Emerging Role of Non-Coding RNAs in Esophageal Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Role of Non-Coding RNAs in ESCC Progression
3. Non-Coding RNAs Regulate Cell Proliferation and Apoptosis during ESCC Development
4. Non-Coding RNAs and EMT and Metastasis in ESCC
5. Non-coding RNAs Influence Chemoresistance and Radioresistance in ESCC
6. Experimental Approaches of Studying Non-Coding RNAs in ESCC
7. Prospects
Funding
Conflicts of Interest
References
- Kauppila, J.H.; Selander, K.S. Toll-like receptors in esophageal cancer. Front. Immunol. 2014, 5, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, F.L.; Yu, S.J. Esophageal cancer: Risk factors, genetic association, and treatment. Asian J. Surg. 2018, 41, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Kwong, D.L.; Cao, T.; Hu, Q.; Zhang, L.; Ming, X.; Chen, J.; Fu, L.; Guan, X. Esophageal squamous cell carcinoma (ESCC): Advance in genomics and molecular genetics. Dis. Esophagus Off. J. Int. Soc. Dis. Esophagus 2015, 28, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Abnet, C.C.; Arnold, M.; Wei, W.Q. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology 2018, 154, 360–373. [Google Scholar] [CrossRef]
- Chen, J.L.; Lin, Z.X.; Qin, Y.S.; She, Y.Q.; Chen, Y.; Chen, C.; Qiu, G.D.; Zheng, J.T.; Chen, Z.L.; Zhang, S.Y. Overexpression of long noncoding RNA LINC01419 in esophageal squamous cell carcinoma and its relation to the sensitivity to 5-fluorouracil by mediating GSTP1 methylation. Ther. Adv. Med. Oncol. 2019, 11. [Google Scholar] [CrossRef] [Green Version]
- Jackie Oh, S.; Han, S.; Lee, W.; Lockhart, A.C. Emerging immunotherapy for the treatment of esophageal cancer. Expert Opin. Investig. Drugs 2016, 25, 667–677. [Google Scholar] [CrossRef]
- Deng, H.Y.; Wang, Y.C.; Ni, P.Z.; Lin, Y.D.; Chen, L.Q. Long noncoding RNAs are novel potential prognostic biomarkers for esophageal squamous cell carcinoma: An overview. J. Thorac. Dis. 2016, 8, E653–E659. [Google Scholar] [CrossRef] [Green Version]
- Sundaram, G.M.; Veera Bramhachari, P. Molecular interplay of pro-inflammatory transcription factors and non-coding RNAs in esophageal squamous cell carcinoma. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2017, 39. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.H.; Wu, M.H.; Yeh, C.T.; Lin, K.H. Long non-coding RNAs as mediators of tumor microenvironment and liver cancer cell communication. Int. J. Mol. Sci. 2018, 19, 3742. [Google Scholar] [CrossRef] [Green Version]
- Lei, B.; Tian, Z.; Fan, W.; Ni, B. Circular RNA: A novel biomarker and therapeutic target for human cancers. Int. J. Med. Sci. 2019, 16, 292–301. [Google Scholar] [CrossRef] [Green Version]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Tomar, D.; Yadav, A.S.; Kumar, D.; Bhadauriya, G.; Kundu, G.C. Non-coding RNAs as potential therapeutic targets in breast cancer. Biochim. Et Biophys. Acta Gene Regul. Mech. 2019. [Google Scholar] [CrossRef] [PubMed]
- Botti, G.; Giordano, A.; Feroce, F.; De Chiara, A.R.; Cantile, M. Noncoding RNAs as circulating biomarkers in osteosarcoma patients. J. Cell. Physiol. 2019, 234, 19249–19255. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Y.; Wang, N.; Li, X.; Zheng, J.; Ge, L. UPF1 inhibits the hepatocellular carcinoma progression by targeting long non-coding RNA UCA1. Sci. Rep. 2019, 9, 6652. [Google Scholar] [CrossRef] [PubMed]
- Tano, K.; Akimitsu, N. Long non-coding RNAs in cancer progression. Front. Genet. 2012, 3, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, G.; He, Q.; Wang, J. Clinical values of long non-coding rnas in bladder cancer: A systematic review. Front. Physiol. 2018, 9, 652. [Google Scholar] [CrossRef] [Green Version]
- Shafiee, M.; Aleyasin, S.A.; Vasei, M.; Semnani, S.S.; Mowla, S.J. Down-regulatory effects of miR-211 on long non-coding RNA SOX2OT and SOX2 genes in esophageal squamous cell carcinoma. Cell J. 2016, 17, 593–600. [Google Scholar]
- Song, H.; Xu, D.; Shi, P.; He, B.; Li, Z.; Ji, Y.; Agbeko, C.K.; Wang, J. Upregulated circ RNA hsa_circ_0000337 promotes cell proliferation, migration, and invasion of esophageal squamous cell carcinoma. Cancer Manag. Res. 2019, 11, 1997–2006. [Google Scholar] [CrossRef] [Green Version]
- Sugihara, H.; Ishimoto, T.; Miyake, K.; Izumi, D.; Baba, Y.; Yoshida, N.; Watanabe, M.; Baba, H. Noncoding RNA expression aberration is associated with cancer progression and is a potential biomarker in esophageal squamous cell carcinoma. Int. J. Mol. Sci. 2015, 16, 27824–27834. [Google Scholar] [CrossRef]
- Chen, M.; Liu, P.; Chen, Y.; Chen, Z.; Shen, M.; Liu, X.; Li, X.; Li, A.; Lin, Y.; Yang, R.; et al. Long noncoding RNA FAM201A mediates the radiosensitivity of esophageal squamous cell cancer by regulating ATM and mTOR expression via miR-101. Front. Genet. 2018, 9, 611. [Google Scholar] [CrossRef]
- Fan, L.; Cao, Q.; Liu, J.; Zhang, J.; Li, B. Circular RNA profiling and its potential for esophageal squamous cell cancer diagnosis and prognosis. Mol. Cancer 2019, 18, 16. [Google Scholar] [CrossRef] [PubMed]
- Salzman, J.; Gawad, C.; Wang, P.L.; Lacayo, N.; Brown, P.O. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS ONE 2012, 7, e30733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greene, J.; Baird, A.M.; Brady, L.; Lim, M.; Gray, S.G.; McDermott, R.; Finn, S.P. Circular RNAs: biogenesis, function and role in human diseases. Front. Mol. Biosci. 2017, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Bach, D.H.; Lee, S.K.; Sood, A.K. Circular RNAs in cancer. Mol. Ther. Nucleic Acids 2019, 16, 118–129. [Google Scholar] [CrossRef] [Green Version]
- Meng, S.; Zhou, H.; Feng, Z.; Xu, Z.; Tang, Y.; Li, P.; Wu, M. CircRNA: Functions and properties of a novel potential biomarker for cancer. Mol. Cancer 2017, 16, 94. [Google Scholar] [CrossRef]
