Fibrin Sealant Derived from Human Plasma as a Scaffold for Bone Grafts Associated with Photobiomodulation Therapy
Abstract
1. Introduction
2. Results
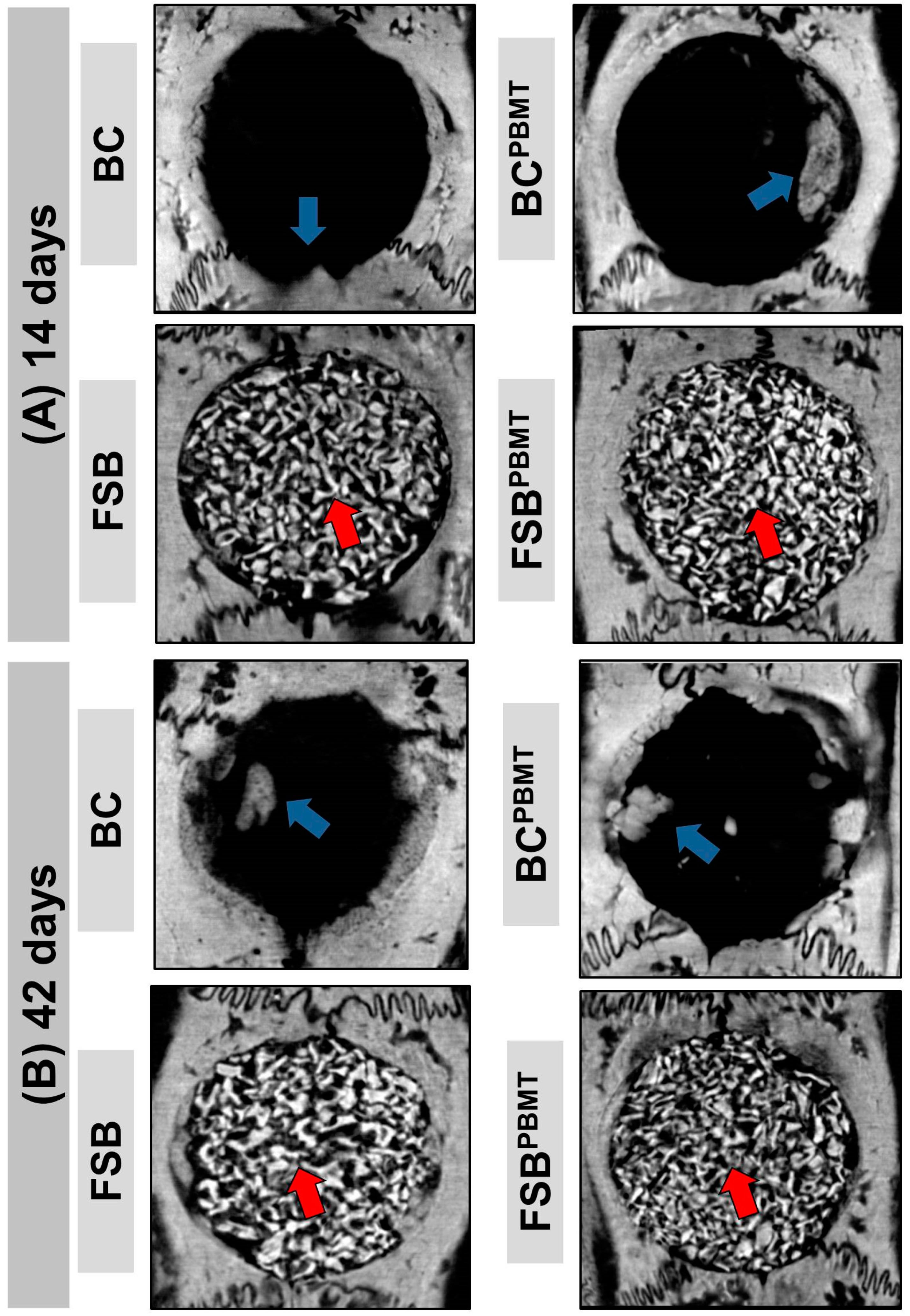
2.1. Microtomographic Analysis
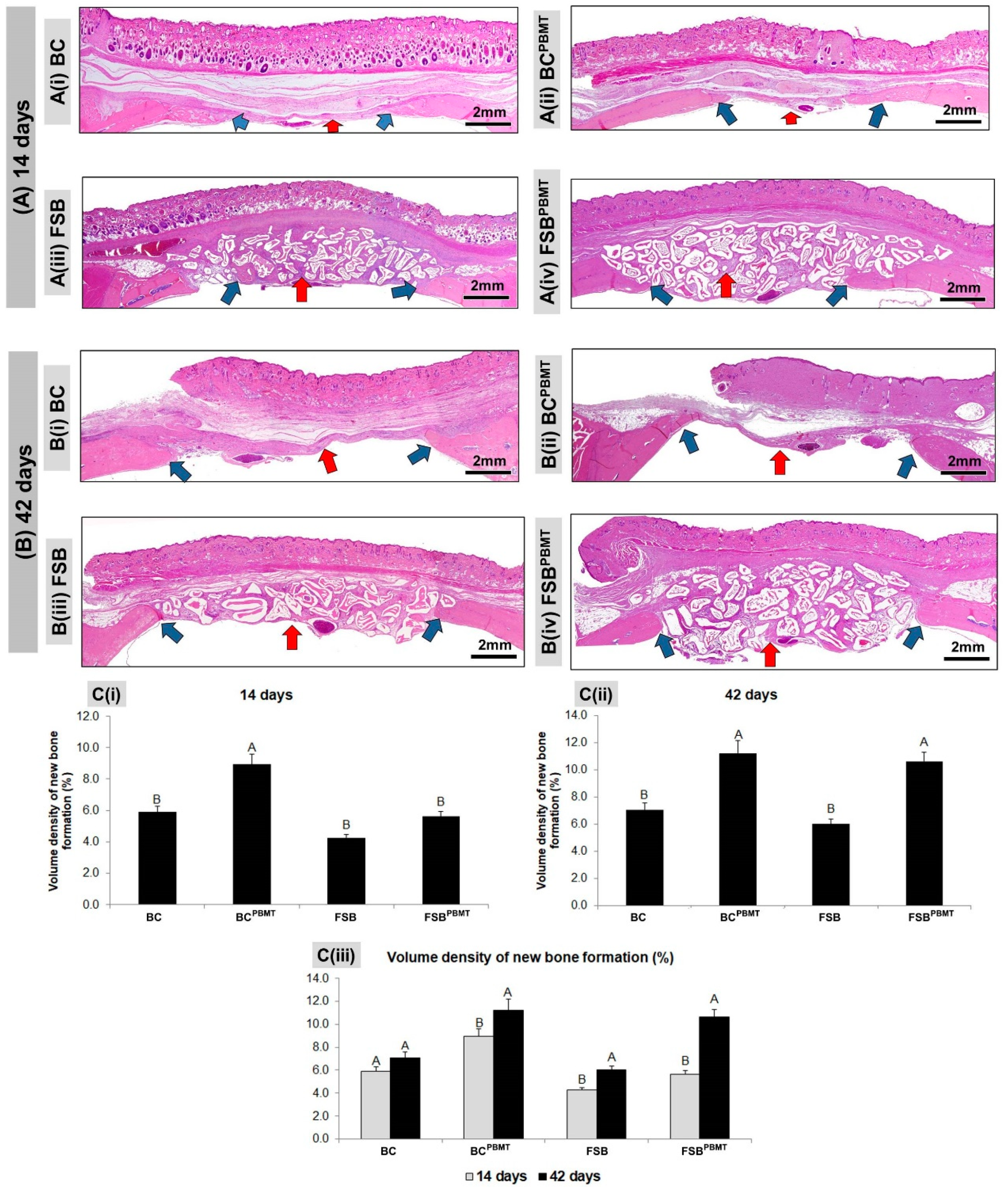
2.2. Histological Evaluation
2.3. Histomorphometric Evaluation
3. Discussion
3.1. Strengths
3.2. Limitations
4. Materials and Methods
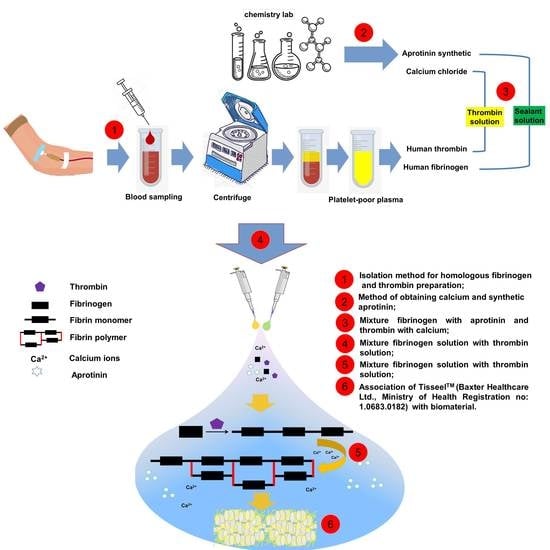
4.1. Blood-Derived Biomaterials—Fibrin Sealant
4.2. Biomaterial—Xenograft
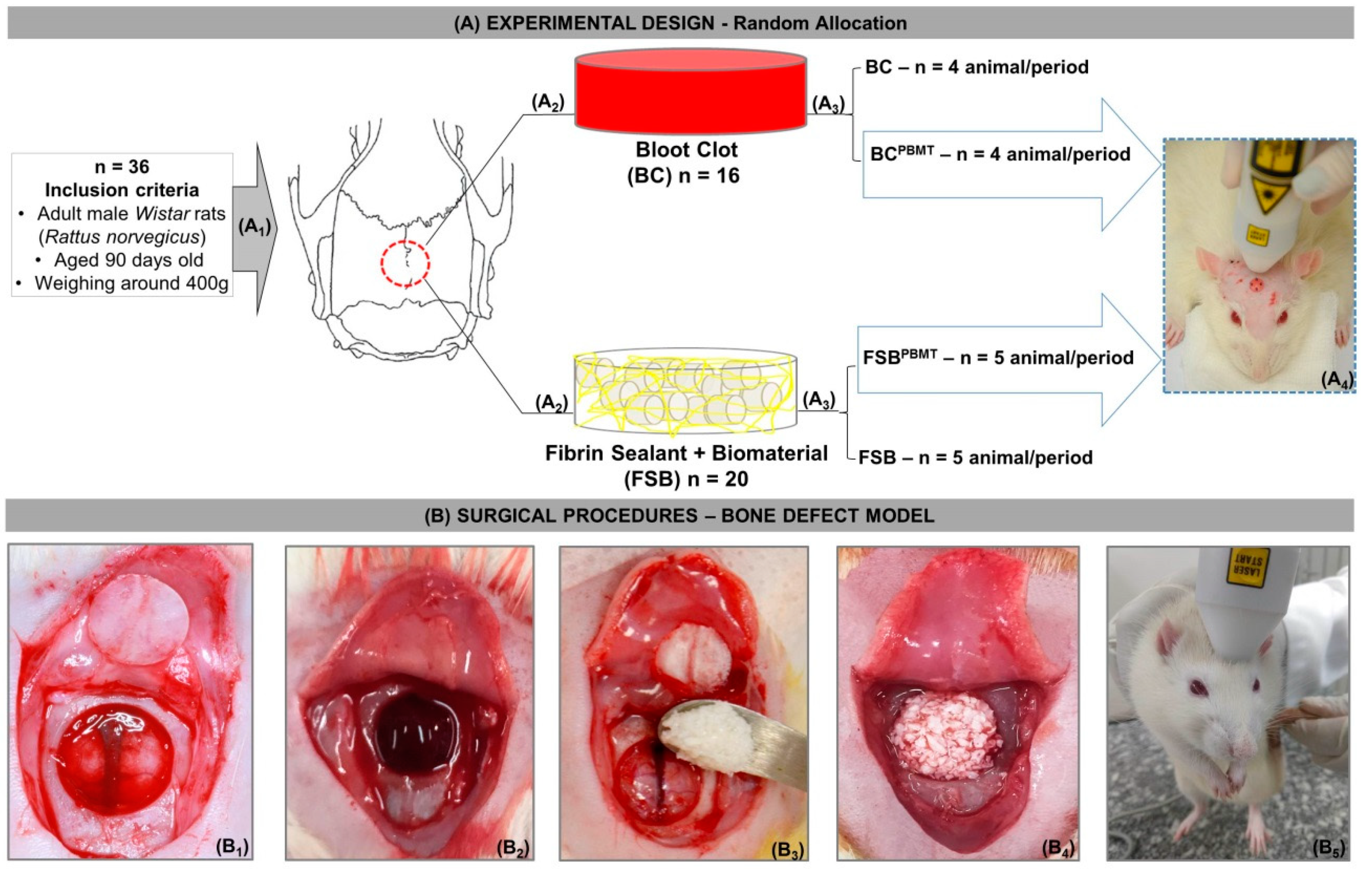
4.3. Experimental Design
4.4. Surgical Procedures
4.5. Photobiomodulation Therapy Protocol
4.6. Collection of Samples and Histological Procedures
4.7. MicroCT Scan (μ-CT)
4.8. Histotechnical Processing
4.9. Histological and Histomorphometric Evaluation of Defects Bone Healing
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nee, E.A.; Chrzanowski, W.; Salih, V.; Kim, H.; Knowles, J. Tissue engineering in dentistry. J. Dent. 2014, 42, 915–928. [Google Scholar] [CrossRef]
- Buchaim, D.V.; Bueno, P.C.D.S.; Andreo, J.C.; Roque, D.D.; Roque, J.S.; Zilio, M.G.; Salatin, J.A.; Kawano, N.; Furlanette, G.; Buchaim, R.L. Action of a deproteinized xenogenic biomaterial in the process of bone repair in rats submitted to inhalation of cigarette smoke. Acta Cir. Bras. 2018, 33, 324–332. [Google Scholar] [CrossRef]
- Pomini, K.; Cestari, M.; German, I.; Rosso, M.; Gonçalves, J.; Buchaim, D.; Pereira, M.; Andreo, J.; Júnior, G.M.R.; della Coletta, B.; et al. Influence of experimental alcoholism on the repair process of bone defects filled with beta-tricalcium phosphate. Drug Alcohol Depend. 2019, 197, 315–325. [Google Scholar] [CrossRef]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yeung, K.W.K. Bioactive Materials Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E.; Jones, E.; Einhorn, T.A. Bone substitutes: An update. Injury 2005, 42, 549–550. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Jones, E.; Einhorn, T.A. Fracture healing and bone repair. Injury 2011, 42, 549–550. [Google Scholar] [CrossRef]
