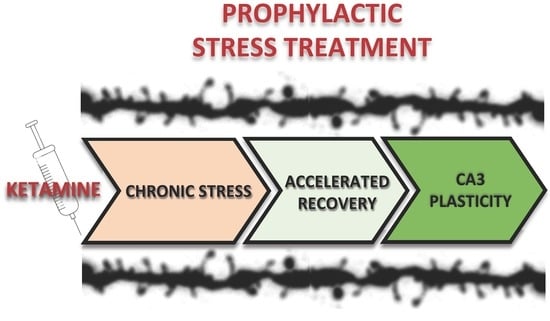
Prophylactic Ketamine Treatment Promotes Resilience to Chronic Stress and Accelerates Recovery: Correlation with Changes in Synaptic Plasticity in the CA3 Subregion of the Hippocampus
Abstract
:1. Introduction
2. Results
2.1. Ketamine Treatment before the Chronic Unpredictable Stress Procedure Altered Sucrose Preference in C57BL6J Mice
2.2. Ketamine Treatment before the Chronic Unpredictable Stress Procedure Affected the Density and Morphology of Dendritic Spines after the 8-day Recovery Period
3. Discussion
4. Materials and Methods
4.1. Animals and Housing Conditions
4.2. Drug Administration
4.3. Experimental Design
4.4. Mouse Model of Depression based on Modified Chronic Unpredictable Stress Protocol
4.5. Behavioral Tests
4.5.1. Sucrose Preference Test
4.5.2. Forced Swim Test
4.5.3. DiI Staining of Brain Slices
4.6. Morphometric Analysis of Dendritic Spines
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMY | Amygdala |
| CUS | Chronic Unpredictable Stress |
| DG | Dentate Gyrus |
| FST | Forced Swimming Test |
| NMDA | N-methyl-d-aspartate |
| SPT | Sucrose Preference Test |
| PFC | Prefrontal Cortex |
References
- Wang, J.; Wu, X.; Lai, W.; Long, E.; Zhang, X.; Li, W.; Zhu, Y.; Chen, C.; Zhong, X.; Liu, Z.; et al. Prevalence of depression and depressive symptoms among outpatients: A systematic review and meta-analysis. BMJ Open 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Bromet, E.J. The Epidemiology of Depression Across Cultures. Annu. Rev. Public Health 2013, 34, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Constance Hammen Stress and depression. Annu. Rev. Clin. Psychol. 2005, 1, 293–319. [CrossRef]
- Grunewald, M.; Johnson, S.; Lu, D.; Wang, Z.; Lomberk, G.; Albert, P.R.; Stockmeier, C.A.; Meyer, J.H.; Urrutia, R.; Miczek, K.A.; et al. Mechanistic role for a novel glucocorticoid-KLF11 (TIEG2) protein pathway in stress-induced monoamine oxidase A expression. J. Biol. Chem. 2012, 287, 24195–24206. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; O’Sullivan, A.J.; Diamond, T.; Gerard, A.; Campbell, P. Psychiatric symptoms as a clinical presentation of Cushing’s syndrome. Ann. Gen. Psychiatry 2013, 12. [Google Scholar] [CrossRef]
- Kvarta, M.D.; Thompson, S.M.; Bailey, A.M.; Dantrassy, H.M.; Bradbrook, K.E. Corticosterone mediates the synaptic and behavioral effects of chronic stress at rat hippocampal temporoammonic synapses. J. Neurophysiol. 2015, 114, 1713–1724. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Ge, T.; Leng, Y.; Pan, Z.; Fan, J.; Yang, W.; Cui, R. The Role of Neural Plasticity in Depression: From Hippocampus to Prefrontal Cortex. Neural Plast. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Li, M.X.; Xu, C.; Chen, H.B.; An, S.C.; Ma, X.M. Dendritic Spines in Depression: What We Learned from Animal Models. Neural Plast. 2016. [Google Scholar] [CrossRef] [PubMed]
