Tonic Activation of Extrasynaptic NMDA Receptors Decreases Intrinsic Excitability and Promotes Bistability in a Model of Neuronal Activity
Abstract
1. Introduction
2. Results
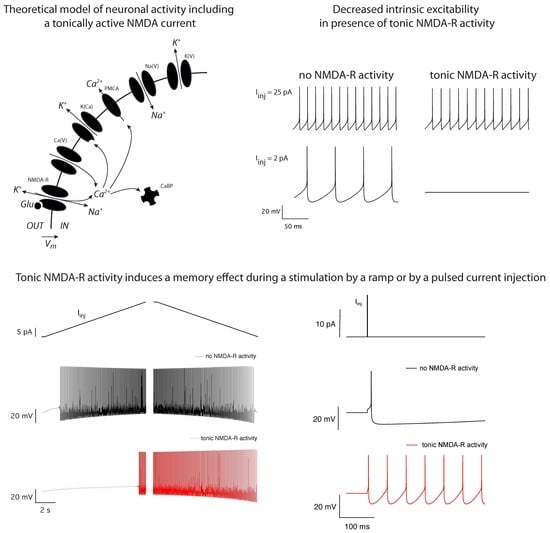
2.1. Theoretical Model
2.2. Tonic NMDA-R Activity Decreases Neuronal Activity
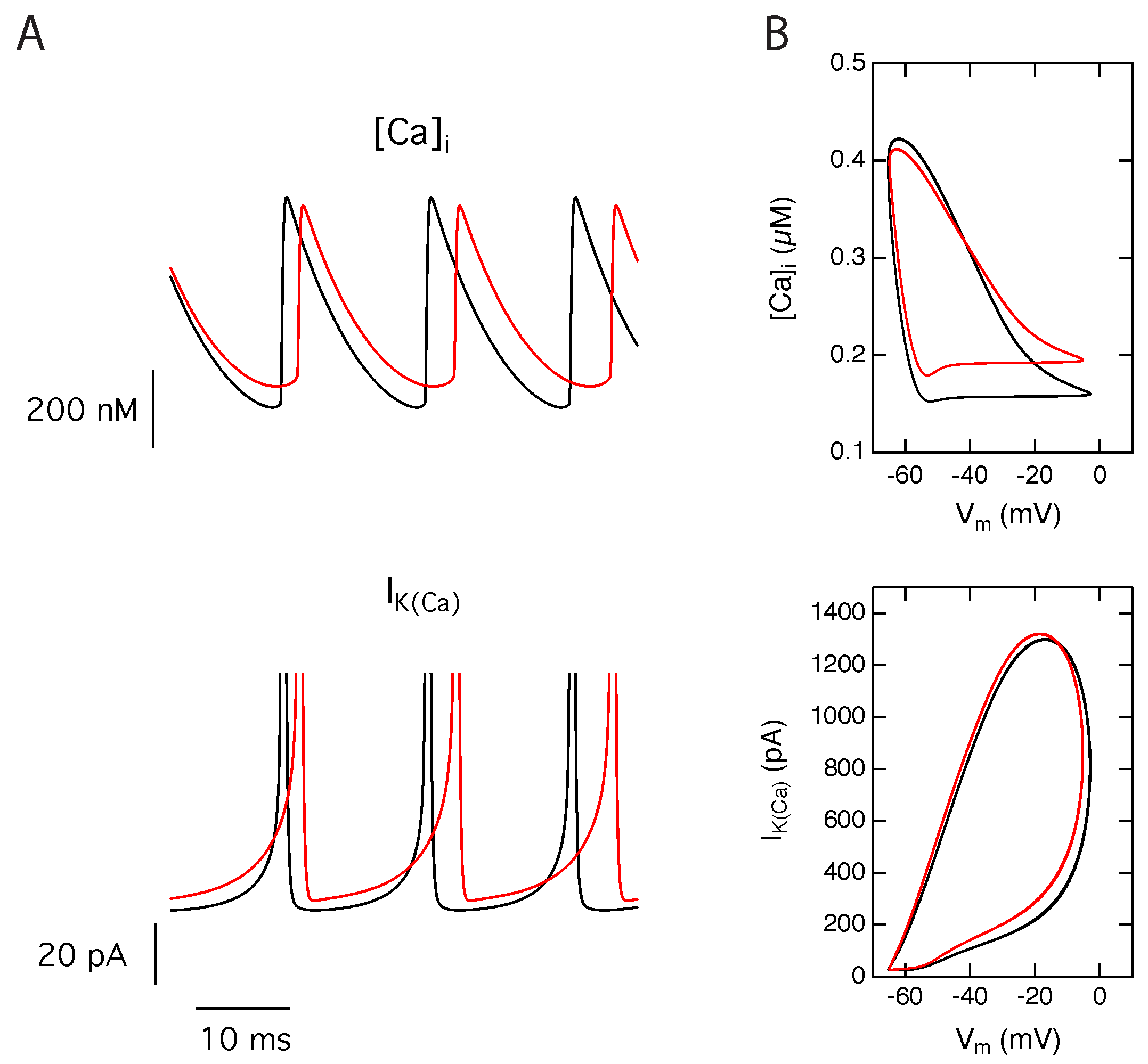
2.3. Activation of Calcium Activated Potassium Channels Couples Excitability and Tonic NMDA-R Activity
2.4. Tonic NMDA-R Activity Induces Bistability in Neuronal Firing
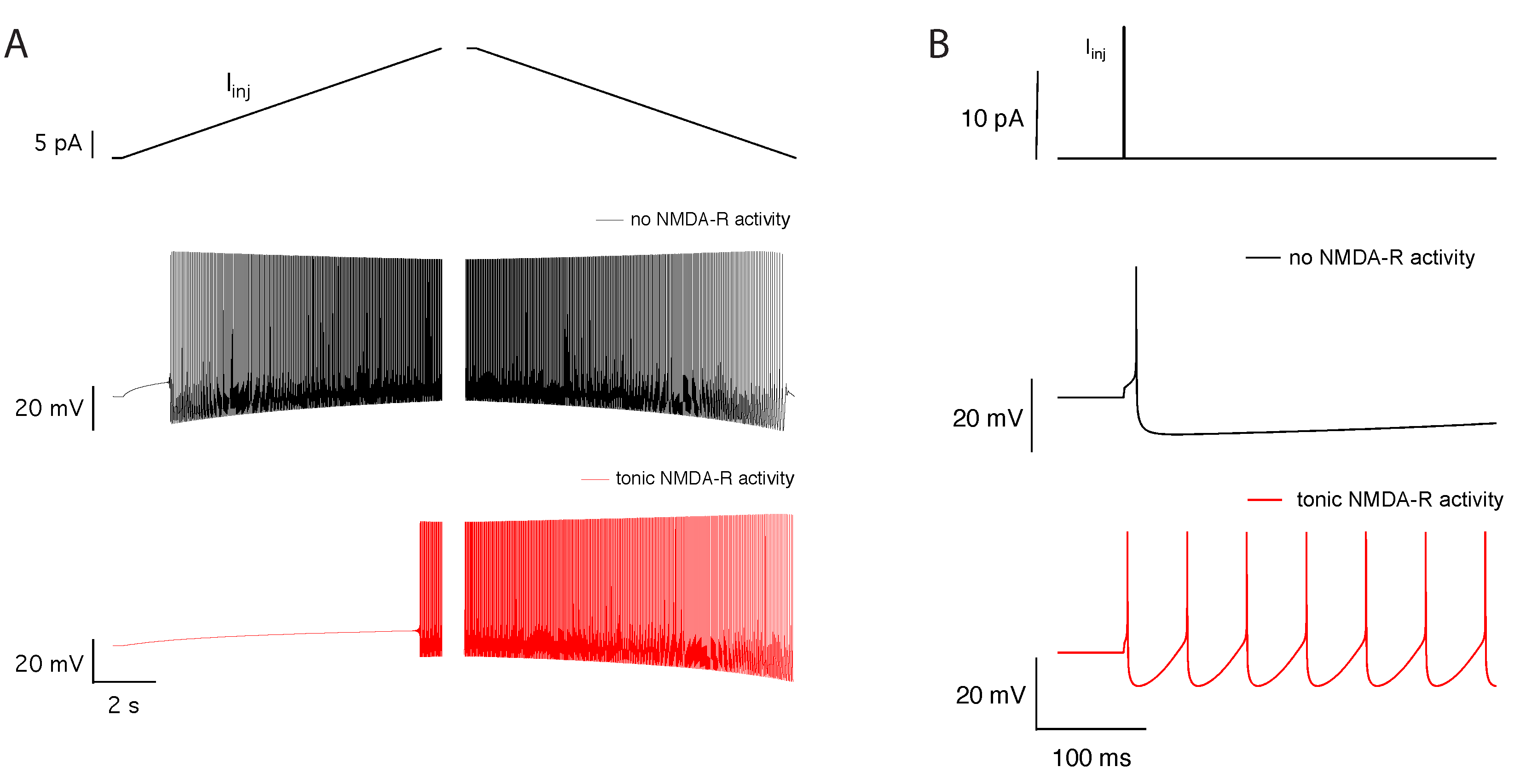
2.5. Tonic NMDA-R Activity Increases Hysteresis in Neuronal Firing
3. Discussion
4. Conclusions
5. Materials and Methods
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Malenka, R.C.; Nicoll, R.A. NMDA-receptor-dependent synaptic plasticity: Multiple forms and mechanisms. Trends Neurosci. 1993, 16, 521–527. [Google Scholar] [CrossRef]
- Singer, W. Development and plasticity of cortical processing architectures. Science 1995, 270, 758–764. [Google Scholar] [CrossRef]
- Pittenger, C.; Kandel, E.R. In search of general mechanisms for long-lasting plasticity: Aplysia and the hippocampus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003, 358, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Jörntell, H.; Hansel, C. Synaptic memories upside down: Bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses. Neuron 2006, 52, 227–238. [Google Scholar] [CrossRef]
- Sah, P.; Hestrin, S.; Nicoll, R.A. Tonic activation of NMDA receptors by ambient glutamate enhances excitability of neurons. Science 1989, 246, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Le Meur, K.; Galante, M.; Angulo, M.C.; Audinat, E. Tonic activation of NMDA receptors by ambient glutamate of non-synaptic origin in the rat hippocampus. J. Physiol. 2007, 580, 373–383. [Google Scholar] [CrossRef]
- Povysheva, N.V.; Johnson, J.W. Tonic NMDA receptor-mediated current in prefrontal cortical pyramidal cells and fast-spiking interneurons. J. Neurophysiol. 2012, 107, 2232–2243. [Google Scholar] [CrossRef]
