European Patent in Immunoncology: From Immunological Principles of Implantation to Cancer Treatment
Abstract
1. Introduction
2. Non-Classical HLA Groups E to G (class Ib)
2.1. HLA-E
2.2. HLA-F
2.3. HLA-G
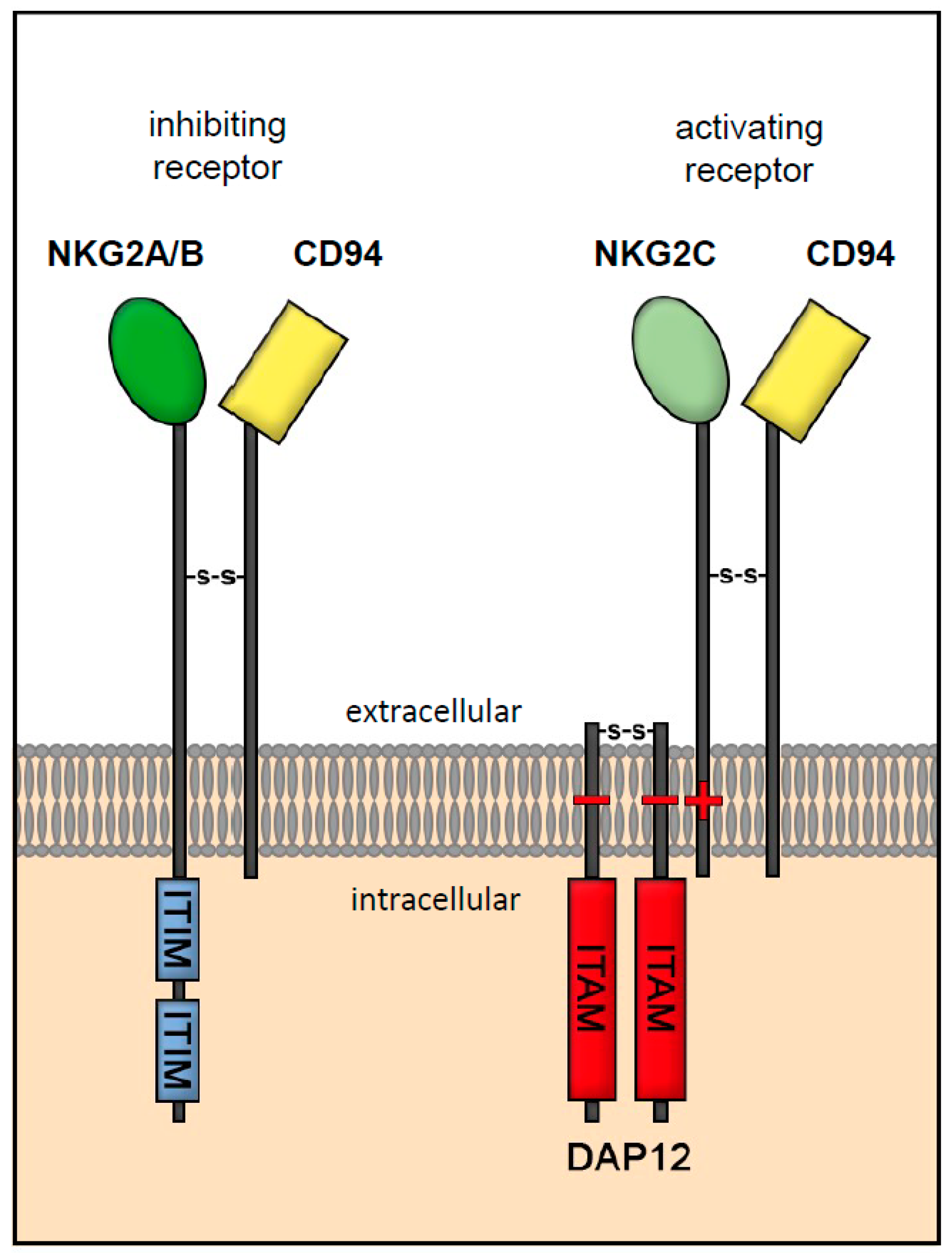
3. Interaction of HLA-E to -G with Receptor Families of Immunocompetent Cells
3.1. Receptor Interaction of HLA-E
3.2. Receptor Interaction of HLA-F
3.3. Receptor Interaction of HLA-G
3.4. HLA-C—A Special Case
3.5. Significance of HLA-E to -G for Implantation/Pregnancy
4. “Embryonic“ HLA Genes in Tumors
4.1. HLA-G Expression in Cancer
4.2. HLA-E Expression in Cancer
4.3. HLA-F Expression in Cancer
4.4. HLA-C Expression in Cancer
4.5. Soluble “Embryonic” HLAs in Cancer
4.6. Metastases
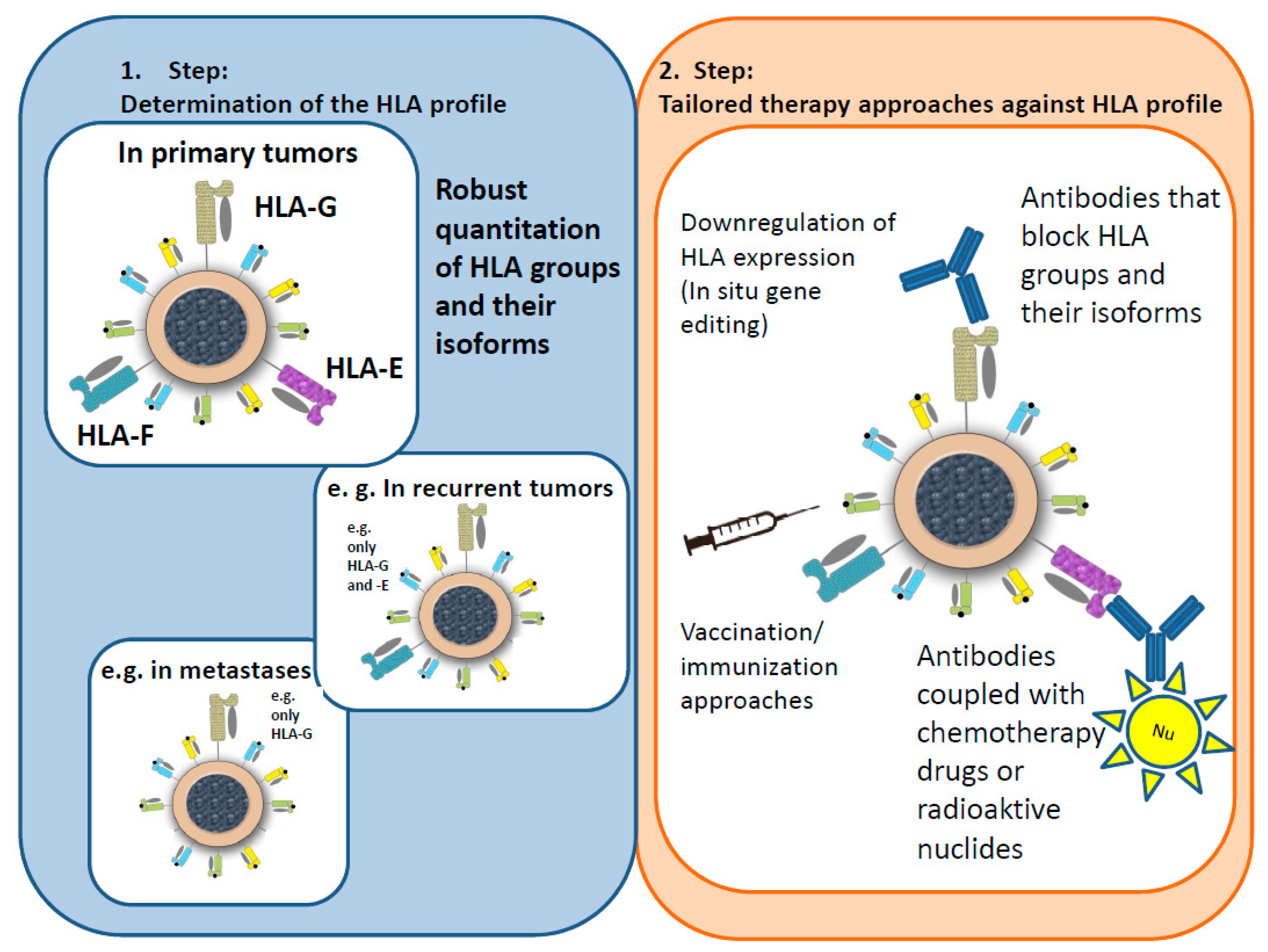
5. Significance for Immunoncology
General Considerations
6. Hypotheses for an Immunological Tumor Therapy Concept (ITTC)
- Masking antibodies mask the corresponding HLA groups/isoforms, blocking the “escape mechanism” of the tumor cells and allowing the immune system to attack the tumor as normal.
- The concept of special antibodies, e.g., coupled with receptors, weak radiation, or chemotherapy drugs, is based on activating the defenses of the immune system as well as attacking the tumor cells directly.
- As radiation and, to a lesser extent, chemotherapy drugs can change the steric structure of an antibody, the antibody would be administered first, followed by an application of the radiation particle or the drug.