- Melek, L.N. ScienceDirect Tissue engineering in oral and maxillofacial reconstruction. Tanta Dent. J. 2015, 12, 211–223. [Google Scholar] [CrossRef]
- Chen, F.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2017, 53, 86–168. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, S.; Yun, P. Autogenous teeth used for bone grafting: A comparison with tradicional grafting materials. Oral Maxillofac. Surg. 2014, 117, e39–e45. [Google Scholar] [CrossRef] [PubMed]
- Burnouf, T.; Su, C.; Radosevich, M.; Goubran, H. Blood-derived biomaterials: Fibrin sealant. Platelet Gel Platelet Fibrin Glue 2009, 4, 136–142. [Google Scholar]
- Pomini, K.T.; Andreo, J.C.; de Rodrigues, A.C.; de Gonçalves, J.B.O.; Daré, L.R.; German, I.J.S.; Rosa, G.M.; Buchaim, R.L. Effect of low-intensity pulsed ultrasound on bone regeneration biochemical and radiologic analyses. J. Ultrasound Med. 2014, 33, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Bayat, M.; Virdi, A.; Jalalifirouzkouhi, R.; Rezaei, F. Comparison of effects of LLLT and LIPUS on fracture healing in animal models and patients: A systematic review. Prog. Biophys. Mol. Biol. 2018, 132, 3–22. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.; de Araújo, A.; Júnior, R.d.; Barboza, C.; Borges, B.; da Silva, J. Low-level laser therapy (780 nm) combined with collagen sponge scaffold promotes repair of rat cranial critical-size defects and increases TGF-b, FGF-2, OPG/RANK and osteocalcin expression. Int. J. Exp. Pathol. 2017, 98, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Meer, M.; George, R.; George, R. Efficacy of photobiomodulation on accelerating bone healing after tooth extraction: A systematic review. Lasers Med. Sci. 2018. [Google Scholar] [CrossRef]
