The Effect of Maternal Intact Protein- and Amino Acid-Based Diets on Development of Food Intake Regulatory Systems and Body Weight in Dams and Male Offspring of Wistar Rats
Abstract
:1. Introduction
2. Results
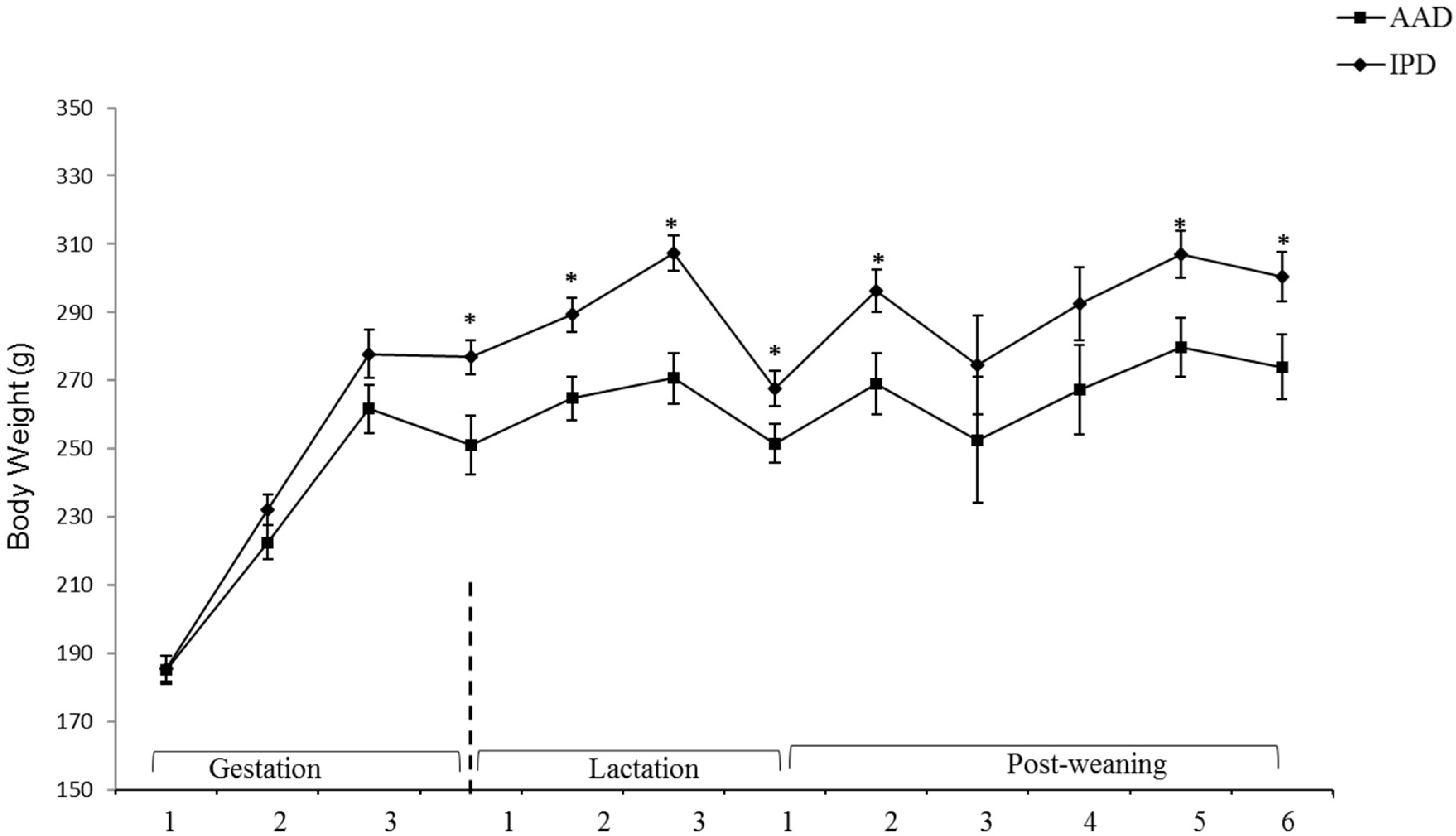
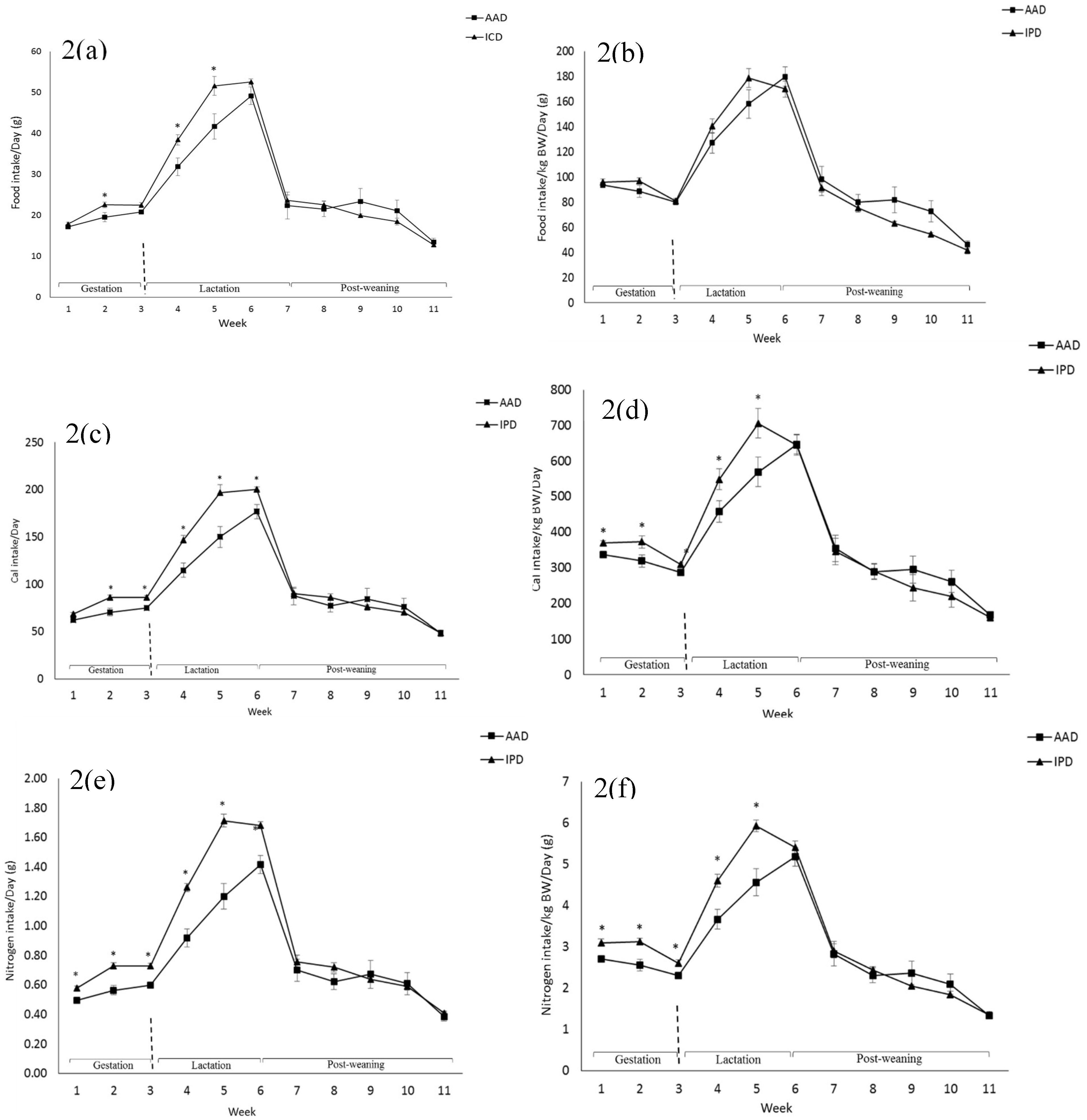
2.1. Dams
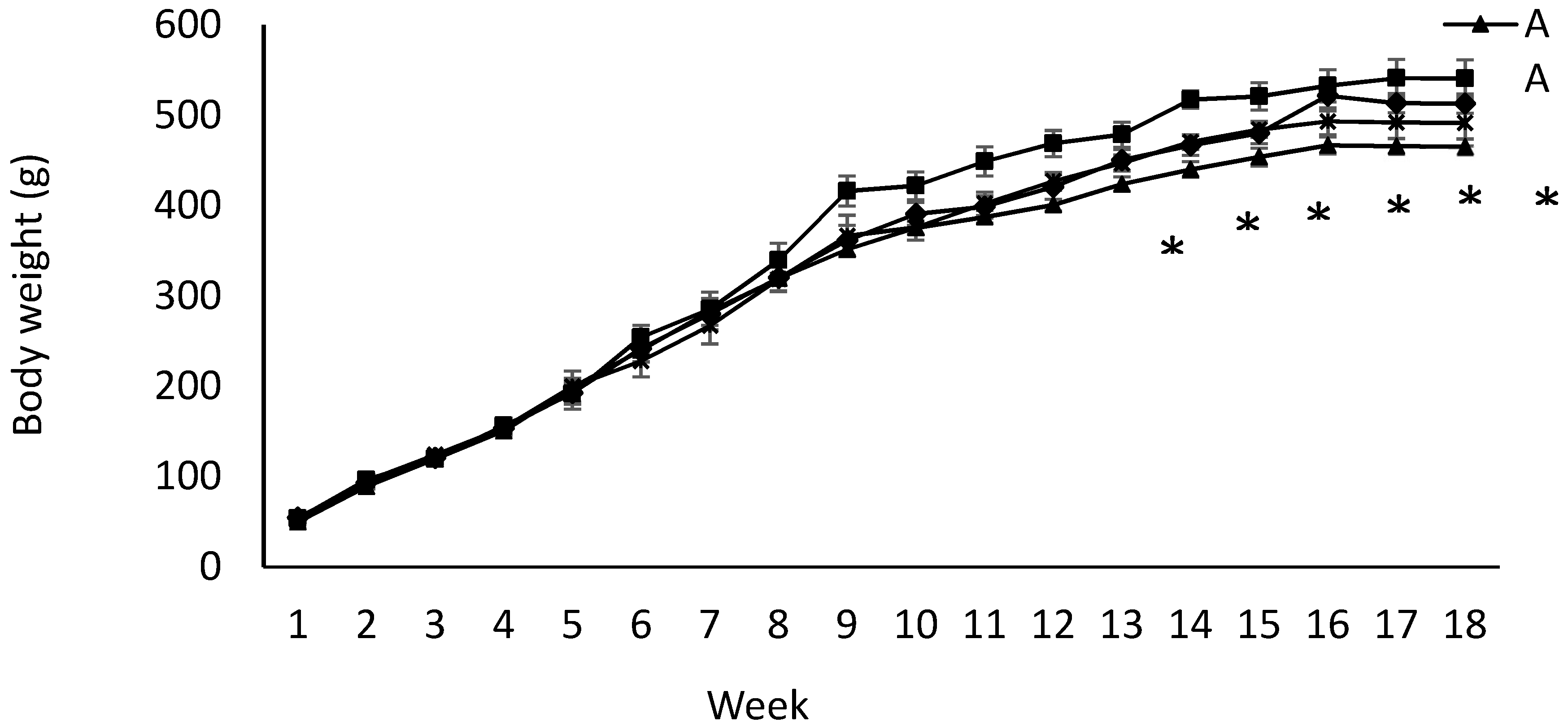
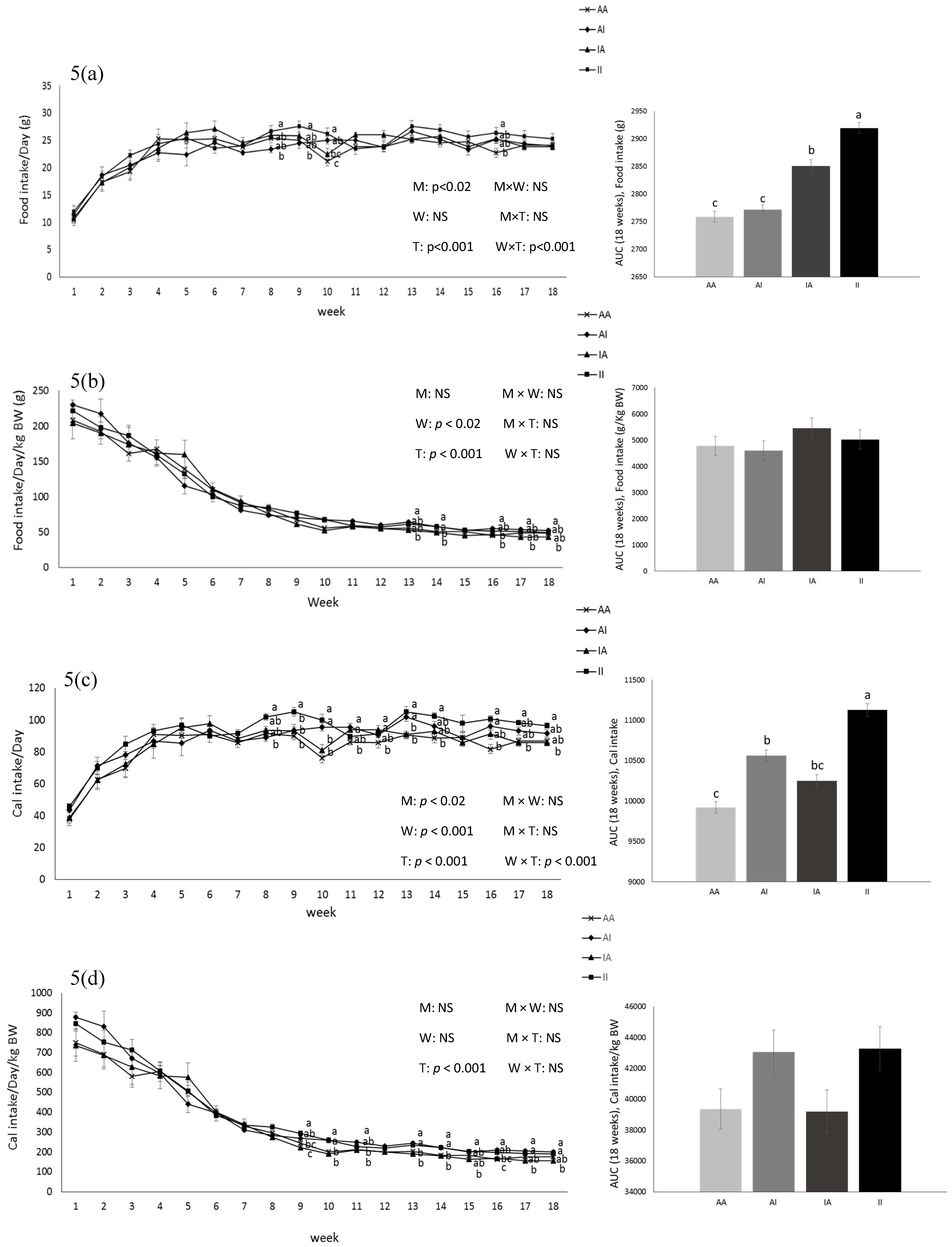
2.2. Pups
3. Discussion
4. Materials and Methods
4.1. Animals and Diets
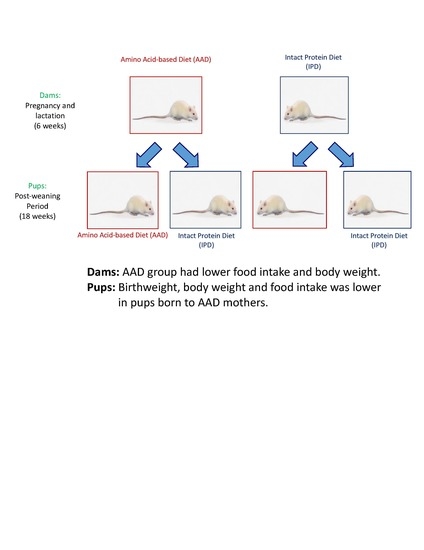
4.2. Experimental Design
4.3. Long- and Short-Term Food Intake
4.4. Protein Preloads
4.5. Blood Glucose
4.6. Blood Collection
4.7. Hormone Assays
4.8. Body Composition
4.9. Statistical Analyses
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | amino acid |
| AAD | amino acid-based AIN-93 G diet |
| BAPs | bioactive peptides |
| BG | blood glucose |
| BW | body weight |
| ELISA | enzyme-linked immunosorbent assay |
| FI | food intake |
| FM | fat mass |
| IPD | intact protein-based AIN-93 G diet |
| PW | post-weaning |
| PYY | peptide YY |
References
- Jahan-Mihan, A.; Rodriguez, J.; Christie, C.; Sadeghi, M.; Zerbe, T. The Role of Maternal Dietary Proteins in Development of Metabolic Syndrome in Offspring. Nutrients 2015, 7, 9185–9217. [Google Scholar] [CrossRef] [Green Version]
- Lucas, A. The developmental origins of adult health and well-being. Adv. Exp. Med. Biol. 2005, 569, 13–15. [Google Scholar] [PubMed]
- Jahan-mihan, A.; Luhovyy, B.L.; el Khoury, D.; Anderson, G.H. Dietary proteins as determinants of metabolic and physiologic functions of the gastrointestinal tract. Nutrients 2011, 3, 574–603. [Google Scholar] [CrossRef]
- Zambrano, E.; Bautista, C.J.; Deas, M.; Martinez-Samayoa, P.M.; Gonzalez-Zamorano, M.; Ledesma, H.; Morales, J.; Larrea, F.; Nathanielsz, P.W. A low maternal protein diet during pregnancy and lactation has sex- and window of exposure-specific effects on offspring growth and food intake, glucose metabolism and serum leptin in the rat. J. Physiol. 2006, 571, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Rees, W.D.; Hay, S.M.; Brown, D.S.; Antipatis, C.; Palmer, R.M. Maternal protein deficiency causes hypermethylation of DNA in the livers of rat fetuses. J. Nutr. 2000, 130, 1821–1826. [Google Scholar] [CrossRef] [PubMed]
