Drosophila Temperature Preference Rhythms: An Innovative Model to Understand Body Temperature Rhythms
Abstract
:1. Introduction
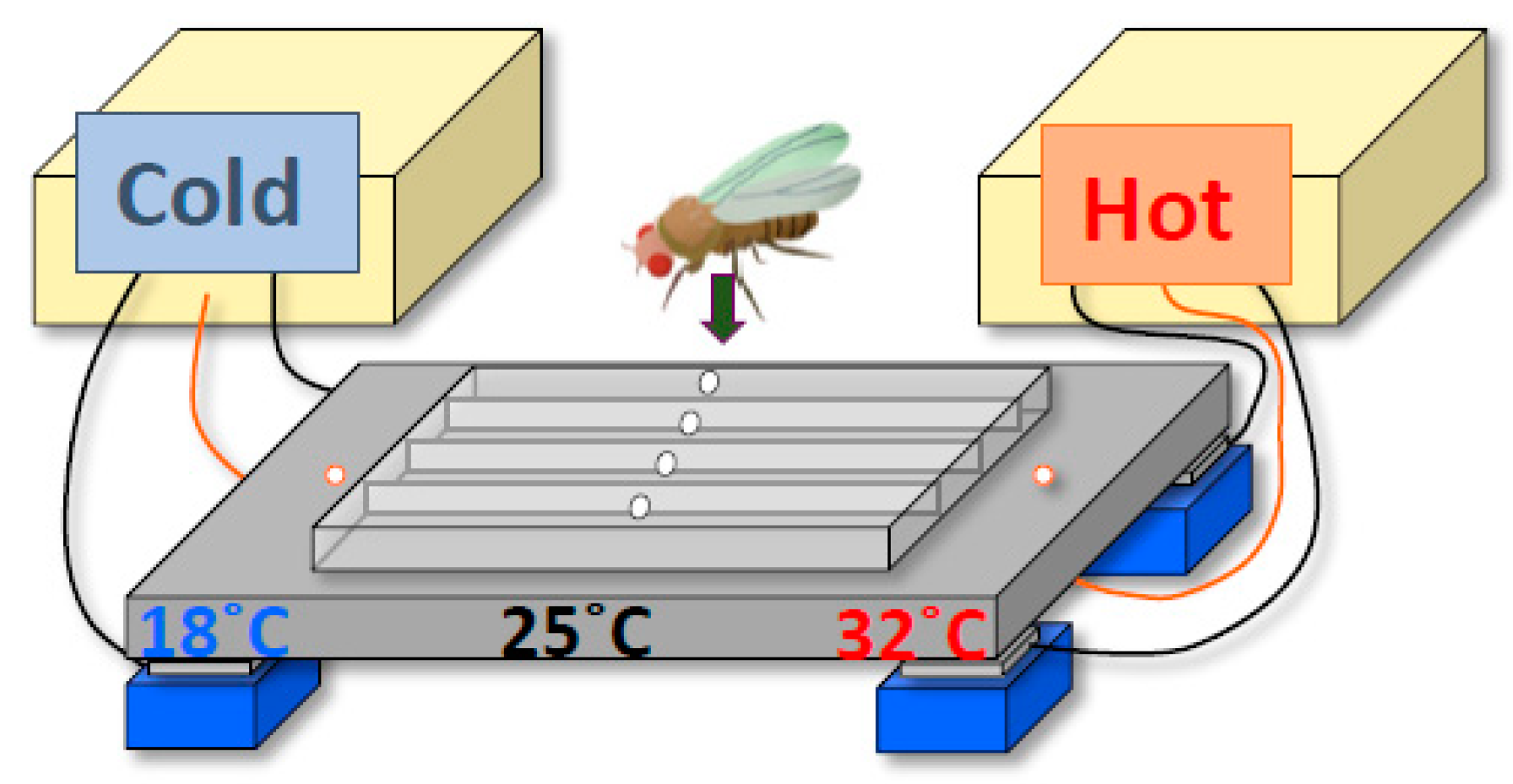
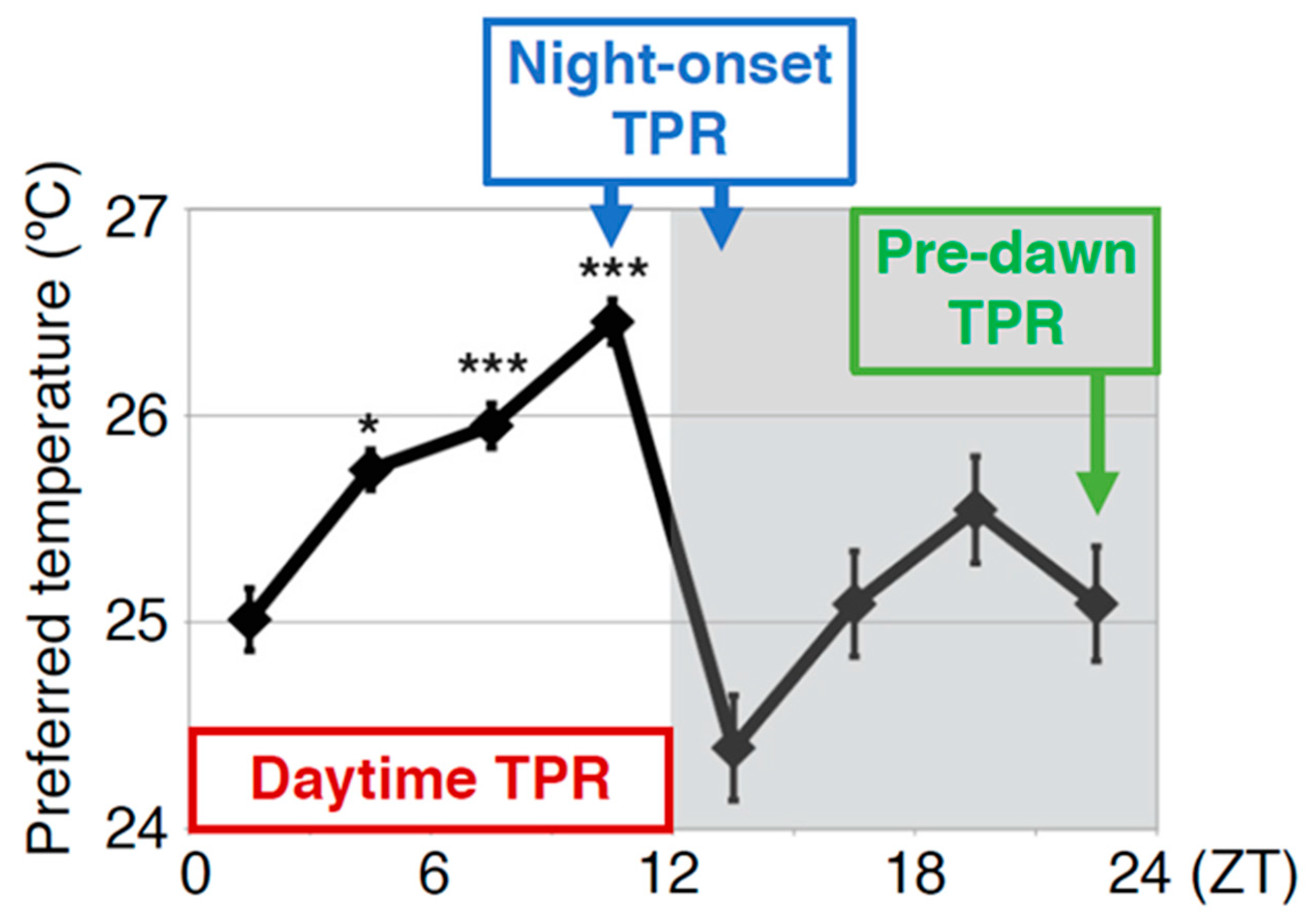
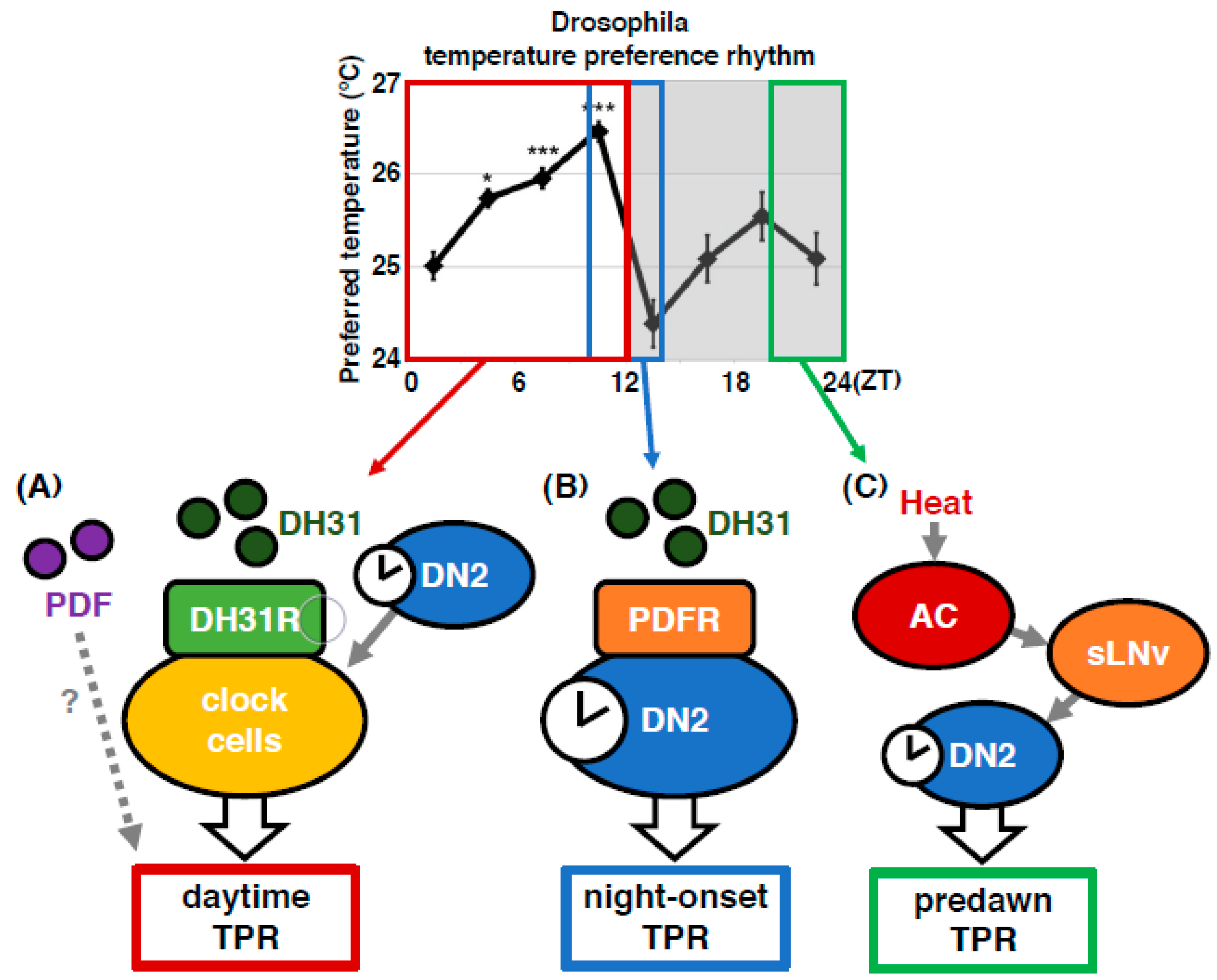
2. Drosophila Exhibit a Robust Temperature Preference Rhythm (TPR)
3. TPR is Controlled by the Noncanonical Clock Circuits via Dorsal Neurons 2 (DN2s)
4. Molecular and Neural Mechanisms Regulating TPR
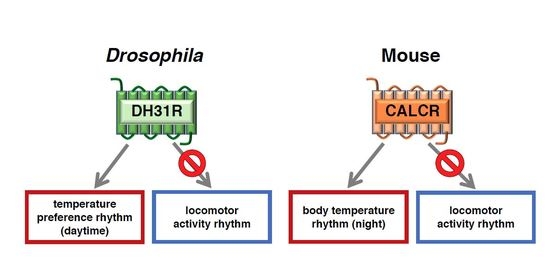
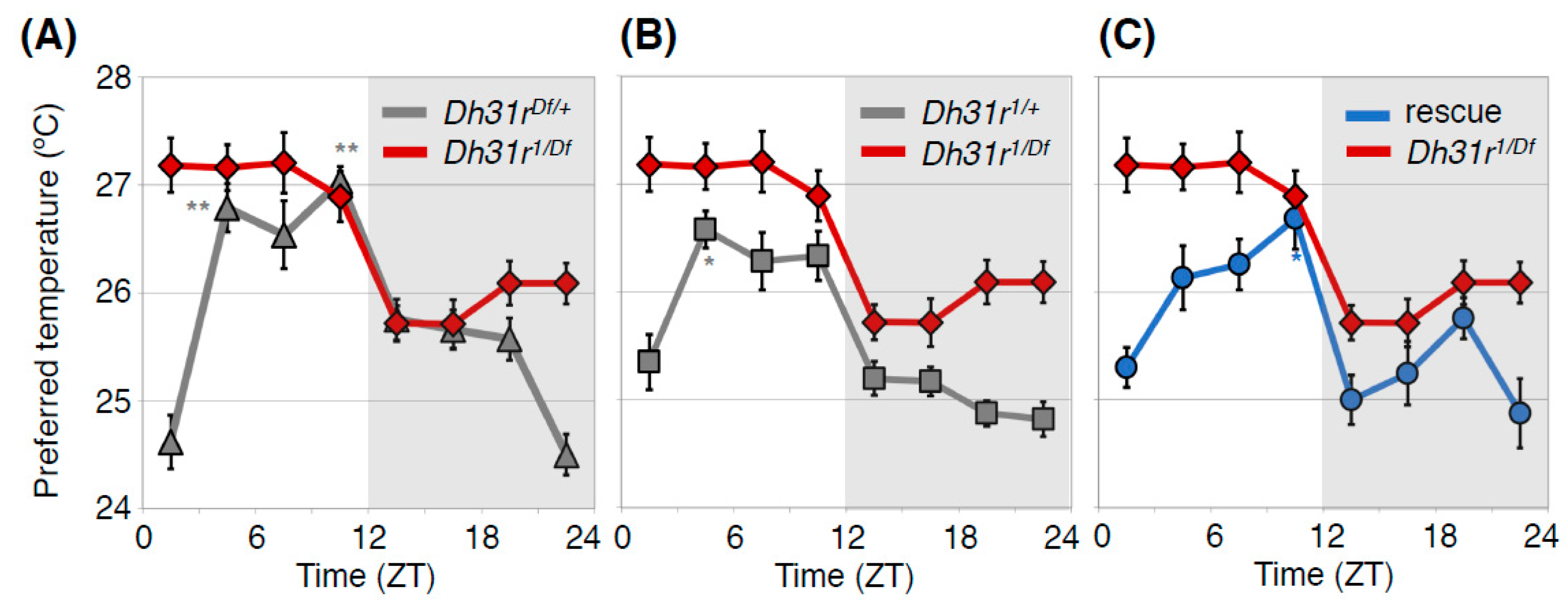
4.1. Diuretic Hormone 31 Receptor (DH31R) Regulates the Daytime TPR (ZT 1-12)
4.2. Both DH31 and PDF Contribute to the Regulation of the Daytime TPR (ZT 1-12)
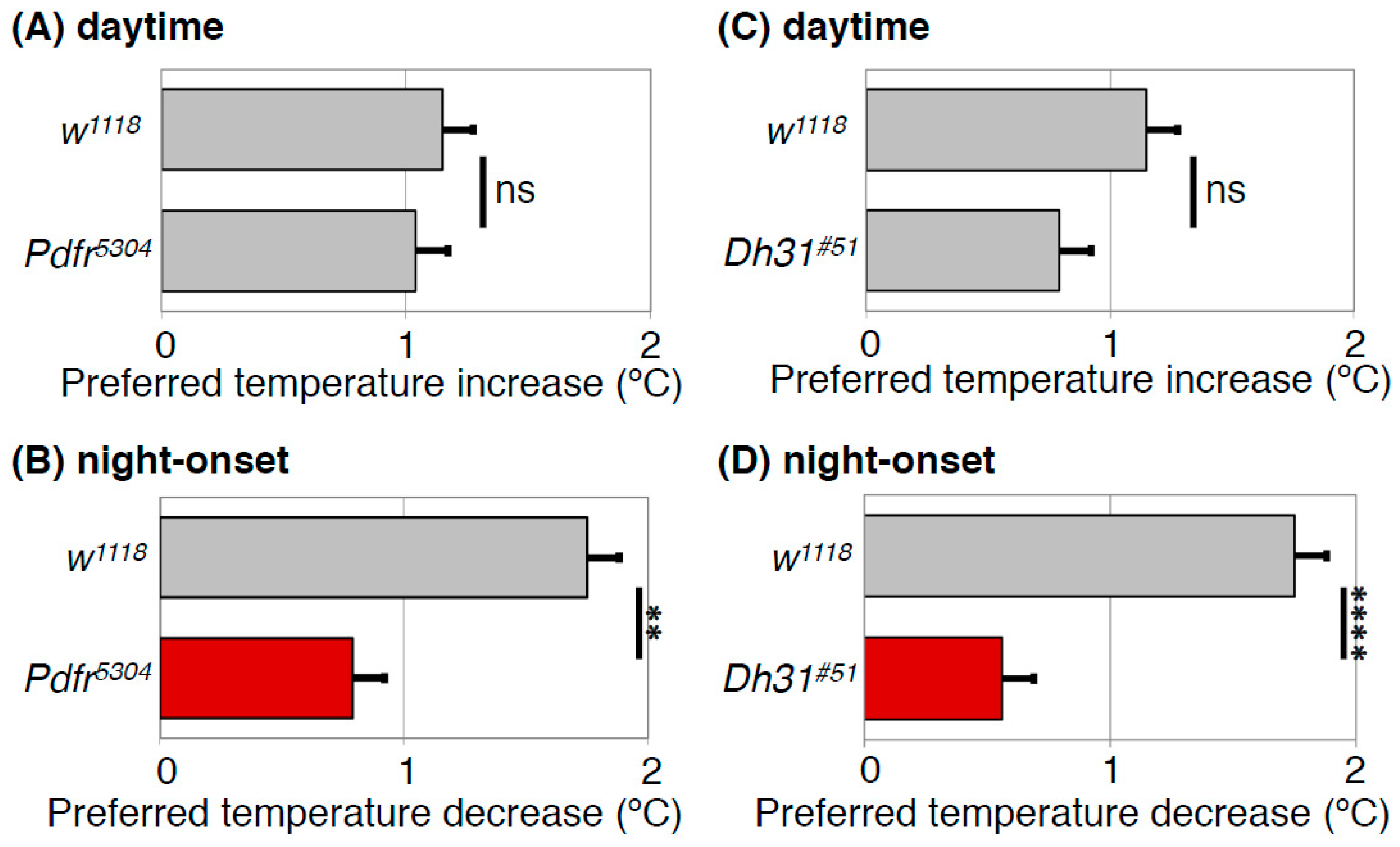
4.3. DH31 Acts on DN2s Through PDFR, but not DH31R, to Regulate the Night-onset TPR (ZT 10-15)
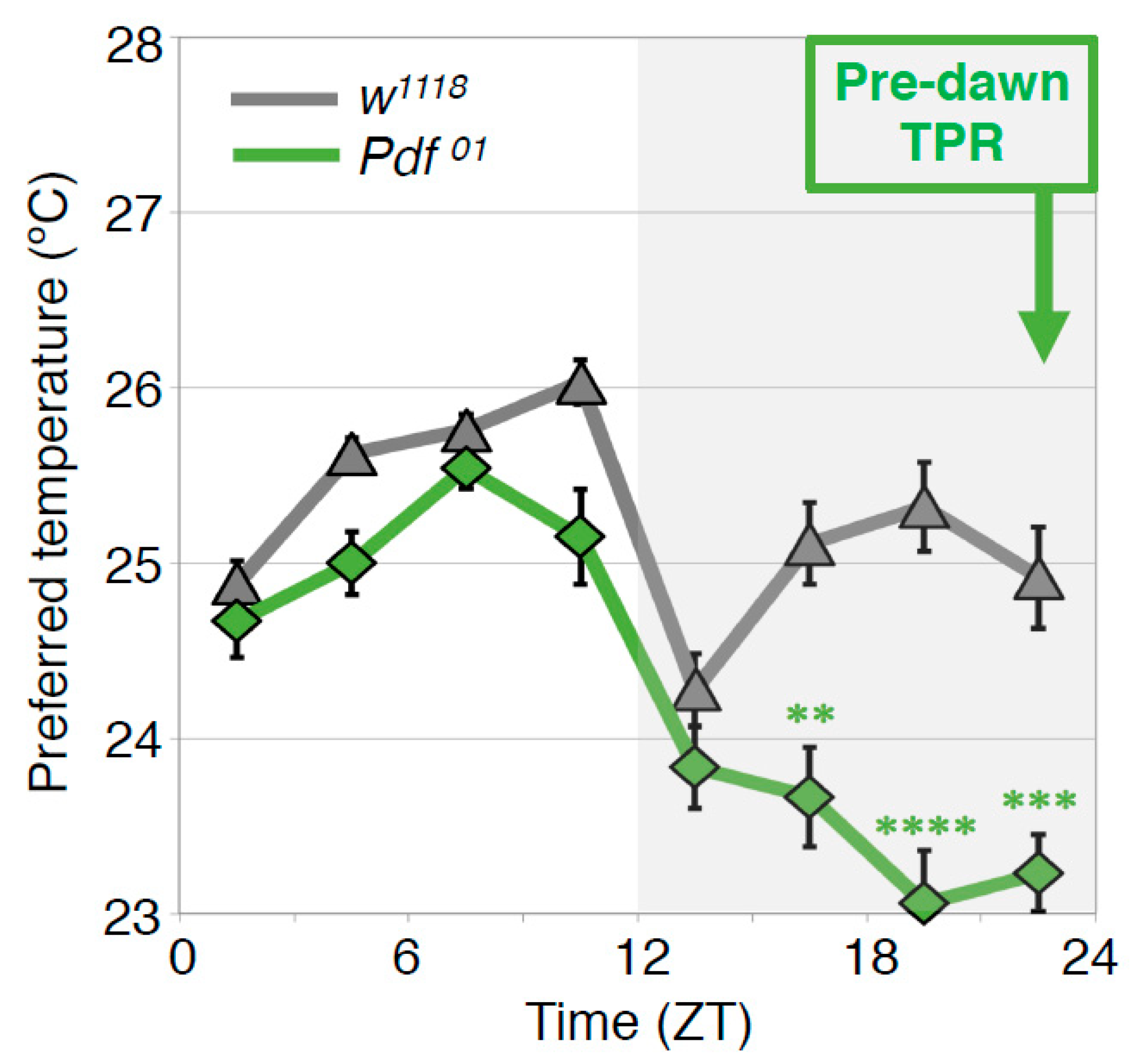
4.4. PDF Neurons Regulate the Predawn TPR via DN2s (ZT 22-24)
4.5. Anterior Cell (AC) Neurons Regulate the Predawn TPR via sLNvs (ZT 22-24)
5. Mammalian BTRs
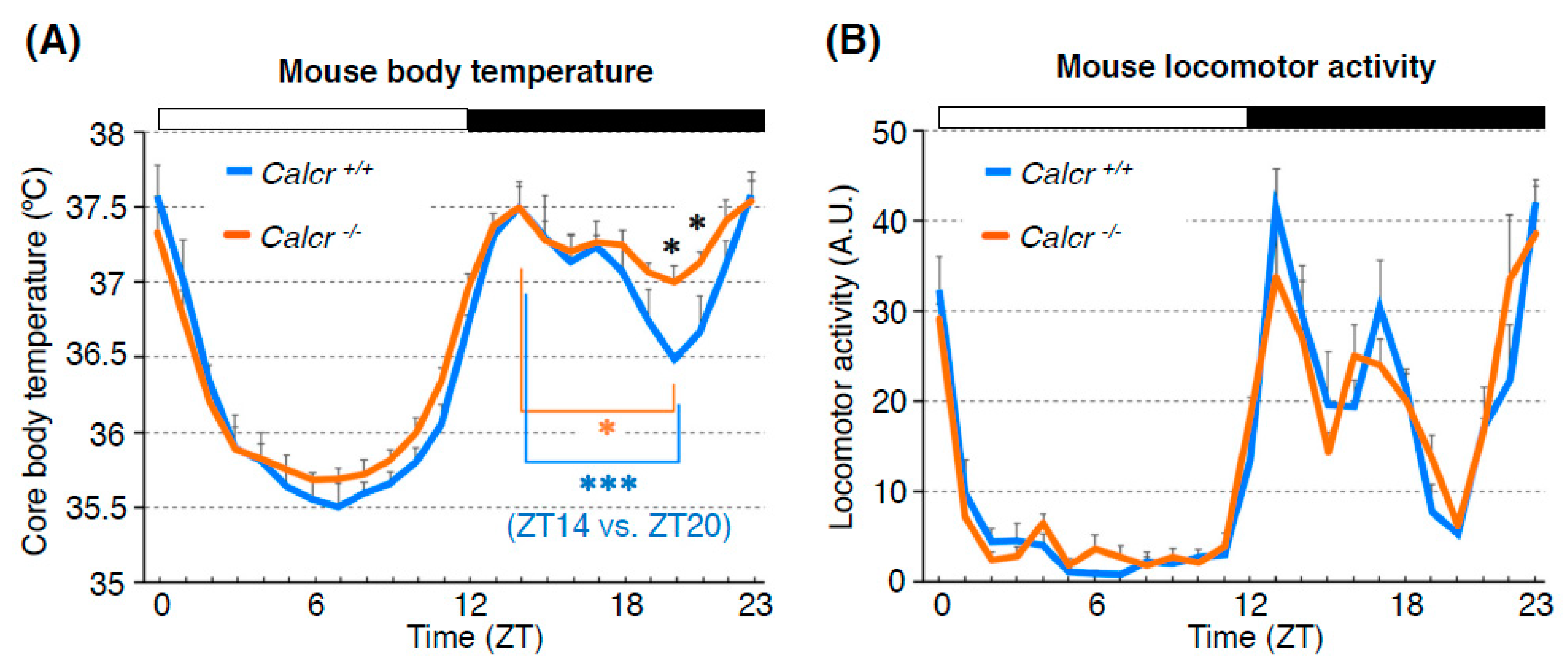
6. The Calcitonin Receptor Regulates the BTR in Mice
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Partch, C.L.; Green, C.B.; Takahashi, J.S. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014, 24, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Dubowy, C.; Sehgal, A. Circadian Rhythms and Sleep in Drosophila melanogaster. Genetics 2017, 205, 1373–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffy, J.F.; Dijk, D.J.; Klerman, E.B.; Czeisler, C.A. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. Am. J. Physiol. 1998, 275, R1478–R1487. [Google Scholar] [CrossRef]
- Aschoff, J. Circadian Control of Body-Temperature. J. Therm. Biol. 1983, 8, 143–147. [Google Scholar] [CrossRef]
- Krauchi, K. How is the circadian rhythm of core body temperature regulated? Clin. Auton. Res. 2002, 12, 147–149. [Google Scholar] [CrossRef]