Funding
Acknowledgments
Conflicts of Interest
References
- Murray, M.J.; Lessey, B.A. Embryo implantation and tumor metastasis: Common pathways of invasion and angiogenesis. Semin. Reprod. Endocrinol. 1999, 17, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.K.; Lins, R.J.; Lobie, P.E.; Mitchell, M.D. Regulation of invasive growth: Similar epigenetic mechanisms underpin tumour progression and implantation in human pregnancy. Clin. Sci. 2009, 118, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Kurlak, L.O.; Knofler, M.; Mistry, H.D. Lumps & bumps: Common features between placental development and cancer growth. Placenta 2017, 56, 2–4. [Google Scholar] [PubMed]
- Hiby, S.E.; Walker, J.J.; O’Shaughnessy, K.M.; Redman, C.W.; Carrington, M.; Trowsdale, J.; Moffett, A. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J. Exp. Med. 2004, 200, 957–965. [Google Scholar] [CrossRef]
- Varla-Leftherioti, M. Role of a KIR/HLA-C allorecognition system in pregnancy. J. Reprod. Immunol. 2004, 62, 19–27. [Google Scholar] [CrossRef]
- Blais, M.E.; Dong, T.; Rowland-Jones, S. HLA-C as a mediator of natural killer and T-cell activation: Spectator or key player? Immunology 2011, 133, 1–7. [Google Scholar] [CrossRef]
- Chazara, O.; Xiong, S.; Moffett, A. Maternal KIR and fetal HLA-C: A fine balance. J. Leukoc. Biol. 2011, 90, 703–716. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Allan, D.S.; Bowen, M.; Powis, S.J.; Joseph, S.; Verma, S.; Hiby, S.E.; McMichael, A.J.; Loke, Y.W.; Braud, V.M. HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur. J. Immunol. 2000, 30, 1623–1631. [Google Scholar] [CrossRef]
- Vargas, R.G.; Sarturi, P.R.; Mattar, S.B.; Bompeixe, E.P.; Silva Jdos, S.; Pirri, A.; Bicalho Mda, G. Association of HLA-G alleles and 3′ UTR 14 bp haplotypes with recurrent miscarriage in Brazilian couples. Hum. Immunol. 2011, 72, 479–485. [Google Scholar] [CrossRef]
- Ishitani, A.; Sageshima, N.; Hatake, K. The involvement of HLA-E and -F in pregnancy. J. Reprod. Immunol. 2006, 69, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Shobu, T.; Sageshima, N.; Tokui, H.; Omura, M.; Saitom, K.; Nagatsuka, Y.; Nakanishi, M.; Hayashi, Y.; Hatake, K.; Ishitani, A. The surface expression of HLA-F on decidual trophoblasts increases from mid to term gestation. J. Reprod. Immunol. 2006, 72, 18–32. [Google Scholar] [CrossRef]
- Kovats, S.; Main, E.K.; Librach, C.; Stubblebine, M.; Fisher, S.J.; DeMars, R. A class I antigen, HLA-G, expressed in human trophoblasts. Science 1990, 248, 220–223. [Google Scholar] [CrossRef]
- Hunt, J.S.; Petroff, M.G.; McIntire, R.H.; Ober, C. HLA-G and immune tolerance in pregnancy. FASEB J. 2005, 19, 681–693. [Google Scholar] [CrossRef]
- Roussev, R.G.; Coulam, C.B. HLA-G and its role in implantation (review). J. Assist. Reprod. Genet. 2007, 24, 288–295. [Google Scholar] [CrossRef]
- Apps, R.; Gardner, L.; Moffett, A. A critical look at HLA-G. Trends Immunol. 2008, 29, 313–321. [Google Scholar] [CrossRef]
- Shaikly, V.R.; Morrison, I.E.; Taranissi, M.; Noble, C.V.; Withey, A.D.; Cherry, R.J.; Blois, S.M.; Fernandez, N. Analysis of HLA-G in maternal plasma, follicular fluid, and preimplantation embryos reveal an asymmetric pattern of expression. J. Immunol. 2008, 180, 4330–4337. [Google Scholar] [CrossRef]
- Rizzo, R.; Andersen, A.S.; Lassen, M.R.; Sorensen, H.C.; Bergholt, T.; Larsen, M.H.; Melchiorri, L.; Stignani, M.; Baricordi, O.R.; Hviid, T.V. Soluble human leukocyte antigen-G isoforms in maternal plasma in early and late pregnancy. Am. J. Reprod. Immunol. 2009, 62, 320–338. [Google Scholar] [CrossRef]
- Rebmann, V.; da Silva Nardi, F.; Wagner, B.; Horn, P.A. HLA-G as a tolerogenic molecule in transplantation and pregnancy. J. Immunol. Res. 2014, 2014, 297073. [Google Scholar] [CrossRef]
- Foroni, I.; Couto, A.R.; Bettencourt, B.F.; Santos, M.; Lima, M.; Bruges-Armas, J. HLA-E, HLA-F and HLA-G-The Non-Classical Side of the MHC Cluster. HLA and Associated Important Diseases; InTech: Rijeka, Croatia, 2014. [Google Scholar]
- Kraemer, T.; Blasczyk, R.; Bade-Doeding, C. HLA-E: A novel player for histocompatibility. J. Immunol. Res. 2014, 2014, 7. [Google Scholar] [CrossRef]
- Robinson, J.; Halliwell, J.A.; Hayhurst, J.D.; Flicek, P.; Parham, P.; Marsh, S.G. The IPD and IMGT/HLA database: Allele variant databases. Nucleic. Acids Res. 2015, 43, D423–D431. [Google Scholar] [CrossRef]
- Zheng, H.; Lu, R.; Xie, S.; Wen, X.; Wang, H.; Gao, X.; Guo, L. Human leukocyte antigen-E alleles and expression in patients with serous ovarian cancer. Cancer Sci. 2015, 106, 522–528. [Google Scholar] [CrossRef]
- Strong, R.K.; Holmes, M.A.; Li, P.; Braun, L.; Lee, N.; Geraghty, D.E. HLA-E Allelic variants: Correlating differential expression, peptide affinities, crystal structures, and thermal stabilities. J. Biol. Chem. 2003, 278, 5082–5090. [Google Scholar] [CrossRef]
- Lee, N.; Goodlett, D.R.; Ishitani, A.; Marquardt, H.; Geraghty, D.E. HLA-E surface expression depends on binding of TAP-dependent peptides derived from certain HLA-F class I signal sequences. J. Immunol. 1998, 160, 4951–4960. [Google Scholar]
- Llano, M.; Lee, N.; Navarro, F.; Garcia, P.; Albar, J.P.; Geraghty, D.E.; Lopez-Botet, M. HLA-E-bound peptides influence recognition by inhibitory and triggering CD94/NKG2 receptors: Preferential response to an HLA-G-derived nonamer. Eur. J. Immunol. 1998, 28, 2854–2863. [Google Scholar] [CrossRef]
- O’Callaghan, C.A.; Tormo, J.; Willcox, B.E.; Braud, V.M.; Jakobsen, B.K.; Stuart, D.I.; McMichael, A.J.; Bell, J.I.; Jones, E.Y. Structural features impose tight peptide binding specificity in the nonclassical MHC molecule HLA-E. Mol. Cell 1998, 1, 531–541. [Google Scholar] [CrossRef]
- Pietra, G.; Romagnani, C.; Moretta, L.; Mingari, M.C. HLA-E and HLA-E-bound peptides: Recognition by subsets of NK and T cells. Curr. Pharm. Des. 2009, 15, 3336–3344. [Google Scholar] [CrossRef]
- Michaelsson, J.; Teixeira de Matos, C.; Achour, A.; Lanier, L.L.; Karre, K.; Soderstrom, K. A signal peptide derived from hsp60 binds HLA-E and interferes with CD94/NKG2A recognition. J. Exp. Med. 2002, 196, 1403–1414. [Google Scholar] [CrossRef]
- Rolle, A.; Meyer, M.; Calderazzo, S.; Jager, D.; Momburg, F. Distinct HLA-E peptide complexes modify antibody-driven effector functions of adaptive NK cells. Cell Rep. 2018, 24, 1967–1976. [Google Scholar] [CrossRef]
- Hackmon, R.; Pinnaduwage, L.; Zhang, J.; Lye, S.J.; Geraghty, D.E.; Dunk, C.E. Definitive class I human leukocyte antigen expression in gestational placentation: HLA-F, HLA-E, HLA-C, and HLA-G in extravillous trophoblast invasion on placentation, pregnancy, and parturition. Am. J. Reprod. Immunol. 2017, 77, e12643. [Google Scholar] [CrossRef]
- Lima, T.H.A.; Buttura, R.V.; Donadi, E.A.; Veiga-Castelli, L.C. Mendes-Junior CT3, Castelli EC4. HLA-F coding and regulatory segments variability determined by massively parallel sequencing procedures in a Brazilian population sample. Hum. Immunol. 2016, 77, 841–853. [Google Scholar] [CrossRef]
- Sim, M.J.W.; Sun, P.D. HLA-F: A New kid licensed for peptide presentation. Immunity 2017, 46, 972–974. [Google Scholar] [CrossRef]
- Goodridge, J.P.; Burian, A.; Lee, N.; Geraghty, D.E. HLA-F complex without peptide binds to MHC class I protein in the open conformer form. J. Immunol. 2010, 184, 6199–6208. [Google Scholar] [CrossRef]
- Lepin, E.J.; Bastin, J.M.; Allan, D.S.; Roncador, G.; Braud, V.M.; Mason, D.Y.; van der Merwe, P.A.; McMichael, A.J.; Bell, J.I.; Powis, S.H. Functional characterization of HLA-F and binding of HLA-F tetramers to ILT2 and ILT4 receptors. Eur. J. Immunol. 2000, 30, 3552–3561. [Google Scholar] [CrossRef]
- Castelli, E.C.; Veiga-Castelli, L.C.; Yaghi, L.; Moreau, P.; Donadi, E.A. Transcriptional and posttranscriptional regulations of the HLA-G gene. J. Immunol. Res. 2014, 2014, 15. [Google Scholar] [CrossRef]
- Carosella, E.D.; Rouas-Freiss, N.; Roux, D.T.-L.; Moreau, P.; LeMaoult, J. HLA-G: An immune checkpoint molecule. In Advances in Immunology; Frederick, W.A., Ed.; Academic Press: Cambridge, MA, USA, 2015; Chapter Two; pp. 33–144. [Google Scholar]
- Donadi, E.A.; Castelli, E.C.; Arnaiz-Villena, A.; Roger, M.; Rey, D.; Moreau, P. Implications of the polymorphism of HLA-G on its function, regulation, evolution and disease association. Cell Mol. Life Sci. 2011, 68, 369–395. [Google Scholar] [CrossRef]
- Ishitani, A.; Geraghty, D.E. Alternative splicing of HLA-G transcripts yields proteins with primary structures resembling both class I and class II antigens. Proc. Natl. Acad. Sci. USA 1992, 89, 3947–3951. [Google Scholar] [CrossRef]
- Carosella, E.D.; Favier, B.; Rouas-Freiss, N.; Moreau, P.; Lemaoult, J. Beyond the increasing complexity of the immunomodulatory HLA-G molecule. Blood 2008, 111, 4862–4870. [Google Scholar] [CrossRef]
- Fujii, T.; Ishitani, A.; Geraghty, D.E. A soluble form of the HLA-G antigen is encoded by a messenger ribonucleic acid containing intron 4. J. Immunol. 1994, 153, 5516–5524. [Google Scholar]
- Sangrouber, D.; Marcou, C.; Le Discorde, M.; Chang, C.C.; Carosella, E.D.; Moreau, P. Cellular co-localization of intron-4 containing mRNA and HLA-G soluble protein in melanoma analyzed by fluorescence in situ hybridization. J. Immunol. Methods 2007, 326, 54–62. [Google Scholar] [CrossRef]
- Paul, P.; Cabestre, F.A.; Ibrahim, E.C.; Lefebvre, S.; Khalil-Daher, I.; Vazeux, G.; Quiles, R.M.; Bermond, F.; Dausset, J.; Carosella, E.D. Identification of HLA-G7 as a new splice variant of the HLA-G mRNA and expression of soluble HLA-G5, -G6, and -G7 transcripts in human transfected cells. Hum. Immunol. 2000, 61, 1138–1149. [Google Scholar] [CrossRef]
- Liepe, J.; Marino, F.; Sidney, J.; Jeko, A.; Bunting, D.E.; Sette, A.; Kloetzel, P.M.; Stumpf, M.P.; Heck, A.J.; Mishto, M. A large fraction of HLA-F class I ligands are proteasome-generated spliced peptides. Science 2016, 354, 354–358. [Google Scholar] [CrossRef]