- Barbizan, R.; Castro, M.; Junior, R.F.; Barraviera, B.; Oliveira, A. Long-Term Spinal Ventral Root Reimplantation, but not Bone Marrow Mononuclear Cell Treatment, Positively Influences Ultrastructural Synapse Recovery and Motor Axonal Regrowth. Int. J. Mol. Sci. 2014, 15, 19535–19551. [Google Scholar] [CrossRef] [PubMed]
- Noori, A.; Ashrafi, S.; Vaez-Ghaemi, R.; Hatamian-Zaremi, A.; Webster, T. A review of fibrin and fibrin composites for bone tissue engineering. Int. J. Nanomed. 2017, 12, 4937–4961. [Google Scholar] [CrossRef] [PubMed]
- Taniyama, K.; Shirakata, Y.; Yoshimoto, T.; Takeuchi, N.; Yoshihara, Y.; Noguchi, K. Bone formation using β-tricalcium phosphate/carboxymethyl-chitin composite scaffold in rat calvarial defects. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, e450–e456. [Google Scholar] [CrossRef]
- Tani, A.; Chellini, F.; Giannelli, M.; Zecchi-orlandini, S.; Sassoli, C. Red (635 nm), Near-Infrared (808 nm) and Violet-Blue (405 nm) Photobiomodulation Potentiality on Human Osteoblasts and Mesenchymal Stromal Cells: A Morphological and Molecular In Vitro Study. Int. J. Mol. Sci. 2018, 19, 1946. [Google Scholar] [CrossRef]
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef]
- De Gonçalves, J.; Buchaim, D.; de Bueno, C.; Pomini, K.; Barraviera, B.; Júnior, R.; Andreo, J.; de Rodrigues, A.; Cestari, T.; Buchaim, R.L. Effects of low-level laser therapy on autogenous bone graft stabilized with a new heterologous fibrin sealant. J. Photochem. Photobiol. B Biol. 2016, 162, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Basford, J.R. Low intensity laser therapy: Still not an established clinical tool. Lasers Surg. Med. 1995, 16, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Karu, T.I.; Afanas’eva, N.I. Cytochrome c oxidase as the primary photoacceptor upon laser exposure of cultured cells to visible and near IR-range light. Dokl Akad Nauk. 1995, 342, 693–695. [Google Scholar]
