Missing Link Between Molecular Aspects of Ventricular Arrhythmias and QRS Complex Morphology in Left Ventricular Hypertrophy
Abstract
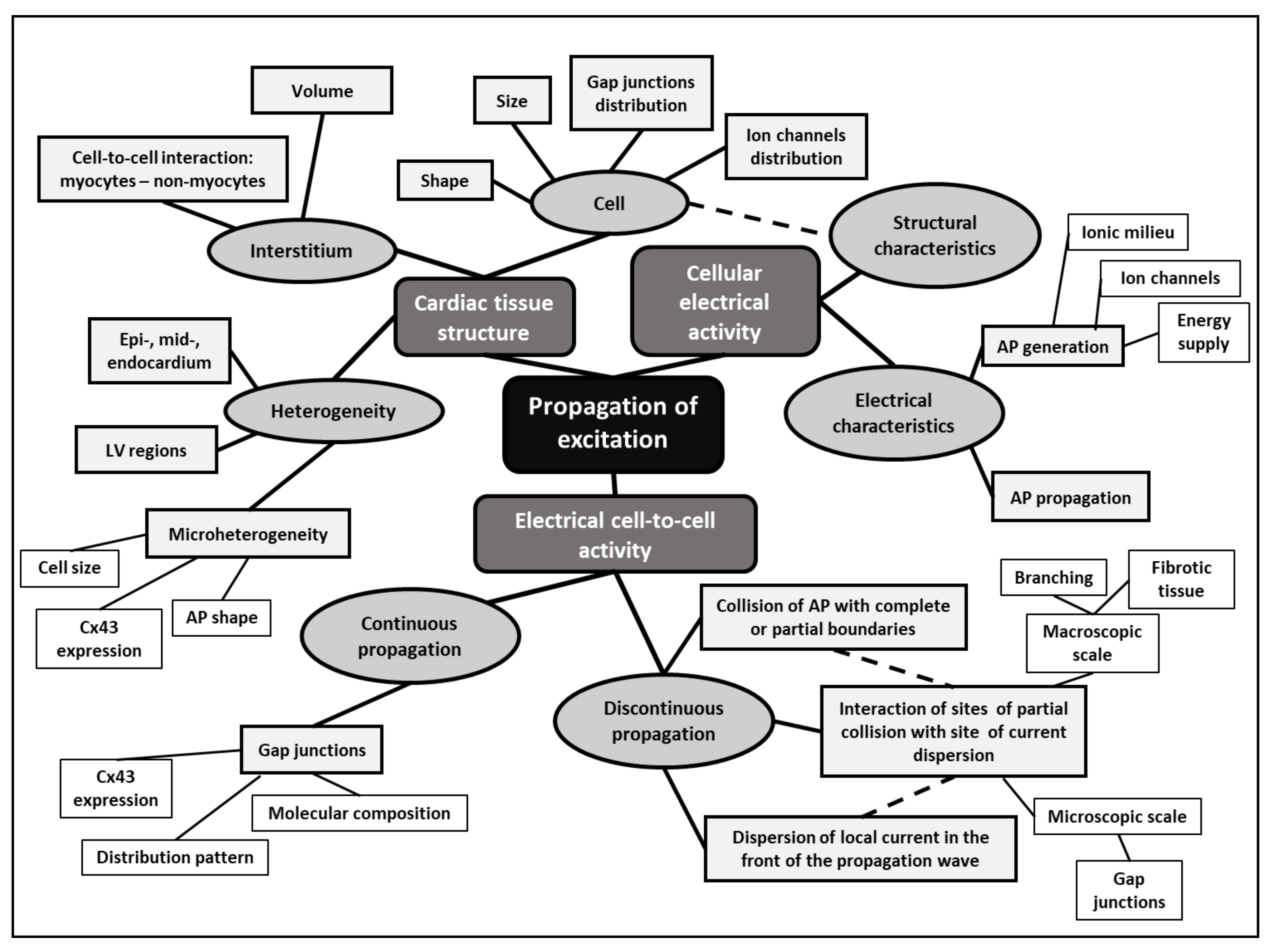
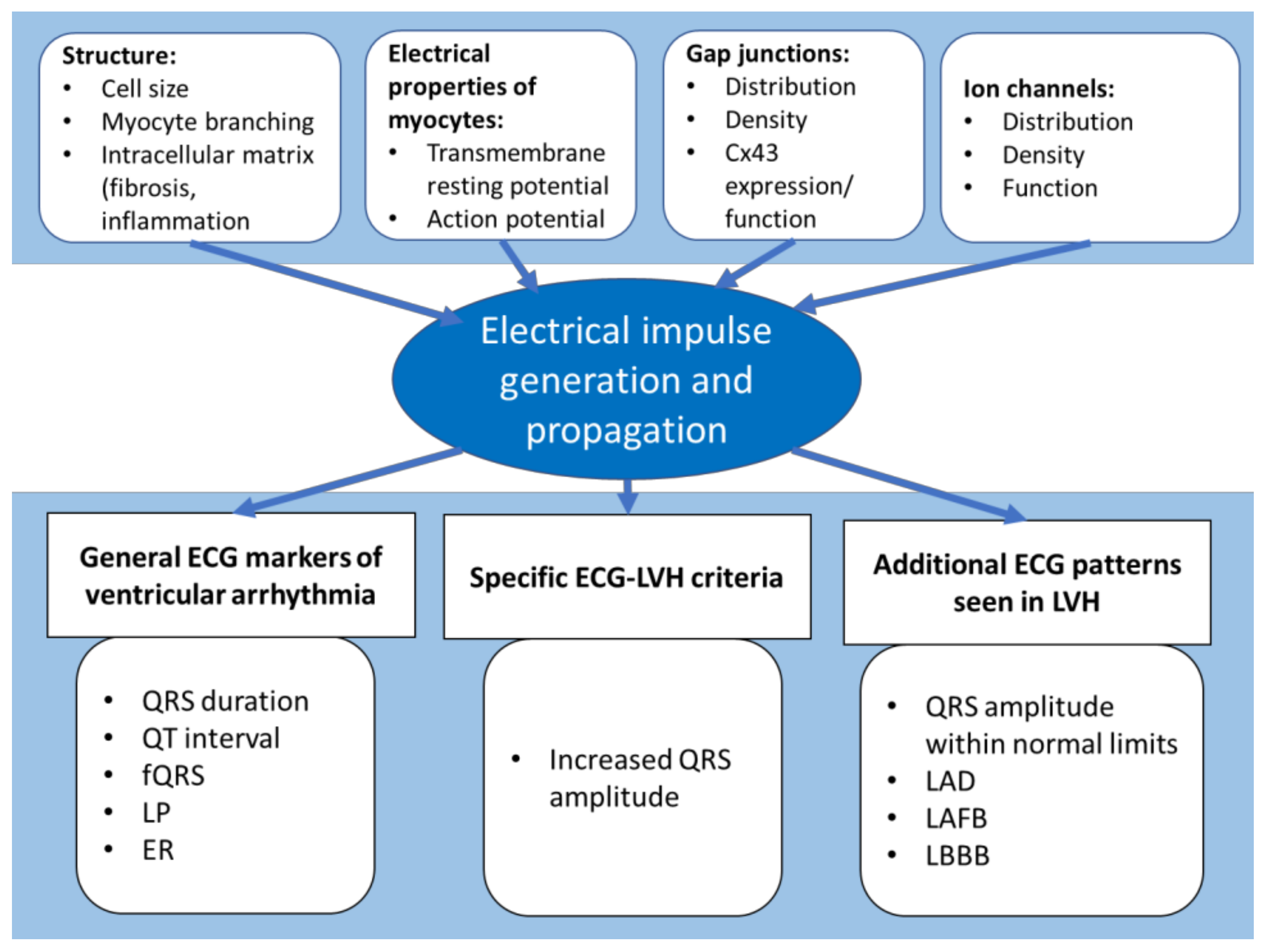
:1. Introduction
2. Left Ventricular Hypertrophy
3. Non-Specific ECG Predictors of Ventricular Arrhythmia in LVH
3.1. QRS Complex Duration
3.2. QT Interval
3.3. Early Repolarization
3.4. Late Ventricular Potentials/Signal Averaged ECG
3.5. Fragmented QRS Complex
3.6. Body Surface Potential Mapping
4. Waveform Interpretation in LVH
4.1. ECG Criteria on LVH
4.2. ECG Findings in LVH Patients
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Calkins, H.; Corrado, D.; Marcus, F. Risk stratification in arrhythmogenic right ventricular cardiomyopathy. Circulation 2017, 136, 2068–2082. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm 2018, 15, e73–e189. [Google Scholar] [PubMed] [Green Version]
- Epstein, A.E.; DiMarco, J.P.; Ellenbogen, K.A.; Estes, N.A., III; Freedman, R.A.; Gettes, L.S.; Gillinov, A.M.; Gregoratos, G.; Hammill, S.C.; Hayes, D.L.; et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2013, 61, e6–75. [Google Scholar] [PubMed]
- Tang, A.S.; Ross, H.; Simpson, C.S.; Mitchell, L.B.; Dorian, P.; Goeree, R.; Hoffmaster, B.; Arnold, M.; Talajic, M. Canadian Heart Rhythm S, Canadian Cardiovascular, S. Canadian Cardiovascular Society/Canadian Heart Rhythm Society position paper on implantable cardioverter defibrillator use in Canada. Can. J. Cardiol. 2005, 21 (Suppl. SA), 11A–18A. [Google Scholar]
- Goldenberg, I.; Vyas, A.K.; Hall, W.J.; Moss, A.J.; Wang, H.; He, H.; Zareba, W.; McNitt, S.; Andrews, M.L.; Madit-II Investigators. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J. Am. Coll. Cardiol. 2008, 51, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.; Bavishi, C.; Sardar, P.; Agarwal, V.; Krishnamoorthy, P.; Grodzicki, T.; Messerli, F.H. Meta-analysis of left ventricular hypertrophy and sustained arrythmias. Am. J. Cardiol. 2014, 114, 1049–1052. [Google Scholar] [CrossRef]
- Levy, D.; Anderson, K.M.; Savage, D.D.; Balkus, A.; Kannel, W.B.; Castelli, W.P. Risk of ventricular arrhythmias in left ventricular hypertrophy: The Framingham Heart Study. Am. J. Cardiol. 1987, 60, 560–565. [Google Scholar] [CrossRef]
- Shenasa, M.; Shenasa, H.; El-Sherif, N. Left ventricular hypertrophy and arrhythmogenesis. Card Electrophysiol. Clin. 2015, 7, 207–220. [Google Scholar] [CrossRef]
- Franchi, F.; Lazzeri, C.; La Villa, G.; Barletta, G.; Del Bene, R.; Buzzelli, G. Cardiac autonomic modulation and incidence of late potentials in essential hypertension: Role of age, sex, ventricular mass and remodeling. J. Hum. Hypertens. 1998, 12, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Sorgato, A.; Faggiano, P.; Aurigemma, G.P.; Rusconi, C.; Gaasch, W.H. Ventricular arrhythmias in adult aortic stenosis: Prevalence, mechanisms, and clinical relevance. Chest 1998, 113, 482–491. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, R.R.; Driscoll, D.J.; Gersony, W.M.; Hayes, C.J.; Keane, J.F.; Kidd, L.; O’Fallon, W.M.; Pieroni, D.R.; Weidman, W.H. Arrhythmias in patients with valvar aortic stenosis, valvar pulmonary stenosis, and ventricular septal defect. Results of 24-h ECG monitoring. Circulation 1993, 87 (Suppl. S2), I89–101. [Google Scholar] [PubMed]
- Tempio, D.; Pruiti, G.P.; Conti, S.; Romano, S.A.; Tavano, E.; Capodanno, D.; Liotta, C.; Di Grazia, A.; Tamburino, C.; Calvi, V. Ventricular arrhythmias in aortic valve stenosis before and after transcatheter aortic valve implantation. Europace 2015, 17, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Kashii, C.; Imamura, K. Ultrastructural changes in hypertrophied myocardium of spontaneously hypertensive rats. Jpn Cicr. J. 1976, 40, 1119–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCrossan, Z.A.; Billeter, R.; White, E. Transmural changes in size, contractile and electrical properties of SHR left ventricular myocytes during compensated hypertrophy. Cardiovasc. Res. 2004, 63, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Main, M.C.; Bryant, S.M.; Hart, G. Regional differences in action potential characteristics and membrane currents of guinea-pig left ventricular myocytes. Exp. Physiol. 1998, 83, 747–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutstein, D.E.; Morley, G.E.; Tammadon, H.; Vaidaya, D.; Schneider, M.D.; Chen, J.; Chien, K.R.; Stuhlman, H.; Fishman, G.I. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ. Res. 2001, 88, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Van Rijen, H.V.M.; Eckardt, D.; Degen, J.; Theis, M.; Ott, T.; Willecke, K.; Jongsma, H.J.; Opthof, T.; de Bakker, J.M. Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation 2004, 109, 1048–1055. [Google Scholar] [CrossRef] [Green Version]