- Mishto, M.; Liepe, J. Post-translational peptide splicing and T Cell responses. Trends Immunol. 2017, 38, 904–915. [Google Scholar] [CrossRef]
- Hunt, J.S.; Morales, P.J.; Pace, J.L.; Fazleabas, A.T.; Langat, D.K. A commentary on gestational programming and functions of HLA-G in pregnancy. Placenta 2007, 28, S57–S63. [Google Scholar] [CrossRef][Green Version]
- Young, N.T.; Waller, E.C.; Patel, R.; Roghanian, A.; Austyn, J.M.; Trowsdale, J. The inhibitory receptor LILRB1 modulates the differentiation and regulatory potential of human dendritic cells. Blood 2008, 111, 3090–3096. [Google Scholar] [CrossRef]
- Rouas-Freiss, N.; Moreau, P.; Menier, C.; Carosella, E.D. HLA-G in cancer: A way to turn off the immune system. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Braud, V.M.; Allan, D.S.; O’Callaghan, C.A.; Soderstrom, K.; D’Andrea, A.; Ogg, G.S.; Lazetic, S.; Young, N.T.; Bell, J.I.; Phillips, J.H.; et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 1998, 391, 795–799. [Google Scholar] [CrossRef]
- Brostjan, C.; Sobanov, Y.; Glienke, J.; Hayer, S.; Lehrach, H.; Francis, F.; Hofer, E. The NKG2 natural killer cell receptor family: Comparative analysis of promoter sequences. Genes Immun. 2000, 1, 504–508. [Google Scholar] [CrossRef][Green Version]
- Carretero, M.; Palmieri, G.; Llano, M.; Tullio, V.; Santoni, A.; Geraghty, D.E.; Lopez-Botet, M. Specific engagement of the CD94/NKG2-A killer inhibitory receptor by the HLA-E class Ib molecule induces SHP-1 phosphatase recruitment to tyrosine-phosphorylated NKG2-A: Evidence for receptor function in heterologous transfectants. Eur. J. Immunol. 1998, 28, 1280–1291. [Google Scholar] [CrossRef]
- Farag, S.S.; Fehniger, T.A.; Ruggeri, L.; Velardi, A.; Caligiuri, M.A. Natural killer cell receptors: New biology and insights into the graft-versus-leukemia effect. Blood 2002, 100, 1935–1947. [Google Scholar] [CrossRef]
- Petrie, E.J.; Clements, C.S.; Lin, J.; Sullivan, L.C.; Johnson, D.; Huyton, T.; Heroux, A.; Hoare, H.L.; Beddoe, T.; Reid, H.H.; et al. CD94-NKG2A recognition of human leukocyte antigen (HLA-F)-E bound to an HLA-F class I leader sequence. J. Exp. Med. 2008, 205, 725–735. [Google Scholar] [CrossRef]
- Lazetic, S.; Chang, C.; Houchins, J.P.; Lanier, L.L.; Phillips, J.H. Human natural killer cell receptors involved in MHC class I recognition are disulfide-linked heterodimers of CD94 and NKG2 subunits. J. Immunol. 1996, 157, 4741–4745. [Google Scholar]
- Campbell, K.S.; Colonna, M. DAP12: A key accessory protein for relaying signals by natural killer cell receptors. Int. J. Biochem. Cell Biol. 1999, 31, 631–636. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Long, E. KIR2DL4 (CD158d): An activation receptor for HLA-G. Front. Immunol. 2012, 3, 258. [Google Scholar] [CrossRef]
- Lanier, L.L. Natural killer cell receptor signaling. Curr. Opin. Immunol. 2003, 15, 308–314. [Google Scholar] [CrossRef]
- Shwetank; Date, O.S.; Carbone, E.; Manjunath, R. Inhibition of ERK and proliferation in NK cell lines by soluble HLA-E released from Japanese encephalitis virus infected cells. Immunol. Lett. 2014, 162, 94–100. [Google Scholar] [CrossRef]
- Lauterbach, N.; Wieten, L.; Popeijus, H.E.; Voorter, C.E.; Tilanus, M.G. HLA-E regulates NKG2C+ natural killer cell function through presentation of a restricted peptide repertoire. Hum. Immunol. 2015, 76, 578–586. [Google Scholar] [CrossRef]
- Allan, D.S.; Lepin, E.J.; Braud, V.M.; O’Callaghan, C.A.; McMichael, A.J. Tetrameric complexes of HLA-E, HLA-F, and HLA-G. J. Immunol. Methods 2002, 268, 43–50. [Google Scholar] [CrossRef]
- Hirayasu, K.; Arase, H. Functional and genetic diversity of leukocyte immunoglobulin-like receptor and implication for disease associations. J. Hum. Genet. 2015, 60, 703–708. [Google Scholar] [CrossRef]
- Ketroussi, F.; Giuliani, M.; Bahri, R.; Azzarone, B.; Charpentier, B.; Durrbach, A. Lymphocyte cell-cycle inhibition by HLA-G is mediated by phosphatase SHP-2 and acts on the mTOR pathway. PLoS ONE 2011, 6, e22776. [Google Scholar] [CrossRef]
- Lesport, E.; Baudhuin, J.; Sousa, S.; LeMaoult, J.; Zamborlini, A.; Rouas-Freiss, N.; Carosella, E.D.; Favier, B. Inhibition of human gamma delta [corrected] T-cell antitumoral activity through HLA-G: Implications for immunotherapy of cancer. Cell Mol. Life Sci. 2011, 68, 3385–3399. [Google Scholar] [CrossRef]
- Thomas, R.; Matthias, T.; Witte, T. Leukocyte immunoglobulin-like receptors as new players in autoimmunity. Clin. Rev. Allerg. Immunol. 2010, 38, 159–162. [Google Scholar] [CrossRef]
- Morel, E.; Bellon, T. HLA-F class I molecules regulate IFN-gamma production induced in NK cells by target cells, viral products, or immature dendritic cells through the inhibitory receptor ILT2/CD85j. J. Immunol. 2008, 181, 2368–2381. [Google Scholar] [CrossRef]
- Cortesini, N.S.-F.; Colovai, A.I.; Manavalan, J.S.; Galluzzo, S.; Naiyer, A.J.; Liu, J.; Vlad, G.; Kim-Schulze, S.; Scotto, L.; Fan, J.; et al. Role of regulatory and suppressor T-cells in the induction of ILT3+ ILT4+ tolerogenic endothelial cells in organ allografts. Transplant Immunol. 2004, 13, 73–82. [Google Scholar] [CrossRef]
- Goodridge, J.P.; Burian, A.; Lee, N.; Geraghty, D.E. HLA-F and MHC class I open conformers are ligands for NK cell Ig-like receptors. J. Immunol. 2013, 191, 3553–3562. [Google Scholar] [CrossRef]
- Burian, A.; Wang, K.L.; Finton, K.A.; Lee, N.; Ishitani, A.; Strong, R.K.; Geraghty, D.E. HLA-F and MHC-I open conformers bind natural killer cell Ig-like receptor KIR3DS1. PLoS ONE 2016, 11, e0163297. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Holzemer, A.; Martrus, G.; Chung, A.W.; Pacheco, Y.; Simoneau, C.R.; Rucevic, M.; Lamothe-Molina, P.A.; Pertel, T.; Kim, T.E.; et al. Open conformers of HLA-F are high-affinity ligands of the activating NK-cell receptor KIR3DS1. Nat. Immunol. 2016, 17, 1067–1074. [Google Scholar] [CrossRef]
- Moradi, S.; Berry, R.; Pymm, P.; Hitchen, C.; Beckham, S.A.; Wilce, M.C.J.; Walpole, N.G.; Clements, C.S.; Reid, H.H.; Perugini, M.A.; et al. The structure of the atypical killer cell immunoglobulin-like receptor, KIR2DL4. J. Biol. Chem. 2015, 290, 10460–10471. [Google Scholar] [CrossRef]
- Burshtyn, D.N.; Scharenberg, A.M.; Wagtmann, N.; Rajagopalan, S.; Berrada, K.; Yi, T.; Kinet, J.P.; Long, E.O. Recruitment of tyrosine phosphatase HCP by the killer cell inhibitor receptor. Immunity 1996, 4, 77–85. [Google Scholar] [CrossRef]
- Yusa, S.-I.; Catina, T.L.; Campbell, K.S. SHP-1- and phosphotyrosine-independent inhibitory signaling by a killer cell Ig-like receptor cytoplasmic domain in human NK cells. J. Immunol. 2002, 168, 5047–5057. [Google Scholar] [CrossRef]
- Lunemann, S.; Schobel, A.; Kah, J.; Fittje, P.; Holzemer, A.; Langeneckert, A.E.; Hess, L.U.; Poch, T.; Martrus, G.; Garcia-Beltran, W.F.; et al. Interactions between KIR3DS1 and HLA-F activate natural killer cells to control HCV replication in cell culture. Gastroenterology 2018, 155, 1366–1371. [Google Scholar] [CrossRef]
- Kiani, Z.; Dupuy, F.P.; Bruneau, J.; Lebouche, B.; Zhang, C.X.; Jackson, E.; Lisovsky, I.; da Fonseca, S.; Geraghty, D.E.; Bernard, N.F. HLA-F on HLA-null 721.221 cells activates primary NK cells expressing the activating killer Ig-like receptor KIR3DS1. J. Immunol. 2018, 201, 113–123. [Google Scholar] [CrossRef]
- Brown, D.; Trowsdale, J.; Allen, R. The LILR family: Modulators of innate and adaptive immune pathways in health and disease. Tissue Antigens 2004, 64, 215–225. [Google Scholar] [CrossRef]
- Gonen-Gross, T.; Achdout, H.; Arnon, T.I.; Gazit, R.; Stern, N.; Horejsi, V.; Goldman-Wohl, D.; Yagel, S.; Mandelboim, O. The CD85J/leukocyte inhibitory receptor-1 distinguishes between conformed and beta 2-microglobulin-free HLA-G molecules. J. Immunol. 2005, 175, 4866–4874. [Google Scholar] [CrossRef]
- Shiroishi, M.; Kuroki, K.; Ose, T.; Rasubala, L.; Shiratori, I.; Arase, H.; Tsumoto, K.; Kumagai, I.; Kohda, D.; Maenaka, K. Efficient leukocyte Ig-like receptor signaling and crystal structure of disulfide-linked HLA-G dimer. J. Biol. Chem. 2006, 281, 10439–10447. [Google Scholar] [CrossRef]
- Nardi Fda, S.; Konig, L.; Wagner, B.; Giebel, B.; Santos Manvailer, L.F.; Rebmann, V. Soluble monomers, dimers and HLA-G-expressing extracellular vesicles: The three dimensions of structural complexity to use HLA-G as a clinical biomarker. HLA 2016, 88, 77–86. [Google Scholar] [CrossRef]
- Chen, B.G.; Xu, D.P.; Lin, A.; Yan, W.H. NK cytolysis is dependent on the proportion of HLA-G expression. Hum. Immunol. 2013, 74, 286–289. [Google Scholar] [CrossRef]
- Lin, A.; Yan, W.H.; Xu, H.H.; Gan, M.F.; Cai, J.F.; Zhu, M.; Zhou, M.Y. HLA-G expression in human ovarian carcinoma counteracts NK cell function. Ann. Oncol. 2007, 18, 1804–1809. [Google Scholar] [CrossRef]
- Bainbridge, D.R.; Ellis, S.A.; Sargent, I.L. HLA-G suppresses proliferation of CD4 (+) T-lymphocytes. J. Reprod. Immunol. 2000, 48, 17–26. [Google Scholar] [CrossRef]
- Naji, A.; Menier, C.; Morandi, F.; Agaugue, S.; Maki, G.; Ferretti, E.; Bruel, S.; Pistoia, V.; Carosella, E.D.; Rouas-Freiss, N. Binding of HLA-G to ITIM-bearing Ig-like transcript 2 receptor suppresses B cell responses. J. Immunol. 2014, 192, 1536–1546. [Google Scholar] [CrossRef]
- Amodio, G.; Gregori, S. Human tolerogenic DC-10: Perspectives for clinical applications. Transpl. Res. 2012, 1, 14. [Google Scholar] [CrossRef]
- Gregori, S.; Tomasoni, D.; Pacciani, V.; Scirpoli, M.; Battaglia, M.; Magnani, C.F.; Hauben, E.; Roncarolo, M.G. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood 2010, 116, 935–944. [Google Scholar] [CrossRef]
- Djurisic, S.; Skibsted, L.; Hviid, T.V.F. A phenotypic analysis of regulatory T cells and uterine NK Cells from first trimester pregnancies and associations with HLA-G. Am. J. Reprod. Immunol. 2015, 74, 427–444. [Google Scholar] [CrossRef]