- Weinert, D. Circadian temperature variation and ageing. Ageing Res. Rev. 2010, 9, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Refinetti, R.; Menaker, M. The circadian rhythm of body temperature. Physiol. Behav. 1992, 51, 613–637. [Google Scholar] [CrossRef]
- Gilbert, S.S.; van den Heuvel, C.J.; Ferguson, S.A.; Dawson, D. Thermoregulation as a sleep signalling system. Sleep Med. Rev. 2004, 8, 81–93. [Google Scholar] [CrossRef]
- Krauchi, K. The thermophysiological cascade leading to sleep initiation in relation to phase of entrainment. Sleep Med. Rev. 2007, 11, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Krauchi, K. The human sleep-wake cycle reconsidered from a thermoregulatory point of view. Physiol. Behav. 2007, 90, 236–245. [Google Scholar] [CrossRef]
- Buhr, E.D.; Yoo, S.H.; Takahashi, J.S. Temperature as a universal resetting cue for mammalian circadian oscillators. Science 2010, 330, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Morf, J.; Schibler, U. Body temperature cycles: Gatekeepers of circadian clocks. Cell Cycle 2013, 12, 539–540. [Google Scholar] [CrossRef]
- Brown, S.A.; Zumbrunn, G.; Fleury-Olela, F.; Preitner, N.; Schibler, U. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr. Biol. 2002, 12, 1574–1583. [Google Scholar] [CrossRef]
- Stevenson, R.D. The Relative Importance of Behavioral and Physiological Adjustments Controlling Body-Temperature in Terrestrial Ectotherms. Am. Nat. 1985, 126, 362–386. [Google Scholar] [CrossRef]
- Ellis, D.J.; Firth, B.T.; Belan, I. Circadian rhythms of locomotor activity and temperature selection in sleepy lizards, Tiliqua rugosa. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2007, 193, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Dillon, M.E.; Wang, G.; Garrity, P.A.; Huey, R.B. Review: Thermal preference in Drosophila. J. Therm. Biol. 2009, 34, 109–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrity, P.A.; Goodman, M.B.; Samuel, A.D.; Sengupta, P. Running hot and cold: Behavioral strategies, neural circuits, and the molecular machinery for thermotaxis in C. elegans and Drosophila. Genes Dev. 2010, 24, 2365–2382. [Google Scholar] [CrossRef]