- Lappalainen, O.; Korpi, R.; Haapea, M.; Korpi, J.; Ylikontiola, L.P.; Kallio-pulkkinen, S.; Serlo, W.S.; Lehenkari, P.; Sándor, G.K. Healing of rabbit calvarial critical-sized defects using autogenous bone grafts and fibrin glue. Child’s Nerv. Syst. 2015, 31, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, A.; Song, S.; Susanto, E.; Chuan, P.; Lam, C.; Woodruff, M.; Hutmacher, D.; Cool, S. Biomaterials The stimulation of healing within a rat calvarial defect by mPCL–TCP/collagen scaffolds loaded with rhBMP-2. Biomaterials 2009, 30, 2479–2488. [Google Scholar] [CrossRef]
- Brown, A.; Barker, T. Fibrin-based biomaterials: Modulation of macroscopic properties through rational design at the molecular level Ashley. Acta Biomater. 2015, 10, 1502–1514. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, F.; Travan, A.; Rustighi, I.; Tarchi, P.; Palmisano, S.; Marsich, E.; Borgogna, M.; Donati, I.; de Manzini, N.; Paoletti, S. Review Article Adhesive and sealant interfaces for general surgery applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 626–639. [Google Scholar] [CrossRef]
- Ruvalcaba-Paredes, E.K.; Hidalgo-Bastida, L.A.; Sesman-Bernal, A.L.; Garciadiego-Cazares, D.; Pérez-Dosal, M.R.; Martínez-López, V.; Vargas-Sandoval, B.; Pichardo-Bahena, R.; Ibarra, C.; Velasquillo, C. Osteogenic potential of murine periosteum for critical-size cranial defects. Br. J. Oral Maxillofac. Surg. 2016, 54, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.A.; Cestari, T.M.; Vidotti, H.A.; de Assis, G.F.; Garlet, G.P.; Taga, R. Sintered anorganic bone graft increases autocrine expression of VEGF, MMP-2 and MMP-9 during repair of critical-size bone defects. J. Mol. Histol. 2014, 45, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Maciel, J.; Momesso, G.; Ramalho-Ferreira, G.; Consolaro, R.; de Carvalho, P.P.; Faverani, L.; Bassi, A.F.; Gosain, A.; Song, L.; Yu, P.; et al. Bone Healing Evaluation in Critical-Size Defects Treated With Xenogenous Bone Plus Porcine Collagen. Implant Dent. 2017, 26, 296–302. [Google Scholar] [CrossRef]