- Langley-Evans, S.C.; Gardner, D.S.; Jackson, A.A. Maternal protein restriction influences the programming of the rat hypothalamic-pituitary-adrenal axis. J. Nutr. 1996, 126, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Langley-Evans, S.C.; Phillips, G.J.; Jackson, A.A. In utero exposure to maternal low protein diets induces hypertension in weanling rats, independently of maternal blood pressure changes. Clin. Nutr. (Edinb. Scotl.) 1994, 13, 319–324. [Google Scholar] [CrossRef]
- Sasaki, A.; Nakagawa, I.; Kajimoto, M. Effect of protein nutrition throughout gestation and lactation ongrowth, morbidity and life span of rat progeny. J. Nutr. Sci. Vitaminol. (Tokyo) 1982, 28, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, C.; Terroni, P.L.; Cagampang, F.R.; Hanson, M.; Byrne, C.D. High-unsaturated-fat, high-protein, and low-carbohydrate diet during pregnancy and lactation modulates hepatic lipid metabolism in female adult offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R112–R118. [Google Scholar] [CrossRef] [Green Version]
- Thone-Reineke, C.; Kalk, P.; Dorn, M.; Klaus, S.; Simon, K.; Pfab, T.; Godes, M.; Persson, P.; Unger, T.; Hocher, B. High-protein nutrition during pregnancy and lactation programs blood pressure, food efficiency, and body weight of the offspring in a sex-dependent manner. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1025–R1030. [Google Scholar] [CrossRef]
- Beyer, M.; Jentsch, W.; Kuhla, S.; Wittenburg, H.; Kreienbring, F.; Scholze, H.; Rudolph, P.E.; Metges, C.C. Effects of dietary energy intake during gestation and lactation on milk yield and composition of first, second and fourth parity sows. Arch. Anim. Nutr. 2007, 61, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Daenzer, M.; Ortmann, S.; Klaus, S.; Metges, C.C. Prenatal high protein exposure decreases energy expenditure and increases adiposity in young rats. J. Nutr. 2002, 132, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Jahan-mihan, A.; Szeto, M.; Luhovyy, B.; Huot, P.; Anderson, G. Soy