- Kaneko, H.; Head, L.M.; Ling, J.; Tang, X.; Liu, Y.; Hardin, P.E.; Emery, P.; Hamada, F.N. Circadian rhythm of temperature preference and its neural control in Drosophila. Curr. Biol. 2012, 22, 1851–1857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saper, C.B.; Lu, J.; Chou, T.C.; Gooley, J. The hypothalamic integrator for circadian rhythms. Trends Neurosci. 2005, 28, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.E. Circadian variations in human thermoregulatory responses. J. Appl. Physiol. 1969, 26, 554–560. [Google Scholar] [CrossRef]
- Gander, P.H.; Connell, L.J.; Graeber, R.C. Masking of the circadian rhythms of heart rate and core temperature by the rest-activity cycle in man. J. Biol. Rhythms 1986, 1, 119–135. [Google Scholar] [CrossRef]
- Lavie, P. Sleep-wake as a biological rhythm. Annu. Rev. Psychol. 2001, 52, 277–303. [Google Scholar] [CrossRef]
- Goda, T.; Doi, M.; Umezaki, Y.; Murai, I.; Shimatani, H.; Chu, M.L.; Nguyen, V.H.; Okamura, H.; Hamada, F.N. Calcitonin receptors are ancient modulators for rhythms of preferential temperature in insects and body temperature in mammals. Genes Dev. 2018, 32, 140–155. [Google Scholar] [CrossRef]
- Sayeed, O.; Benzer, S. Behavioral genetics of thermosensation and hygrosensation in Drosophila. Proc. Natl. Acad. Sci. USA 1996, 93, 6079–6084. [Google Scholar] [CrossRef]
- Hamada, F.N.; Rosenzweig, M.; Kang, K.; Pulver, S.R.; Ghezzi, A.; Jegla, T.J.; Garrity, P.A. An internal thermal sensor controlling temperature preference in Drosophila. Nature 2008, 454, 217–220. [Google Scholar] [CrossRef] [Green Version]
- Goda, T.; Leslie, J.R.; Hamada, F.N. Design and analysis of temperature preference behavior and its circadian rhythm in Drosophila. J. Vis. Exp. 2014, e51097. [Google Scholar] [CrossRef]
- Morrison, S.F.; Nakamura, K.; Madden, C.J. Central control of thermogenesis in mammals. Exp. Physiol. 2008, 93, 773–797. [Google Scholar] [CrossRef] [Green Version]