- Colle, R.; Cury, C.; Chupin, M.; Deflesselle, E.; Hardy, P.; Nasser, G.; Falissard, B.; Ducreux, D.; Colliot, O.; Corruble, E. Hippocampal volume predicts antidepressant efficacy in depressed patients without incomplete hippocampal inversion. NeuroImage Clin. 2016, 12, 949–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conrad, C.D.; Ortiz, J.B.; Judd, J.M. Chronic stress and hippocampal dendritic complexity: Methodological and functional considerations. Physiol. Behav. 2017, 178, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.D.; Narayan, M.; Anderson, E.R.; Staib, L.H.; Miller, H.L.; Charney, D.S. Hippocampal volume reduction in major depression. Am. J. Psychiatry 2000, 157, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R. The prefrontal-limbic network in depression: A core pathology of synapse regression. Prog. Neurobiol. 2011, 93, 457–467. [Google Scholar] [CrossRef]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet 2018, 391, 1357–1366. [Google Scholar] [CrossRef]
- Autry, A.E.; Adachi, M.; Nosyreva, E.; Na, E.S.; Los, M.F.; Cheng, P.F.; Kavalali, E.T.; Monteggia, L.M. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 2011, 475, 91–96. [Google Scholar] [CrossRef]
- Zanos, P.; Moaddel, R.; Morris, P.J.; Georgiou, P.; Fischell, J.; Elmer, G.I.; Alkondon, M.; Yuan, P.; Pribut, H.J.; Singh, N.S.; et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 2016, 533, 481–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastrodonato, A.; Martinez, R.; Pavlova, I.P.; LaGamma, C.T.; Brachman, R.A.; Robison, A.J.; Denny, C.A. Ventral CA3 Activation Mediates Prophylactic Ketamine Efficacy Against Stress-Induced Depressive-like Behavior. Biol. Psychiatry 2018, 84, 846–856. [Google Scholar] [CrossRef] [PubMed]
- McGowan, J.C.; Hill, C.; Mastrodonato, A.; Lagamma, C.T.; Kitayev, A.; Brachman, R.A.; Narain, N.R.; Kiebish, M.A.; Denny, C.A. Prophylactic ketamine alters nucleotide and neurotransmitter metabolism in brain and plasma following stress. Neuropsychopharmacology 2018, 43, 1813–1821. [Google Scholar] [CrossRef]
- McGowan, J.C.; LaGamma, C.T.; Lim, S.C.; Tsitsiklis, M.; Neria, Y.; Brachman, R.A.; Denny, C.A. Prophylactic Ketamine Attenuates Learned Fear. Neuropsychopharmacology 2017, 42, 1577–1589. [Google Scholar] [CrossRef]
- Brachman, R.A.; McGowan, J.C.; Perusini, J.N.; Lim, S.C.; Pham, T.H.; Faye, C.; Gardier, A.M.; Mendez-David, I.; David, D.J.; Hen, R.; et al. Ketamine as a Prophylactic Against Stress-Induced Depressive-like Behavior. Biol. Psychiatry 2016, 79, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Donahue, R.J.; Muschamp, J.W.; Russo, S.J.; Nestler, E.J.; Carlezon, W.A. Effects of striatal ΔFosB over expression and ketamine on social defeat stress-induced anhedonia in mice. Biol. Psychiatry 2014, 76, 550–558. [Google Scholar] [CrossRef]
- Liu, M.Y.; Yin, C.Y.; Zhu, L.J.; Zhu, X.H.; Xu, C.; Luo, C.X.; Chen, H.; Zhu, D.Y.; Zhou, Q.G. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat. Protoc. 2018. [Google Scholar] [CrossRef]
