Suppression of β1-Adrenoceptor Autoantibodies is Involved in the Antiarrhythmic Effects of Omega-3 Fatty Acids in Male and Female Hypertensive Rats
Abstract
:1. Introduction
2. Results
2.1. Biometric Parameters of Examined Rats
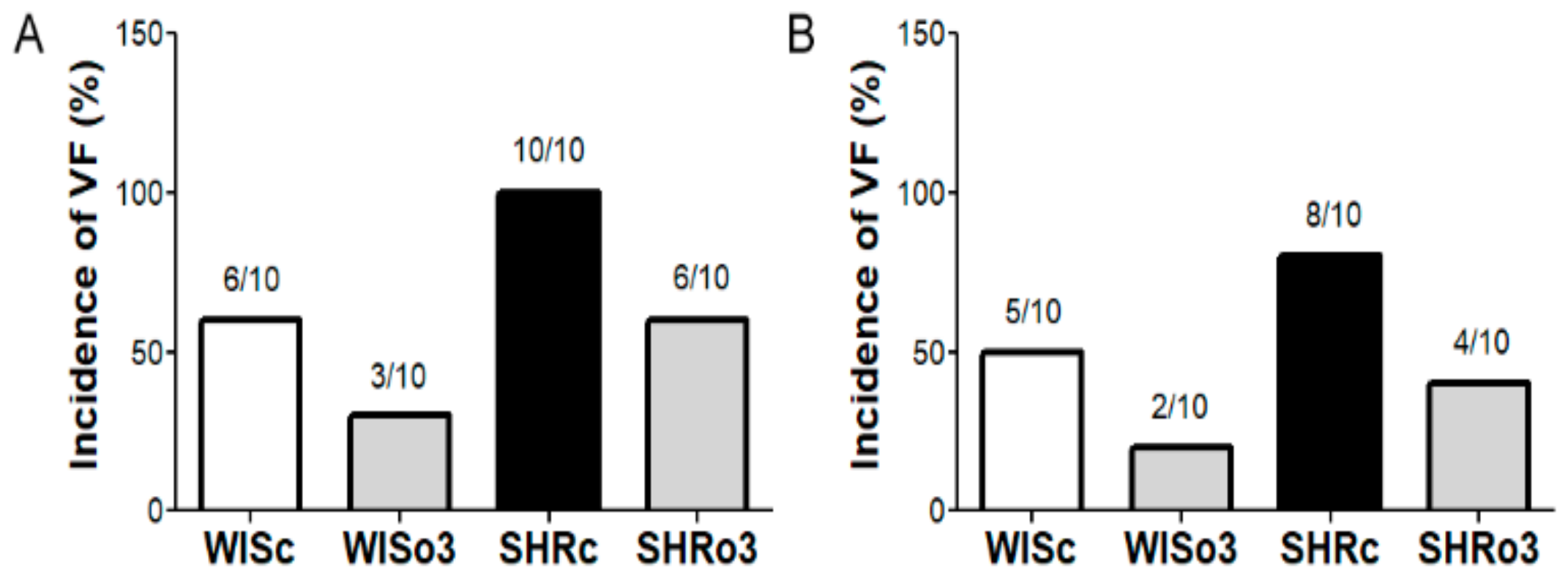
2.2. Incidence of Electrically-Induced Sustained VF
2.3. Identification of β1-AA Activity
2.4. Myocardial MMP-2 Activity
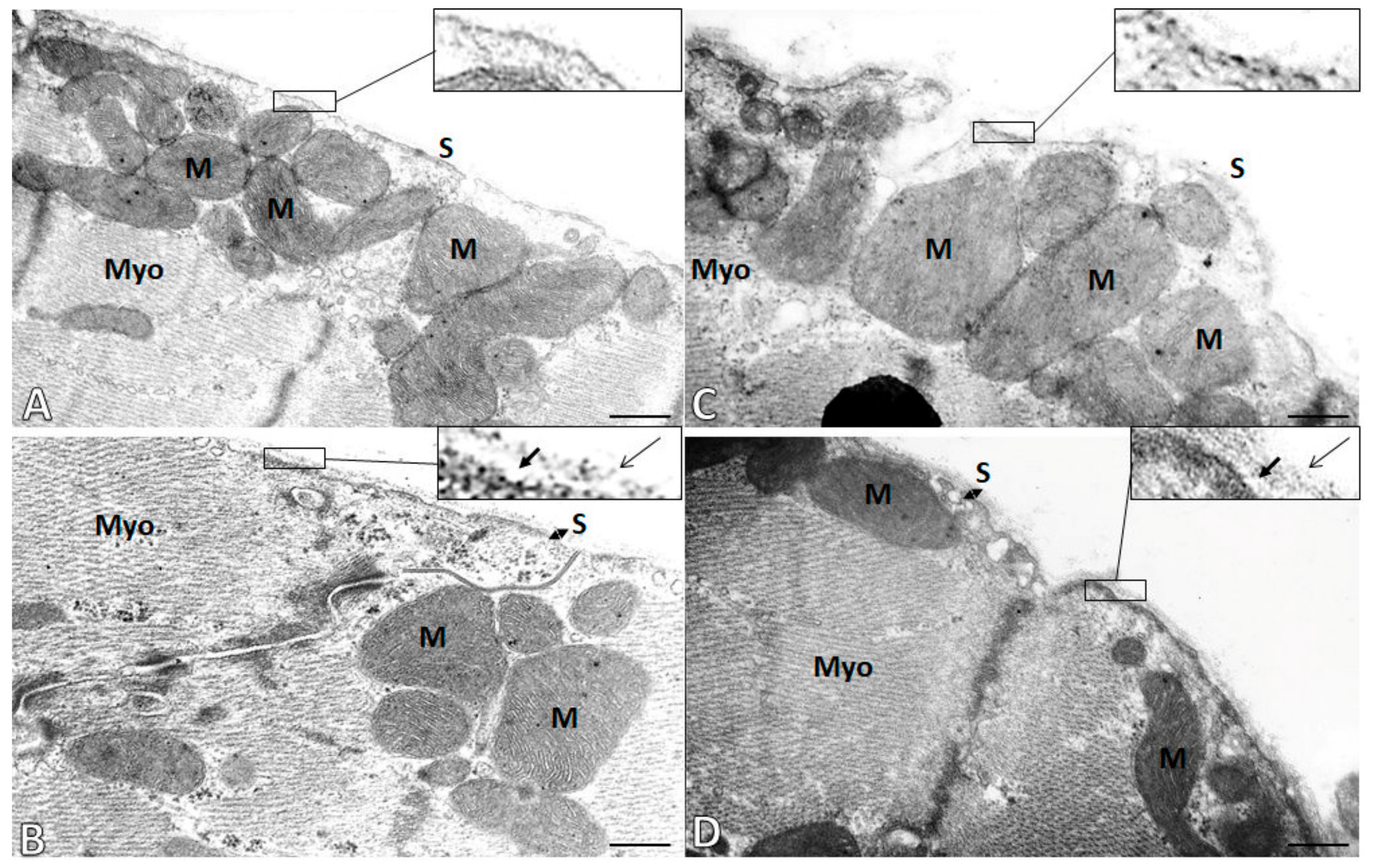
2.5. Subcellular Integrity of the Cardiac Cell Membrane (Sarcolemma)
2.6. Topology of Myocardial Cx43
2.7. Myocardial Cx43 Protein Levels
2.8. Myocardial PKC-ε Protein Levels
2.9. Myocardial PKC-δ Protein Levels
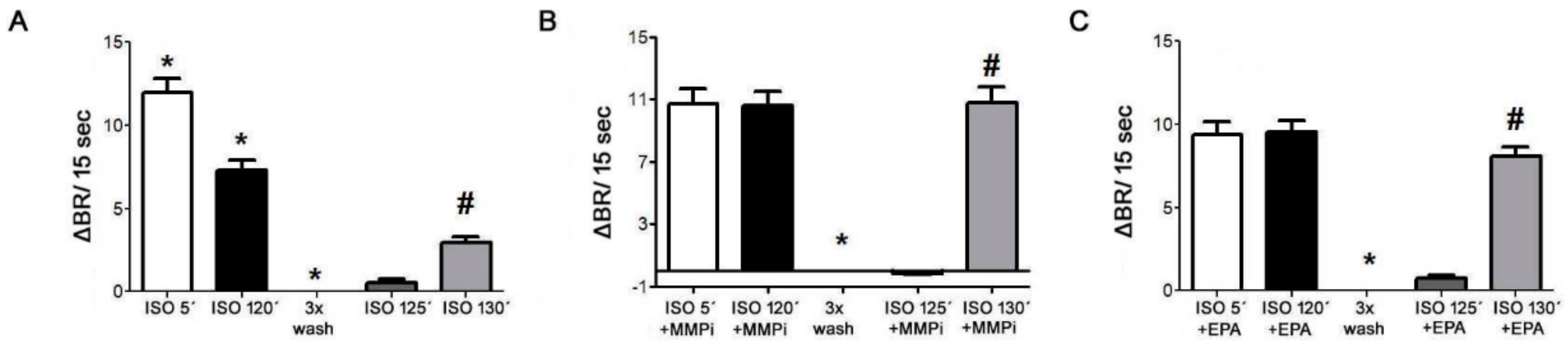
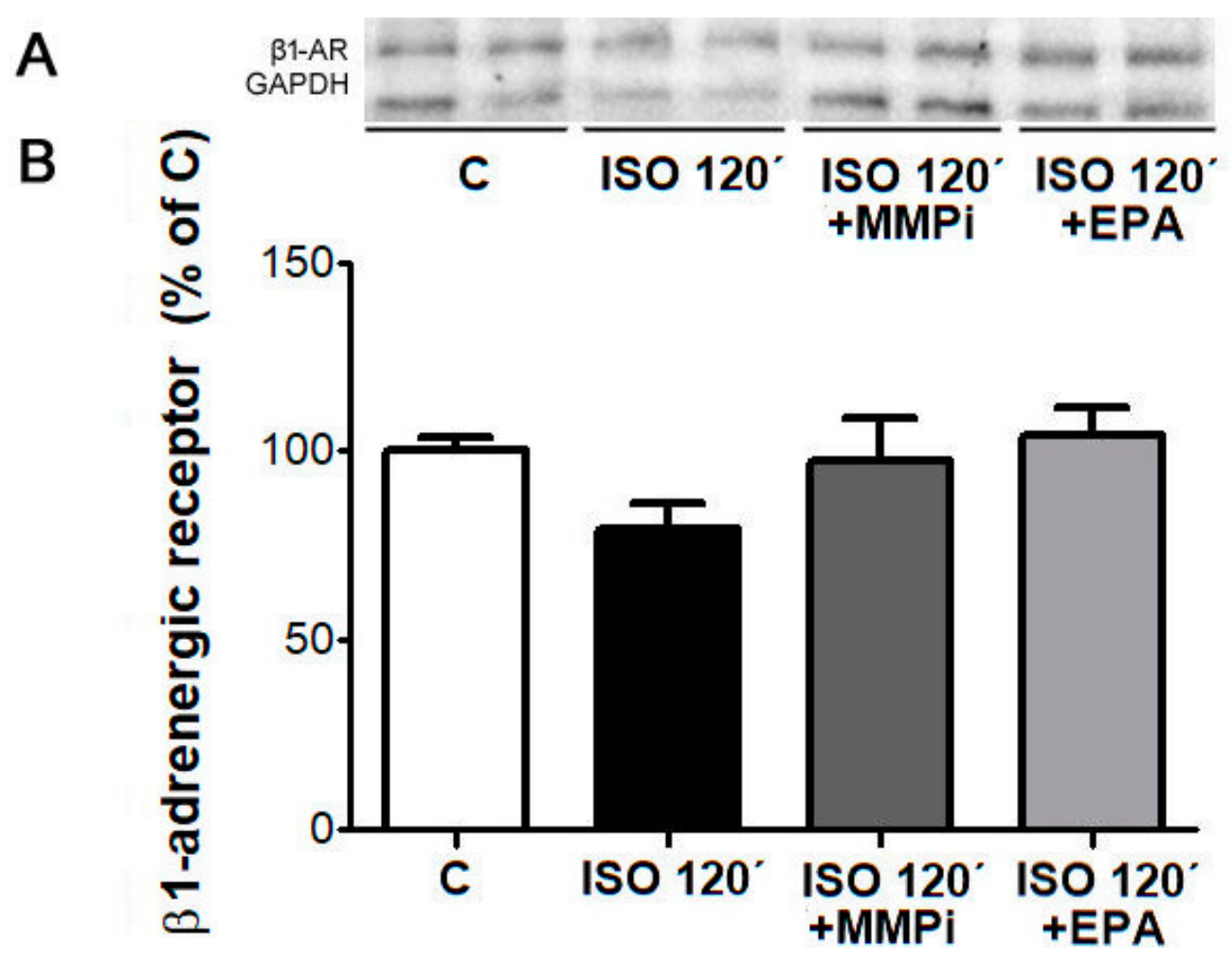
2.10. Desensitization of β1-AR and Effects of MMP Inhibitor and EPA in Cultured Neonatal Cardiomyocytes
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