Protein and Casein Based Nutritionally Complete Diets Fed During Gestation And Lactation Differ in Effects on Characteristics of Metabolic Syndrome in Male Offspring of Wistar Rat. Br. J. Nutr. 2012, 107, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Jahan-Mihan, A.; Smith, C.; Anderson, G.H. The Effect of Protein Source in Diets Fed during Gestation and Lactation on Food Intake Regulation in Male Offspring of Wistar Rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R1175–R1184. [Google Scholar] [CrossRef] [PubMed]
- Jahan-mihan, A.; Smith, C.E.; Hamedani, A.; Anderson, G.H. Soy Protein, Compared with Casein Based Diets Fed During Gestation and Lactation Increase Food Intake and Risk of Developing Characteristics of Metabolic Syndrome in Female Rat Offspring. Nutrients 2011, 31, 644–651. [Google Scholar]
- Bautista, C.J.; Guzman, C.; Rodriguez-Gonzalez, G.L.; Zambrano, E. Improvement in metabolic effects by dietary intervention is dependent on the precise nature of the developmental programming challenge. J. Dev. Orig. Health Dis. 2015, 10, 1–8. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Bendsen, N.T.; Tremblay, A.; Astrup, A. Effect of proteins from different sources on body composition. Nutr. Metab. Cardiovasc. Dis. 2011, 21 (Suppl. 2), B16–B31. [Google Scholar] [CrossRef]
- Mellinkoff, S.M.; Frankland, M.; Boyled, D.; Greipel, M. Relationship between serum amino acid concentration and fluctuations in appetite. J. Appl. Physiol. 1956, 8, 535–538. [Google Scholar] [CrossRef]
- Jahan-mihan, A.; Labyak, C.; Arikawa, A.; Ertemin-Pearson, E. The Effect of Devazepide (CCK-1 Receptor Blocker) on food intake suppression induced by whey protein and glycomacropeptide in wistar rats. Curr. Nutr. Food Sci. 2017, 13, 198–203. [Google Scholar]