- Welsh, D.K.; Takahashi, J.S.; Kay, S.A. Suprachiasmatic nucleus: Cell autonomy and network properties. Annu. Rev. Physiol. 2010, 72, 551–577. [Google Scholar] [CrossRef]
- Dubruille, R.; Emery, P. A plastic clock: How circadian rhythms respond to environmental cues in Drosophila. Mol. Neurobiol. 2008, 38, 129–145. [Google Scholar] [CrossRef]
- Renn, S.C.; Park, J.H.; Rosbash, M.; Hall, J.C.; Taghert, P.H. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 1999, 99, 791–802. [Google Scholar] [CrossRef]
- Lin, Y.; Stormo, G.D.; Taghert, P.H. The neuropeptide pigment-dispersing factor coordinates pacemaker interactions in the Drosophila circadian system. J. Neurosci. 2004, 24, 7951–7957. [Google Scholar] [CrossRef]
- Lear, B.C.; Zhang, L.; Allada, R. The neuropeptide PDF acts directly on evening pacemaker neurons to regulate multiple features of circadian behavior. PLoS Biol. 2009, 7, e1000154. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.; Lee, Y.; Hong, S.T.; Bang, S.; Paik, D.; Kang, J.; Shin, J.; Lee, J.; Jeon, K.; Hwang, S.; et al. Drosophila GPCR Han is a receptor for the circadian clock neuropeptide PDF. Neuron 2005, 48, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Mertens, I.; Vandingenen, A.; Johnson, E.C.; Shafer, O.T.; Li, W.; Trigg, J.S.; De Loof, A.; Schoofs, L.; Taghert, P.H. PDF receptor signaling in Drosophila contributes to both circadian and geotactic behaviors. Neuron 2005, 48, 213–219. [Google Scholar] [CrossRef]
- Lear, B.C.; Merrill, C.E.; Lin, J.M.; Schroeder, A.; Zhang, L.; Allada, R. A G protein-coupled receptor, groom-of-PDF, is required for PDF neuron action in circadian behavior. Neuron 2005, 48, 221–227. [Google Scholar] [CrossRef]
- Kunst, M.; Tso, M.C.; Ghosh, D.D.; Herzog, E.D.; Nitabach, M.N. Rhythmic control of activity and sleep by class B1 GPCRs. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 18–30. [Google Scholar] [CrossRef]