- Sawada, K.; Kawamura, K. Architecture of myocardial cells in human cardiac ventricles with concentric and eccentric hypertrophy as demonstrated by quantitative scanning electron microscopy. Heart Vessel. 1991, 6, 129–142. [Google Scholar] [CrossRef]
- Yamamoto, S.; James, T.N.; Sawada, K.; Okabe, M.; Kawamura, K. Generation of new intercellular junctions between cardiocytes. A possible mechanism compensating for mechanical overload in the hypertrophied human adult myocardium. Circ. Res. 1996, 78, 362–370. [Google Scholar] [CrossRef]
- Okabe, M.; Kawamura, K.; Terasaki, F.; Hayashi, T. Remodeling of cardiomyocytes and their branches in juvenile, adult, and senescent spontaneously hypertensive rats and Wistar Kyoto rats: Comparative morphometric analyses by scanning electron microscopy. Heart Vessel. 1999, 14, 15–28. [Google Scholar] [CrossRef]
- Rithalia, A.; Hopkins, P.M.; Harrison, S.M. Effects of halothane on action potential configuration in subendocardial and sub-epicardial myocytes from normotensive and hypertensive rat left ventricle. Br. J. Anaesth. 2001, 90, 501–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spach, M.S.; Heidlage, J.F.; Dolber, P.C.; Barr, R.C. Electrophysiological effects of remodeling cardiac gap junctions and cell size. Experimental and model studies of normal cardiac growth. Circ. Res. 2000, 86, 302–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joyner, R.W. Effects of the discrete pattern of electrical coupling on propagation through an electrical syncytium. Circ. Res. 1982, 50, 192–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucera, J.P.; Rudy, Y. Mechanistic insight into very slow conduction in branching cardiac tissue. A model study. Circ. Res. 2001, 89, 799–806. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, M.; Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018, 15, 387–407. [Google Scholar] [CrossRef]
- Dweck, M.R.; Joshi, S.; Murigu, T.; Alpendurada, F.; Jabbour, A.; Melina, G.; Banya, W.; Gulati, A.; Roussin, I.; Raza, S.; et al. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J. Am. Coll. Cardiol. 2011, 58, 1271–1279. [Google Scholar] [CrossRef] [Green Version]
- Leyva, F.; Taylor, R.J.; Foley, P.W.; Umar, F.; Mulligan, L.J.; Patel, K.; Stegemann, B.; Haddad, T.; Smith, R.E.; Prasad, S.K. Left ventricular midwall fibrosis as a predictor of mortality and morbidity after cardiac resynchronization therapy in patients with nonischemic cardiomyopathy. J. Am. Coll. Cardiol. 2012, 60, 1659–1667. [Google Scholar] [CrossRef] [Green Version]
- Shenasa, M.; Shenasa, H. Hypertension, left ventricular hypertrophy, and sudden cardiac death. Int J. Cardiol. 2017, 237, 60–63. [Google Scholar] [CrossRef]
- Salvador, A.M.; Nevers, T.; Velázquez, F.; Aronovitz, M.; Wang, B.; Abadía Molina, A.; Jaffe, I.Z.; Karas, R.H.; Blanton, R.M.; Alcaide, P. Intercellular adhesion molecule 1 regulates left ventricular leukocyte infiltration, cardiac remodeling, and function in pressure overload-induced heart failure. J. Am. Heart Assoc. 2016, 5, e003126. [Google Scholar] [CrossRef] [Green Version]
- Disertori, M.; Rigoni, M.; Pace, N.; Casolo, G.; Masè, M.; Gonzini, L.; Lucci, D.; Nollo, G.; Ravelli, F. Myocardial Fibrosis Assessment by LGE is a powerful predictor of ventricular tachyarrhythmias in ischemic and nonischemic LV dysfunction: A meta-analysis. JACC Cardiovasc. Imaging 2016, 9, 1046–1055. [Google Scholar] [CrossRef]
- Morita, N.; Mandel, W.J.; Kobayashi, Y.; Karagueuzian, H.S. Cardiac fibrosis as a determinant of ventricular tachyarrhythmias. J. Arrhythm. 2014, 30, 389–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacharova, L. Electrical and structural remodeling in left ventricular hypertrophy-a substrate for a decrease in QRS voltage? Ann. Noninvasive Electrocardiol. 2007, 12, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Chorvatova, A.; Hart, G.; Hussain, M. Na+/Ca2+ exchange current (I(Na/Ca)) and sarcoplasmatic reticulum Ca2+ release in catecholamine-induced cardiac hypertrophy. Cardiovasc. Res. 2004, 61, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, W. Electrical remodelling of membrane ionic channels of hypertrophied ventricular myocytes from spontaneously hypertensive rats. Chin. Med. J. 2000, 113, 584–587. [Google Scholar] [PubMed]