- Huang, Y.; Shen, W.; Su, J.; Cheng, B.; Li, D.; Liu, G.; Zhou, W.X.; Zhang, Y.X. Modulating the Balance of Synaptic and Extrasynaptic NMDA Receptors Shows Positive Effects against Amyloid-β-Induced Neurotoxicity. J. Alzheimers Dis. 2017, 57, 885–897. [Google Scholar] [CrossRef]
- Rush, T.; Buisson, A. Reciprocal disruption of neuronal signaling and Aβ production mediated by extrasynaptic NMDA receptors: A downward spiral. Cell Tissue Res. 2014, 356, 279–286. [Google Scholar] [CrossRef]
- Pluta, R.; Salínska, E.; Puka, M.; Stafiej, A.; Łazarewicz, J.W. Early changes in extracellular amino acids and calcium concentrations in rabbit hippocampus following complete 15-min cerebral ischemia. Resuscitation 1988, 16, 193–210. [Google Scholar] [CrossRef]
- Salińska, E.; Pluta, R.; Puka, M.; Łazarewicz, J.W. Blockade of N-methyl-D-aspartate-sensitive excitatory amino acid receptors with 2-amino-5-phosphonovalerate reduces ischemia-evoked calcium redistribution in rabbit hippocampus. Exp. Neurol. 1991, 112, 89–94. [Google Scholar] [CrossRef]
- Ishikawa, M.; Mu, P.; Moyer, J.T.; Wolf, J.A.; Quock, R.M.; Davies, N.M.; Hu, X.T.; Schlüter, O.M.; Dong, Y. Homeostatic synapse-driven membrane plasticity in nucleus accumbens neurons. J. Neurosci. 2009, 29, 5820–5831. [Google Scholar] [CrossRef] [PubMed]
- Lambot, L.; Chaves Rodriguez, E.; Houtteman, D.; Li, Y.; Schiffmann, S.N.; Gall, D.; de Kerchove d’Exaerde, A. Striatopallidal Neuron NMDA Receptors Control Synaptic Connectivity, Locomotor, and Goal-Directed Behaviors. J. Neurosci. 2016, 36, 4976–4992. [Google Scholar] [CrossRef] [PubMed]
- Pagadala, P.; Park, C.K.; Bang, S.; Xu, Z.Z.; Xie, R.G.; Liu, T.; Han, B.X.; Tracey, W.D.; Wang, F.; Ji, R.R. Loss of NR1 subunit of NMDARs in primary sensory neurons leads to hyperexcitability and pain hypersensitivity: Involvement of Ca2+-activated small conductance potassium channels. J. Neurosci. 2013, 33, 13425–13430. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, J.S.; Murphy, G.J. Glutamate-mediated extrasynaptic inhibition: Direct coupling of NMDA receptors to Ca2+-activated K+ channels. Neuron 2001, 31, 1027–1034. [Google Scholar] [CrossRef]