- Goodridge, J.P.; Lathbury, L.J.; John, E.; Charles, A.K.; Christiansen, F.T.; Witt, C.S. The genotype of the NK cell receptor, KIR2DL4, influences INFγ secretion by decidual natural killer cells. Mol. Hum. Reprod. 2009, 15, 489–497. [Google Scholar] [CrossRef]
- Melsen, J.E.; Lugthart, G.; Lankester, A.C.; Schilham, M.W. Human circulating and tissue-resident CD56 (bright) natural killer cell populations. Front Immunol. 2016, 7, 262. [Google Scholar] [CrossRef]
- van der Meer, A.; Lukassen, H.G.; van Lierop, M.J.; Wijnands, F.; Mosselman, S.; Braat, D.D.; Joosten, I. Membrane-bound HLA-G activates proliferation and interferon-gamma production by uterine natural killer cells. Mol. Hum. Reprod. 2004, 10, 189–195. [Google Scholar] [CrossRef]
- Contini, P.; Ghio, M.; Poggi, A.; Filaci, G.; Indiveri, F.; Ferrone, S.; Puppo, F. Soluble HLA-F-A,-B,-C and -G molecules induce apoptosis in T and NK CD8+ cells and inhibit cytotoxic T cell activity through CD8 ligation. Eur. J. Immunol. 2003, 33, 125–134. [Google Scholar] [CrossRef]
- Le Bouteiller, P.; Tabiasco, J.; Polgar, B.; Kozma, N.; Giustiniani, J.; Siewiera, J.; Berrebi, A.; Aguerre-Girr, M.; Bensussan, A.; Jabrane-Ferrat, N. CD160: A unique activating NK cell receptor. Immunol. Lett. 2011, 138, 93–96. [Google Scholar] [CrossRef]
- Burton, G. Human implantation: Cell biology and immunology. J. Anat. 1997, 190, 473–475. [Google Scholar] [CrossRef]
- Fournel, S.; Aguerre-Girr, M.; Huc, X.; Lenfant, F.; Alam, A.; Toubert, A.; Bensussan, A.; Le Bouteiller, P. Cutting edge: Soluble HLA-G1 triggers CD95/CD95 ligand-mediated apoptosis in activated CD8+ cells by interacting with CD8. J. Immunol. 2000, 164, 6100–6104. [Google Scholar] [CrossRef]
- Fons, P.; Chabot, S.; Cartwright, J.E.; Lenfant, F.; L’Faqihi, F.; Giustiniani, J.; Herault, J.-P.; Gueguen, G.; Bono, F.; Savi, P.; et al. Soluble HLA-G1 inhibits angiogenesis through an apoptotic pathway and by direct binding to CD160 receptor expressed by endothelial cells. Blood 2006, 108, 2608–2615. [Google Scholar] [CrossRef]
- King, A.; Burrows, T.D.; Hiby, S.E.; Bowen, J.M.; Joseph, S.; Verma, S.; Lim, P.B.; Gardner, L.; Le Bouteiller, P.; Ziegler, A.; et al. Surface expression of HLA-C antigen by human extravillous trophoblast. Placenta 2000, 21, 376–387. [Google Scholar] [CrossRef]
- Sun, H.S.; Liu, D.X.; Bai, Y.Y.; Hu, N.W. Disease-association of different killer cell immunoglobulin-like receptors (KIR) and HLA-C gene combinations in reactive arthritis. Mod. Rheumatol. 2018, 180, 1–7. [Google Scholar] [CrossRef]
- Colonna, M.; Samaridis, J. Cloning of immunoglobulin-superfamily members associated with HLA-C and HLA-F-B recognition by human natural killer cells. Science 1995, 268, 405–408. [Google Scholar] [CrossRef]
- Male, V.; Sharkey, A.; Masters, L.; Kennedy, P.R.; Farrell, L.E.; Moffett, A. The effect of pregnancy on the uterine NK cell KIR repertoire. Eur. J. Immunol. 2011, 41, 3017–3027. [Google Scholar] [CrossRef]
- Sharkey, A.M.; Gardner, L.; Hiby, S.; Farrell, L.; Apps, R.; Masters, L.; Goodridge, J.; Lathbury, L.; Stewart, C.A.; Verma, S.; et al. Killer Ig-like receptor expression in uterine NK cells is biased toward recognition of HLA-C and alters with gestational age. J. Immunol. 2008, 181, 39–46. [Google Scholar] [CrossRef]
- Faas, M.M.; de Vos, P. Uterine NK cells and macrophages in pregnancy. Placenta 2017, 56, 44–52. [Google Scholar] [CrossRef]
- Amodio, G.; Mugione, A.; Sanchez, A.M.; Vigano, P.; Candiani, M.; Somigliana, E.; Roncarolo, M.G.; Panina-Bordignon, P.; Gregori, S. HLA-G expressing DC-10 and CD4 (+) T cells accumulate in human decidua during pregnancy. Hum. Immunol. 2013, 74, 406–411. [Google Scholar] [CrossRef]
- Lash, G.E.; Pitman, H.; Morgan, H.L.; Innes, B.A.; Agwu, C.N.; Bulmer, J.N. Decidual macrophages: Key regulators of vascular remodeling in human pregnancy. J. Leukoc. Biol. 2016, 100, 315–325. [Google Scholar] [CrossRef]
- Lila, N.; Rouas-Freiss, N.; Dausset, J.; Carpentier, A.; Carosella, E.D. Soluble HLA-G protein secreted by allo-specific CD4+ T cells suppresses the allo-proliferative response: A CD4+ T cell regulatory mechanism. Proc. Natl. Acad. Sci. USA 2001, 98, 12150–12155. [Google Scholar] [CrossRef]
- Persson, G.; Melsted, W.N.; Nilsson, L.L.; Hviid, T.V.F. HLA class Ib in pregnancy and pregnancy-related disorders. Immunogenetics 2017, 69, 581–595. [Google Scholar] [CrossRef]
- Rizzo, R.; Vercammen, M.; van de Velde, H.; Horn, P.A.; Rebmann, V. The importance of HLA-G expression in embryos, trophoblast cells, and embryonic stem cells. Cell Mol. Life Sci. 2011, 68, 341–352. [Google Scholar] [CrossRef]
- Steinborn, A.; Varkonyi, T.; Scharf, A.; Bahlmann, F.; Klee, A.; Sohn, C. Early detection of decreased soluble HLA-G levels in the maternal circulation predicts the occurrence of preeclampsia and intrauterine growth retardation during further course of pregnancy. Am. J. Reprod. Immunol. 2007, 57, 277–286. [Google Scholar] [CrossRef]
- Drukker, M.; Katz, G.; Urbach, A.; Schuldiner, M.; Markel, G.; Itskovitz-Eldor, J.; Reubinoff, B.; Mandelboim, O.; Benvenisty, N. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2002, 99, 9864–9869. [Google Scholar] [CrossRef]
- Fuzzi, B.; Rizzo, R.; Criscuoli, L.; Noci, I.; Melchiorri, L.; Scarselli, B.; Bencini, E.; Menicucci, A.; Baricordi, O.R. HLA-G expression in early embryos is a fundamental prerequisite for the obtainment of pregnancy. Eur. J. Immunol. 2002, 32, 311–315. [Google Scholar] [CrossRef]
- Verloes, A.; Van de Velde, H.; LeMaoult, J.; Mateizel, I.; Cauffman, G.; Horn, P.A.; Carosella, E.D.; Devroey, P.; De Waele, M.; Rebmann, V.; et al. HLA-G expression in human embryonic stem cells and preimplantation embryos. J. Immunol. 2011, 186, 2663–2671. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, N.; Li, T.; Guo, J.; Wang, Z.; Yang, M.; Gao, L. Human umbilical cord Wharton’s jelly stem cells: Immune property genes assay and effect of transplantation on the immune cells of heart failure patients. Cell Immunol. 2012, 276, 83–90. [Google Scholar] [CrossRef]
- Giacomini, E.; Vago, R.; Sanchez, A.M.; Podini, P.; Zarovni, N.; Murdica, V.; Rizzo, R.; Bortolotti, D.; Candiani, M.; Vigano, P. Secretome of in vitro cultured human embryos contains extracellular vesicles that are uptaken by the maternal side. Sci. Rep. 2017, 7, 5210. [Google Scholar] [CrossRef]
- Noci, I.; Fuzzi, B.; Rizzo, R.; Melchiorri, L.; Criscuoli, L.; Dabizzi, S.; Biagiotti, R.; Pellegrini, S.; Menicucci, A.; Baricordi, O.R. Embryonic soluble HLA-G as a marker of developmental potential in embryos. Hum. Reprod. 2005, 20, 138–146. [Google Scholar] [CrossRef]
- Jabeen, A.; Miranda-Sayago, J.M.; Obara, B.; Spencer, P.S.; Dealtry, G.B.; Hayrabedyan, S.; Shaikly, V.; Laissue, P.P.; Fernández, N. Quantified colocalization reveals heterotypic histocompatibility class I antigen associations on trophoblast cell membranes: Relevance for human pregnancy. Biol. Reprod. 2013, 89, 1–10. [Google Scholar] [CrossRef][Green Version]
- Sher, G.; Keskintepe, L.; Nouriani, M.; Roussev, R.; Batzofin, J. Expression of sHLA-G in supernatants of individually cultured 46-h embryos: A potentially valuable indicator of ‘embryo competency’ and IVF outcome. Reprod. Biomed. Online 2004, 9, 74–78. [Google Scholar] [CrossRef]
- Tabiasco, J.; Perrier d’Hauterive, S.; Thonon, F.; Parinaud, J.; Leandri, R.; Foidart, J.M.; Chaouat, G.; Munaut, C.; Lombroso, R.; Selva, J.; et al. Soluble HLA-G in IVF/ICSI embryo culture supernatants does not always predict implantation success: A multicentre study. Reprod. Biomed. Online 2009, 18, 374–381. [Google Scholar] [CrossRef]
- Niu, Z.; Wang, L.; Pang, R.T.K.; Guo, Y.; Yeung, W.S.B.; Yao, Y. A meta-analysis of the impact of human leukocyte antigen-G on the outcomes of IVF/ICSI. Reprod. Biomed. Online 2017, 34, 611–618. [Google Scholar] [CrossRef]
- Mosaferi, E.; Majidi, J.; Mohammadian, M.; Babaloo, Z.; Monfaredan, A.; Baradaran, B. HLA-G expression pattern: Reliable assessment for pregnancy outcome prediction. Adv. Pharm. Bull. 2013, 3, 443–446. [Google Scholar]
- Koc, A.; Kirbiyik, O.; Kutbay, Y.B.; Ozyilmaz, B.; Ozdemir, T.R.; Kaya, O.O.; Kubat, G.; Koc, Z.P. Fetal HLA-G alleles and their effect on miscarriage. Adv. Clin. Exp. Med. 2018, 27, 1233–1237. [Google Scholar] [CrossRef]
- Jassem, R.M.; Shani, W.S.; Loisel, D.A.; Sharief, M.; Billstrand, C.; Ober, C. HLA-G polymorphisms and soluble HLA-G protein levels in women with recurrent pregnancy loss from Basrah province in Iraq. Hum. Immunol. 2012, 73, 811–817. [Google Scholar] [CrossRef]
- Abediankenari, S.; Farzad, F.; Rahmani, Z.; Hashemi-Soteh, M.B. HLA-G5 and G7 isoforms in pregnant women. Iran J. Allerg. Asthma Immunol. 2015, 14, 217–221. [Google Scholar]
- Zidi, I.; Laaribi, A.B.; Bortolotti, D.; Belhadj, M.; Mehri, A.; Yahia, H.B.; Babay, W.; Chaouch, H.; Zidi, N.; Letaief, A.; et al. HLA-E polymorphism and soluble HLA-E plasma levels in chronic hepatitis B patients. HLA-F 2016, 87, 153–159. [Google Scholar] [CrossRef]
- Enghelabifar, M.; Allafan, S.; Khayatzadeh, J.; Shahrokh Abadi, K.; Hasanzadeh Nazarabadi, M.; Moradi, F.; Musavifar, N.; Jalali, M.; Mojarrad, M. Association of the maternal 14-bp insertion/deletion polymorphism in the histocompatibility leukocyte antigen G gene with recurrent implantation failure. Iran J. Reprod. Med. 2014, 12, 641–646. [Google Scholar]
- Hashemi, M.; Mokhtari, M.; Khazaeian, S.; Bahari, G.; Rezaei, M.; Nakhaee, A.; Taheri, M. Evaluation of HLA-G 14-bp ins/del and +3142G>C polymorphisms with susceptibility to recurrent spontaneous abortion. Taiwan J. Obstet. Gynecol. 2017, 56, 276–280. [Google Scholar] [CrossRef]
- Amodio, G.; Canti, V.; Maggio, L.; Rosa, S.; Castiglioni, M.T.; Rovere-Querini, P.; Gregori, S. Association of genetic variants in the 3′UTR of HLA-G with recurrent pregnancy loss. Hum. Immunol. 2016, 77, 886–891. [Google Scholar] [CrossRef]