- Ahmmed, G.U.; Dong, P.H.; Song, G.; Ball, N.A.; Xu, Y.; Walsh, R.A.; Chiamvimonvat, N. Changes in Ca2+ cycling proteins underlie cardiac action potential prolongation in apressure-overload guinea pig model with cardiac hypertrophy and failure. Circ. Res. 2000, 86, 558–570. [Google Scholar] [CrossRef] [Green Version]
- Ming, Z.; Nordin, C.; Siri, F.; Aronson, R.S. Reduced calcium current density in single myocytes isolated from hypertrophied failing guinea pig hearts. J. Mol. Cell. Cardiol. 1994, 26, 1133–1143. [Google Scholar] [CrossRef]
- Ouadid, H.; Albat, B.; Nargeot, J. Calcium currents in diseased human cardiac cells. J. Cardiovasc. Pharm. 1995, 25, 282–291. [Google Scholar] [CrossRef]
- Wang, Z.; Kutschke, W.; Richardson, K.E.; Karimi, M.; Hill, J.A. Electrical remodeling in pressure-overload cardiac hypertrophy. Role of calcineurin. Circulation 2001, 104, 1657–1663. [Google Scholar] [CrossRef] [Green Version]
- Ryder, K.O.; Bryant, S.M.; Hart, G. Membrane current changes in left ventricular myocytes isolated from guinea pig after abdominal aortic coarctation. Cardiovasc. Res. 1993, 27, 1278–1287. [Google Scholar] [CrossRef]
- Brooksby, P.; Levi, A.J.; Jones, J.V. The electrophysiological characteristics of hypertrophied ventricular myocytes from spontaneously hypertensive rat. J. Hypertens. 1993, 11, 611–622. [Google Scholar] [CrossRef]
- Cerbai, E.; Barbieri, M.; Li, Q.; Mugelli, A. Ionic basis of action potential prolongation of hypertrophied cardiac myocytes isolated from hypertensive rats of different ages. Cardiovasc. Res. 1994, 28, 1180–1187. [Google Scholar] [CrossRef]
- Meszaros, J.; Khananshvili, D.; Hart, G. Mechanisms underlying delayed afterdepolarizations in hypertrophied left ventricular myocytes of rats. Am. J. Physiol. Heart. Circ. Physiol 2001, 281, H903–H914. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.A. Electrical remodeling in cardiac hypertrophy. Trends Cardiovasc. Med. 2003, 13, 316–322. [Google Scholar] [CrossRef]
- Nattel, S.; Li, D. Ionic remodeling in the heart. Pathophysiological significance and new therapeutic opportunities for atrial fibrillation. Circ. Res. 2000, 87, 440–447. [Google Scholar] [CrossRef] [Green Version]
- Shaw, R.M.; Rudy, Y. Ionic mechanisms of propagation in cardiac tissue. Roles of sodium and L-type calcium currents during reduced excitability and decreased gap junction coupling. Circ. Res. 1997, 81, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Sipido, K.R.; Volders, P.G.A.; Vos, M.A.; Verdonck, F. Altered Na/Ca exchange activity in cardiac hypertrophy and heart failure: A new target for therapy? Cardiovasc. Res. 2002, 53, 782–805. [Google Scholar] [CrossRef]
- Hicks, M.N.; McIntosh, M.A.; Kane, K.A.; Rankin, A.C.; Cobbe, S.M. The electrophysiology of rabbit hearts with left ventricular hypertrophy under normal and ischemic conditions. Cardiovasc. Res. 1995, 30, 181–186. [Google Scholar] [CrossRef]
- Winterton, S.J.; Turner, M.A.; O’Gorman, D.J.; Flores, N.A.; Sheridan, D.J. Hypertrophy causes delayed conduction in human and guinea pig myocardium: Accentuation during ischemic perfusion. Cardiovasc. Res. 1994, 28, 47–54. [Google Scholar] [CrossRef]
- Yokoshiki, H.; Kohya, T.; Tomita, F.; Tohse, N.; Nakaya, H.; Kanno, M.; Kitabatake, A. Restoration of action potential duration and transient outward current by regression of left ventricular hypertrophy. J. Mol. Cell Cardiol. 1997, 29, 1331–1339. [Google Scholar] [CrossRef]
- Aiello, E.A.; Villa-Abrille, M.C.; Escudero, E.M.; Portiansky, E.L.; Perez, N.G.; de Hurtado, M.C.C.; Cingolami, H.E. Myocardial hypertrophy of normotensive Wistar-Kyoto rats. Am. J. Physiol Heart Circ. Physiol. 2004, 286, H1229–H1235. [Google Scholar] [CrossRef]