- Basu, S.; Saha, P.K.; Roszkowska, M.; Magnowska, M.; Baczynska, E.; Das, N.; Plewczynski, D.; Wlodarczyk, J. Quantitative 3-D morphometric analysis of individual dendritic spines. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Bonanno, G.A. Loss, Trauma, and Human Resilience: Have We Underestimated the Human Capacity to Thrive after Extremely Aversive Events? Am. Psychol. 2004, 59, 20–28. [Google Scholar] [CrossRef]
- Feder, A.; Nestler, E.J.; Charney, D.S. Psychobiology and molecular genetics of resilience. Nat. Rev. Neurosci. 2009, 10, 446–457. [Google Scholar] [CrossRef] [Green Version]
- Pfau, M.L.; Russo, S.J. Peripheral and central mechanisms of stress resilience. Neurobiol. Stress 2015, 1, 66–79. [Google Scholar] [CrossRef]
- Friedman, A.K.; Juarez, B.; Ku, S.M.; Zhang, H.; Calizo, R.C.; Walsh, J.J.; Chaudhury, D.; Zhang, S.; Hawkins, A.; Dietz, D.M.; et al. KCNQ channel openers reverse depressive symptoms via an active resilience mechanism. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef]
- Krishnan, V.; Han, M.H.; Graham, D.L.; Berton, O.; Renthal, W.; Russo, S.J.; LaPlant, Q.; Graham, A.; Lutter, M.; Lagace, D.C.; et al. Molecular Adaptations Underlying Susceptibility and Resistance to Social Defeat in Brain Reward Regions. Cell 2007, 131, 391–404. [Google Scholar] [CrossRef] [Green Version]
- Montgomery, S.A.; Dufour, H.; Brion, S.; Gailledreau, J.; Laqueille, X.; Ferrey, G.; Moron, P.; Parant-Lucena, N.; Singer, L.; Danion, J.M.; et al. The prophylactic efficacy of fluoxetine in unipolar depression. Br. J. Psychiatry 1988, 153, 69–76. [Google Scholar] [CrossRef]
- Gilaberte, I.; Montejo, A.L.; De La Gandara, J.; Perez-Sola, V.; Bernardo, M.; Massana, J.; Martin-Santos, R.; Santiso, A.; Noguera, R.; Casais, L.; et al. Fluoxetine in the prevention of depressive recurrences: A double-blind study. J. Clin. Psychopharmacol. 2001, 21, 417–424. [Google Scholar] [CrossRef]
- Charney, D.S. Psychobiological Mechanism of Resilience and Vulnerability: Implications for Successful Adaptation to Extreme Stress. Am. J. Psychiatry 2004, 161, 195–216. [Google Scholar] [CrossRef]
- Roque, A.P. Pharmacotherapy as Prophylactic Treatment of Post-Traumatic Stress Disorder: A Review of the Literature. Issues Ment. Health Nurs. 2015, 36, 740–751. [Google Scholar] [CrossRef]
- Taylor, M.J.; Goodwin, G.M. Long-term prophylaxis in bipolar disorder. CNS Drugs 2006, 20, 303–310. [Google Scholar] [CrossRef]
- Abdallah, C.G.; Averill, L.A.; Krystal, J.H. Ketamine as a promising prototype for a new generation of rapid-acting antidepressants. Ann. N. Y. Acad. Sci. 2015, 1344, 66–77. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.C.; Dang, Y.H.; Jia, M.; Ma, R.; Wang, F.; Wu, J.; Gao, C.G.; Hashimoto, K. Long-Lasting Antidepressant Action of Ketamine, but Not Glycogen Synthase Kinase-3 Inhibitor SB216763, in the Chronic Mild Stress Model of Mice. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Katalinic, N.; Lai, R.; Somogyi, A.; Mitchell, P.B.; Glue, P.; Loo, C.K. Ketamine as a new treatment for depression: A review of its efficacy and adverse effects. Aust. N. Z. J. Psychiatry 2013, 47, 710–727. [Google Scholar] [CrossRef]