- Faber, E.S.L.; Delaney, A.J.; Sah, P. SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nat. Neurosci. 2005, 8, 635–641. [Google Scholar] [CrossRef]
- Gall, D.; Roussel, C.; Susa, I.; D’Angelo, E.; Rossi, P.; Bearzatto, B.; Galas, M.; Blum, D.; Schurmans, S.; Schiffmann, S.N. Altered neuronal excitability in cerebellar granule cells of mice lacking calretinin. J. Neurosci. 2003, 23, 9320–9327. [Google Scholar] [CrossRef]
- Roussel, C.; Erneux, T.; Schiffmann, S.N.; Gall, D. Modulation of neuronal excitability by intracellular calcium buffering: From spiking to bursting. Cell Calcium 2006, 39, 455–466. [Google Scholar] [CrossRef]
- D’Angelo, E.; Filippi, G.D.; Rossi, P.; Taglietti, V. Ionic mechanism of electroresponsiveness in cerebellar granule cells implicates the action of a persistent sodium current. J. Neurophysiol. 1998, 80, 493–503. [Google Scholar] [CrossRef]
- Narayanan, R.; Johnston, D. The h current is a candidate mechanism for regulating the sliding modification threshold in a BCM-like synaptic learning rule. J. Neurophysiol. 2010, 104, 1020–1033. [Google Scholar] [CrossRef]
- Gall, D.; Prestori, F.; Sola, E.; D’Errico, A.; Roussel, C.; Forti, L.; Rossi, P.; D’Angelo, E. Intracellular calcium regulation by burst discharge determines bidirectional long-term synaptic plasticity at the cerebellum input stage. J. Neurosci. 2005, 25, 4813–4822. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.L.; Westbrook, G.L. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J. Physiol. 1987, 394, 501–527. [Google Scholar] [CrossRef] [PubMed]
- Jahr, C.E.; Stevens, C.F. Voltage dependence of NMDA-activated macroscopic conductances predicted by single-channel kinetics. J. Neurosci. 1990, 10, 3178–3182. [Google Scholar] [CrossRef] [PubMed]
- Allbritton, N.L.; Meyer, T.; Stryer, L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science 1992, 258, 1812–1815. [Google Scholar] [CrossRef]