- Johnson, E.C.; Shafer, O.T.; Trigg, J.S.; Park, J.; Schooley, D.A.; Dow, J.A.; Taghert, P.H. A novel diuretic hormone receptor in Drosophila: Evidence for conservation of CGRP signaling. J. Exp. Biol. 2005, 208, 1239–1246. [Google Scholar] [CrossRef]
- Shafer, O.T.; Kim, D.J.; Dunbar-Yaffe, R.; Nikolaev, V.O.; Lohse, M.J.; Taghert, P.H. Widespread receptivity to neuropeptide PDF throughout the neuronal circadian clock network of Drosophila revealed by real-time cyclic AMP imaging. Neuron 2008, 58, 223–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunst, M.; Hughes, M.E.; Raccuglia, D.; Felix, M.; Li, M.; Barnett, G.; Duah, J.; Nitabach, M.N. Calcitonin gene-related peptide neurons mediate sleep-specific circadian output in Drosophila. Curr. Biol. 2014, 24, 2652–2664. [Google Scholar] [CrossRef]
- Goda, T.; Tang, X.; Umezaki, Y.; Chu, M.L.; Kunst, M.; Nitabach, M.N.N.; Hamada, F.N. Drosophila DH31 Neuropeptide and PDF Receptor Regulate Night-Onset Temperature Preference. J. Neurosci. 2016, 36, 11739–11754. [Google Scholar] [CrossRef] [Green Version]
- Choi, C.; Fortin, J.P.; McCarthy, E.; Oksman, L.; Kopin, A.S.; Nitabach, M.N. Cellular dissection of circadian peptide signals with genetically encoded membrane-tethered ligands. Curr. Biol. 2009, 19, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chung, B.Y.; Lear, B.C.; Kilman, V.L.; Liu, Y.; Mahesh, G.; Meissner, R.A.; Hardin, P.E.; Allada, R. DN1(p) circadian neurons coordinate acute light and PDF inputs to produce robust daily behavior in Drosophila. Curr. Biol. 2010, 20, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Roessingh, S.; Hayley, S.E.; Chu, M.L.; Tanaka, N.K.; Wolfgang, W.; Song, S.; Stanewsky, R.; Hamada, F.N. The role of PDF neurons in setting the preferred temperature before dawn in Drosophila. Elife 2017, 6, e23206. [Google Scholar] [CrossRef]
- Baines, R.A.; Uhler, J.P.; Thompson, A.; Sweeney, S.T.; Bate, M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J. Neurosci. 2001, 21, 1523–1531. [Google Scholar] [CrossRef]