- Zhang, H.D.; Jiang, L.H.; Sun, D.W.; Hou, J.C.; Ji, Z.L. CircRNA: A novel type of biomarker for cancer. Breast Cancer 2018, 25, 1–7. [Google Scholar] [CrossRef]
- Shi, N.; Shan, B.; Gu, B.; Song, Y.; Chu, H.; Qian, L. Circular RNA circ-PRKCI functions as a competitive endogenous RNA to regulate AKT3 expression by sponging miR-3680-3p in esophageal squamous cell carcinoma. J. Cell. Biochem. 2019, 120, 10021–10030. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Chen, G.; Yan, L.; Li, L.; Huang, X. Contribution of dysregulated circRNA_100876 to proliferation and metastasis of esophageal squamous cell carcinoma. Oncotargets Ther. 2018, 11, 7385–7394. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Ren, G.; Zhu, L.; Liu, X.; He, X. The upregulation of miRNA-146a inhibited biological behaviors of ESCC through inhibition of IRS2. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 4641–4647. [Google Scholar] [CrossRef] [PubMed]
- Hemmatzadeh, M.; Mohammadi, H.; Karimi, M.; Musavishenas, M.H.; Baradaran, B. Differential role of microRNAs in the pathogenesis and treatment of Esophageal cancer. Biomed. Pharmacother. Biomed. Pharmacother. 2016, 82, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Han, L.; Yin, D.; He, X.; Hong, L.; Si, X.; Qiu, M.; Xu, T.; De, W.; Xu, L.; et al. H3K27 acetylation activated-long non-coding RNA CCAT1 affects cell proliferation and migration by regulating SPRY4 and HOXB13 expression in esophageal squamous cell carcinoma. Nucleic Acids Res. 2017, 45, 3086–3101. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, J.; Meng, X.; Li, T.; Wang, S.; Bao, Y. The pharmacological effects of spatholobi caulis tannin in cervical cancer and its precise therapeutic effect on related circRNA. Mol. Ther. Oncolytics 2019, 14, 121–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, G.; Dai, C.; He, Y.; Shi, J.; Xu, C. Effects of miR106b3p on cell proliferation and epithelialmesenchymal transition, and targeting of ZNRF3 in esophageal squamous cell carcinoma. Int. J. Mol. Med. 2019, 43, 1817–1829. [Google Scholar] [PubMed]
- Eichelmann, A.K.; Matuszcak, C.; Lindner, K.; Haier, J.; Hussey, D.J.; Hummel, R. Complex role of miR-130a-3p and miR-148a-3p balance on drug resistance and tumor biology in esophageal squamous cell carcinoma. Sci. Rep. 2018, 8, 17553. [Google Scholar] [CrossRef]
- Huang, H.; Xu, Y.; Guo, Z.; Chen, X.; Ji, S.; Xu, Z. MicroRNA-133b inhibits cell proliferation and promotes apoptosis by targeting cullin 4B in esophageal squamous cell carcinoma. Exp. Ther. Med. 2018, 15, 3743–3750. [Google Scholar] [CrossRef]
- Park, M.; Yoon, H.J.; Kang, M.C.; Kwon, J.; Lee, H.W. MiR-338-5p enhances the radiosensitivity of esophageal squamous cell carcinoma by inducing apoptosis through targeting survivin. Sci. Rep. 2017, 7, 10932. [Google Scholar] [CrossRef]
- Luo, H.L.; Huang, M.D.; Guo, J.N.; Fan, R.H.; Xia, X.T.; He, J.D.; Chen, X.F. AFAP1-AS1 is upregulated and promotes esophageal squamous cell carcinoma cell proliferation and inhibits cell apoptosis. Cancer Med. 2016, 5, 2879–2885. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Zhang, Y.; Wang, H.; Pan, T.; Zhang, Y.; Li, C. LINC00473/miR-374a-5p regulates esophageal squamous cell carcinoma via targeting SPIN1 to weaken the effect of radiotherapy. J. Cell. Biochem. 2019, 120, 14562–14572. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, J.H.; Shi, Z.X.; Li, Z.; Liu, H.T.; Lu, P. MicroRNA-133b suppresses cell proliferation and invasion of esophageal squamous cell carcinoma via downregulating TAGLN2 expression. Zhonghua Zhong Liu Za Zhi Chin. J. Oncol. 2019, 41, 91–96. [Google Scholar]
- Ma, Q. MiR-219-5p suppresses cell proliferation and cell cycle progression in esophageal squamous cell carcinoma by targeting CCNA2. Cell. Mol. Biol. Lett. 2019, 24, 4. [Google Scholar] [CrossRef]
- Zhang, J.; Fa, X.; Zhang, Q. MicroRNA206 exerts antioncogenic functions in esophageal squamous cell carcinoma by suppressing the cMet/AKT/mTOR pathway. Mol. Med. Rep. 2019, 19, 1491–1500. [Google Scholar] [PubMed]
- Yu, H.X.; Wang, X.L.; Zhang, L.N.; Zhang, J.; Zhao, W. MicroRNA-384 inhibits the progression of esophageal squamous cell carcinoma through blockade of the LIMK1/cofilin signaling pathway by binding to LIMK1. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 109, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, Y.; Li, P. Inhibitory effect of microRNA-455-5p on biological functions of esophageal squamous cell carcinoma Eca109 cells via Rab31. Exp. Ther. Med. 2018, 16, 4959–4966. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Guo, T.; Guo, Y.; Liu, J.; Qu, F.; He, Y. Methylationassociated silencing of miR128 promotes the development of esophageal cancer by targeting COX2 in areas with a high incidence of esophageal cancer. Int. J. Oncol. 2019, 54, 644–654. [Google Scholar] [PubMed] [Green Version]
- Mei, L.L.; Wang, W.J.; Qiu, Y.T.; Xie, X.F.; Bai, J.; Shi, Z.Z. miR-145-5p Suppresses Tumor Cell Migration, Invasion and Epithelial to Mesenchymal Transition by Regulating the Sp1/NF-kappaB Signaling Pathway in Esophageal Squamous Cell Carcinoma. Int. J. Mol. Sci. 2017, 18, 1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimonosono, M.; Idichi, T.; Seki, N.; Yamada, Y.; Arai, T.; Arigami, T.; Sasaki, K.; Omoto, I.; Uchikado, Y.; Kita, Y.; et al. Molecular pathogenesis of esophageal squamous cell carcinoma: Identification of the antitumor effects of miR1453p on gene regulation. Int. J. Oncol. 2019, 54, 673–688. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.F.; Yu, J.R.; Yang, Z.; Zhu, G.X.; Gao, P.; Wang, H.; Chen, S.Y.; Zhang, J.; Liu, M.Y.; Niu, Y.; et al. Promoter hypomethylation mediated upregulation of MicroRNA-10b-3p targets FOXO3 to promote the progression of esophageal squamous cell carcinoma (ESCC). J. Exp. Clin. Cancer Res. 2018, 37, 301. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.B.; Zhang, S.H.; He, Y.; Zhang, X.Y.; Zhang, Y.B. MiR-874-3p is an independent prognostic factor and functions as an anti-oncomir in esophageal squamous cell carcinoma via targeting STAT3. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7265–7273. [Google Scholar]