- Manuel, M.; Zytnicki, D.; Meunier, C. The dendritic location of the L-type current and its deactivation by the somatic AHP current both contribute to firing bistability in motoneurons. Front. Comput. Neurosci. 2014, 8, 4. [Google Scholar] [CrossRef]
- Wang, X.J. Calcium coding and adaptive temporal computation in cortical pyramidal neurons. J. Neurophysiol. 1998, 79, 1549–1566. [Google Scholar] [CrossRef]
- Booth, V.; Rinzel, J.; Kiehn, O. Compartmental model of vertebrate motoneurons for Ca2+-dependent spiking and plateau potentials under pharmacological treatment. J. Neurophysiol. 1997, 78, 3371–3385. [Google Scholar] [CrossRef]
- Goldbeter, A. Biochemical Oscillations and Cellular Rhythms; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar] [CrossRef]
- Marder, E.; Abbott, L.F.; Turrigiano, G.G.; Liu, Z.; Golowasch, J. Memory from the dynamics of intrinsic membrane currents. Proc. Natl. Acad. Sci. USA 1996, 93, 13481–13486. [Google Scholar] [CrossRef]
- Cowansage, K.K.; Shuman, T.; Dillingham, B.C.; Chang, A.; Golshani, P.; Mayford, M. Direct reactivation of a coherent neocortical memory of context. Neuron 2014, 84, 432–441. [Google Scholar] [CrossRef]
- Tanaka, K.Z.; Pevzner, A.; Hamidi, A.B.; Nakazawa, Y.; Graham, J.; Wiltgen, B.J. Cortical representations are reinstated by the hippocampus during memory retrieval. Neuron 2014, 84, 347–354. [Google Scholar] [CrossRef]
- Ryan, T.J.; Roy, D.S.; Pignatelli, M.; Arons, A.; Tonegawa, S. Engram cells retain memory under retrograde amnesia. Science 2015, 348, 1007–1013. [Google Scholar] [CrossRef]
- De Caluwé, J.; Dupont, G. The progression towards Alzheimer’s disease described as a bistable switch arising from the positive loop between amyloids and Ca2+. J. Theor. Biol. 2013, 331, 12–18. [Google Scholar] [CrossRef]
- Texidó, L.; Martín-Satué, M.; Alberdi, E.; Solsona, C.; Matute, C. Amyloid β peptide oligomers directly activate NMDA receptors. Cell Calcium 2011, 49, 184–190. [Google Scholar] [CrossRef]