- Michita, R.T.; Zambra, F.M.B.; Fraga, L.R.; Sanseverino, M.T.V.; Callegari-Jacques, S.M.; Vianna, P.; Chies, J.A.B. A tug-of-war between tolerance and rejection—New evidence for 3′UTR HLA-G haplotypes influence in recurrent pregnancy loss. Hum. Immunol. 2016, 77, 892–897. [Google Scholar] [CrossRef]
- Fan, W.; Huang, Z.; Li, S.; Xiao, Z. The HLA-G 14-bp polymorphism and recurrent implantation failure: A meta-analysis. J. Assist. Reprod. Genet. 2017, 34, 1559–1565. [Google Scholar] [CrossRef]
- Aldrich, C.L.; Stephenson, M.D.; Karrison, T.; Odem, R.R.; Branch, D.W.; Scott, J.R.; Schreiber, J.R.; Ober, C. HLA-G genotypes and pregnancy outcome in couples with unexplained recurrent miscarriage. Mol. Hum. Reprod. 2001, 7, 1167–1172. [Google Scholar] [CrossRef][Green Version]
- Kuroshli, Z.; Gourabi, H.; Bazrgar, M.; Sanati, M.; Zamani Esteki, M. The relationship between HLA-G gene polymorphisms and repeated implantation failure in infertile couples undergoing assisted reproductive technique. Iran J. Allerg. Asthma Immunol. 2015, 14, 535–542. [Google Scholar]
- Fotoohi, M.; Ghasemi, N.; Mirghanizadeh, S.A.; Vakili, M.; Samadi, M. Association between HLA-E gene polymorphism and unexplained recurrent spontaneous abortion (RSA) in Iranian women. Int. J. Reprod. Biomed. 2016, 14, 477–482. [Google Scholar] [CrossRef]
- Lin, A.; Yan, W.-H. Heterogeneity of HLA-G expression in cancers: Facing the challenges. Front. Immunol. 2018, 9, 2164. [Google Scholar] [CrossRef]
- Amiot, L.; Ferrone, S.; Grosse-Wilde, H.; Seliger, B. Biology of HLA-G in cancer: A candidate molecule for therapeutic intervention? Cell Mol. Life Sci. 2011, 68, 417–431. [Google Scholar] [CrossRef]
- Jeong, S.; Park, S.; Park, B.W.; Park, Y.; Kwon, O.J.; Kim, H.S. Human leukocyte antigen-G (HLA-G) polymorphism and expression in breast cancer patients. PLoS ONE 2014, 9, e98284. [Google Scholar] [CrossRef]
- Rolfsen, G.B.; Castelli, E.C.; Donadi, E.A.; Duarte, R.A.; Soares, C.P. HLA-G polymorphism and breast cancer. Int. J. Immunogenet. 2014, 41, 143–148. [Google Scholar] [CrossRef]
- da Silva, G.B.; Silva, T.G.; Duarte, R.A.; Neto, N.L.; Carrara, H.H.; Donadi, E.A.; Goncalves, M.A.; Soares, E.G.; Soares, C.P. Expression of the classical and nonclassical HLA-F molecules in breast cancer. Int. J. Breast Cancer 2013, 2013, 250435. [Google Scholar] [CrossRef]
- He, X.; Dong, D.-D.; Yie, S.-M.; Yang, H.; Cao, M.; Ye, S.-R.; Li, K.; Liu, J.; Chen, J. HLA-G expression in human breast cancer: Implications for diagnosis and prognosis, and effect on allocytotoxic lymphocyte response after hormone treatment in vitro. Ann. Surg. Oncol. 2010, 17, 1459–1469. [Google Scholar] [CrossRef]
- Kleinberg, L.; Florenes, V.A.; Skrede, M.; Dong, H.P.; Nielsen, S.; McMaster, M.T.; Nesland, J.M.; Shih Ie, M.; Davidson, B. Expression of HLA-G in malignant mesothelioma and clinically aggressive breast carcinoma. Virchows Arch. 2006, 449, 31–39. [Google Scholar] [CrossRef]
- Palmisano, G.L.; Pistillo, M.P.; Fardin, P.; Capanni, P.; Nicolo, G.; Salvi, S.; Spina, B.; Pasciucco, G.; Ferrara, G.B. Analysis of HLA-G expression in breast cancer tissues. Hum. Immunol. 2002, 63, 969–976. [Google Scholar] [CrossRef]
- Ferguson, R.; Ramanakumar, A.V.; Koushik, A.; Coutlee, F.; Franco, E.; Roger, M. Human leukocyte antigen G polymorphism is associated with an increased risk of invasive cancer of the uterine cervix. Int. J. Cancer 2012, 131, E312–E319. [Google Scholar] [CrossRef]
- Singer, G.; Rebmann, V.; Chen, Y.C.; Liu, H.T.; Ali, S.Z.; Reinsberg, J.; McMaster, M.T.; Pfeiffer, K.; Chan, D.W.; Wardelmann, E.; et al. HLA-G is a potential tumor marker in malignant ascites. Clin. Cancer Res. 2003, 9, 4460–4464. [Google Scholar]
- Zhang, X.; Han, Q.Y.; Li, J.B.; Ruan, Y.Y.; Yan, W.H.; Lin, A. Lesion HLA-G5/-G6 isoforms expression in patients with ovarian cancer. Hum. Immunol. 2016, 77, 780–784. [Google Scholar] [CrossRef]
- Rutten, M.J.; Dijk, F.; Savci-Heijink, C.D.; Buist, M.R.; Kenter, G.G.; van de Vijver, M.J.; Jordanova, E.S. HLA-G expression is an independent predictor for improved survival in high grade ovarian carcinomas. J. Immunol. Res. 2014, 2014, 274584. [Google Scholar] [CrossRef]
- Lin, A.; Xu, H.H.; Xu, D.P.; Zhang, X.; Wang, Q.; Yan, W.H. Multiple steps of HLA-G in ovarian carcinoma metastasis: Alter NK cytotoxicity and induce matrix metalloproteinase-15 (MMP-15) expression. Hum. Immunol. 2013, 74, 439–446. [Google Scholar] [CrossRef]
- Bijen, C.B.; Bantema-Joppe, E.J.; de Jong, R.A.; Leffers, N.; Mourits, M.J.; Eggink, H.F.; van der Zee, A.G.; Hollema, H.; de Bock, G.H.; Nijman, H.W. The prognostic role of classical and nonclassical MHC class I expression in endometrial cancer. Int. J. Cancer 2010, 126, 1417–1427. [Google Scholar] [CrossRef]
- Gimenes, F.; Teixeira, J.J.; de Abreu, A.L.; Souza, R.P.; Pereira, M.W.; da Silva, V.R.; Boer, C.G.; Maria-Engler, S.S.; Bonini, M.G.; Borelli, S.D.; et al. Human leukocyte antigen (HLA-F)-G and cervical cancer immunoediting: A candidate molecule for therapeutic intervention and prognostic biomarker? Biochim. Biophys. Acta 2014, 1846, 576–589. [Google Scholar] [CrossRef]
- Li, X.J.; Zhang, X.; Lin, A.; Ruan, Y.Y.; Yan, W.H. Human leukocyte antigen-G (HLA-G) expression in cervical cancer lesions is associated with disease progression. Hum. Immunol. 2012, 73, 946–949. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Galeano, L.; Palacios, D.M.; Gomez, C.; Serrano, M.L.; Bravo, M.M.; Combita, A.L. Altered HLA-F class I and HLA-G expression is associated with IL-10 expression in patients with cervical cancer. Pathobiology 2012, 79, 72–83. [Google Scholar] [CrossRef]
- Zheng, N.; Wang, C.X.; Zhang, X.; Du, L.T.; Zhang, J.; Kan, S.F.; Zhu, C.B.; Dong, Z.G.; Wang, L.L.; Wang, S.; et al. Up-regulation of HLA-G expression in cervical premalignant and malignant lesions. Tissue Antigens 2011, 77, 218–224. [Google Scholar] [CrossRef]
- Langat, D.K.; Sue Platt, J.; Tawfik, O.; Fazleabas, A.T.; Hunt, J.S. Differential expression of human leukocyte antigen-G (HLA-G) messenger RNAs and proteins in normal human prostate and prostatic adenocarcinoma. J. Reprod. Immunol. 2006, 71, 75–86. [Google Scholar] [CrossRef]
- Montilla, D.; Perez, M.; Borges, L.; Bianchi, G.; Cova, J.A. Soluble human leukocyte antigen-G in the bronchoalveolar lavage of lung cancer patients. Arch. Bronconeumol. 2016, 52, 420–424. [Google Scholar] [CrossRef]
- Urosevic, M.; Kurrer, M.O.; Kamarashev, J.; Mueller, B.; Weder, W.; Burg, G.; Stahel, R.A.; Dummer, R.; Trojan, A. Human leukocyte antigen G up-regulation in lung cancer associates with high-grade histology, human leukocyte antigen class I loss and interleukin-10 production. Am. J. Pathol. 2001, 159, 817–824. [Google Scholar] [CrossRef]
- Wisniewski, A.; Kowal, A.; Wyrodek, E.; Nowak, I.; Majorczyk, E.; Wagner, M.; Pawlak-Adamska, E.; Jankowska, R.; Slesak, B.; Frydecka, I.; et al. Genetic polymorphisms and expression of HLA-G and its receptors, KIR2DL4 and LILRB1, in non-small cell lung cancer. Tissue Antigens 2015, 85, 466–475. [Google Scholar] [CrossRef]
- Yan, W.H.; Liu, D.; Lu, H.Y.; Li, Y.Y.; Zhang, X.; Lin, A. Significance of tumour cell HLA-G5/-G6 isoform expression in discrimination for adenocarcinoma from squamous cell carcinoma in lung cancer patients. J. Cell Mol. Med. 2015, 19, 778–785. [Google Scholar] [CrossRef]
- Yie, S.M.; Yang, H.; Ye, S.R.; Li, K.; Dong, D.D.; Lin, X.M. Expression of human leucocyte antigen G (HLA-G) is associated with prognosis in non-small cell lung cancer. Lung Cancer 2007, 58, 267–274. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Qiu, L.; Zhang, P.; Li, J.; Yang, D.; Wei, X.; Han, Y.; Nie, S.; Sun, Y. Co-expression of ILT4/HLA-G in human non-small cell lung cancer correlates with poor prognosis and ILT4-HLA-G interaction activates ERK signaling. Tumour. Biol. 2016, 37, 11187–11198. [Google Scholar] [CrossRef]
- Carosella, E.D.; Ploussard, G.; LeMaoult, J.; Desgrandchamps, F. A systematic review of immunotherapy in urologic cancer: Evolving roles for targeting of CTLA-4, PD-1/PD-L1, and HLA-G. Eur. Urol. 2015, 68, 267–279. [Google Scholar] [CrossRef]
- Castelli, E.C.; Mendes-Junior, C.T.; Viana de Camargo, J.L.; Donadi, E.A. HLA-G polymorphism and transitional cell carcinoma of the bladder in a Brazilian population. Tissue Antigens 2008, 72, 149–157. [Google Scholar] [CrossRef]
- El-Chennawi, F.A.; Auf, F.A.; El-Diasty, A.M.; El-Daim, M.A.; El-Sherbiny, S.M.; Ali, A.; El-Baz, M.; El-Hameed, M.A.; Paul, P.; Ibrahim, E.C.; et al. Expression of HLA-G in cancer bladder. Egypt J. Immunol. 2005, 12, 57–64. [Google Scholar]
- Gan, L.H.; Huang, L.F.; Zhang, X.; Lin, A.; Xu, D.P.; Wang, Q.; Wang, T.J.; Yan, W.H. Tumor-specific upregulation of human leukocyte antigen-G expression in bladder transitional cell carcinoma. Hum. Immunol. 2010, 71, 899–904. [Google Scholar] [CrossRef]
- Swets, M.; Konig, M.H.; Zaalberg, A.; Dekker-Ensink, N.G.; Gelderblom, H.; van de Velde, C.J.; van den Elsen, P.J.; Kuppen, P.J. HLA-G and classical HLA-F class I expression in primary colorectal cancer and associated liver metastases. Hum. Immunol. 2016, 77, 773–779. [Google Scholar] [CrossRef]
- Guo, Z.Y.; Lv, Y.G.; Wang, L.; Shi, S.J.; Yang, F.; Zheng, G.X.; Wen, W.H.; Yang, A.G. Predictive value of HLA-G and HLA-E in the prognosis of colorectal cancer patients. Cell Immunol. 2015, 293, 10–16. [Google Scholar] [CrossRef]
- Reimers, M.S.; Engels, C.C.; Putter, H.; Morreau, H.; Liefers, G.J.; van de Velde, C.J.; Kuppen, P.J. Prognostic value of HLA class I, HLA-E, HLA-G and tregs in rectal cancer: A retrospective cohort study. BMC Cancer 2014, 14, 486. [Google Scholar] [CrossRef]
- Zeestraten, E.C.; Reimers, M.S.; Saadatmand, S.; Goossens-Beumer, I.J.; Dekker, J.W.; Liefers, G.J.; van den Elsen, P.J.; van de Velde, C.J.; Kuppen, P.J. Combined analysis of HLA-F class I, HLA-E and HLA-G predicts prognosis in colon cancer patients. Br. J. Cancer 2014, 110, 459–468. [Google Scholar] [CrossRef]