- Liu, Y.; Wang, X.; Jiang, X.; Yan, P.; Zhan, L.; Zhu, H.; Wang, T.; Wen, J. Tumor-suppressive microRNA-10a inhibits cell proliferation and metastasis by targeting Tiam1 in esophageal squamous cell carcinoma. J. Cell. Biochem. 2018, 120, 7845–7857. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, X.; Chen, Y.; Jia, Q.; Yang, J.; Shu, Y. MicroRNA-365 suppresses cell growth and invasion in esophageal squamous cell carcinoma by modulating phosphoserine aminotransferase 1. Cancer Manag. Res. 2018, 10, 4581–4590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, H.; Wu, Y.; Fang, Y.; Shen, L.; Fei, Z.; Xie, C.; Chen, M. MicroRNA301a targets WNT1 to suppress cell proliferation and migration and enhance radiosensitivity in esophageal cancer cells. Oncol. Rep. 2019, 41, 599–607. [Google Scholar] [PubMed]
- Meng, L.; Liu, F.; Ju, Y.; Ding, P.; Liu, S.; Chang, S.; Liu, S.; Zhang, Y.; Lian, Y.; Gu, L.; et al. Tumor suppressive miR-6775-3p inhibits ESCC progression through forming a positive feedback loop with p53 via MAGE-A family proteins. Cell Death Dis. 2018, 9, 1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, W.; Zhang, J.; Wei, Y.; Feng, J.; Ma, M.; Zhao, H.; Wang, L.; Jiao, W. MiR-139-5p regulates VEGFR and downstream signaling pathways to inhibit the development of esophageal cancer. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2019, 51, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Xing, G. miR-516b functions as a tumor suppressor by directly modulating CCNG1 expression in esophageal squamous cell carcinoma. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 106, 1650–1660. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Liu, J.; Mu, J. Downregulation of microRNA449a5p promotes esophageal squamous cell carcinoma cell proliferation via cyclin D1 regulation. Mol. Med. Rep. 2018, 18, 848–854. [Google Scholar] [PubMed] [Green Version]
- Fan, Y.X.; Bian, X.H.; Qian, P.D.; Chen, Z.Z.; Wen, J.; Luo, Y.H.; Yan, P.W.; Zhang, Q. MicroRNA-125b inhibits cell proliferation and induces cell apoptosis in esophageal squamous cell carcinoma by targeting BMF. Oncol. Rep. 2018, 40, 61–72. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, Y.; Mu, Y.; Wang, X.; Fan, Q. MiR-433-3p Inhibits Proliferation and Invasion of Esophageal Squamous Cell Carcinoma by Targeting GRB2. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 46, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xia, Y.; Tan, Y.; Jiang, G.; Jin, H.; Chen, Y. Downregulation of microRNA-370 in esophageal squamous-cell carcinoma is associated with cancer progression and promotes cancer cell proliferation via upregulating PIN1. Gene 2018, 661, 68–77. [Google Scholar] [CrossRef]
- Liu, W.; Li, M.; Chen, X.; Zhu, S.; Shi, H.; Zhang, D.; Cheng, C.; Li, B. MicroRNA-1 suppresses proliferation, migration and invasion by targeting Notch2 in esophageal squamous cell carcinoma. Sci. Rep. 2018, 8, 5183. [Google Scholar] [CrossRef] [Green Version]
- Qi, B.; Wang, Y.; Chen, Z.J.; Li, X.N.; Qi, Y.; Yang, Y.; Cui, G.H.; Guo, H.Z.; Li, W.H.; Zhao, S. Down-regulation of miR-30a-3p/5p promotes esophageal squamous cell carcinoma cell proliferation by activating the Wnt signaling pathway. World J. Gastroenterol. 2017, 23, 7965–7977. [Google Scholar] [CrossRef]
- Cui, X.B.; Peng, H.; Li, R.R.; Mu, J.Q.; Yang, L.; Li, N.; Liu, C.X.; Hu, J.M.; Li, S.G.; Wei, Y.; et al. MicroRNA-34a functions as a tumor suppressor by directly targeting oncogenic PLCE1 in Kazakh esophageal squamous cell carcinoma. Oncotarget 2017, 8, 92454–92469. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Li, J.; Xiong, G.; He, G.; Guan, X.; Yang, K.; Bai, Y. Negative regulation of lncRNA GAS5 by miR-196a inhibits esophageal squamous cell carcinoma growth. Biochem. Biophys. Res. Commun. 2018, 495, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.L.; Wang, W.J.; Qiu, Y.T.; Xie, X.F.; Bai, J.; Shi, Z.Z. miR-125b-5p functions as a tumor suppressor gene partially by regulating HMGA2 in esophageal squamous cell carcinoma. PLoS ONE 2017, 12, e0185636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Xia, H.B. Lentiviral-mediated overexpression of MicroRNA-141 promotes cell Proliferation and inhibits apoptosis in human esophageal squamous cell carcinoma. Recent Pat. Anti-Cancer Drug Discov. 2019, 14, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cai, S.; Li, B.; Zhang, X.; Li, W.; Liang, H.; Cao, X.; Wang, L.; Wu, Z. MicroRNA21 regulates the biological behavior of esophageal squamous cell carcinoma by targeting RASA1. Oncol. Rep. 2019, 41, 1627–1637. [Google Scholar] [PubMed] [Green Version]
- Wen, J.; Hu, Y.; Liu, Q.; Ling, Y.; Zhang, S.; Luo, K.; Xie, X.; Fu, J.; Yang, H. miR-424 coordinates multilayered regulation of cell cycle progression to promote esophageal squamous cell carcinoma cell proliferation. EBioMedicine 2018, 37, 110–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Q.; Chen, T.; Wu, Y.; Wu, W.; Xu, Y.; Gong, Z.; Chen, S. MicroRNA6753p promotes esophageal squamous cell cancer cell migration and invasion. Mol. Med. Rep. 2018, 18, 3631–3640. [Google Scholar] [PubMed] [Green Version]