- Bicca, M.A.; Figueiredo, C.P.; Piermartiri, T.C.; Meotti, F.C.; Bouzon, Z.L.; Tasca, C.I.; Medeiros, R.; Calixto, J.B. The selective and competitive N-methyl-D-aspartate receptor antagonist, (-)-6-phosphonomethyl-deca-hydroisoquinoline-3-carboxylic acid, prevents synaptic toxicity induced by amyloid-β in mice. Neuroscience 2011, 192, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Brito-Moreira, J.; Paula-Lima, A.C.; Bomfim, T.R.; Oliveira, F.B.; Sepúlveda, F.J.; De Mello, F.G.; Aguayo, L.G.; Panizzutti, R.; Ferreira, S.T. Aβ oligomers induce glutamate release from hippocampal neurons. Curr. Alzheimer Res. 2011, 8, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Kaczorowski, C.C.; Sametsky, E.; Shah, S.; Vassar, R.; Disterhoft, J.F. Mechanisms underlying basal and learning-related intrinsic excitability in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2011, 32, 1452–1465. [Google Scholar] [CrossRef] [PubMed]
- Busche, M.A.; Eichhoff, G.; Adelsberger, H.; Abramowski, D.; Wiederhold, K.H.; Haass, C.; Staufenbiel, M.; Konnerth, A.; Garaschuk, O. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science 2008, 321, 1686–1689. [Google Scholar] [CrossRef]
- Folch, J.; Busquets, O.; Ettcheto, M.; Sanchez-Lopez, E.; Castro-Torres, R.D.; Verdaguer, E.; Garcia, M.L.; Olloquequi, J.; Casadesus, G.; Beas-Zarate, C.; et al. Memantine for the Treatment of Dementia: A Review on its Current and Future Applications. J. Alzheimers Dis. 2018, 62, 1223–1240. [Google Scholar] [CrossRef]
- Kocki, J.; Ułamek-Kozioł, M.; Bogucka-Kocka, A.; Januszewski, S.; Jabłołski, M.; Gil-Kulik, P.; Brzozowska, J.; Petniak, A.; Furmaga-Jabłołska, W.; Bogucki, J.; et al. Dysregulation of Amyloid-β Protein Precursor, β-Secretase, Presenilin 1 and 2 Genes in the Rat Selectively Vulnerable CA1 Subfield of Hippocampus Following Transient Global Brain Ischemia. J. Alzheimers Dis. 2015, 47, 1047–1056. [Google Scholar] [CrossRef]
- Pluta, R.; Kocki, J.; Ułamek-Kozioł, M.; Petniak, A.; Gil-Kulik, P.; Januszewski, S.; Bogucki, J.; Jabłołski, M.; Brzozowska, J.; Furmaga-Jabłołska, W.; et al. Discrepancy in Expression of β-Secretase and Amyloid-β Protein Precursor in Alzheimer-Related Genes in the Rat Medial Temporal Lobe Cortex Following Transient Global Brain Ischemia. J. Alzheimers Dis. 2016, 51, 1023–1031. [Google Scholar] [CrossRef]