- Cooklin, M.; Wallis, W.R.J.; Sheridan, D.J.; Fry, C.H. Changes in cell-to-cell electrical coupling associated with left ventricular hypertrophy. Circ. Res. 1997, 80, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Wiegerinck, R.F.; Verkerk, A.O.; Belterman, C.N.; van Veen, T.A.; Baartscheer, A.; Opthof, T.; Wilders, R.; de Bakker, J.M.; Coronel, R. Larger cell size in rabbits with heart failure increases myocardial conduction velocity and QRS duration. Circulation 2006, 113, 806–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostin, S.; Dammer, S.; Hein, S.; Klovekom, W.P.; Bauer, E.P.; Schaper, J. Connexin43 expression and distribution in compensated and decompensated cardiac hypertrophy in patients with aortic stenosis. Cardiovasc Res. 2004, 62, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Peters, N.S.; Green, C.R.; Poole-Wilson, P.A.; Severs, N.J. Reduced content of connexin43 gap junctions in ventricular myocardium from hypertrophied and ischemic human hearts. Circulation 1993, 88, 864–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danik, S.B.; Liu, F.; Zhang, J.; Suk, H.J.; Morley, G.E.; Fishman, G.I.; Gutstein, D.E. Modulation of cardiac gap junction expression and arrhythmic susceptibility. Circ. Res. 2004, 95, 1035–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodge, S.M.; Beardslee, M.A.; Darrow, B.J.; Green, K.G.; Beyer, E.C.; Saffitz, J.E. Effect of angiotensin II on expression of the gap junction channel protein connexin43 in neonatal rat ventricular myocytes. J. Am. Coll. Cardiol. 1998, 32, 800–807. [Google Scholar] [CrossRef] [Green Version]
- Egan Benova, T.; Szeiffova Bacova, B.; Viczenczova, C.; Diez, E.; Barancik, M.; Tribulova, N. Protection of cardiac cell-to-cell coupling attenuate myocardial remodeling and proarrhythmia induced by hypertension. Physiol. Res. 2016, 65 (Suppl. S1), S29–S42. [Google Scholar]
- Tribulova, N.; Szeiffova Bacova, B.; Benova, T.; Viczenczova, C. Can we protect from malignant arrhythmias by modulation of cardiac cell-to-cell coupling? J. Electrocardiol. 2015, 48, 434–440. [Google Scholar] [CrossRef]
- Radosinska, J.; Bacova, B.; Knezl, V.; Benova, T.; Zurmanova, J.; Soukup, T.; Arnostova, P.; Slezak, J.; Gonçalvesova, E.; Tribulova, N. Dietary omega-3 fatty acids attenuate myocardial arrhythmogenic factors and propensity of the heart to lethal arrhythmias in a rodent model of human essential hypertension. J. Hypertens. 2013, 31, 1876–1885. [Google Scholar] [CrossRef]
- Firek, L.; Weingart, R. Modification of gap junction conductance by divalent cations and protons in neonatal rat heart cells. J. Mol. Cell Cardiol. 1995, 27, 1633–1643. [Google Scholar] [CrossRef]
- Marionneau, C.; Brunet, S.; Flagg, T.P.; Pilgram, T.K.; Demolombe, S.; Nerbonne, J.M. Distinct cellular and molecular mechanisms underlie functional remodeling of repolarizing K+ currents with left ventricular hypertrophy. Circ. Res. 2008, 102, 1406–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antzelevitch, C.; Belardinelli, L. The role of sodium channel current in modulating transmural dispersion of repolarization and arrhythmogenesis. J. Cardiovasc. Electrophysiol. 2006, 17, S79–S85. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.P.; Kucera, J.P.; Bircher-Lehmann, L.; Rudy, Y.; Saffitz, J.E.; Kleber, A.G. Impulse propagation in synthetic strands of neonatal cardiac myocytes with genetically reduced levels of connexin43. Circ. Res. 2003, 92, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Josephson, M.E.; Anter, E. Substrate mapping for ventricular tachycardia: Assumptions and misconceptions. JACC Clin. Electrophysiol. 2015, 1, 341–352. [Google Scholar] [CrossRef]
- Chávez-González, E.; Rodríguez Jiménez, A.E.; Moreno-Martínez, F.L. QRS duration and dispersion for predicting ventricular arrhythmias in early stage of acute myocardial infraction. Med. Intensiva 2017, 41, 347–355. [Google Scholar] [CrossRef]
- Udink ten Cate, F.E.; Sreeram, N.; Brockmeier, K. The pathophysiologic aspects and clinical implications of electrocardiographic parameters of ventricular conduction delay in repaired tetralogy of Fallot. J. Electrocardiol. 2014, 47, 618–624. [Google Scholar] [CrossRef]
- Dao, D.T.; Hollander, S.A.; Rosenthal, D.N.; Dubin, A.M. QRS prolongation is strongly associated with life-threatening ventricular arrhythmias in children with dilated cardiomyopathy. J. Heart Lung Transpl. 2013, 32, 1013–1019. [Google Scholar] [CrossRef]
- Morin, D.P.; Oikarinen, L.; Viitasalo, M.; Toivonen, L.; Nieminen, M.S.; Kjeldsen, S.E.; Dahlöf, B.; John, M.; Devereux, R.B.; Okin, P.M. QRS duration predicts sudden cardiac death in hypertensive patients undergoing intensive medical therapy: The LIFE study. Eur. Heart J. 2009, 30, 2908–2914. [Google Scholar] [CrossRef]
- Romhilt, D.W.; Estes, E.H., Jr. A point-score system for the ECG diagnosis of left ventricular hypertrophy. Am. Heart J. 1968, 75, 752–758. [Google Scholar] [CrossRef]