- Dardano, A.; Rizzo, R.; Polini, A.; Stignani, M.; Tognini, S.; Pasqualetti, G.; Ursino, S.; Colato, C.; Ferdeghini, M.; Baricordi, O.R.; et al. Soluble human leukocyte antigen-G and its insertion/deletion polymorphism in papillary thyroid carcinoma: Novel potential biomarkers of disease? J. Clin. Endocrinol. Metab. 2012, 97, 4080–4086. [Google Scholar] [CrossRef]
- Nunes, L.M.; Ayres, F.M.; Francescantonio, I.C.; Saddi, V.A.; Avelino, M.A.; Alencar Rde, C.; Silva, R.C.; Meneghini, A.J.; Wastowski, I.J. Association between the HLA-G molecule and lymph node metastasis in papillary thyroid cancer. Hum. Immunol. 2013, 74, 447–451. [Google Scholar] [CrossRef]
- Cao, M.; Yie, S.M.; Liu, J.; Ye, S.R.; Xia, D.; Gao, E. Plasma soluble HLA-G is a potential biomarker for diagnosis of colorectal, gastric, esophageal and lung cancer. Tissue Antigens 2011, 78, 120–128. [Google Scholar] [CrossRef]
- Lin, A.; Zhang, X.; Zhou, W.J.; Ruan, Y.Y.; Xu, D.P.; Wang, Q.; Yan, W.H. Human leukocyte antigen-G expression is associated with a poor prognosis in patients with esophageal squamous cell carcinoma. Int. J. Cancer 2011, 129, 1382–1390. [Google Scholar] [CrossRef]
- Degenhardt, Y.; Huang, J.; Greshock, J.; Horiates, G.; Nathanson, K.; Yang, X.; Herlyn, M.; Weber, B. Distinct MHC gene expression patterns during progression of melanoma. Genes Chromosom. Cancer 2010, 49, 144–154. [Google Scholar]
- Bezuhly, M.; Howlett, A.; Colp, P.; Conrad, D.M.; Walsh, N.; Rowden, G.; Morris, S.F.; Langley, R.G. Quantitative HLA-G expression in metastasising and non-metastasising primary thin cutaneous melanomas. Dermatology 2008, 217, 281–283. [Google Scholar] [CrossRef][Green Version]
- Paul, P.; Cabestre, F.A.; Le Gal, F.A.; Khalil-Daher, I.; Le Danff, C.; Schmid, M.; Mercier, S.; Avril, M.F.; Dausset, J.; Guillet, J.G.; et al. Heterogeneity of HLA-G gene transcription and protein expression in malignant melanoma biopsies. Cancer Res. 1999, 59, 1954–1960. [Google Scholar]
- Paul, P.; Rouas-Freiss, N.; Khalil-Daher, I.; Moreau, P.; Riteau, B.; Le Gal, F.A.; Avril, M.F.; Dausset, J.; Guillet, J.G.; Carosella, E.D. HLA-G expression in melanoma: A way for tumor cells to escape from immunosurveillance. Proc. Natl. Acad. Sci. USA 1998, 95, 4510–4515. [Google Scholar] [CrossRef]
- Urosevic, M.; Willers, J.; Mueller, B.; Kempf, W.; Burg, G.; Dummer, R. HLA-G protein up-regulation in primary cutaneous lymphomas is associated with interleukin-10 expression in large cell T-cell lymphomas and indolent B-cell lymphomas. Blood 2002, 99, 609–617. [Google Scholar] [CrossRef]
- Diepstra, A.; Poppema, S.; Boot, M.; Visser, L.; Nolte, I.M.; Niens, M.; Te Meerman, G.J.; van den Berg, A. HLA-G protein expression as a potential immune escape mechanism in classical Hodgkin’s lymphoma. Tissue Antigens 2008, 71, 219–226. [Google Scholar] [CrossRef]
- Tronik-Le Roux, D.; Renard, J.; Verine, J.; Renault, V.; Tubacher, E.; LeMaoult, J.; Rouas-Freiss, N.; Deleuze, J.F.; Desgrandschamps, F.; Carosella, E.D. Novel landscape of HLA-G isoforms expressed in clear cell renal cell carcinoma patients. Mol. Oncol. 2017, 11, 1561–1578. [Google Scholar] [CrossRef]
- Ibrahim, E.C.; Guerra, N.; Lacombe, M.J.; Angevin, E.; Chouaib, S.; Carosella, E.D.; Caignard, A.; Paul, P. Tumor-specific up-regulation of the nonclassical class I HLA-G antigen expression in renal carcinoma. Cancer Res. 2001, 61, 6838–6845. [Google Scholar]
- Hanak, L.; Slaby, O.; Lauerova, L.; Kren, L.; Nenutil, R.; Michalek, J. Expression pattern of HLA-F class I antigens in renal cell carcinoma and primary cell line cultures: Methodological implications for immunotherapy. Med. Sci. Monit. 2009, 15, CR638–CR643. [Google Scholar]
- Kren, L.; Muckova, K.; Lzicarova, E.; Sova, M.; Vybihal, V.; Svoboda, T.; Fadrus, P.; Smrcka, M.; Slaby, O.; Lakomy, R.; et al. Production of immune-modulatory nonclassical molecules HLA-G and HLA-E by tumor infiltrating ameboid microglia/macrophages in glioblastomas: A role in innate immunity? J. Neuroimmunol. 2010, 220, 131–135. [Google Scholar] [CrossRef]
- Kren, L.; Slaby, O.; Muckova, K.; Lzicarova, E.; Sova, M.; Vybihal, V.; Svoboda, T.; Fadrus, P.; Lakomy, R.; Vanhara, P.; et al. Human leukocyte antigen-G is frequently expressed in glioblastoma and may be induced in vitro by combined 5-aza-2′-deoxycytidine and interferon-gamma treatments: Results from a multicentric study. Am. J. Pathol. 2013, 182, 540–552. [Google Scholar]
- Wastowski, I.J.; Simoes, R.T.; Yaghi, L.; Donadi, E.A.; Pancoto, J.T.; Poras, I.; Lechapt-Zalcman, E.; Bernaudin, M.; Valable, S.; Carlotti, C.G., Jr.; et al. Human leukocyte antigen-G is frequently expressed in glioblastoma and may be induced in vitro by combined 5-aza-2′-deoxycytidine and interferon-gamma treatments: Results from a multicentric study. Am. J. Pathol. 2013, 182, 540–552. [Google Scholar] [CrossRef]
- Wiendl, H.; Mitsdoerffer, M.; Hofmeister, V.; Wischhusen, J.; Bornemann, A.; Meyermann, R.; Weiss, E.H.; Melms, A.; Weller, M. A functional role of HLA-G expression in human gliomas: An alternative strategy of immune escape. J. Immunol. 2002, 168, 4772–4780. [Google Scholar] [CrossRef]
- Wischhusen, J.; Waschbisch, A.; Wiendl, H. Immune-refractory cancers and their little helpers—An extended role for immunetolerogenic MHC molecules HLA-G and HLA-E? Semin. Cancer Biol. 2007, 17, 459–468. [Google Scholar] [CrossRef]
- Karagoz, B.; Haholu, A.; Ozgun, A.; Bilgi, O.; Tuncel, T.; Emirzeoglu, L.; Celik, S.; Demirel, D. HLA-G in testicular germ cell tumors. Oncol. Res. Treat. 2014, 37, 245–248. [Google Scholar] [CrossRef]
- Zhou, L.; Niu, Z.Y.; Liang, Z.Y.; Zhou, W.X.; You, L.; Wang, M.Y.; Yao, L.T.; Liao, Q.; Zhao, Y.P. HLA-G impairs host immune response and predicts poor prognosis in pancreatic cancer. Am. J. Transl. Res. 2015, 7, 2036–2044. [Google Scholar]
- Sheu, J.; Shih Ie, M. HLA-G and immune evasion in cancer cells. J. Formos. Med. Assoc. 2010, 109, 248–257. [Google Scholar] [CrossRef]
- Bossard, C.; Bézieau, S.; Matysiak-Budnik, T.; Volteau, C.; Laboisse, C.L.; Jotereau, F.; Mosnier, J.-F. HLA-E/β2 microglobulin overexpression in colorectal cancer is associated with recruitment of inhibitory immune cells and tumor progression. Int. J. Cancer 2012, 131, 855–863. [Google Scholar] [CrossRef]
- Curigliano, G.; Criscitiello, C.; Gelao, L.; Goldhirsch, A. Molecular pathways: Human leukocyte antigen G (HLA-G). Clin. Cancer Res. 2013, 19, 5564–5571. [Google Scholar] [CrossRef]
- Xu, Y.; Han, H.; Zhang, F.; Lv, S.; Li, Z.; Fang, Z. Lesion human leukocyte antigen-F expression is associated with a poor prognosis in patients with hepatocellular carcinoma. Oncol. Lett. 2015, 9, 300–304. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, A.; Zhang, J.G.; Bao, W.G.; Xu, D.P.; Ruan, Y.Y.; Yan, W.H. Alteration of HLA-F and HLA-F I antigen expression in the tumor is associated with survival in patients with esophageal squamous cell carcinoma. Int. J. Cancer 2013, 132, 82–89. [Google Scholar] [CrossRef]
- Lin, A.; Zhang, X.; Ruan, Y.Y.; Wang, Q.; Zhou, W.J.; Yan, W.H. HLA-F expression is a prognostic factor in patients with non-small-cell lung cancer. Lung Cancer 2011, 74, 504–509. [Google Scholar] [CrossRef]
- Wan, R.; Wang, Z.W.; Li, H.; Peng, X.D.; Liu, G.Y.; Ou, J.M.; Cheng, A.Q. Human leukocyte antigen-g inhibits the anti-tumor effect of natural killer cells via immunoglobulin-like transcript 2 in gastric cancer. Cell Physiol. Biochem. 2017, 44, 1828–1841. [Google Scholar] [CrossRef]
- Ueshima, C.; Kataoka, T.R.; Hirata, M.; Furuhata, A.; Suzuki, E.; Toi, M.; Tsuruyama, T.; Okayama, Y.; Haga, H.; Ueshima, C.; et al. The killer cell Ig-like receptor 2DL4 expression in human mast cells and its potential role in breast cancer invasion. Cancer Immunol. Res. 2015, 3, 871–880. [Google Scholar] [CrossRef]
- LeMaoult, J.; Caumartin, J.; Daouya, M.; Favier, B.; Le Rond, S.; Gonzalez, A.; Carosella, E.D. Immune regulation by pretenders: Cell-to-cell transfers of HLA-G make effector T cells act as regulatory cells. Blood 2007, 109, 2040–2048. [Google Scholar] [CrossRef]
- LeMaoult, J.; Krawice-Radanne, I.; Dausset, J.; Carosella, E.D. HLA-G1-expressing antigen-presenting cells induce immunosuppressive CD4+ T cells. Proc. Natl. Acad. Sci. USA 2004, 101, 7064–7069. [Google Scholar] [CrossRef]
- Solinas, G.; Germano, G.; Mantovani, A.; Allavena, P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J. Leukoc. Biol. 2009, 86, 1065–1073. [Google Scholar] [CrossRef]
- Moreau, P.; Adrian-Cabestre, F.; Menier, C.; Guiard, V.; Gourand, L.; Dausset, J.; Carosella, E.D.; Paul, P. IL-10 selectively induces HLA-G expression in human trophoblasts and monocytes. Int. Immunol. 1999, 11, 803–811. [Google Scholar] [CrossRef]
- Goncalves, A.S.; Oliveira, J.P.; Oliveira, C.F.; Silva, T.A.; Mendonca, E.F.; Wastowski, I.J.; Batista, A.C. Relevance of HLA-G, HLA-E and IL-10 expression in lip carcinogenesis. Hum. Immunol. 2016, 77, 785–790. [Google Scholar] [CrossRef]
- Dong, D.D.; Yie, S.M.; Li, K.; Li, F.; Xu, Y.; Xu, G.; Song, L.; Yang, H. Importance of HLA-G expression and tumor infiltrating lymphocytes in molecular subtypes of breast cancer. Hum. Immunol. 2012, 73, 998–1004. [Google Scholar] [CrossRef]
- Ibrahim, E.C.; Aractingi, S.; Allory, Y.; Borrini, F.; Dupuy, A.; Duvillard, P.; Carosella, E.D.; Avril, M.F.; Paul, P. Analysis of HLA-F antigen expression in benign and malignant melanocytic lesions reveals that upregulation of HLA-G expression correlates with malignant transformation, high inflammatory infiltration and HLA-F-A1 genotype. Int. J. Cancer 2004, 108, 243–250. [Google Scholar] [CrossRef]
- Hofmeister, V. Expression und Immunmodulatorische Funktion von HLA-G und Seinen Verkürzten Isoformen in Tumorzellinien. Thesis 2004, 1, 1. [Google Scholar]