- Zhao, H.; Diao, C.; Wang, X.; Xie, Y.; Liu, Y.; Gao, X.; Han, J.; Li, S. MiR-543 promotes migration, invasion and epithelial-mesenchymal transition of esophageal cancer cells by targeting phospholipase A2 group IVA. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 48, 1595–1604. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, S.; Yuan, F.; Zhang, K.; Fan, Y.; Zheng, S.; Gao, Z.; Zhao, J.; Mu, T.; Zhao, S.; et al. miR-135 promotes proliferation and stemness of oesophageal squamous cell carcinoma by targeting RERG. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1210–1219. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Tan, X.; Zhang, Q.; Liu, C.; Zhang, Y. MiR-23b-3p induces the proliferation and metastasis of esophageal squamous cell carcinomas cells through the inhibition of EBF3. Acta Biochim. Biophys. Sin. 2018, 50, 605–614. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Pan, X.; Hu, Z. MiR-502 mediates esophageal cancer cell TE1 proliferation by promoting AKT phosphorylation. Biochem. Biophys. Res. Commun. 2018, 501, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, D.; Li, M.; Gao, X.; Shi, G.; Zhao, H. The CADM2/Akt pathway is involved in the inhibitory effect of miR-21-5p downregulation on proliferation and apoptosis in esophageal squamous cell carcinoma cells. Chem. Biol. Interact. 2018, 288, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lin, J.; Wu, S.; Xu, C.; Chen, F.; Huang, Z. Up-regulated miR-548k promotes esophageal squamous cell carcinoma progression via targeting long noncoding RNA-LET. Exp. Cell Res. 2018, 362, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jin, X.; Wang, Z.; Zhang, X.; Liu, S.; Liu, G. Downregulation of SNHG1 suppresses cell proliferation and invasion by regulating Notch signaling pathway in esophageal squamous cell cancer. Cancer Biomark. Sect. A Dis. Markers 2017, 21, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Fan, Q.; Wang, L.; Zhou, Y.; Li, J.; Zhou, K. LncRNA Snhg1, a non-degradable sponge for miR-338, promotes expression of proto-oncogene CST3 in primary esophageal cancer cells. Oncotarget 2017, 8, 35750–35760. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, R.; Ding, X.; Zhang, K.; Qin, W. Upregulation of long non-coding RNA SNHG6 promote esophageal squamous cell carcinoma cell malignancy and its diagnostic value. Am. J. Transl. Res. 2019, 11, 1084–1091. [Google Scholar]
- Zhang, K.; Chen, J.; Song, H.; Chen, L.B. SNHG16/miR-140-5p axis promotes esophagus cancer cell proliferation, migration and EMT formation through regulating ZEB1. Oncotarget 2018, 9, 1028–1040. [Google Scholar] [CrossRef] [Green Version]
- Han, G.H.; Lu, K.J.; Wang, P.; Ye, J.; Ye, Y.Y.; Huang, J.X. LncRNA SNHG16 predicts poor prognosis in ESCC and promotes cell proliferation and invasion by regulating Wnt/beta-catenin signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3795–3803. [Google Scholar]
- Ma, J.; Li, T.F.; Han, X.W.; Yuan, H.F. Downregulated MEG3 contributes to tumour progression and poor prognosis in oesophagal squamous cell carcinoma by interacting with miR-4261, downregulating DKK2 and activating the Wnt/beta-catenin signalling. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1513–1523. [Google Scholar] [CrossRef]
- Zhang, S.; Liang, Y.; Wu, Y.; Chen, X.; Wang, K.; Li, J.; Guan, X.; Xiong, G.; Yang, K.; Bai, Y. Upregulation of a novel lncRNA LINC01980 promotes tumor growth of esophageal squamous cell carcinoma. Biochem. Biophys. Res. Commun. 2019, 513, 73–80. [Google Scholar] [CrossRef]
- Li, W.; Zhang, L.; Guo, B.; Deng, J.; Wu, S.; Li, F.; Wang, Y.; Lu, J.; Zhou, Y. Exosomal FMR1-AS1 facilitates maintaining cancer stem-like cell dynamic equilibrium via TLR7/NFkappaB/c-Myc signaling in female esophageal carcinoma. Mol. Cancer 2019, 18, 22. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, Y.; Yang, Y.; Liu, X.; Zang, M.; Li, Y.; Yang, K.; Yang, W.; Zhang, S. Long noncoding RNA DLX6AS1 is associated with malignant progression and promotes proliferation and invasion in esophageal squamous cell carcinoma. Mol. Med. Rep. 2019, 19, 1942–1950. [Google Scholar] [PubMed] [Green Version]
- Zhong, Y.B.; Shan, A.J.; Lv, W.; Wang, J.; Xu, J.Z. Long non-coding RNA LINC00675 inhibits tumorigenesis and EMT via repressing Wnt/beta-catenin signaling in esophageal squamous cell carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8288–8297. [Google Scholar] [PubMed]
- Wang, Z.; Ren, B.; Huang, J.; Yin, R.; Jiang, F.; Zhang, Q. LncRNA DUXAP10 modulates cell proliferation in esophageal squamous cell carcinoma through epigenetically silencing p21. Cancer Biol. Ther. 2018, 19, 998–1005. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Liang, T.; Li, J. Long noncoding RNA LINC01296 is associated with poor prognosis in ESCC and promotes ESCC cell proliferation, migration and invasion. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4524–4531. [Google Scholar]
- Shi, H.; Shi, J.; Zhang, Y.; Guan, C.; Zhu, J.; Wang, F.; Xu, M.; Ju, Q.; Fang, S.; Jiang, M. Long non-coding RNA DANCR promotes cell proliferation, migration, invasion and resistance to apoptosis in esophageal cancer. J. Thorac. Dis. 2018, 10, 2573–2582. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, X.; Liang, Y.; Li, J.; Zhang, K.; Dai, L.; Guan, X.; Wang, K.; Bai, Y. Overexpression of long non-coding RNA SOX2OT promotes esophageal squamous cell carcinoma growth. Cancer Cell Int. 2018, 18, 76. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Luo, M.; Zhou, C.; Shi, X.; Yang, W.; Lu, Z.; Chen, Z.; Sun, N.; He, J. Novel long noncoding RNA NMR promotes tumor progression via NSUN2 and BPTF in esophageal squamous cell carcinoma. Cancer Lett. 2018, 430, 57–66. [Google Scholar] [CrossRef]
- Sun, K.; Zhao, X.; Wan, J.; Yang, L.; Chu, J.; Dong, S.; Yin, H.; Ming, L.; He, F. The diagnostic value of long non-coding RNA MIR31HG and its role in esophageal squamous cell carcinoma. Life Sci. 2018, 202, 124–130. [Google Scholar] [CrossRef]
- Zong, M.Z.; Shao, Q.; An, X.S. Expression and prognostic significance of long noncoding RNA AK001796 in esophageal squamous cell carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 181–186. [Google Scholar]