- Li, Z.; Dahlöf, B.; Okin, P.M.; Kjeldsen, S.E.; Wachtell, K.; Ibsen, H.; Nieminen, M.S.; Jern, S.; Devereux, R.B. Left bundle branch block and cardiovascular morbidity and mortality in hypertensive patients with left ventricular hypertrophy: The Losartan Intervention for Endpoint Reduction in Hypertension study. J. Hypertens 2008, 26, 1244–1249. [Google Scholar] [CrossRef]
- Prihadi, E.A.; Leung, M.; Vollema, E.M.; Ng, A.C.T.; Ajmone Marsan, N.; Bax, J.J.; Delgado, V. Prevalence and prognostic relevance of ventricular conduction disturbances in patients with aortic stenosis. Am. J. Cardiol. 2017, 120, 2226–2232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erne, P.; Iglesias, J.F.; Urban, P.; Eberli, F.R.; Rickli, H.; Simon, R.; Fischer, T.A.; Radovanovic, D. Left bundle-branch block in patients with acute myocardial infarction: Presentation, treatment, and trends in outcome from 1997 to 2016 in routine clinical practice. Am. Heart J. 2017, 84, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Bacharova, L.; Szathmary, V.; Kovalcik, M.; Mateasik, A. Effect of changes in left ventricular anatomy and conduction velocity on the QRS voltage and morphology in left ventricular hypertrophy: A model study. J. Electrocardiol. 2010, 43, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Bacharova, L.; Szathmary, V.; Mateasik, A. Electrocardiographic patterns of left bundle-branch block caused by intraventricular conduction impairment in working myocardium: A model study. J. Electrocardiol. 2011, 44, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Zhong, A.; You, T.; Chen, J.; Xu, W.; Shi, M. Prognostic significance of right bundle branch block for patients with acute myocardial infarction: A Systematic Review and Meta-Analysis. Med. Sci. Monit. 2016, 22, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Al Rajoub, B.; Noureddine, S.; El Chami, S.; Haidar, M.H.; Itani, B.; Zaiter, A.; Akl, E.A. The prognostic value of a new left bundle branch block in patients with acute myocardial infarction: A systematic review and meta-analysis. Heart Lung. 2017, 46, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.B.; Wachtell, K.; Okin, P.M.; Gerdts, E.; Liu, J.E.; Nieminen, M.S.; Jern, S.; Dahlöf, B.; Devereux, R.B. Association of left bundle branch block with left ventricular structure and function in hypertensive patients with left ventricular hypertrophy: The LIFE study. J. Hum. Hypertens 2004, 18, 397–402. [Google Scholar] [CrossRef] [Green Version]
- Buxton, A.E.; Sweeney, M.O.; Wathen, M.S.; Josephson, M.E.; Otterness, M.F.; Hogan-Miller, E.; Stark, A.J.; Degroot, P.J.; Pain, F.R., III. QRS duration does not predict occurrence of ventricular tachyarrhythmias in patients with implanted cardioverter-defibrillators. J. Am. Coll. Cardiol. 2005, 46, 310–316. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.P.; Hall, W.J.; McNitt, S.; Wang, H.; Daubert, J.P.; Zareba, W.; Ruskin, J.N.; Moss, A.J.; Madit-II Investigators. Investigators M-I. Factors influencing appropriate firing of the implanted defibrillator for ventricular tachycardia/fibrillation: Findings from the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II). J. Am. Coll. Cardiol. 2005, 46, 1712–1720. [Google Scholar] [CrossRef] [Green Version]
- Niemeijer, M.N.; van den Berg, M.E.; Eijgelsheim, M.; van Herpen, G.; Stricker, B.H.; Kors, J.A.; Rijnbeek, P.R. Short-term QT variability markers for the prediction of ventricular arrhythmias and sudden cardiac death: A systematic review. Heart 2014, 100, 1831–1836. [Google Scholar] [CrossRef]
- Buğra, Z.; Koylan, N.; Vural, A.; Erzengin, F.; Umman, B.; Yilmaz, E.; Meriç, M.; Büyüköztürk, K. Left ventricular geometric patterns and QT dispersion in untreated essential hypertension. Am. J. Hypertens 1998, 11, 1164–1170. [Google Scholar] [CrossRef] [Green Version]
- Kunisek, J.; Zaputovic, L.; Cubranic, Z.; Kunisek, L.; Zuvic Butora, M.; Lukin-Eskinja, K.; Karlavaris, R. Influence of the left ventricular types on QT intervals in hypertensive patients. Anatol. J. Cardiol. 2015, 15, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Oikarinen, L.; Nieminen, M.S.; Toivonen, L.; Viitasalo, M.; Wachtell, K.; Papademetriou, V.; Jern, S.; Dahlöf, B.; Devereux, RB.; Okin, P.M. LIFE Study Investigators. Relation of QT interval and QT dispersion to regression of echocardiographic and electrocardiographic left ventricular hypertrophy in hypertensive patients: The Losartan Intervention for Endpoint Reduction (LIFE) study. Am. Heart J. 2003, 145, 919–925. [Google Scholar] [CrossRef]