- Gray, J.D.; Rubin, T.G.; Kogan, J.F.; Marrocco, J.; Weidmann, J.; Lindkvist, S.; Lee, F.S.; Schmidt, E.F.; McEwen, B.S. Translational profiling of stress-induced neuroplasticity in the CA3 pyramidal neurons of BDNF Val66Met mice. Mol. Psychiatry 2018, 23, 904–913. [Google Scholar] [CrossRef]
- Carreno, F.R.; Donegan, J.J.; Boley, A.M.; Shah, A.; DeGuzman, M.; Frazer, A.; Lodge, D.J. Activation of a ventral hippocampus-medial prefrontal cortex pathway is both necessary and sufficient for an antidepressant response to ketamine. Mol. Psychiatry 2016, 21, 1298–1308. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, T.; Yuan, Z.; Wei, Z.; Yamaki, V.N.; Huang, M.; Huganir, R.L.; Cai, X. Essential roles of AMPA receptor GluA1 phosphorylation and presynaptic HCN channels in fast-Acting antidepressant responses of ketamine. Sci. Signal. 2016, 9. [Google Scholar] [CrossRef]
- Ardalan, M.; Wegener, G.; Polsinelli, B.; Madsen, T.M.; Nyengaard, J.R. Neurovascular plasticity of the hippocampus one week after a single dose of ketamine in genetic rat model of depression. Hippocampus 2016, 26, 1414–1423. [Google Scholar] [CrossRef]
- Sałat, K.; Siwek, A.; Starowicz, G.; Librowski, T.; Nowak, G.; Drabik, U.; Gajdosz, R.; Popik, P. Antidepressant-like effects of ketamine, norketamine and dehydronorketamine in forced swim test: Role of activity at NMDA receptor. Neuropharmacology 2015, 99, 301–307. [Google Scholar] [CrossRef]
- Popik, P.; Kos, T.; Sowa-Kućma, M.; Nowak, G. Lack of persistent effects of ketamine in rodent models of depression. Psychopharmacology 2008, 198, 421–430. [Google Scholar] [CrossRef]
- Franceschelli, A.; Sens, J.; Herchick, S.; Thelen, C.; Pitychoutis, P.M. Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naïve and “depressed” mice exposed to chronic mild stress. Neuroscience 2015, 290, 49–60. [Google Scholar] [CrossRef]
- Bechtholt-Gompf, A.J.; Smith, K.L.; John, C.S.; Kang, H.H.; Carlezon, W.A.; Cohen, B.M.; Öngür, D. CD-1 and Balb/cJ mice do not show enduring antidepressant-like effects of ketamine in tests of acute antidepressant efficacy. Psychopharmacology 2011, 215, 689–695. [Google Scholar] [CrossRef]
- Li, N.; Liu, R.J.; Dwyer, J.M.; Banasr, M.; Lee, B.; Son, H.; Li, X.Y.; Aghajanian, G.; Duman, R.S. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol. Psychiatry 2011, 69, 754–761. [Google Scholar] [CrossRef]
- Strekalova, T.; Steinbusch, H.W.M. Measuring behavior in mice with chronic stress depression paradigm. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2010, 34, 348–361. [Google Scholar] [CrossRef]
- Tse, Y.C.; Montoya, I.; Wong, A.S.; Mathieu, A.; Lissemore, J.; Lagace, D.C.; Wong, T.P. A longitudinal study of stress-induced hippocampal volume changes in mice that are susceptible or resilient to chronic social defeat. Hippocampus 2014, 24, 1120–1128. [Google Scholar] [CrossRef]
- Chan, S.W.Y.; Harmer, C.J.; Norbury, R.; O’Sullivan, U.; Goodwin, G.M.; Portella, M.J. Hippocampal volume in vulnerability and resilience to depression. J. Affect. Disord. 2016, 189, 199–202. [Google Scholar] [CrossRef]