- Yudkoff, M.; Daikhin, Y.; Nissim, I.; Horyn, O.; Luhovyy, B.; Lazarow, A.; Nissim, I. Brain amino acid requirements and toxicity: The example of leucine. J. Nutr. 2005, 135, 1531S–1538S. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, K.; LeBlanc, R.E.; Loh, D.; Schwartz, G.J.; Yu, Y.H. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes 2007, 56, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Wellman, PJ. Modulation of eating by central catecholamine systems. Curr. Drug Targets 2005, 6, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Yamamoto, Y.; Yamatodani, A. Brain histamine and feeding behavior. Behav. Brain Res. 2001, 124, 145–150. [Google Scholar] [CrossRef]
- Devkota, S.; Layman, D.K. Protein metabolic roles in treatment of obesity. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M.; Food and Nutrition Board of the Institute of Medicine, The National Academies. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet. Assoc. 2003, 103, 563. [Google Scholar]
- Jackson, A.A.; Dunn, R.L.; Marchand, M.C.; Langley-Evans, S.C. Increased systolic blood pressure in rats induced by a maternal low-protein diet is reversed by dietary supplementation with glycine. Clin. Sci. (Lond.) 2002, 103, 633–663. [Google Scholar] [CrossRef] [PubMed]
- Cherif, H.; Reusens, B.; Ahn, M.T.; Hoet, J.J.; Remacle, C. Effects of taurine on the insulin secretion of rat fetal islets from dams fed a low-protein diet. J. Endocrinol. 1998, 159, 341–348. [Google Scholar] [CrossRef] [Green Version]
- Korhonen, H.; Pihlanto, A. Food-derived bioactive peptides–opportunities for designing future foods. Curr. Pharm. Des. 2003, 9, 1297–1308. [Google Scholar] [CrossRef]
- Meisel, H. Casokinins as bioactive peptides in the primary structure of casein. In Food Proteins: Structure Functionality; Schwenke, K.D., Mothes, R., Eds.; VCH-Weinheim: New York, NY, USA, 1993; pp. 67–75. [Google Scholar]
- Pupovac, J.; Anderson, G.H. Dietary peptides induce satiety via cholecystokinin-A and peripheral opioid receptors in rats. J. Nutr. 2002, 132, 2775–2780. [Google Scholar] [CrossRef]
- Daniel, H.; Vohwinkel, M.; Rehner, G. Effect of casein and beta-casomorphins on gastrointestinal motility in rats. J. Nutr. 1990, 120, 252–257. [Google Scholar] [CrossRef]
- Paroli, E. Opioid peptides from food (the exorphins). World Rev. Nutr. Diet. 1988, 55, 58–97. [Google Scholar] [PubMed]