- Lin, A.; Zhang, X.; Zhang, R.-L.; Zhang, J.-G.; Zhou, W.-J.; Yan, W.-H. Clinical significance of potential unidentified HLA-G isoforms without α1 domain but containing intron 4 in colorectal cancer patients. Front. Oncol. 2018, 8, 361. [Google Scholar] [CrossRef]
- Rizzo, R.; Trentini, A.; Bortolotti, D.; Manfrinato, M.C.; Rotola, A.; Castellazzi, M.; Melchiorri, L.; Di Luca, D.; Dallocchio, F.; Fainardi, E.; et al. Matrix metalloproteinase-2 (MMP-2) generates soluble HLA-G1 by cell surface proteolytic shedding. Mol. Cell. Biochem. 2013, 381, 243–255. [Google Scholar] [CrossRef]
- Li, T.; Huang, H.; Liao, D.; Ling, H.; Su, B.; Cai, M. Genetic polymorphism in HLA-G 3′UTR 14-bp ins/del and risk of cancer: A meta-analysis of case-control study. Mol. Genet. Genom. 2015, 290, 1235–1245. [Google Scholar] [CrossRef]
- Haghi, M.; Hosseinpour Feizi, M.A.; Sadeghizadeh, M.; Lotfi, A.S. 14-bp insertion/deletion polymorphism of the HLA-G gene in breast cancer among women from North Western Iran. Asian Pac. J. Cancer Prev. 2015, 16, 6155–6158. [Google Scholar] [CrossRef]
- Benevolo, M.; Mottolese, M.; Tremante, E.; Rollo, F.; Diodoro, M.G.; Ercolani, C.; Sperduti, I.; Lo Monaco, E.; Cosimelli, M.; Giacomini, P. High expression of HLA-E in colorectal carcinoma is associated with a favorable prognosis. J. Transl. Med. 2011, 9, 184. [Google Scholar] [CrossRef]
- Seliger, B.; Jasinski-Bergner, S.; Quandt, D.; Stoehr, C.; Bukur, J.; Wach, S.; Legal, W.; Taubert, H.; Wullich, B.; Hartmann, A. HLA-E expression and its clinical relevance in human renal cell carcinoma. Oncotarget 2016, 7, 67360–67372. [Google Scholar] [CrossRef]
- Kren, L.; Valkovsky, I.; Dolezel, J.; Capak, I.; Pacik, D.; Poprach, A.; Lakomy, R.; Redova, M.; Fabian, P.; Krenova, Z.; et al. HLA-G and HLA-E specific mRNAs connote opposite prognostic significance in renal cell carcinoma. Diagn. Pathol. 2012, 7, 58. [Google Scholar] [CrossRef][Green Version]
- Talebian Yazdi, M.; van Riet, S.; van Schadewijk, A.; Fiocco, M.; van Hall, T.; Taube, C.; Hiemstra, P.S.; van der Burg, S.H. The positive prognostic effect of stromal CD8+ tumor-infiltrating T cells is restrained by the expression of HLA-E in non-small cell lung carcinoma. Oncotarget 2016, 7, 3477–3488. [Google Scholar]
- Tremante, E.; Ginebri, A.; Lo Monaco, E.; Benassi, B.; Frascione, P.; Grammatico, P.; Cappellacci, S.; Catricala, C.; Arcelli, D.; Natali, P.G.; et al. A melanoma immune response signature including human leukocyte antigen-E. Pigment Cell Melanoma Res. 2014, 27, 103–112. [Google Scholar] [CrossRef]
- Allard, M.; Oger, R.; Vignard, V.; Percier, J.M.; Fregni, G.; Perier, A.; Caignard, A.; Charreau, B.; Bernardeau, K.; Khammari, A.; et al. Serum soluble HLA-E in melanoma: A new potential immune-related marker in cancer. PLoS ONE 2011, 6, e21118. [Google Scholar] [CrossRef]
- Ishigami, S.; Arigami, T.; Okumura, H.; Uchikado, Y.; Kita, Y.; Kurahara, H.; Maemura, K.; Kijima, Y.; Ishihara, Y.; Sasaki, K.; et al. Human leukocyte antigen (HLA-F)-E and HLA-F expression in gastric cancer. Anticancer Res. 2015, 35, 2279–2285. [Google Scholar]
- Sasaki, T.; Ravindranath, M.H.; Terasaki, P.I.; Freitas, M.C.; Kawakita, S.; Jucaud, V. Gastric cancer progression may involve a shift in HLA-E profile from an intact heterodimer to β2-microglobulin-free monomer. Int. J. Cancer 2014, 134, 1558–1570. [Google Scholar] [CrossRef]
- Spaans, V.M.; Peters, A.A.; Fleuren, G.J.; Jordanova, E.S. HLA-E expression in cervical adenocarcinomas: Association with improved long-term survival. J. Transl. Med. 2012, 10, 184. [Google Scholar] [CrossRef]
- Wolpert, F.; Roth, P.; Lamszus, K.; Tabatabai, G.; Weller, M.; Eisele, G. HLA-E contributes to an immune-inhibitory phenotype of glioblastoma stem-like cells. J. Neuroimmunol. 2012, 250, 27–34. [Google Scholar] [CrossRef][Green Version]
- Chen, A.; Shen, Y.; Xia, M.; Xu, L.; Pan, N.; Yin, Y.; Miao, F.; Shen, C.; Xie, W.; Zhang, J. Expression of the nonclassical HLA-F class I and MICA/B molecules in human hepatocellular carcinoma. Neoplasma 2011, 58, 371–376. [Google Scholar] [CrossRef]
- Kren, L.; Fabian, P.; Slaby, O.; Janikova, A.; Soucek, O.; Sterba, J.; Krenova, Z.; Michalek, J.; Kral, Z. Multifunctional immune-modulatory protein HLA-E identified in classical Hodgkin lymphoma: Possible implications. Pathol. Res. Pract. 2012, 208, 45–49. [Google Scholar] [CrossRef]
- Zanetti, B.R.; Carvalho-Galano, D.F.; Feitosa, N.L.; Hassumi-Fukasawa, M.K.; Miranda-Camargo, F.A.; Maciel, L.M.; Ribeiro-Silva, A.; Soares, E.G. Differential expression of immune-modulatory molecule HLA-E in non-neoplastic and neoplastic lesions of the thyroid. Int. J. Immunopathol. Pharmacol. 2013, 26, 889–896. [Google Scholar] [CrossRef]
- Xu, Y.P.; Wieten, L.; Wang, S.X.; Cai, Y.; Olieslagers, T.; Zhang, L.; He, L.M.; Tilanus, M.G.J.; Hong, W.X. Clinical significance of HLA-E genotype and surface/soluble expression levels between healthy individuals and patients with acute leukemia. Leuk. Lymphoma 2018, 60, 1–8. [Google Scholar] [CrossRef]
- Pietra, G.; Romagnani, C.; Manzini, C.; Moretta, L.; Mingari, M.C. The emerging role of HLA-E-restricted CD8+ T lymphocytes in the adaptive immune response to pathogens and tumors. J. Biomed. Biotechnol. 2010, 2010, 907092. [Google Scholar] [CrossRef]
- Gooden, M.; Lampen, M.; Jordanova, E.S.; Leffers, N.; Trimbos, J.B.; van der Burg, S.H.; Nijman, H.; van Hall, T. HLA-E expression by gynecological cancers restrains tumor-infiltrating CD8 (+) T lymphocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 10656–10661. [Google Scholar] [CrossRef]
- Derre, L.; Corvaisier, M.; Charreau, B.; Moreau, A.; Godefroy, E.; Moreau-Aubry, A.; Jotereau, F.; Gervois, N. Expression and release of HLA-E by melanoma cells and melanocytes: Potential impact on the response of cytotoxic effector cells. J. Immunol. 2006, 177, 3100–3107. [Google Scholar] [CrossRef]
- Wagner, B.; da Silva Nardi, F.; Schramm, S.; Kraemer, T.; Celik, A.A.; Durig, J.; Horn, P.A.; Duhrsen, U.; Nuckel, H.; Rebmann, V. HLA-E allelic genotype correlates with HLA-E plasma levels and predicts early progression in chronic lymphocytic leukemia. Cancer 2017, 123, 814–823. [Google Scholar] [CrossRef]
- Harada, A.; Ishigami, S.; Kijima, Y.; Nakajo, A.; Arigami, T.; Kurahara, H.; Kita, Y.; Yoshinaka, H.; Natsugoe, S. Clinical implication of human leukocyte antigen (HLA-F)-F expression in breast cancer. Pathol. Int. 2015, 65, 569–574. [Google Scholar] [CrossRef]
- Ishigami, S.; Arigami, T.; Setoyama, T.; Okumura, H.; Sasaki, K.; Uchikado, Y.; Kurahara, H.; Kijima, Y.; Nishizono, Y.; Nakajo, A.; et al. Clinical-pathological implication of human leukocyte antigen-F-positive gastric adenocarcinoma. J. Surg. Res. 2013, 184, 802–806. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.; Feng, N.; Dong, J.; Deng, X.; Yue, Y.; Guo, Y.; Hou, J. Human HLA-F F adjacent transcript 10 promotes the formation of cancer initiating cells and cisplatin resistance in bladder cancer. Mol. Med. Rep. 2018, 18, 308–314. [Google Scholar] [CrossRef]
- Wu, B.; Yang, H.; Ying, S.; Lu, H.; Wang, W.; Lv, J.; Xiong, H.; Hu, W. High HLA-F expression is a poor prognosis factor in patients with nasopharyngeal carcinoma. Anal. Cell Pathol. 2018, 2018, 7691704. [Google Scholar] [CrossRef]
- Morandi, F.; Cangemi, G.; Barco, S.; Amoroso, L.; Giuliano, M.; Gigliotti, A.R.; Pistoia, V.; Corrias, M.V. Plasma levels of soluble HLA-E and HLA-F at diagnosis may predict overall survival of neuroblastoma patients. BioMed. Res. Int. 2013, 2013, 9. [Google Scholar] [CrossRef]
- Zhang, J.G.; Zhang, X.; Lin, A.; Yan, W.H. Lesion HLA-F expression is irrelevant to prognosis for patients with gastric cancer. Hum. Immunol. 2013, 74, 828–832. [Google Scholar] [CrossRef]
- Melsted, W.N.; Johansen, L.L.; Lock-Andersen, J.; Behrendt, N.; Eriksen, J.O.; Bzorek, M.; Scheike, T.; Hviid, T.V.F. HLA-F class Ia and Ib molecules and FOXP3+ TILs in relation to the prognosis of malignant melanoma patients. Clin. Immunol. 2017, 183, 191–197. [Google Scholar] [CrossRef]
- Campillo, J.A.; Martinez-Escribano, J.A.; Moya-Quiles, M.R.; Marin, L.A.; Muro, M.; Guerra, N.; Parrado, A.; Campos, M.; Frias, J.F.; Minguela, A.; et al. Natural killer receptors on CD8 T cells and natural killer cells from different HLA-C phenotypes in melanoma patients. Clin. Cancer Res. 2006, 12, 4822–4831. [Google Scholar] [CrossRef]
- Rios, A.; Rodriguez, J.M.; Moya, M.R.; Galindo, P.J.; Canteras, M.; Alvarez, M.R.; Parrilla, P. Frequency of HLA-C alleles in differentiated thyroid carcinoma in Southeastern Spain. Cancer 2004, 100, 264–269. [Google Scholar] [CrossRef]
- Laoui, D.; Movahedi, K.; Van Overmeire, E.; Van den Bossche, J.; Schouppe, E.; Mommer, C.; Nikolaou, A.; Morias, Y.; De Baetselier, P.; Van Ginderachter, J.A. Tumor-associated macrophages in breast cancer: Distinct subsets, distinct functions. Int. J. Dev. Biol. 2011, 55, 861–867. [Google Scholar] [CrossRef]
- Quatromoni, J.G.; Eruslanov, E. Tumor-associated macrophages: Function, phenotype, and link to prognosis in human lung cancer. Am. J. Transl. Res. 2012, 4, 376–389. [Google Scholar]
- Marchesi, M.; Andersson, E.; Villabona, L.; Seliger, B.; Lundqvist, A.; Kiessling, R.; Masucci, G.V. HLA-F-dependent tumour development: A role for tumour associate macrophages? J. Transl. Med. 2013, 11, 247. [Google Scholar] [CrossRef]
- Ugurel, S.; Rebmann, V.; Ferrone, S.; Tilgen, W.; Grosse-Wilde, H.; Reinhold, U. Soluble human leukocyte antigen—G serum level is elevated in melanoma patients and is further increased by interferon-alpha immunotherapy. Cancer 2001, 92, 369–376. [Google Scholar] [CrossRef]
- Konig, L.; Kasimir-Bauer, S.; Hoffmann, O.; Bittner, A.K.; Wagner, B.; Manvailer, L.F.; Schramm, S.; Bankfalvi, A.; Giebel, B.; Kimmig, R.; et al. The prognostic impact of soluble and vesicular HLA-G and its relationship to circulating tumor cells in neoadjuvant treated breast cancer patients. Hum. Immunol. 2016, 77, 791–799. [Google Scholar] [CrossRef]