- Xie, J.J.; Jiang, Y.Y.; Jiang, Y.; Li, C.Q.; Lim, M.C.; An, O.; Mayakonda, A.; Ding, L.W.; Long, L.; Sun, C.; et al. Super-enhancer-driven long non-coding RNA LINC01503, regulated by TP63, is over-expressed and oncogenic in squamous cell carcinoma. Gastroenterology 2018, 154, 2137–2151.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.H.; You, B.H.; Park, C.H.; Kim, Y.J.; Nam, J.W.; Lee, S.K. The long noncoding RNA LUCAT1 promotes tumorigenesis by controlling ubiquitination and stability of DNA methyltransferase 1 in esophageal squamous cell carcinoma. Cancer Lett. 2018, 417, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, X.; Zhang, Z.; Pang, L.; Xu, J.; Jiang, J.; Liang, W.; Chai, Y.; Hou, J.; Li, F. Linc-ROR promotes esophageal squamous cell carcinoma progression through the derepression of SOX9. J. Exp. Clin. Cancer Res. 2017, 36, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Fan, Y.; Feng, T.; Chen, F.; Xu, Z.; Li, S.; Lin, Q.; He, X.; Shi, W.; Liu, Y.; et al. HOTAIR regulates HK2 expression by binding endogenous miR-125 and miR-143 in oesophageal squamous cell carcinoma progression. Oncotarget 2017, 8, 86410–86422. [Google Scholar] [CrossRef]
- Wu, X.; Dinglin, X.; Wang, X.; Luo, W.; Shen, Q.; Li, Y.; Gu, L.; Zhou, Q.; Zhu, H.; Li, Y.; et al. Long noncoding RNA XIST promotes malignancies of esophageal squamous cell carcinoma via regulation of miR-101/EZH2. Oncotarget 2017, 8, 76015–76028. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhen, Q.; Fan, Y. LncRNA GHET1 promotes esophageal squamous cell carcinoma cells proliferation and invasion via induction of EMT. Int. J. Biol. Markers 2017, 32, e403–e408. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wu, X.; Gu, L.; Shen, Q.; Luo, W.; Deng, C.; Zhou, Q.; Chen, X.; Li, Y.; Lim, Z.; et al. Long non-coding RNA ATB promotes malignancy of esophageal squamous cell carcinoma by regulating miR-200b/Kindlin-2 axis. Cell Death Dis. 2017, 8, e2888. [Google Scholar] [CrossRef]
- Lin, C.; Wang, Y.; Wang, Y.; Zhang, S.; Yu, L.; Guo, C.; Xu, H. Transcriptional and posttranscriptional regulation of HOXA13 by lncRNA HOTTIP facilitates tumorigenesis and metastasis in esophageal squamous carcinoma cells. Oncogene 2017, 36, 5392–5406. [Google Scholar] [CrossRef]
- Li, P.D.; Hu, J.L.; Ma, C.; Ma, H.; Yao, J.; Chen, L.L.; Chen, J.; Cheng, T.T.; Yang, K.Y.; Wu, G.; et al. Upregulation of the long non-coding RNA PVT1 promotes esophageal squamous cell carcinoma progression by acting as a molecular sponge of miR-203 and LASP1. Oncotarget 2017, 8, 34164–34176. [Google Scholar] [CrossRef]
- Yan, Y.; Li, S.; Wang, S.; Rubegni, P.; Tognetti, L.; Zhang, J.; Yan, L. Long noncoding RNA HAND2-AS1 inhibits cancer cell proliferation, migration, and invasion in esophagus squamous cell carcinoma by regulating microRNA-21. J. Cell. Biochem. 2019, 120, 9564–9571. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, S.L.; Qiao, C.H.; Ye, J.F.; Li, M.; Ma, H.M.; Wang, J.H.; Xin, S.Y.; Yuan, Z.L. LncRNA-NEF inhibits proliferation, migration and invasion of esophageal squamous-cell carcinoma cells by inactivating wnt/beta-catenin pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6824–6831. [Google Scholar] [PubMed]
- Wang, G.; Sun, J.; Zhao, H.; Li, H. Long non-coding RNA (lncRNA) growth arrest specific 5 (GAS5) suppresses esophageal squamous cell carcinoma cell proliferation and migration by inactivating phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian Target of Rapamycin (mTOR) signaling pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 7689–7696. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhang, C.Q.; Li, H.L.; Gu, J.; Miao, G.Y.; Cai, H.Y.; Wang, J.K.; Zhang, L.J.; Song, Y.M.; Tian, Y.H.; et al. LncRNA FER1L4 suppressed cancer cell growth and invasion in esophageal squamous cell carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2638–2645. [Google Scholar] [PubMed]
- Rong, J.; Wang, Q.; Zhang, Y.; Zhu, D.; Sun, H.; Tang, W.; Wang, R.; Shi, W.; Cao, X.F. Circ-DLG1 promotes the proliferation of esophageal squamous cell carcinoma. Oncotargets Ther. 2018, 11, 6723–6730. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Wei, L.; Qin, T.; Yang, N.; Li, Z.; Xu, Z. Circular RNA ciRS-7 triggers the migration and invasion of esophageal squamous cell carcinoma via miR-7/KLF4 and NF-kappaB signals. Cancer Biol. Ther. 2019, 20, 73–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, W.; Qiu, M.; Chen, R.; Wang, S.; Leng, X.; Wang, J.; Xu, Y.; Hu, J.; Dong, G.; Xu, P.L.; et al. Circular RNA has_circ_0067934 is upregulated in esophageal squamous cell carcinoma and promoted proliferation. Sci. Rep. 2016, 6, 35576. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Li, R.; Xu, S.; Li, Y.; Zhao, P.; Dong, W.; Liu, Z.; Zhao, Q.; Tan, B. Tumor suppressor miR-128-3p inhibits metastasis and epithelial-mesenchymal transition by targeting ZEB1 in esophageal squamous-cell cancer. Acta Biochim. Et Biophys. Sin. 2018, 50, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.; Zhao, Y.; Lu, Q.; Lu, Y.; Liu, Z.; Xue, T.; Shao, Y. MicroRNA-30c functions as a tumor suppressor via targeting SNAI1 in esophageal squamous cell carcinoma. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 98, 680–686. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.O.; Jin, Q.; Shang, L.; Zhang, J. Function of miR-25 in the invasion and metastasis of esophageal squamous carcinoma cells and bioinformatical analysis of the miR-106b-25 cluster. Exp. Ther. Med. 2018, 15, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Zuo, J.; Zhu, K.; Wang, Y.; Yu, Z. MicroRNA-34a suppresses invasion and metastatic in esophageal squamous cell carcinoma by regulating CD44. Mol. Cell. Biochem. 2018, 443, 139–149. [Google Scholar] [CrossRef]