- Yang, Q.Q.; He, X.Y.; Wu, H.Y.; Zhang, C.Q.; Zou, S.Y.; Lang, T.Q.; Sun, S.S.; Liu, Q.Q. Subchronic feeding study of high-free-lysine transgenic rice in Sprague-Dawley rats. Food Chem. Toxicol. 2017, 105, 214–222. [Google Scholar] [CrossRef]
- Xu, Y.; Zeng, Z.; Xu, X.; Tian, Q.; Ma, X.; Long, S.; Piao, M.; Cheng, Z.; Piao, X. Effects of the standardized ileal digestible valine: Lysine ratio on performance, milk composition and plasma indices of lactating sows. Anim. Sci. J. 2017, 88, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Batistel, F.; Alharthi, A.S.; Wang, L.; Parys, C.; Pan, Y.X.; Cardoso, F.C.; Loor, J.J. Placentome nutrient transporters and mammalian target of rapamycin signaling proteins are altered by the methionine supply during late gestation in dairy cows and are associated with newborn birth weight. J. Nutr. 2017, 147, 1640–1647. [Google Scholar] [CrossRef]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.P.; Maubois, J.L.; Beaufrere, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dangin, M.; Boirie, Y.; Guillet, C.; Beaufrere, B. Influence of the protein digestion rate on protein turnover in young and elderly subjects. J. Nutr. 2002, 132, 3228S–3233S. [Google Scholar] [CrossRef] [PubMed]
- Luiking, Y.C.; Deutz, N.E.; Jakel, M.; Soeters, P.B. Casein and soy protein meals differentially affect whole-body and splanchnic protein metabolism in healthy humans. J. Nutr. 2005, 135, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, D. Soy Proteins. In Proteins in Food Processing; Yada, R.Y., Ed.; Woodhead Publishing: Cambridge, UK, 2004; pp. 100–122. [Google Scholar]
- Shimizu, M. Food-derived peptides and intestinal functions. Biofactors 2004, 21, 43–47. [Google Scholar] [CrossRef]
- Yvon, M.; Beucher, S.; Guilloteau, P.; Le Huerou-Luron, I.; Corring, T. Effects of caseinomacropeptide (CMP) on digestion regulation. Reprod. Nutr. Dev. 1994, 34, 527–537. [Google Scholar] [CrossRef] [Green Version]
- Lackland, D.T.; Egan, B.M.; Ferguson, P.L. Low birth weight as a risk factor for hypertension. J. Clin. Hypertens. (Greenwich) 2003, 5, 133–136. [Google Scholar] [CrossRef]