- Gosain, A.; Song, L.; Yu, P.; Mehrara, B.; Maeda, C.; Gold, L.; Longaker, M. Experimental Osteogenesis in Cranial Defects: Reassessment of the Concept of Critical Size and the Expression of TGF-beta isoforms. Plast. Reconstr. Surg. 2000, 106, 360–371. [Google Scholar] [CrossRef]
- An, Y.; Heo, Y.; Lee, J.; Jung, U.; H, C.S. Dehydrothermally Cross-Linked Collagen Membrane with a Bone Graft Improves Bone Regeneration in a Rat Calvarial Defect Model. Materials 2017, 10, 927. [Google Scholar] [CrossRef]
- De Oliveira, A.; Castro-silva, I.; Vicentis, G.; Fernandes, O. Effectiveness and Acceleration of Bone Repair in Critical-Sized Rat Calvarial Defects Using Low-Level Laser Therapy Effectiveness and Acceleration of Bone Repair in Critical-Sized Rat Calvarial Defects Using Low-Level Laser Therapy. Lasers Surg. Med. 2014, 46, 61–67. [Google Scholar] [CrossRef]
- Ozawa, Y.; Shimizu, N.; Kariya, G.; Abiko, Y. Low-Energy Laser Irradiation Stimulates Bone Nodule Formation at Early Stages of Cell Culture in Rat Calvarial Cells. Bone 1998, 22, 347–354. [Google Scholar] [CrossRef]
- Marques, L.; Holgado, L.A.; Francischone, L.A.; Ximenez, J.P.B.; Okamoto, R.; Kinoshita, A. New LLLT protocol to speed up the bone healing process—Histometric and immunohistochemical analysis in rat calvarial bone defect. Lasers Med. Sci. 2015, 30, 1225–1230. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Layrolle, P.; Daculsi, G. A review of bioceramics and fibrin sealant. Eur. Cell Mater 2004, 8, 1e11. [Google Scholar] [CrossRef]
- Buchta, C.; Christian, H.; Macher, M. Biochemical characterization of autologous fibrin sealants produced by CryoSeal s and Vivostat s in comparison to the homologous fibrin sealant product Tissucol/Tisseel s. Biomaterials 2005, 26, 6233–6241. [Google Scholar] [CrossRef]
- Pavel, Š.; Strnadová, M.; Urban, K. In vivo behaviour of low-temperature calcium-deficient hydroxyapatite: Comparison with deproteinised bovine bone. Int. Orthop. 2011, 35, 1553–1560. [Google Scholar] [CrossRef][Green Version]