- Oikarinen, L.; Nieminen, M.S.; Viitasalo, M.; Toivonen, L.; Jern, S.; Dahlöf, B.; Devereux, R.B.; Okin, P.M. LIFE Study Investigators. QRS duration and QT interval predict mortality in hypertensive patients with left ventricular hypertrophy: The Losartan Intervention for Endpoint Reduction in Hypertension Study. Hypertension 2004, 43, 1029–1034. [Google Scholar] [CrossRef] [Green Version]
- Bacharova, L.; Szathmary, V.; Mateasik, A. Primary and secondary T wave changes in LVH: A model study. J. Electrocardiol. 2010, 43, 624–633. [Google Scholar] [CrossRef]
- Lu, H.R.; Yan, G.X.; Gallacher, D.J. A new biomarker–index of cardiac electrophysiological balance (iCEB) –plays an important role in drug-induced cardiac arrhythmias: Beyond QT prolongation and torsade de pointes (TdPs). J. Pharm. Toxicol. Methods 2013, 68, 250–259. [Google Scholar] [CrossRef]
- Robyns, T.; Lu, H.R.; Gallacher, D.J.; Garweg, C.; Ector, J.; Willems, R.; Janssens, S.; Nuyens, D. Evaluation of cardio-electrophysiological balance (iCEB) as a new biomarker for the identification of patients at increased arrhythmic risk. Ann. Noninvasive Electrocardiol. 2016, 21, 294–304. [Google Scholar] [CrossRef] [Green Version]
- Haigney, M.C.; Zareba, W.; Gentlesk, P.J.; Goldstein, R.E.; Illovsky, M.; McNitt, S.; Andrews, M.L.; Moss, A.J. Multicenter Automatic Defibrillator Implantation Trial IIi. QT interval variability and spontaneous ventricular tachycardia or fibrillation in the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II patients. J. Am. Coll Cardiol. 2004, 44, 1481–1487. [Google Scholar] [CrossRef] [Green Version]
- Miragoli, M.; Goldoni, M.; Demola, P.; Paterlini, A.; Li Calzi, M.; Gioia, M.I.; Visioli, F.; Rossi, S.; Pelà, G. Left ventricular geometry correlates with early repolarization pattern in adolescent athletes. Scand. J. Med. Sci. Sports 2019, 29, 1727–1735. [Google Scholar] [CrossRef]
- Haïssaguerre, M.; Derval, N.; Sacher, F.; Jesel, L.; Deisenhofer, I.; de Roy, L.; Pasquié, J.L.; Nogami, A.; Babuty, D.; Yli-Mayry, S.; et al. Sudden cardiac arrest associated with early repolarization. N Engl. J. Med. 2008, 358, 2016–2023. [Google Scholar] [CrossRef] [Green Version]
- Ali Diab, O.; Abdel-Hafez Allam, R.M.; Mohamed, H.G.; Mohamed, T.R.; Abel-Hafeez Khalid, S. Early repolarization pattern is associated with increased risk of early ventricular arrhythmias during acute ST segment elevation myocardial infarction. Ann. Noninvasive Electrocardiol. 2015, 20, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Pranata, R.; Yonas, E.; Vania, R.; Raharjo, S.B.; Siswanto, B.B.; Setianto, B. Electrocardiographic early repolarization is associated with future ventricular arrhythmia after acute myocardial infarction-Systematic Review and Meta-Analysis. J. Arrhythm. 2019, 35, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Naruse, Y.; Tada, H.; Harimura, Y.; Ishibashi, M.; Noguchi, Y.; Sato, A.; Hoshi, T.; Sekiguchi, Y.; Aonuma, K. Early repolarization increases the occurrence of sustained ventricular tachyarrhythmias and sudden death in the chronic phase of an acute myocardial infarction. Circ. Arrhythm. Electrophysiol. 2014, 7, 626–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalos, D.; Fiedler, L.; Radojevic, J.; Sponder, M.; Dichtl, W.; Schukro, C. Prevalence of early repolarization syndrome and long-term clinical outcome in patients with the diagnosis of idiopathic ventricular fibrillation. Heart Vessel. 2019, 34, 625–631. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.J.; Li, Z.Y.; Yao, F.J.; Xu, X.J.; Ji, C.C.; Chen, X.M.; Liu, L.J.; Lin, X.X.; Yao, H.; Wu, S.H. Early repolarization is associated with a significantly increased risk of ventricular arrhythmias and sudden cardiac death in patients with structural heart diseases. Heart Rhythm. 2017, 14, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.X.; Antzelevitch, C. Cellular basis for the electrocardiographic J wave. Circulation 1996, 93, 372–379. [Google Scholar] [CrossRef]
- Gussak, I.; Antzelevitch, C. Early repolarization syndrome: Clinical characteristics and possible cellular and ionic mechanisms. J. Electrocardiol. 2000, 33, 299–309. [Google Scholar] [CrossRef]