- Picot, M.; Klarsfeld, A.; Chelot, E.; Malpel, S.; Rouyer, F. A role for blind DN2 clock neurons in temperature entrainment of the Drosophila larval brain. J. Neurosci. 2009, 29, 8312–8320. [Google Scholar] [CrossRef]
- Helfrich-Forster, C. The neuroarchitecture of the circadian clock in the brain of Drosophila melanogaster. Microsc. Res. Technol. 2003, 62, 94–102. [Google Scholar] [CrossRef]
- Feinberg, E.H.; Vanhoven, M.K.; Bendesky, A.; Wang, G.; Fetter, R.D.; Shen, K.; Bargmann, C.I. GFP Reconstitution Across Synaptic Partners (GRASP) defines cell contacts and synapses in living nervous systems. Neuron 2008, 57, 353–363. [Google Scholar] [CrossRef]
- Gordon, M.D.; Scott, K. Motor control in a Drosophila taste circuit. Neuron 2009, 61, 373–384. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Zhao, T.; Petralia, R.S.; Yu, Y.; Peng, H.C.; Myers, E.; Magee, J.C. mGRASP enables mapping mammalian synaptic connectivity with light microscopy. Nat. Methods 2012, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Macara, A.M.; Lelito, K.R.; Minosyan, T.Y.; Shafer, O.T. Analysis of functional neuronal connectivity in the Drosophila brain. J. Neurophysiol. 2012, 108, 684–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.; Platt, M.D.; Lagnese, C.M.; Leslie, J.R.; Hamada, F.N. Temperature integration at the AC thermosensory neurons in Drosophila. J. Neurosci. 2013, 33, 894–901. [Google Scholar] [CrossRef] [Green Version]
- Shih, H.W.; Chiang, A.S. Anatomical characterization of thermosensory AC neurons in the adult Drosophila brain. J. Neurogenet. 2011, 25, 1–6. [Google Scholar] [CrossRef]
- Das, A.; Holmes, T.C.; Sheeba, V. dTRPA1 in Non-circadian Neurons Modulates Temperature-Dependent Rhythmic Activity in Drosophila melanogaster. J. Biol. Rhythms 2016. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Lin, F.; Zheng, X.; Sehgal, A. Serotonin modulates circadian entrainment in Drosophila. Neuron 2005, 47, 115–127. [Google Scholar] [CrossRef]