- Aamodt, J.M.; Grainger, D.W. Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials 2016, 86, 68–82. [Google Scholar] [CrossRef]
- De Almeida, A.L.P.F.; Medeiros, I.L.; Cunha, M.J.S.; Sbrana, M.C.; de Oliveira, P.G.F.P.; Esper, L.A. The effect of low-level laser on bone healing in critical size defects treated with or without autogenous bone graft: An experimental study in rat calvaria. Clin. Oral Implant. Res. 2014, 25, 1131–1136. [Google Scholar] [CrossRef]
- Bosco, A.; Faleiros, P.; Carmona, L.; Garcia, V.; Theodoro, L.; de Araujo, N.; Nagata, M.; de Almeida, J. Effects of low-level laser therapy on bone healing of critical-size defects treated with bovine bone graft. J. Photochem. Photobiol. B Biol. 2016, 163, 303–310. [Google Scholar] [CrossRef]
- Kazancioglu, H.O.; Ezirganli, S.; Aydin, M.S.; Dds, Þ. Effects of Laser and Ozone Therapies on Bone Healing in the Calvarial Defects. J. Craniofacial Surg. 2013, 24, 2141–2146. [Google Scholar] [CrossRef]
- De Deco, C.P.; Marchini, A.M.P.d.; Marchini, L.; da Rocha, R.F. Extended Periods of Alcohol Intake Negatively Affects Osseointegration in Rats. J. Oral Implantol. 2015, 41, e44–e49. [Google Scholar] [CrossRef]
- Moreira, G.; Henry, P.; Alves, M.; Esper, L.; Sbrana, M.; Dalben, S.; Neppelenbroek, K.; de Almeida, A. Effect of Low-Level Laser on the Healing of Bone Defects Filled with Autogenous Bone or Bioactive Glass: In Vivo Study. Int. J. Oral Maxillofac. Implant. 2018, 33, 169–174. [Google Scholar] [CrossRef]
- Kushibiki, T.; Hirasawa, T.; Okawa, S.; Ishihara, M. Regulation of miRNA Expression by Low-Level Laser Therapy (LLLT) and Photodynamic Therapy (PDT). Int. J. Mol. Sci. 2013, 14, 13542–13558. [Google Scholar] [CrossRef]
- Migliario, M.; Pittarella, P.; Fanuli, M.; Rizzi, M.; Renò, F. Laser-induced osteoblast proliferation is mediated by ROS production. Lasers Med. Sci. 2014, 29, 1463–1467. [Google Scholar] [CrossRef]