- Zhang, J.C.; Yao, W.; Dong, C.; Yang, C.; Ren, Q.; Ma, M.; Hashimoto, K. Blockade of interleukin-6 receptor in the periphery promotes rapid and sustained antidepressant actions: A possible role of gut-microbiota-brain axis. Transl. Psychiatry 2017, 7, e1138. [Google Scholar] [CrossRef]
- Qu, Y.; Yang, C.; Ren, Q.; Ma, M.; Dong, C.; Hashimoto, K. Regional differences in dendritic spine density confer resilience to chronic social defeat stress. Acta Neuropsychiatr. 2018, 30, 117–122. [Google Scholar] [CrossRef]
- Antoniuk, S.; Bijata, M.; Ponimaskin, E.; Wlodarczyk, J. Chronic Unpredictable Mild Stress for Modeling Depression in Rodents:Meta-analysis of Model Reliability. Neurosci. Biobehav. Rev. 2018, in press. [Google Scholar]
- Conrad, C.D. What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus? Behav. Cogn. Neurosci. Rev. 2006, 5, 41–60. [Google Scholar] [CrossRef]
- Phoumthipphavong, V.; Barthas, F.; Hassett, S.; Kwan, A.C. Longitudinal Effects of Ketamine on Dendritic Architecture In Vivo in the Mouse Medial Frontal Cortex. eNeuro 2011, 3, 91–95. [Google Scholar] [CrossRef]
- Sebastian, V.; Estil, J.B.; Chen, D.; Schrott, L.M.; Serrano, P.A. Acute Physiological Stress Promotes Clustering of Synaptic Markers and Alters Spine Morphology in the Hippocampus. PLoS ONE 2013. [Google Scholar] [CrossRef]
- Magnowska, M.; Gorkiewicz, T.; Suska, A.; Wawrzyniak, M.; Rutkowska-Wlodarczyk, I.; Kaczmarek, L.; Wlodarczyk, J. Transient ECM protease activity promotes synaptic plasticity. Sci. Rep. 2016. [Google Scholar] [CrossRef]
- Basu, S.; Plewczynski, D.; Saha, S.; Roszkowska, M.; Magnowska, M.; Baczynska, E.; Wlodarczyk, J. 2dSpAn: Semiautomated 2-d segmentation, classification and analysis of hippocampal dendritic spine plasticity. Bioinformatics 2016, 32, 2490–2498. [Google Scholar] [CrossRef]
- Radley, J.J. Toward a limbic cortical inhibitory network: Implications for hypothalamic-pituitary-adrenal responses following chronic stress. Front. Behav. Neurosci. 2012. [Google Scholar] [CrossRef]
- Wang, G.; Cheng, Y.; Gong, M.; Liang, B.; Zhang, M.; Chen, Y.; Zhang, C.; Yuan, X.; Xu, J. Systematic correlation between spine plasticity and the anxiety/depression-like phenotype induced by corticosterone in mice. Neuroreport 2013. [Google Scholar] [CrossRef]
- Bloss, E.B.; Janssen, W.G.; Ohm, D.T.; Yuk, F.J.; Wadsworth, S.; Saardi, K.M.; McEwen, B.S.; Morrison, J.H. Evidence for reduced experience-dependent dendritic spine plasticity in the aging prefrontal cortex. J. Neurosci. 2011, 31, 7831–7839. [Google Scholar] [CrossRef] [Green Version]
- Cline, B.H.; Steinbusch, H.W.M.; Malin, D.; Revishchin, A.V.; Pavlova, G.V.; Cespuglio, R.; Strekalova, T. The neuronal insulin sensitizer dicholine succinate reduces stress-induced depressive traits and memory deficit: Possible role of insulin-like growth factor 2. BMC Neurosci. 2012, 13. [Google Scholar] [CrossRef]
- Shors, T.J.; Falduto, J.; Leuner, B. The opposite effects of stress on dendritic spines in male vs. female rats are NMDA receptor-dependent. Eur. J. Neurosci. 2004. [Google Scholar] [CrossRef]