- Gao, Y.; Yi, J.; Zhang, K.; Bai, F.; Feng, B.; Wang, R.; Chu, X.; Chen, L.; Song, H. Downregulation of MiR-31 stimulates expression of LATS2 via the hippo pathway and promotes epithelial-mesenchymal transition in esophageal squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2017, 36, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Xu, W.W.; Han, L.; Chan, K.T.; Tsao, S.W.; Lee, N.P.Y.; Law, S.; Xu, L.Y.; Li, E.M.; Chan, K.W.; et al. MicroRNA-377 suppresses initiation and progression of esophageal cancer by inhibiting CD133 and VEGF. Oncogene 2017, 36, 3986–4000. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhan, Y.; Xu, Z.; Li, Y.; Luo, A.; Ding, F.; Cao, X.; Chen, H.; Liu, Z. ZEB1 induced miR-99b/let-7e/miR-125a cluster promotes invasion and metastasis in esophageal squamous cell carcinoma. Cancer Lett. 2017, 398, 37–45. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, W.; Pan, T.; Wang, H.; Zhang, Y.; Li, C. LBX2-AS1 is activated by ZEB1 and promotes the development of esophageal squamous cell carcinoma by interacting with HNRNPC to enhance the stability of ZEB1 and ZEB2 mRNAs. Biochem. Biophys. Res. Commun. 2019, 511, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Shen, X.; Li, H.; Xu, J.; Shao, S.; Huang, J.X.; Lin, M. LncRNA-ECM is overexpressed in esophageal squamous cell carcinoma and promotes tumor metastasis. Oncol. Lett. 2018, 16, 3935–3942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Sun, K.; Chu, J.; Qu, Y.; Zhao, X.; Yin, H.; Ming, L.; Wan, J.; He, F. Long non-coding RNA FTH1P3 regulated metastasis and invasion of esophageal squamous cell carcinoma through SP1/NF-kB pathway. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 106, 1570–1577. [Google Scholar] [CrossRef]
- Niu, G.; Zhuang, H.; Li, B.; Cao, G. Long noncoding RNA linc-UBC1 promotes tumor invasion and metastasis by regulating EZH2 and repressing E-cadherin in esophageal squamous cell carcinoma. J. B.U.ON. Off. J. Balk. Union Oncol. 2018, 23, 157–162. [Google Scholar]
- Gao, G.D.; Liu, X.Y.; Lin, Y.; Liu, H.F.; Zhang, G.J. LncRNA CASC9 promotes tumorigenesis by affecting EMT and predicts poor prognosis in esophageal squamous cell cancer. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 422–429. [Google Scholar]
- Shang, M.; Wang, X.; Zhang, Y.; Gao, Z.; Wang, T.; Liu, R. LincRNA-ROR promotes metastasis and invasion of esophageal squamous cell carcinoma by regulating miR-145/FSCN1. Oncotargets Ther. 2018, 11, 639–649. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Zhao, W.; Gao, X.; Zhang, D.; Li, Y.; Zhang, Y.; Li, W. HNF1AAS1 promotes growth and metastasis of esophageal squamous cell carcinoma by sponging miR214 to upregulate the expression of SOX-4. Int. J. Oncol. 2017, 51, 657–667. [Google Scholar] [CrossRef]
- Chen, X.; Han, H.; Li, Y.; Zhang, Q.; Mo, K.; Chen, S. Upregulation of long noncoding RNA HOTTIP promotes metastasis of esophageal squamous cell carcinoma via induction of EMT. Oncotarget 2016, 7, 84480–84485. [Google Scholar] [CrossRef] [Green Version]
- Yao, G.L.; Pan, C.F.; Xu, H.; Wei, K.; Liu, B.; Zhai, R.; Chen, Y.J. Long noncoding RNA RP11-766N7.4 functions as a tumor suppressor by regulating epithelial-mesenchymal transition in esophageal squamous cell carcinoma. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 88, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Hong, P.; Zheng, C.C.; Dai, W.; Chen, W.Y.; Yang, Q.S.; Han, L.; Tsao, S.W.; Chan, K.T.; Lee, N.P.Y.; et al. Identification of miR-29c and its Target FBXO31 as a Key Regulatory Mechanism in Esophageal Cancer Chemoresistance: Functional Validation and Clinical Significance. Theranostics 2019, 9, 1599–1613. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ma, K.; Yang, S.; Zhang, X.; Wang, F.; Zhang, X.; Liu, H.; Fan, Q. MicroRNA-125a-5p enhances the sensitivity of esophageal squamous cell carcinoma cells to cisplatin by suppressing the activation of the STAT3 signaling pathway. Int. J. Oncol. 2018, 53, 644–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Q.; Liu, Y.; Wen, C.; Zhao, Y.; Jin, S.; Hu, Y.; Wang, F.; Chen, L.; Zhang, B.; Wang, W.; et al. MicroRNA-1 inhibits tumorigenicity of esophageal squamous cell carcinoma and enhances sensitivity to gefitinib. Oncol. Lett. 2018, 15, 963–971. [Google Scholar] [CrossRef]
- Zheng, R.; Liu, Y.; Zhang, X.; Zhao, P.; Deng, Q. miRNA-200c enhances radiosensitivity of esophageal cancer by cell cycle arrest and targeting P21. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 90, 517–523. [Google Scholar] [CrossRef]
- Chang, Z.W.; Jia, Y.X.; Zhang, W.J.; Song, L.J.; Gao, M.; Li, M.J.; Zhao, R.H.; Li, J.; Zhong, Y.L.; Sun, Q.Z.; et al. LncRNA-TUSC7/miR-224 affected chemotherapy resistance of esophageal squamous cell carcinoma by competitively regulating DESC1. J. Exp. Clin. Cancer Res. 2018, 37, 56. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Liu, L.; Li, J.; Du, Y.; Wang, J.; Liu, J. Effects of long noncoding RNA (linc-VLDLR) existing in extracellular vesicles on the occurrence and multidrug resistance of esophageal cancer cells. Pathol. Res. Pract. 2019, 215, 470–477. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, J.; Pan, S.; Yang, T.; Sun, X.; Wang, Y.; Shi, X.; Zhao, X.; Guo, J.; Zhang, X. LINC00657 played oncogenic roles in esophageal squamous cell carcinoma by targeting miR-615-3p and JunB. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 108, 316–324. [Google Scholar] [CrossRef]