- Carlsson, S.; Persson, P.G.; Alvarsson, M.; Efendic, S.; Norman, A.; Svanström, L.; Ostenson, C.G.; Grill, V. Low birth weight, family history of diabetes, and glucose intolerance in Swedish middle-aged men. Diabetes Care. 1999, 22, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Forsen, T.; Eriksson, J.; Tuomilehto, J.; Reunanen, A.; Osmond, C.; Barker, D. The fetal and childhood growth of persons who develop type 2 diabetes. Ann. Intern. Med. 2000, 133, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Jaquet, D.; Deghmoun, S.; Chevenne, D.; Collin, D.; Czernichow, P.; Levy-Marchal, C. Dynamic change in adiposity from fetal to postnatal life is involved in the metabolic syndrome associated with reduced fetal growth. Diabetologia 2005, 48, 849–855. [Google Scholar] [CrossRef] [Green Version]
- Yajnik, C. Interactions of perturbations in intrauterine growth and growth during childhood on the risk of adult-onset disease. Proc. Nutr. Soc. 2000, 59, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Ong, K.K.; Ahmed, M.L.; Emmett, P.M.; Preece, M.A.; Dunger, D.B. Association between postnatal catch-upgrowth and obesity in childhood: Prospective cohort study. BMJ 2000, 320, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Cole, T.J.; Fewtrell, M.; Kennedy, K.; Stephenson, T.; Elias-Jones, A.; Lucas, A. Promotion of faster weight gain in infants born small for gestational age: Is there an adverse effect on later blood pressure? Circulation 2007, 115, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Holzhauer, S.; Hokken Koelega, A.C.; Ridder, M.D.; Hofman, A.; Moll, H.A.; Steegers, E.A.; Witteman, J.C.; Jaddoe, V.W. Effect of birth weight and postnatal weight gain on body composition in early infancy: The Generation R Study. Early Hum. Dev. 2009, 85, 285–290. [Google Scholar] [CrossRef] [PubMed]


| Maternal Diet | IPD | AAD | p | |||
|---|---|---|---|---|---|---|
| Dams: At wk 9 postpartum | ||||||
| Ghrelin, pg/mL | 3.73 ± 0.07 | 3.31 ± 0.11 | NS | |||
| PYY, ng/mL | 0.87 ± 0.07 | 0.95 ± 0.08 | NS | |||
| Glucose, mM | 5.31 ± 0.28 | 4.90 ± 0.28 | NS | |||
| Insulin, ng/mL | 2.28 ± 0.43 | 2.35 ± 0.65 | NS | |||
| Pups: At Birth | ||||||