- Pekkan, G.; Aktas, A.; Pekkan, K. Comparative radiopacity of bone graft materials Comparative radiopacity of bone graft materials. J. Cranio-Maxillofacial Surg. 2011, 40, e1–e4. [Google Scholar] [CrossRef]
- Carinci, F.; Piattelli, A.; Degidi, M.; Palmieri, A.; Perrotti, V.; Scapoli, L.; Martinelli, M.; Laino, G.; Pezzetti, F. Genetic effects of anorganic bovine bone (Bio-OssW) on osteoblast-like MG63 cells. Arch. Oral Biol. 2006, 51, 154–163. [Google Scholar] [CrossRef]
- Lee, J.; Ryu, M.; Baek, H.; Lee, K.; Seo, J.; Lee, H. Fabrication and Evaluation of Porous Beta-Tricalcium Phosphate/Hydroxyapatite (60/40) Composite as a Bone Graft Extender Using Fabrication and Evaluation of Porous Beta-Tricalcium Phosphate/Hydroxyapatite (60/40) Composite as a Bone Graft Exte. Sci. World J. 2013, 481789. [Google Scholar] [CrossRef]
- Parasuraman, S.; Raveendran, R.; Kesavan, R. Blood sample collection in small laboratory animals. J. Pharmacol. Pharmacother. 2010, 1, 87. [Google Scholar] [CrossRef]
- Neagu, T.P.; Ţigliş, M.; Cocoloş, I.; Jecan, C.R. The relationship between periosteum and fracture healing, Rom. J. Morphol. Embryol. 2016, 57, 1215–1220. [Google Scholar]
- Warshawsky, H.; Moore, G. elect U41. J. Histochem. Cytochem. 1967, 15, 542–549. [Google Scholar] [CrossRef]
- Weibel, E.R. Stereological Principles for Morphometry in Electron Microscopic Cytology. Int. Rev. Cytol. 1969, 26, 235–302. [Google Scholar] [CrossRef]

| Parameter | Unit/Explanation |
|---|---|
| Optical Power | 30 mW |
| Wavelength | 830 nm |
| Density of Power or Irradiance | 258.6 mW/cm2 |
| Fluency or Density of Energy or Dosimetry | 6 J/cm2 |
| Beam Area | 0.116 cm2 |
| Total Power | 2.9 J |
| Type of Beam | Positioned for laser irradiation at perpendicular incidence to the skull |
| Emission Mode | Continuous (laser power remains constant at all times) |
| Form of Application | Four points surrounding the surgical area, north, south, east, and west |