- Kang, M.; Ren, M.; Li, Y.; Fu, Y.; Deng, M.; Li, C. Exosome-mediated transfer of lncRNA PART1 induces gefitinib resistance in esophageal squamous cell carcinoma via functioning as a competing endogenous RNA. J. Exp. Clin. Cancer Res. 2018, 37, 171. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Tu, B.; Bu, Y.; Liu, A.; Kong, J. Long noncoding RNA MALAT1 affects the efficacy of radiotherapy for esophageal squamous cell carcinoma by regulating Cks1 expression. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2017, 46, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.; Su, H.X.; Zhao, D.; Guan, Q.L. Plasma miRNA-506 as a Prognostic Biomarker for Esophageal Squamous Cell Carcinoma. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 2195–2201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, W.; Luo, W.; Fang, W.; Wang, Y.; Wang, L.; Shen, Q.; Liu, W.; Zhang, H. miR-145 expression level in tissue predicts prognosis of patients with esophageal squamous cell carcinoma. Pathol. Res. Pract. 2019, 215, 152401. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.S.; Wu, D.H. Identification of miR-375 as a potential prognostic biomarker for esophageal squamous cell cancer: A bioinformatics analysis based on TCGA and meta-analysis. Pathol. Res. Pract. 2019, 215, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Kiuchi, J.; Komatsu, S.; Imamura, T.; Nishibeppu, K.; Shoda, K.; Arita, T.; Kosuga, T.; Konishi, H.; Shiozaki, A.; Okamoto, K.; et al. Low levels of tumour suppressor miR-655 in plasma contribute to lymphatic progression and poor outcomes in oesophageal squamous cell carcinoma. Mol. Cancer 2019, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Liang, X.; Wu, X.; Kang, X.; Guo, Y.; Shen, S.; Liang, J.; Guo, W. Promoter hypermethylation-mediated downregulation of tumor suppressor gene SEMA3B and lncRNA SEMA3B-AS1 correlates with progression and prognosis of esophageal squamous cell carcinoma. Clin. Exp. Metastasis 2019, 36, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Sadeghpour, S.; Ghorbian, S. Evaluation of the potential clinical prognostic value of lncRNA-BANCR gene in esophageal squamous cell carcinoma. Mol. Biol. Rep. 2019, 46, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Huang, Y.H.; Li, H.P.; Guo, S.M. Expression of UCA1 and MALAT1 long-chain non-coding RNAs in esophageal squamous cell carcinoma tissues is predictive of patient prognosis. Arch. Med. Sci. AMS 2018, 14, 752–759. [Google Scholar] [CrossRef]
- Bao, J.; Zhou, C.; Zhang, J.; Mo, J.; Ye, Q.; He, J.; Diao, J. Upregulation of the long noncoding RNA FOXD2-AS1 predicts poor prognosis in esophageal squamous cell carcinoma. Cancer Biomark. Sect. A Dis. Markers 2018, 21, 527–533. [Google Scholar] [CrossRef]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef]
- Cao, J.; Lv, W.; Wang, L.; Xu, J.; Yuan, P.; Huang, S.; He, Z.; Hu, J. Ricolinostat (ACY-1215) suppresses proliferation and promotes apoptosis in esophageal squamous cell carcinoma via miR-30d/PI3K/AKT/mTOR and ERK pathways. Cell Death Dis. 2018, 9, 817. [Google Scholar] [CrossRef] [Green Version]
- Peng, W.X.; Koirala, P.; Mo, Y.Y. LncRNA-mediated regulation of cell signaling in cancer. Oncogene 2017, 36, 5661–5667. [Google Scholar] [CrossRef]
- Li, Y.; Chen, D.; Gao, X.; Li, X.; Shi, G. LncRNA NEAT1 Regulates Cell Viability and Invasion in Esophageal Squamous Cell Carcinoma through the miR-129/CTBP2 Axis. Dis. Markers 2017, 2017, 5314649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, C.; Xu, D.; You, Z.; Xu, K.; Tian, W. Dysregulated circRNAs and ceRNA network in esophageal squamous cell carcinoma. Front. Biosci. 2019, 24, 277–290. [Google Scholar]
- Diepenbruck, M.; Christofori, G. Epithelial-mesenchymal transition (EMT) and metastasis: Yes, no, maybe? Curr. Opin. Cell Biol. 2016, 43, 7–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, W.; Kang, Y. Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Dev. Cell 2019, 49, 361–374. [Google Scholar] [CrossRef]
- Hiroyuki, K.; Masanobu, N. Treatments for esophageal cancer: A review. Gen. Thorac. Cardiovasc. Surg. 2013, 61, 330–335. [Google Scholar]
- Haenisch, S.; Cascorbi, I. miRNAs as mediators of drug resistance. Epigenomics 2012, 4, 369–381. [Google Scholar] [CrossRef]
- Chen, Q.N.; Wei, C.C.; Wang, Z.X.; Sun, M. Long non-coding RNAs in anti-cancer drug resistance. Oncotarget 2017, 8, 1925–1936. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wei, C.; Li, P.; Wang, L.; Li, W.; Chen, K.; Zhang, J.; Zhang, W.; Jiang, G. Integrative analysis of mRNA and lncRNA profiles identified pathogenetic lncRNAs in esophageal squamous cell carcinoma. Gene 2018, 661, 169–175. [Google Scholar] [CrossRef]
- Rao, D.D.; Vorhies, J.S.; Senzer, N.; Nemunaitis, J. siRNA vs. shRNA: Similarities and differences. Adv. Drug Deliv. Rev. 2009, 61, 746–759. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hu, L.; Liang, Y.; Li, J.; Wang, K.; Chen, X.; Meng, H.; Guan, X.; Yang, K.; Bai, Y. Up-regulation of lncRNA CASC9 promotes esophageal squamous cell carcinoma growth by negatively regulating PDCD4 expression through EZH2. Mol. Cancer 2017, 16, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Chen, X.; Wu, Y.; Li, J.; Zhang, S.; Wang, K.; Guan, X.; Yang, K.; Bai, Y. LncRNA CASC9 promotes esophageal squamous cell carcinoma metastasis through upregulating LAMC2 expression by interacting with the CREB-binding protein. Cell Death Differ. 2018, 25, 1980–1995. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadou, E.; Jacob, L.S.; Slack, F.J. Non-coding RNA networks in cancer. Nat. Rev. Cancer 2018, 18, 5–18. [Google Scholar] [CrossRef] [PubMed]
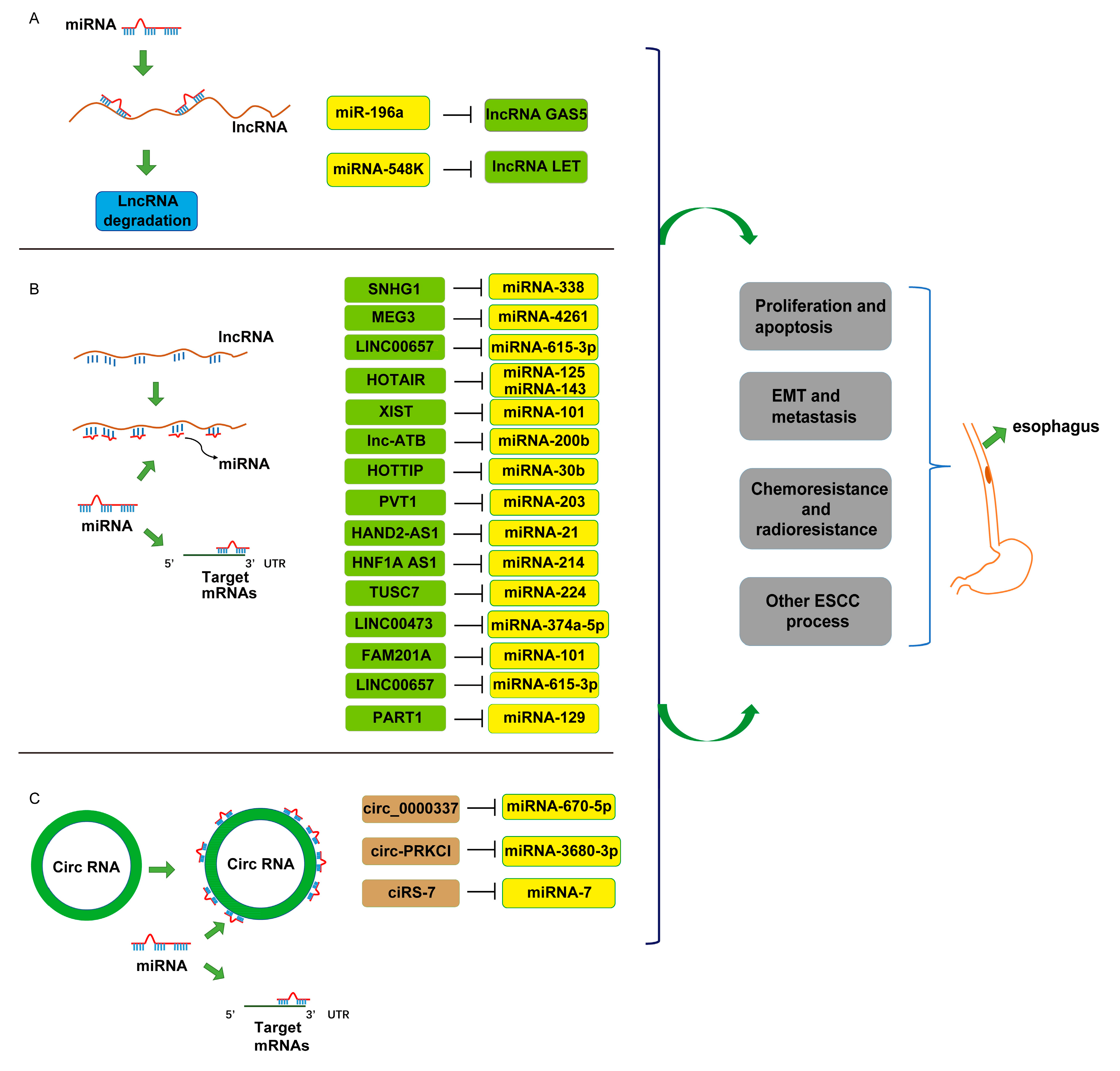
| Role of ncRNAs in ESCC | ncRNAs | Identified TARGETS or Signaling Pathways | Role | Reference |
|---|---|---|---|---|
| Cell proliferation and apoptosis | miRNA: | |||
| miRNA-146a | IRS2 | − | [29] | |
| miRNA-133b | TAGLN2 | − | [39] | |
| miRNA-106b-3p | ZNRF3 | − | [33] | |
| miRNA−219-5p | CCNA2 | − | [40] | |
| miRNA-206 | c-MET | − | [41] | |
| miRNA-384 | LIMK1 | − | [42] | |
| miRNA-455-5p | Rab31 | − | [43] | |
| miRNA-128 | COX2 | − | [44] | |
| miRNA 145 3p, 5p | DHRS2 and MYO1B, Sp1 | − | [45,46] | |
| miRNA-10b-3p | FOXO3 | − | [47] | |
| miRNA-874-3p | STAT3 | − | [48] | |
| miRNA-10a | Tiam1 | − | [49] | |
| miRNA-365 | PSAT1 | − | [50] | |
| miRNA-301a | WNT1 | − | [51] | |
| miRNA-6775-3p | MAGE-A and SLC7A5 | − | [52] | |
| miRNA-139-5p | VEGFR | − | [53] | |
| miRNA-516b | CCNG1 | − | [54] | |
| miRNA-449a-5p | Cyclin D1 | − | [55] | |