| Ghrelin, pg/mL | 2.00 ± 0.73 | 2.61 ± 0.40 | NS | |||
| PYY, ng/mL | 1.02 ± 0.05 | 1.17 ± 0.15 | NS | |||
| Glucose, mM | 5.25 ± 0.78 | 5.36 ± 0.38 | NS | |||
| Insulin, ng/mL | 1.13 ± 0.30 | 1.18 ± 0.14 | NS | |||
| Weaning | ||||||
| Ghrelin, pg/mL | 3.62 ± 0.10 | 3.78 ± 0.06 | NS | |||
| PYY, ng/mL | 1.08 ± 0.07 | 0.79 ± 0.05 | p < 0.05 | |||
| Glucose, mM | 5.41 ± 0.50 | 4.88 ± 0.56 | NS | |||
| Insulin, ng/mL | 1.66 ± 0.06 | 1.91 ± 0.24 | NS | |||
| At wk 18 PW | ||||||
| Ghrelin, pg/mL | 9.55 ± 0.97 | 7.75 ± 1.13 | NS | |||
| PYY, ng/mL | 1.25 ± 0.35 | 1.17 ± 0.03 | NS | |||
| Glucose, mM | 4.64 ± 0.15 | 5.52 ± 0.20 | p < 0.05 | |||
| Insulin, ng/mL | 2.17 ± 0.42 | 3.32 ± 0.10 | NS | |||
| Maternal Diet | IPD | AAD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Weaning Diet | IPD | AAD | IPD | AAD | |||||||
| Intact | AA-based | Intact | AA-based | Intact | AA-based | Intact | AA-based | ||||
| Casein | Casein | Casein | Casein | Casein | Casein | Casein | Casein | p | |||
| 0–1 h | |||||||||||
| Control (water) | 7.18 ± 0.62 | 6.23 ± 0.87 | 5.70 ± 0.47 | 6.77 ± 0.88 | M: p = 0.05 | ||||||
| Protein | 4.47 ± 0.67 | 3.68 ± 0.61 | 6.48 ± 0.85 | 5.17 ± 0.43 | 3.30 ± 0.81 | 3.80 ± 0.60 | 4.15 ± 0.30 | 5.30 ± 0.33 | W: NS | ||
| Water-Protein | 2.77 ± 0.42 * | 3.50 ± 0.44 * | 0.25 ± 1.63 | 0.80 ± 0.21 | 2.80 ± 1.06 * | 1.90 ± 0.50 * | 2.7 ± 1.21 * | 1.40 ± 1.34 * | P: NS | ||
| T: p < 0.0001 | |||||||||||
| 0–2 h | M × T: p < 0.05 | ||||||||||
| Control (water) | 5.20 ± 1.70 b | 10.68 ± 2.62 a | 7.53 ± 0.49 b | 6.90 ± 0.68 b | M × W × T: p < 0.05 | ||||||
| Protein | 7.83 ± 1.97 | 7.08 ± 2.45 | 11.08 ± 1.19 | 9.90 ± 2.50 | 7.17 ± 1.96 | 5.13 ± 0.63 | 7.55 ± 1.68 | 6.40 ± 1.03 | W × T: p < 0.05 | ||
| Water-Protein | 2.93 ± 4.14 | 1.88 ± 3.70 | 0.40 ± 1.71 | 1.17 ± 0.55 | 0.70 ± 2.31 | 2.40 ± 0.58 | 1.03 ± 2.98 | 1.13 ± 0.94 | P × T: p < 0.05 | ||
| 0–12 h | |||||||||||
| Control (water) | 16.58 ± 0.82 | 20.95 ± 2.69 | 19.05 ± 2.35 | 18.83 ± 0.97 | |||||||
| Protein | 19.30 ± 3.15 | 15.25 ± 2.04 | 22.10 ± 0.95 | 21.53 ± 1.92 | 20.10 ± 1.96 | 17.03 ± 1.36 | 20.03 ± 2.26 | 17.93 ± 1.42 | |||