- Morrison, S.F.; Nakamura, K. Central Mechanisms for Thermoregulation. Annu. Rev. Physiol. 2018. [Google Scholar] [CrossRef]
- Tan, C.L.; Knight, Z.A. Regulation of Body Temperature by the Nervous System. Neuron 2018, 98, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Cambras, T.; Weller, J.R.; Angles-Pujoras, M.; Lee, M.L.; Christopher, A.; Diez-Noguera, A.; Krueger, J.M.; de la Iglesia, H.O. Circadian desynchronization of core body temperature and sleep stages in the rat. Proc. Natl. Acad. Sci. USA 2007, 104, 7634–7639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, F.A.; King, R.; Smillie, S.J.; Kodji, X.; Brain, S.D. Calcitonin gene-related peptide: Physiology and pathophysiology. Physiol. Rev. 2014, 94, 1099–1142. [Google Scholar] [CrossRef]
- Kee, Z.; Kodji, X.; Brain, S.D. The Role of Calcitonin Gene Related Peptide (CGRP) in Neurogenic Vasodilation and Its Cardioprotective Effects. Front. Physiol. 2018, 9, 1249. [Google Scholar] [CrossRef] [PubMed]
- Masi, L.; Brandi, M.L. Calcitonin and calcitonin receptors. Clin. Cases Miner. Bone Metab. 2007, 4, 117–122. [Google Scholar]
- Nakamoto, H.; Soeda, Y.; Takami, S.; Minami, M.; Satoh, M. Localization of calcitonin receptor mRNA in the mouse brain: Coexistence with serotonin transporter mRNA. Brain Res. Mol. Brain Res. 2000, 76, 93–102. [Google Scholar] [CrossRef]
- Becskei, C.; Riediger, T.; Zund, D.; Wookey, P.; Lutz, T.A. Immunohistochemical mapping of calcitonin receptors in the adult rat brain. Brain Res. 2004, 1030, 221–233. [Google Scholar] [CrossRef]
- Doi, M.; Murai, I.; Kunisue, S.; Setsu, G.; Uchio, N.; Tanaka, R.; Kobayashi, S.; Shimatani, H.; Hayashi, H.; Chao, H.W.; et al. Gpr176 is a Gz-linked orphan G-protein-coupled receptor that sets the pace of circadian behaviour. Nat. Commun. 2016, 7, 10583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrahamson, E.E.; Moore, R.Y. Suprachiasmatic nucleus in the mouse: Retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001, 916, 172–191. [Google Scholar] [CrossRef]