- Antzelevitch, C.; Yan, G.X. J wave syndromes. Heart Rhythm. 2010, 7, 549–558. [Google Scholar] [CrossRef]
- Trenkwalder, T.; King, R.; Kaess, B.M.; Hengstenberg, C.; Schunkert, H.; Ittermann, T.; Felix, S.B.; Busch, M.; Dörr, M.; Reinhard, W. Ventricular and supraventricular ectopy in subjects with early repolarization. Am. J. Cardiol. 2017, 120, 92–97. [Google Scholar] [CrossRef]
- Wellens, H.J. Early repolarization revisited. N. Engl. J. Med. 2008, 358, 2063–2065. [Google Scholar] [CrossRef]
- Ghosh, S.; Cooper, D.H.; Vijayakumar, R.; Zhang, J.; Pollak, S.; Haïssaguerre, M.; Rudy, Y. Early repolarization associated with sudden death: Insights from noninvasive electrocardiographic imaging. Heart Rhythm 2010, 7, 534–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sherif, N.; Turitto, G.; Fontaine, J.M. Risk stratification of patients with complex ventricular arrhythmias. Value of ambulatory electrocardiographic recording, programmed electrical stimulation and the signal-averaged electrocardiogram. Herz 1988, 13, 204–214. [Google Scholar] [PubMed]
- Marstrand, P.; Axelsson, A.; Thune, J.J.; Vejlstrup, N.; Pehrson, S.; Bundgaard, H.; Theilade, J. Late potentials and their correlation with ventricular structure in patients with ventricular arrhythmias. Pacing Clin. Electrophysiol. 2017, 40, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Irie, T.; Yu, R.; Bradfield, J.S.; Vaseghi, M.; Buch, E.F.; Ajijola, O.; Macias, C.; Fujimura, O.; Mandapati, R.; Boyle, N.G.; et al. Relationship between sinus rhythm late activation zones and critical sites for scar-related ventricular tachycardia: Systematic analysis of isochronal late activation mapping. Circ. Arrhythm. Electrophysiol. 2015, 8, 390–399. [Google Scholar] [CrossRef] [Green Version]
- Santangeli, P.; Marchlinski, F.E. Substrate mapping for unstable ventricular tachycardia. Heart Rhythm 2016, 13, 569–583. [Google Scholar] [CrossRef] [Green Version]
- Wojszwiłło, A.; Łoboz-Grudzień, K.; Jaroch, J. Signal averaged ECG in different patterns of left ventricular hypertrophy and geometry in hypertension. Kardiol. Pol. 2003, 58, 335–343. [Google Scholar]
- Maffei, P.; Martini, C.; Milanesi, A.; Corfini, A.; Mioni, R.; de Carlo, E.; Menegazzo, C.; Scanarini, M.; Vettor, R.; Federspil, G.; et al. Late potentials and ventricular arrhythmias in acromegaly. Int. J. Cardiol. 2005, 104, 197–203. [Google Scholar] [CrossRef]
- Palmiero, P.; Maiello, M. Arrhythmic risk in essential hypertension: Late potentials. Minerva Cardioangiol. 2004, 52, 1–8. [Google Scholar]
- Goldberger, J.J.; Cain, M.E.; Hohnloser, S.H.; Kadish, A.H.; Knight, B.P.; Lauer, M.S.; Maron, B.J.; Page, R.L.; Passman, R.S.; Siscovick, D.; et al. American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society Scientific Statement on Noninvasive Risk Stratification Techniques for Identifying Patients at Risk for Sudden Cardiac Death. A scientific statement from the American Heart Association Council on Clinical Cardiology Committee on Electrocardiography and Arrhythmias and Council on Epidemiology and Prevention. J. Am. Coll. Cardiol. 2008, 52, 1179–1199. [Google Scholar]
- Deyell, M.W.; Krahn, A.D.; Goldberger, J.J. Sudden cardiac death risk stratification. Circ. Res. 2015, 116, 1907–1918. [Google Scholar] [CrossRef] [Green Version]
- Bokma, J.P.; Winter, M.M.; Vehmeijer, J.T.; Vliegen, H.W.; van Dijk, A.P.; van Melle, J.P.; Meijboom, F.J.; Post, M.C.; Zwinderman, A.H.; Mulder, B.J.; et al. QRS fragmentation is superior to QRS duration in predicting mortality in adults with tetralogy of Fallot. Heart 2017, 103, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Pietrasik, G.; Zaręba, W. QRS fragmentation: Diagnostic and prognostic significance. Cardiol. J. 2012, 19, 114–1121. [Google Scholar] [CrossRef] [PubMed]
- Das, M.K.; Maskoun, W.; Shen, C.; Michael, M.A.; Suradi, H.; Desai, M.; Subbarao, R.; Bhakta, D. Fragmented QRS on twelve-lead electrocardiogram predicts arrhythmic events in patients with ischemic and nonischemic cardiomyopathy. Heart Rhythm. 2010, 7, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Kadi, H.; Kevser, A.; Ozturk, A.; Koc, F.; Ceyhan, K. Fragmented QRS complexes are associated with increased left ventricular mass in patients with essential hypertension. Ann. Noninvasive Electrocardiol. 2013, 18, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Eyuboglu, M. Fragmented QRS as a marker of myocardial fibrosis in hypertension: A systematic review. Curr. Hypertens Rep. 2019, 21, 73. [Google Scholar] [CrossRef] [PubMed]
