3 ubiquitin-protein ligase UBR7 | Modification-related protein | 2.90 | — | 2.679 |
| LOC107959749 | RUB2 | A0A1U8PJK7 | ubiquitin-NEDD8-like protein RUB2 | Modification-related protein | 0.26 | — | 0.286 |
| LOC107938100 | — | A0A1U8MII2 | Phosphotransferase | Modification-related protein | 0.42 | — | 0.476 |
| LOC107898863 | CYP86B1 | A0A1U8IP72 | Cytochrome P450 86B1-like | Fatty acid | 4.51 | — | 2.388 |
| LOC107922796 | CYP86A8 | A0A1U8L513 | Cytochrome P450 86A8-like | Fatty acid | 2.02 | — | — |
| LOC107915850 | PIP2-5 | A0A1U8KIL6 | probable aquaporin PIP2-5 | Aquaporins | — | 2.58 | — |
| LOC107898442 | TIP3-2 | A0A1U8IU16 | probable aquaporin TIP3-2 | Aquaporins | — | 2.29 | 9.086 |
| LOC107963873 | GhPIP2;10 | D8FSK4 | Aquaporin PIP210 | Aquaporins | 0.14 | — | 0.102 |
| — | GhTIP1;4 | D8FSK6 | Aquaporin TIP14 | Aquaporins | 0.20 | — | 0.195 |
| LOC107934987 LOC107944588 | PIP1;4 | G8XV51 | PIP protein | Aquaporins | 0.22 | — | 0.109 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Guo, H.; Zhang, L.; Fan, Y.; Fan, Y.; Tang, Z.; Zeng, F. Dynamic TMT-Based Quantitative Proteomics Analysis of Critical Initiation Process of Totipotency during Cotton Somatic Embryogenesis Transdifferentiation. Int. J. Mol. Sci. 2019, 20, 1691. https://doi.org/10.3390/ijms20071691
Guo H, Guo H, Zhang L, Fan Y, Fan Y, Tang Z, Zeng F. Dynamic TMT-Based Quantitative Proteomics Analysis of Critical Initiation Process of Totipotency during Cotton Somatic Embryogenesis Transdifferentiation. International Journal of Molecular Sciences. 2019; 20(7):1691. https://doi.org/10.3390/ijms20071691
Chicago/Turabian StyleGuo, Haixia, Huihui Guo, Li Zhang, Yijie Fan, Yupeng Fan, Zhengmin Tang, and Fanchang Zeng. 2019. "Dynamic TMT-Based Quantitative Proteomics Analysis of Critical Initiation Process of Totipotency during Cotton Somatic Embryogenesis Transdifferentiation" International Journal of Molecular Sciences 20, no. 7: 1691. https://doi.org/10.3390/ijms20071691
APA StyleGuo, H., Guo, H., Zhang, L., Fan, Y., Fan, Y., Tang, Z., & Zeng, F. (2019). Dynamic TMT-Based Quantitative Proteomics Analysis of Critical Initiation Process of Totipotency during Cotton Somatic Embryogenesis Transdifferentiation. International Journal of Molecular Sciences, 20(7), 1691. https://doi.org/10.3390/ijms20071691