- Shiromani, P.J.; Xu, M.; Winston, E.M.; Shiromani, S.N.; Gerashchenko, D.; Weaver, D.R. Sleep rhythmicity and homeostasis in mice with targeted disruption of mPeriod genes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R47–R57. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, K.; Matsue, K.; Konishi, M.; Iidaka, C.; Miyazaki, K.; Ishida, N.; Kanosue, K. The involvement of Cry1 and Cry2 genes in the regulation of the circadian body temperature rhythm in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R329–R335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerhart-Hines, Z.; Feng, D.; Emmett, M.J.; Everett, L.J.; Loro, E.; Briggs, E.R.; Bugge, A.; Hou, C.; Ferrara, C.; Seale, P.; et al. The nuclear receptor Rev-erbalpha controls circadian thermogenic plasticity. Nature 2013, 503, 410–413. [Google Scholar] [CrossRef]
- Wolff, G.; Duncan, M.J.; Esser, K.A. Chronic phase advance alters circadian physiological rhythms and peripheral molecular clocks. J. Appl. Physiol. 2013, 115, 373–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goda, T.; Hamada, F.N. Drosophila Temperature Preference Rhythms: An Innovative Model to Understand Body Temperature Rhythms. Int. J. Mol. Sci. 2019, 20, 1988. https://doi.org/10.3390/ijms20081988
Goda T, Hamada FN. Drosophila Temperature Preference Rhythms: An Innovative Model to Understand Body Temperature Rhythms. International Journal of Molecular Sciences. 2019; 20(8):1988. https://doi.org/10.3390/ijms20081988
Chicago/Turabian StyleGoda, Tadahiro, and Fumika N. Hamada. 2019. "Drosophila Temperature Preference Rhythms: An Innovative Model to Understand Body Temperature Rhythms" International Journal of Molecular Sciences 20, no. 8: 1988. https://doi.org/10.3390/ijms20081988
APA StyleGoda, T., & Hamada, F. N. (2019). Drosophila Temperature Preference Rhythms: An Innovative Model to Understand Body Temperature Rhythms. International Journal of Molecular Sciences, 20(8), 1988. https://doi.org/10.3390/ijms20081988