- Bekar, L.; Kalçık, M.; Kilci, H.; Çelik, O.; Yetim, M.; Doğan, T.; Önalan, O. Presence of fragmented QRS may be associated with complex ventricular arrhythmias in patients with essential hypertension. J. Electrocardiol. 2019, 55, 20–25. [Google Scholar] [CrossRef]
- De Ambroggi, L.; Corlan, A.D. Clinical use of body surface potential mapping in cardiac arrhythmias. Anatol. J. Cardiol. 2007, 7 (Suppl. S1), 8–10. [Google Scholar]
- Burnes, J.E.; Taccardi, B.; Rudy, Y. A noninvasive imaging modality for cardiac arrhythmias. Circulation 2000, 102, 2152–2158. [Google Scholar] [CrossRef]
- Hancock, E.W.; Deal, B.J.; Mirvis, D.M.; Okin, P.; Kligfield, P.; Gettes, L.S.; Bailey, J.J.; Childers, R.; Gorgels, A.; Josephson, M.; et al. AHA/ACCF/HRS Recommendations for the Standardization and Interpretation of the Electrocardiogram. Part V: Electrocardiogram changes associated with cardiac chamber hypertrophy A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2009, 53, 992–1002. [Google Scholar]
- Pewsner, D.; Jüni, P.; Egger, M.; Battaglia, M.; Sundström, J.; Bachmann, L.M. Accuracy of electrocardiography in diagnosis of left ventricular hypertrophy in arterial hypertension: Systematic review. BMJ 2007, 335, 711. [Google Scholar] [CrossRef] [Green Version]
- Bacharova, L.; Chen, H.; Estes, E.H.; Mateasik, A.; Bluemke, D.A.; Lima, J.A.; Burke, G.L.; Soliman, E.Z. Determinants of discrepancies in detection and comparison of the prognostic significance of left ventricular hypertrophy by electrocardiogram and cardiac magnetic resonance imaging. Am. J. Cardiol. 2015, 115, 515–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estes, E.H.; Zhang, Z.M.; Li, Y.; Tereshchenko, L.G.; Soliman, E.Z. Individual components of the Romhilt-Estes left ventricular hypertrophy score differ in their prediction of cardiovascular events: The Atherosclerosis Risk in Communities (ARIC) study. Am. Heart J. 2015, 170, 1220–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIntyre, H.; Fry, C.H. Abnormal action potential conduction in isolated human hypertrophied left ventricular myocardium. J. Cardiovasc. Electrophysiol. 1997, 8, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Cooklin, M.; Wallis, W.R.; Sheridan, D.J.; Fry, C.H. Conduction velocity and gap junction resistance in hypertrophied, hypoxic guinea-pig left ventricular myocardium. Exp. Physiol. 1998, 83, 763–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carey, P.A.; Turner, M.; Fry, C.H.; Sheridan, D.J. Reduced anisotropy of action potential conduction in left ventricular hypertrophy. J. Cardiovasc. Electrophysiol. 2001, 12, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Moreo, A.; Ambrosio, G.; De Chiara, B.; Tran, T.; Raman, S.V. Influence of midwall fibrosis on diastolic dysfunction in non-ischemic cardiomyopathy. Int. J. Cardiol. 2013, 163, 342–344. [Google Scholar] [CrossRef] [Green Version]
- Bacharova, L.; Szathmary, V.; Svehlikova, J.; Mateasik, A.; Gyhagen, J.; Tysler, M. The effect of conduction velocity slowing in left ventricular midwall on the QRS complex morphology: A simulation study. J. Electrocardiol. 2016, 49, 164–170. [Google Scholar] [CrossRef]
- Brooks, J.E.; Soliman, E.Z.; Upadhya, B. Is left ventricular hypertrophy a valid therapeutic target? Curr. Hypertens Rep. 2019, 21, 47. [Google Scholar] [CrossRef]


© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bacharova, L. Missing Link Between Molecular Aspects of Ventricular Arrhythmias and QRS Complex Morphology in Left Ventricular Hypertrophy. Int. J. Mol. Sci. 2020, 21, 48. https://doi.org/10.3390/ijms21010048
Bacharova L. Missing Link Between Molecular Aspects of Ventricular Arrhythmias and QRS Complex Morphology in Left Ventricular Hypertrophy. International Journal of Molecular Sciences. 2020; 21(1):48. https://doi.org/10.3390/ijms21010048
Chicago/Turabian StyleBacharova, Ljuba. 2020. "Missing Link Between Molecular Aspects of Ventricular Arrhythmias and QRS Complex Morphology in Left Ventricular Hypertrophy" International Journal of Molecular Sciences 21, no. 1: 48. https://doi.org/10.3390/ijms21010048
APA StyleBacharova, L. (2020). Missing Link Between Molecular Aspects of Ventricular Arrhythmias and QRS Complex Morphology in Left Ventricular Hypertrophy. International Journal of Molecular Sciences, 21(1), 48. https://doi.org/10.3390/ijms21010048