- Provatopoulou, X.; Kalogera, E.; Sagkriotis, A.; Zagouri, F.; Nonni, A.; Zografos, G.C.; Gounaris, A. Soluble human leukocyte antigen-G expression in patients with ductal and lobular breast malignancy. Anticancer Res. 2012, 32, 1021–1026. [Google Scholar]
- Morandi, F.; Levreri, I.; Bocca, P.; Galleni, B.; Raffaghello, L.; Ferrone, S.; Prigione, I.; Pistoia, V. Human neuroblastoma cells trigger an immunosuppressive program in monocytes by stimulating soluble HLA-G release. Cancer Res. 2007, 67, 6433–6441. [Google Scholar] [CrossRef]
- Locafaro, G.; Amodio, G.; Tomasoni, D.; Tresoldi, C.; Ciceri, F.; Gregori, S. HLA-G expression on blasts and tolerogenic cells in patients affected by acute myeloid leukemia. J. Immunol. Res. 2014, 2014, 636292. [Google Scholar] [CrossRef]
- Grange, C.; Tapparo, M.; Tritta, S.; Deregibus, M.C.; Battaglia, A.; Gontero, P.; Frea, B.; Camussi, G. Role of HLA-G and extracellular vesicles in renal cancer stem cell-induced inhibition of dendritic cell differentiation. BMC Cancer 2015, 15, 1009. [Google Scholar] [CrossRef]
- Zhou, K.; Xia, M.; Tang, B.; Yang, D.; Liu, N.; Tang, D.; Xie, H.; Wang, X.; Zhu, H.; Liu, C.; et al. Isolation and comparison of mesenchymal stem celllike cells derived from human gastric cancer tissues and corresponding ovarian metastases. Mol. Med. Rep. 2016, 13, 1788–1794. [Google Scholar] [CrossRef]
- Santana-Codina, N.; Carretero, R.; Sanz-Pamplona, R.; Cabrera, T.; Guney, E.; Oliva, B.; Clezardin, P.; Olarte, O.E.; Loza-Alvarez, P.; Mendez-Lucas, A.; et al. A transcriptome-proteome integrated network identifies endoplasmic reticulum thiol oxidoreductase (ERp57) as a hub that mediates bone metastasis. Mol. Cell Proteom. 2013, 12, 2111–2125. [Google Scholar] [CrossRef]
- Rouas-Freiss, N.; Moreau, P.; Ferrone, S.; Carosella, E.D. HLA-G proteins in cancer: Do they provide tumor cells with an escape mechanism? Cancer Res. 2005, 65, 10139–10144. [Google Scholar] [CrossRef]
- Kochan, G.; Escors, D.; Breckpot, K.; Guerrero-Setas, D. Role of non-classical MHC class I molecules in cancer immunosuppression. Oncoimmunology 2013, 2, e26491. [Google Scholar] [CrossRef]
- Allen, C.T.; Clavijo, P.E.; Van Waes, C.; Chen, Z. Anti-tumor immunity in head and neck cancer: Understanding the evidence, how tumors escape and immunotherapeutic approaches. Cancers 2015, 7, 2397–2414. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X. Immunosuppressive cells in tumor immune escape and metastasis. J. Mol. Med. 2016, 94, 509–522. [Google Scholar] [CrossRef]
- Rosa, B.; de Jesus, J.P.; de Mello, E.L.; Cesar, D.; Correia, M.M. Effectiveness and safety of monoclonal antibodies for metastatic colorectal cancer treatment: Systematic review and meta-analysis. Ecancermedicalscience 2015, 9, 582. [Google Scholar] [CrossRef][Green Version]
- Shaza, L.; Charette, N.; Hendlisz, A. Colorectal cancer: Ten years of illusion of progress but advances are on the horizon. Rev. Med. Brux 2015, 36, 263–266. [Google Scholar]
- Tchekmedyian, N.; Gray, J.E.; Creelan, B.C.; Chiappori, A.A.; Beg, A.A.; Soliman, H.; Perez, B.A.; Antonia, S.J. Propelling immunotherapy combinations into the clinic. Oncology 2015, 29, 990–1002. [Google Scholar]
- Lopes, M.; Gonzaga, A.K.G.; Mosconi, C.; Palomino, G.M.; Mendonca, E.F.; Batista, A.C.; Silveira, E. Immune response and evasion mechanisms in lip carcinogenesis: An immunohistochemical study. Arch. Oral. Biol. 2019, 98, 99–107. [Google Scholar] [CrossRef]
- Mosconi, C.; Arantes, D.A.C.; Goncalves, A.S.; Alencar, R.C.G.; Oliveira, J.C.; Silva, T.A.; Mendonca, E.F.; Batista, A.C. Immunohistochemical investigations on the expression of programmed cell death ligand 1, human leukocyte antigens G and E, and granzyme B in intraoral mucoepidermoid carcinoma. Arch. Oral. Biol. 2017, 83, 55–62. [Google Scholar] [CrossRef]
- Rodriguez, J.A. HLA-F-mediated tumor escape mechanisms that may impair immunotherapy clinical outcomes via T-cell activation. Oncol. Lett. 2017, 14, 4415–4427. [Google Scholar] [CrossRef]
- Koepsell, S.A.; Miller, J.S.; McKenna, D.H., Jr. Natural killer cells: A review of manufacturing and clinical utility. Transfusion 2013, 53, 404–410. [Google Scholar] [CrossRef]
- Stefanovic, S.; Wirtz, R.; Deutsch, T.M.; Hartkopf, A.; Sinn, P.; Varga, Z.; Sobottka, B.; Sotiris, L.; Taran, F.A.; Domschke, C.; et al. Tumor biomarker conversion between primary and metastatic breast cancer: mRNA assessment and its concordance with immunohistochemistry. Oncotarget 2017, 8, 51416–51428. [Google Scholar] [CrossRef]
- O’Brien, C.S.; Farnie, G.; Howell, S.J.; Clarke, R.B. Breast cancer stem cells and their role in resistance to endocrine therapy. Horm. Cancer 2011, 2, 91–103. [Google Scholar] [CrossRef]
- Campoli, M.; Ferrone, S. HLA-F antigen changes in malignant cells: Epigenetic mechanisms and biologic significance. Oncogene 2008, 27, 5869–5885. [Google Scholar]


| Localization | HLA Gene | Authors |
|---|---|---|
| CTs, STs, EVTs | HLA-E, HLA-F, HLA-G | Apps et al., 2008 [15] Hackmon et al., 2017 [30] Rizzo et al., 2011 [103] Shobu et al., 2006 [11] |
| ES | HLA-E, HLA-G | Drukker et al., 2002 [105] |
| PIE | HLA-G | Fuzzi et al., 2002 [106] Shaikly et al., 2008 [16] |
| sHLA-G | Sher, 2004 [112] Tabiasco et al., 2009 [113] | |
| PIE/ES | HLA-G | Rizzo et al., 2011 [103] Verloes et al., 2011 [107] |
| ES (Wharton-jelly) | HLA-E, HLA-F, HLA-G | Chen et al., 2012 [108] |
| Culture medium of PIE | sHLA-G | Noci et al., 2005 [110] |
| Carcinoma | Authors |
|---|---|
| Bladder cancer | Castelli et al., 2008 [154] El-Chennawi et al., 2005 [155] Gan et al., 2010 [156] |
| Breast cancer | Jeong et al., 2014 [130] Rolfsen et al., 2013 [131] Da Silva et al.,2013 [132] He et al.,2010 [133] Kleinberg et al., 2006 [134] Palmisano et al., 2002 [135] Singer et al., 2003 [136] de Kruijf et al., 2010 [137] |
| Cervical cancer | Gimenes et al., 2014 [142] Li et al., 2012 [143] Rodriguez et al., 2012 [144] Zheng et al., 2011 [145] Ferguson et al., 2012 [136] |
| Colon cancer | Zeestraten et al., 2014 [160] |
| Endometrial cancer | Bijen et al., 2010 [141] |
| Esophageal cancer | Cao et al., 2011 [163] Lin et al., 2011b [164] |
| Germ cell tumor (testicular) | Karagoz et al., 2014 [179] |
| Glioblastoma | Kren et al., 2010 [174] Kren et al.,2011 [175] Wastowski et al., 2013 [176] Wiendl et al., 2002 [177] Wischhusen et al., 2007 [178] |
| Hodgkin’s lymphoma | Diepstra et al., 2008 [170] |
| Lymphoma | Urosevic et al., 2002 [169] |
| Lung cancer | Montilla et al., 2016 [147] Urosevic et al., 2001 [148] Wisniewski et al., 2015 [149] Yan et al., 2015 [150] Yie et al., 2007 [151] Zhang et al., 2016 [152] |
| Malignant melanoma | Degenhardt et al., 2010 [165] Bezuhly et al., 2008 [166] Paul et al., 1999 [167] Paul et ak., 1998 [168] |
| Ovarian cancer | Zhang et al., 2016 [138] Rutten et al., 2014 [139] Lin et al., 2013 [140] |
| Pancreatic cancer | Zhou et al., 2015 [180] |
| Prostate cancer | Langat et al., 2006 [146] |
| Rectal cancer | Reimers et al., 2014 [163] Guo et al., 2015 [162] |
| Renal cancer | Tronik-Le Roux, 2017 [171] Ibrahim et al., 2001 [172] Hanak et al., 2009 [173] |
| Thyroid cancer | Dardano et al., 2011 [161] Nunes et al., 2013 [162] |
| Carcinoma | Authors |
|---|---|
| Breast cancer | da Silva et al, 2013 [132] |
| Cervical carcinoma (adenocarcinoma) | Spaans et al., 2012 [213] |
| Colorectal cancer | Guo et al., 2015 [158] |
| Reimer et al., 2014 [159] | |
| Zeestraten et al., 2014 [160] | |
| Benevolo et al., 2011 [201] | |
| Gastric cancer | Ishigami et al., 2015 [207] |
| Sasaki et al., 2014 [208] | |
| Glioblastoma | Kren et al., 2011 [174] Wischhusen et al., 2007 [178] Wolpert et al., 2012 [210] |
| Hepatic carcinoma (hepatocellular) | Chen et al., 2011 [211] |
| Hodgkin’s lymphoma | Kren et al., 2012 [212] |
| Leukemia | Xu et al., 2018 [214] |
| Lung cancer | Talebian et al., 2015 [204] |
| Melanoma | Tremante et al., 2014 [205] |
| Allard et al., 2011 [206] | |
| Renal cancer | Hanak et al., 2009 [173] Seliger et al., 2016 [202] Kren et al., 2012 [203] |
| Thyroid cancer | Zanetti et al., 2013 [213] |
| Carcinoma | Author |
|---|---|
| Breast cancer | Harada et al., 2015 [219] |
| Bladder cancer | Li et al., 2018 [221] |
| Esophageal cancer | Zhang et al., 2013 [185] |
| Gastric cancer | Ishigami et al., 2015 [207] |
| Ishigami et al., 2013 [220] | |
| Zhang et al., 2013 [224] | |
| Hepatic cancer (hepatocellular) | Xu et al., 2015 [184] |
| Lung cancer | Lin et al. 2011a [186] |
| Neuroblastoma | Morandi et al., 2013 [223] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Würfel, F.M.; Winterhalter, C.; Trenkwalder, P.; Wirtz, R.M.; Würfel, W. European Patent in Immunoncology: From Immunological Principles of Implantation to Cancer Treatment. Int. J. Mol. Sci. 2019, 20, 1830. https://doi.org/10.3390/ijms20081830
Würfel FM, Winterhalter C, Trenkwalder P, Wirtz RM, Würfel W. European Patent in Immunoncology: From Immunological Principles of Implantation to Cancer Treatment. International Journal of Molecular Sciences. 2019; 20(8):1830. https://doi.org/10.3390/ijms20081830
Chicago/Turabian StyleWürfel, Franziska M., Christoph Winterhalter, Peter Trenkwalder, Ralph M. Wirtz, and Wolfgang Würfel. 2019. "European Patent in Immunoncology: From Immunological Principles of Implantation to Cancer Treatment" International Journal of Molecular Sciences 20, no. 8: 1830. https://doi.org/10.3390/ijms20081830
APA StyleWürfel, F. M., Winterhalter, C., Trenkwalder, P., Wirtz, R. M., & Würfel, W. (2019). European Patent in Immunoncology: From Immunological Principles of Implantation to Cancer Treatment. International Journal of Molecular Sciences, 20(8), 1830. https://doi.org/10.3390/ijms20081830