- Pluta, R.; Ułamek, M.; Jabłołski, M. Alzheimer’s mechanisms in ischemic brain degeneration. Anat. Rec. 2009, 292, 1863–1881. [Google Scholar] [CrossRef] [PubMed]
- Kiryk, A.; Pluta, R.; Figiel, I.; Mikosz, M.; Ulamek, M.; Niewiadomska, G.; Jablonski, M.; Kaczmarek, L. Transient brain ischemia due to cardiac arrest causes irreversible long-lasting cognitive injury. Behav. Brain Res. 2011, 219, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gavello, D.; Calorio, C.; Franchino, C.; Cesano, F.; Carabelli, V.; Carbone, E.; Marcantoni, A. Early Alterations of Hippocampal Neuronal Firing Induced by Abeta42. Cereb. Cortex 2018, 28, 433–446. [Google Scholar] [CrossRef] [PubMed]



| Changes VS. Default Values | = 56.5 pS | = 50 pS |
|---|---|---|
| − | 0.81 pA | 0.31 pA |
| = 80 nS | 3.71 pA | 1.12 pA |
| = 8 ms | 4.01 pA | 1.43 pA |
| f = 0.1 | 2.81 pA | 1.51 pA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gall, D.; Dupont, G. Tonic Activation of Extrasynaptic NMDA Receptors Decreases Intrinsic Excitability and Promotes Bistability in a Model of Neuronal Activity. Int. J. Mol. Sci. 2020, 21, 206. https://doi.org/10.3390/ijms21010206
Gall D, Dupont G. Tonic Activation of Extrasynaptic NMDA Receptors Decreases Intrinsic Excitability and Promotes Bistability in a Model of Neuronal Activity. International Journal of Molecular Sciences. 2020; 21(1):206. https://doi.org/10.3390/ijms21010206
Chicago/Turabian StyleGall, David, and Geneviève Dupont. 2020. "Tonic Activation of Extrasynaptic NMDA Receptors Decreases Intrinsic Excitability and Promotes Bistability in a Model of Neuronal Activity" International Journal of Molecular Sciences 21, no. 1: 206. https://doi.org/10.3390/ijms21010206
APA StyleGall, D., & Dupont, G. (2020). Tonic Activation of Extrasynaptic NMDA Receptors Decreases Intrinsic Excitability and Promotes Bistability in a Model of Neuronal Activity. International Journal of Molecular Sciences, 21(1), 206. https://doi.org/10.3390/ijms21010206