- Strekalova, T.; Spanagel, R.; Bartsch, D.; Henn, F.A.; Gass, P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology 2004, 29, 2007–2017. [Google Scholar] [CrossRef]
- Conrad, C.D. Chronic stress-induced hippocampal vulnerability: The glucocorticoid vulnerability hypothesis. Rev. Neurosci. 2008, 19, 395–411. [Google Scholar] [CrossRef]
- Shishkina, G.T.; Dygalo, N.N. The glucocorticoid hypothesis of depression: History and prospects. Russ. J. Genet. Appl. Res. 2017, 7, 128–133. [Google Scholar] [CrossRef]
- Strekalova, T.; Couch, Y.; Kholod, N.; Boyks, M.; Malin, D.; Leprince, P.; Steinbusch, H.M.W. Update in the methodology of the chronic stress paradigm: Internal control matters. Behav. Brain Funct. 2011, 7. [Google Scholar] [CrossRef]
- Michaluk, P.; Wawrzyniak, M.; Alot, P.; Szczot, M.; Wyrembek, P.; Mercik, K.; Medvedev, N.; Wilczek, E.; De Roo, M.; Zuschratter, W.; et al. Influence of matrix metalloproteinase MMP-9 on dendritic spine morphology. J. Cell Sci. 2011. [Google Scholar] [CrossRef]
- Yasumatsu, N.; Matsuzaki, M.; Miyazaki, T.; Noguchi, J.; Kasai, H. Principles of Long-Term Dynamics of Dendritic Spines. J. Neurosci. 2008. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzystyniak, A.; Baczynska, E.; Magnowska, M.; Antoniuk, S.; Roszkowska, M.; Zareba-Koziol, M.; Das, N.; Basu, S.; Pikula, M.; Wlodarczyk, J. Prophylactic Ketamine Treatment Promotes Resilience to Chronic Stress and Accelerates Recovery: Correlation with Changes in Synaptic Plasticity in the CA3 Subregion of the Hippocampus. Int. J. Mol. Sci. 2019, 20, 1726. https://doi.org/10.3390/ijms20071726
Krzystyniak A, Baczynska E, Magnowska M, Antoniuk S, Roszkowska M, Zareba-Koziol M, Das N, Basu S, Pikula M, Wlodarczyk J. Prophylactic Ketamine Treatment Promotes Resilience to Chronic Stress and Accelerates Recovery: Correlation with Changes in Synaptic Plasticity in the CA3 Subregion of the Hippocampus. International Journal of Molecular Sciences. 2019; 20(7):1726. https://doi.org/10.3390/ijms20071726
Chicago/Turabian StyleKrzystyniak, Adam, Ewa Baczynska, Marta Magnowska, Svitlana Antoniuk, Matylda Roszkowska, Monika Zareba-Koziol, Nirmal Das, Subhadip Basu, Michal Pikula, and Jakub Wlodarczyk. 2019. "Prophylactic Ketamine Treatment Promotes Resilience to Chronic Stress and Accelerates Recovery: Correlation with Changes in Synaptic Plasticity in the CA3 Subregion of the Hippocampus" International Journal of Molecular Sciences 20, no. 7: 1726. https://doi.org/10.3390/ijms20071726
APA StyleKrzystyniak, A., Baczynska, E., Magnowska, M., Antoniuk, S., Roszkowska, M., Zareba-Koziol, M., Das, N., Basu, S., Pikula, M., & Wlodarczyk, J. (2019). Prophylactic Ketamine Treatment Promotes Resilience to Chronic Stress and Accelerates Recovery: Correlation with Changes in Synaptic Plasticity in the CA3 Subregion of the Hippocampus. International Journal of Molecular Sciences, 20(7), 1726. https://doi.org/10.3390/ijms20071726