| Water-Protein | 2.07 ± 2.28 | 0.68 ± 2.88 | 1.15 ± 2.03 | 1.70 ± 0.85 | 1.57 ± 0.77 | 2.03 ± 1.08 | 1.90 ± 4.00 | 1.1 ± 2.06 | |||
| Intact Protein | Amino Acid-Mixed | ||
|---|---|---|---|
| Diet | Diet | ||
| g/kg | |||
| Alanine | 4.7 | 4.5 | |
| Arginine | 6.5 | 6.3 | |
| Aspartic acid | 11.7 | 11.3 | |
| Cystine | 3.7 * | 3.7 | |
| Glutamic acid | 37.5 | 36.2 | |
| Glycine | 3.2 | 3.1 | |
| Histidine | 4.7 | 4.5 | |
| Isoleucine | 8.6 | 8.4 | |
| Leucine | 15.9 | 15.3 | |
| Lysine | 13.3 | 16.1 | |
| Methionine | 4.7 | 4.5 | |
| Phenylalanine | 9.0 | 8.7 | |
| Proline | 21.1 | 20.4 | |
| Serine | 9.7 | 9.4 | |
| Threonine | 6.8 | 6.6 | |
| Tryptophan | 2.2 | 2.1 | |
| Tyrosine | 9.6 | 9.2 | |
| Valine | 10.3 | 9.9 |
| Intact Protein | Amino Acid-Mixed | ||
|---|---|---|---|
| Diet | Diet | ||
| Cal | 3597 | 3811 | |
| g/kg | |||
| Casein | 200 | 0 | |
| Amino acid Mix | 0 | 180 | |
| Sucrose | 100 | 100 | |
| Soybean oil | 70 | 70 | |
| t-Butyhydroquinone | 0 | 0.014 | |
| Cornstarch | 397.5 | 399.9 | |
| Dyetrose | 132 | 145 | |
| Cellulose | 50 | 50 | |
| Mineral Mix | 35 | 35 | |
| Sodium Bicorbonate | 0 | 7.4 | |
| Vitamin Mix | 10 | 10 | |
| Choline Bitartrate | 2.5 | 2.5 | |
| L-Cystine | 3 | 0 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahan-mihan, A. The Effect of Maternal Intact Protein- and Amino Acid-Based Diets on Development of Food Intake Regulatory Systems and Body Weight in Dams and Male Offspring of Wistar Rats. Int. J. Mol. Sci. 2019, 20, 1690. https://doi.org/10.3390/ijms20071690
Jahan-mihan A. The Effect of Maternal Intact Protein- and Amino Acid-Based Diets on Development of Food Intake Regulatory Systems and Body Weight in Dams and Male Offspring of Wistar Rats. International Journal of Molecular Sciences. 2019; 20(7):1690. https://doi.org/10.3390/ijms20071690
Chicago/Turabian StyleJahan-mihan, Alireza. 2019. "The Effect of Maternal Intact Protein- and Amino Acid-Based Diets on Development of Food Intake Regulatory Systems and Body Weight in Dams and Male Offspring of Wistar Rats" International Journal of Molecular Sciences 20, no. 7: 1690. https://doi.org/10.3390/ijms20071690