| miRNA-125b | BMF | − | [56] | |
| miRNA-433-3p | GRB2 | − | [57] | |
| miRNA-370 | PIN1 | − | [58] | |
| miRNA-133b | Cullin 4B | − | [35] | |
| miRNA-1 | Notch2 | − | [59] | |
| miRNA-30a-3p, 5p | Wnt2, FZD2 | − | [60] | |
| miRNA-34a | PLCE1 | − | [61] | |
| miRNA-196a | lncRNA GAS5 | − | [62] | |
| miRNA-125b-5p | HMGA2 | − | [63] | |
| miRNA-141 | YAP1 and SOX17 | + | [64] | |
| miRNA-21 | RASA1 | + | [65] | |
| miRNA-424 | PRKCD and WEE1 | + | [66] | |
| miRNA-675-3p | NA | + | [67] | |
| miRNA-543 | PLA2G4A | + | [68] | |
| miRNA-135 | RERG | + | [69] | |
| miRNA-23b-3p | EBF3 | + | [70] | |
| miRNA-502 | NA | + | [71] | |
| miRNA-21-5p | CADM2 | + | [72] | |
| miRNA-548k | lncRNA-LET | + | [73] | |
| LncRNAs: | ||||
| LncRNA SNHG1 | miRNA-338/CST3,Notch signaling | + | [74,75] | |
| LncRNA SNHG6 | NA | + | [76] | |
| LncRNA SNHG16 | Wnt/β-catenin,miRNA-140-5p/ZEB | + | [77,78] | |
| LncRNA MEG3 | miRNA-4261 | + | [79] | |
| LINC01980 | GADD45A | + | [80] | |
| FMR1-AS1 (female patients) | TLR7 | + | [81] | |
| DLX6AS1 | NA | + | [82] | |
| LINC00657 | miRNA-615-3p | + | [83] | |
| DUXAP10 | p21 | + | [84] | |
| LINC01296 | NA | + | [85] | |
| LncRNA DANCR | NA | + | [86] | |
| LncRNA SOX2OT | NA | + | [87] | |
| LncRNA NMR | BPTF/ ERK1/2 pathway | + | [88] | |
| MIR31HG | NA | + | [89] | |
| AK001796 | p53 | + | [90] | |
| LINC01503 | EBP1 and DUSP6 | + | [91] | |
| LUCAT1 | DNMT1 | + | [92] | |
| Linc ROR | SOX9 | + | [93] | |
| HOTAIR | miRNA-125 and miRNA-143 | + | [94] | |
| XIST | miRNA-101/EZH2 | + | [95] | |
| LncRNA GHET1 | EMT | + | [96] | |
| Lnc-ATB | miRNA-200b/Kindlin-2 | + | [97] | |
| HOTTIP | miRNA-30b/snail1, HOXA13 | + | [98] | |
| PVT1 | miRNA-203/LASP1 | + | [99] | |
| AFAP1-AS1 | NA | + | [37] | |
| LINC00675 | Wnt/β-catenin | − | [83] | |
| HAND2-AS1 | miRNA-21 | − | [100] | |
| LncRNA NEF | Wnt/β-catenin pathway | − | [101] | |
| LncRNA GAS5 | PI3K/AKT/mTOR | − | [102] | |
| FER1L4 | NA | − | [103] | |
| CircRNAs: | ||||
| Circ_0000337 | miRNA-670-5p | + | [18] | |
| Circ-PRKCI | miRNA-3680-3p | + | [27] | |
| CircRNA_100876 | NA | + | [28] | |
| Circ-DLG1 | miRNAs | + | [104] | |
| CiRS-7 | miRNA-7/KLF4 and NF-κB signals | + | [105] | |
| Circ_0067934 | NA | + | [106] | |
| Cell EMT and metastasis | miRNA: | |||
| miRNA-106b-3p | ZNRF3 | − | [33] | |
| miRNA-455-5p | Rab31 | − | [43] | |
| miRNA-128 | COX 2 | − | [44] | |
| miR-128-3p | ZEB1 | − | [107] | |
| miRNA-10a | Tiam1 | − | [49] | |
| miRNA-6775-3p | MAGE-A and SLC7A5 | − | [52] | |
| miRNA-139-5p | VEGFR | − | [53] | |
| miRNA-30c | SNAI1 | − | [108] | |
| miRNA-25 | NA | − | [109] | |
| miRNA-34a | PLCE1, CD44 | − | [61,110] | |
| miRNA-31 | LATS2 | − | [111] | |
| miRNA-145-5p | Sp1 | − | [45] | |
| miRNA-377 | CD133 and VEGF | − | [112] | |
| miRNA-543 | PLA2G4A | + | [68] | |
| miRNA-23b-3p | EBF3 | + | [70] | |
| miRNA-25 | FBXW7 | + | [109] | |
| miR-99b/let-7e/miR-125a | ARID3A | + | [113] | |
| LncRNA: | ||||
| LBX2-AS1 | ZEB1 and ZEB2 | + | [114] | |
| LncRNA-ECM | ICAMI | + | [115] | |
| FTH1P3 | SP1/NF-kB | + | [116] | |
| NMR | BPTF/ ERK1/2 pathway | + | [88] | |
| Linc-UBC1 | EZH2 and E-cadherin | + | [117] | |
| CASC9 | CBP and LAMC2 | + | [118] | |
| LincRNA-ROR | miR-145/FSCN1 | + | [119] | |
| SNHG16 | miR-140-5p/ZEB1 | + | [77] | |
| LncRNA SNHG1 | Notch signaling | + | [74] | |
| LncRNA GHET1 | EMT | + | [96] | |
| HNF1A-AS1 | miR 214/SOX-4 | + | [120] | |
| Lnc-ATB | miR-200b/Kindlin-2 | + | [97] | |
| HOTTIP | WDR5 and HOXA13 | + | [98,121] | |
| LINC00675 | Wnt/β-catenin | − | [83] | |
| RP11-766N7.4 | NA | − | [122] | |
| Chemosensitivity and radiosensitivity | miRNA: | − | ||
| miRNA-29c (5-fluorouracil) | F-box only protein 31 | − | [123] | |
| miR-130a-3p and miR-148a-3 (Cisplatin and 5-FU) | Bcl-2/Bim and Bcl2/XIAP | − | [34] | |
| miRNA-125a-5p (cisplatin) | STAT3 signaling | − | [124] | |
| miRNA-1 (gefitinib) | PIK3CA signaling | − | [125] | |
| miRNA-338-5p (radiosensitivity) | Apoptosis signaling | − | [36] | |
| miRNA-200c (radiosensitivity) | Cell cycle arrest and p21 | − | [126] | |
| LncRNA TUSC7 (cisplatin or 5-Fu) | miRNA-224/DESC1 | − | [127] | |
| LINC01419 (5-fluorouracil) | GSTP1 methylation | + | [5] | |
| LINC00473 (radiotherapy) | miRNA-374a-5p | + | [38] | |
| Linc-VLDLR in extracellular vesicles (adriamycin) | NA | + | [128] | |
| LnRNA FAM201A (radiotherapy) | miRNA-101/ATM and mTOR | + | [20] | |
| LINC00657 (radiotherapy) | miRNA-615-3p and JunB | + | [129] | |
| LncRNA PART1 (gefitinib) | miRNA-129/Bcl-2 pathway | + | [130] | |
| MALAT1 (radiotherapy) | Cks1 | + | [131] | |
| Prognostic biomarkers | miRNA: | |||
| miRNA-506, miRNA-145, miRNA-375, miRNA-655, miRNA-874-3p, miRNA-9 | [48,132,133,134,135] | |||
| LncRNA: | ||||
| MEG3, SEMA3B-AS1, SNHG6, AK001796, ANRIL, BANCR, UCA1 and MALAT1, MIR31HG, FOXD2-AS1, LINC01296 | [76,79,85,89,90,136,137,138,139] | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Q.; Zhang, H.; Yao, D.; Chen, W.-D.; Wang, Y.-D. Emerging Role of Non-Coding RNAs in Esophageal Squamous Cell Carcinoma. Int. J. Mol. Sci. 2020, 21, 258. https://doi.org/10.3390/ijms21010258
Feng Q, Zhang H, Yao D, Chen W-D, Wang Y-D. Emerging Role of Non-Coding RNAs in Esophageal Squamous Cell Carcinoma. International Journal of Molecular Sciences. 2020; 21(1):258. https://doi.org/10.3390/ijms21010258
Chicago/Turabian StyleFeng, Qingqing, Hongli Zhang, Denglin Yao, Wei-Dong Chen, and Yan-Dong Wang. 2020. "Emerging Role of Non-Coding RNAs in Esophageal Squamous Cell Carcinoma" International Journal of Molecular Sciences 21, no. 1: 258. https://doi.org/10.3390/ijms21010258
APA StyleFeng, Q., Zhang, H., Yao, D., Chen, W.-D., & Wang, Y.-D. (2020). Emerging Role of Non-Coding RNAs in Esophageal Squamous Cell Carcinoma. International Journal of Molecular Sciences, 21(1), 258. https://doi.org/10.3390/ijms21010258