| Duration of Irradiation | 24 s/point |
| Total Time of each Application | 96 s |
| Treatment Time | Immediately after surgery and three times a week until euthanasia |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pomini, K.T.; Buchaim, D.V.; Andreo, J.C.; Rosso, M.P.d.O.; Della Coletta, B.B.; German, Í.J.S.; Biguetti, A.C.C.; Shinohara, A.L.; Rosa Júnior, G.M.; Cosin Shindo, J.V.T.; et al. Fibrin Sealant Derived from Human Plasma as a Scaffold for Bone Grafts Associated with Photobiomodulation Therapy. Int. J. Mol. Sci. 2019, 20, 1761. https://doi.org/10.3390/ijms20071761
Pomini KT, Buchaim DV, Andreo JC, Rosso MPdO, Della Coletta BB, German ÍJS, Biguetti ACC, Shinohara AL, Rosa Júnior GM, Cosin Shindo JVT, et al. Fibrin Sealant Derived from Human Plasma as a Scaffold for Bone Grafts Associated with Photobiomodulation Therapy. International Journal of Molecular Sciences. 2019; 20(7):1761. https://doi.org/10.3390/ijms20071761
Chicago/Turabian StylePomini, Karina Torres, Daniela Vieira Buchaim, Jesus Carlos Andreo, Marcelie Priscila de Oliveira Rosso, Bruna Botteon Della Coletta, Íris Jasmin Santos German, Ana Carolina Cestari Biguetti, André Luis Shinohara, Geraldo Marco Rosa Júnior, João Vitor Tadashi Cosin Shindo, and et al. 2019. "Fibrin Sealant Derived from Human Plasma as a Scaffold for Bone Grafts Associated with Photobiomodulation Therapy" International Journal of Molecular Sciences 20, no. 7: 1761. https://doi.org/10.3390/ijms20071761
APA StylePomini, K. T., Buchaim, D. V., Andreo, J. C., Rosso, M. P. d. O., Della Coletta, B. B., German, Í. J. S., Biguetti, A. C. C., Shinohara, A. L., Rosa Júnior, G. M., Cosin Shindo, J. V. T., Alcalde, M. P., Duarte, M. A. H., de Bortoli Teixeira, D., & Buchaim, R. L. (2019). Fibrin Sealant Derived from Human Plasma as a Scaffold for Bone Grafts Associated with Photobiomodulation Therapy. International Journal of Molecular Sciences, 20(7), 1761. https://doi.org/10.3390/ijms20071761