Animal Monitoring and Tissue Sampling
4.2. Neonatal Rat Cardiomyocytes
4.3. Examination of Ventricular Fibrillation Incidence in Langendorff-Perfused Hearts
4.4. IgG Preparation from Rats Blood Serum
Measurement of Autoantibodies Directed Against the β1-Adrenergic Receptor
4.5. Gelatin Zymography
4.6. EM Myocardial Subcellular Integrity
4.7. Immunolabeling of Cx43
4.8. SDS-PAGE and WB Analysis of Proteins
4.9. Monitoring of β1 Receptors Desensitization and Its Possible Reverse
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kuneš, J.; Kadlecová, M.; Vanecková, I.; Zicha, J. Critical developmental periods in the pathogenesis of hypertension. Physiol. Res. 2012, 61. [Google Scholar]
- Egan Benova, T.; Szeiffova Bacova, B.; Viczenczova, C.; Diez, E.; Barancik, M.; Tribulova, N. Protection of cardiac cell-to-cell coupling attenuate myocardial remodeling and proarrhythmia induced by hypertension. Physiol. Res. 2016, 65, S29–S42. [Google Scholar]
- Ryabkova, V.A.; Shubik, Y.V.; Erman, M.V.; Churilov, L.P.; Kanduc, D.; Shoenfeld, Y. Lethal immunoglobulins: Autoantibodies and sudden cardiac death. Autoimmun. Rev. 2019, 18, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini, P.E.; Capecchi, P.L.; Guideri, F.; Acampa, M.; Selvi, E.; Bisogno, S.; Galeazzi, M.; Laghi-Pasini, F. Autoantibody-mediated cardiac arrhythmias: Mechanisms and clinical implications. Basic Res. Cardiol. 2008, 103, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chiale, P.A.; Garro, H.A.; Schmidberg, J.; Sánchez, R.A.; Acunzo, R.S.; Lago, M.; Levy, G.; Levin, M. Inappropriate sinus tachycardia may be related to an immunologic disorder involving cardiac β andrenergic receptors. Hear. Rhythm 2006, 3, 1182–1186. [Google Scholar] [CrossRef]
- Iwata, M.; Yoshikawa, T.; Baba, A.; Anzai, T.; Mitamura, H.; Ogawa, S. Autoantibodies against the second extracellular loop of beta1-adrenergic receptors predict ventricular tachycardia and sudden death in patients with idiopathic dilated cardiomyopathy. J. Am. Coll. Cardiol. 2001, 37, 418–424. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Kellems, R.E. Receptor-activating autoantibodies and disease: Preeclampsia and beyond. Expert Rev. Clin. Immunol. 2011, 7, 659–674. [Google Scholar] [CrossRef] [Green Version]
- Dandel, M.; Wallukat, G.; Potapov, E.; Hetzer, R. Role of β 1-adrenoceptor autoantibodies in the pathogenesis of dilated cardiomyopathy. Immunobiology 2012, 217, 511–520. [Google Scholar] [CrossRef]
- Wallukat, G.; Boewer, V.; Förster, A.; Wollenberger, A. Anti-β-Adrenoceptor Autoantibodies with β-Adrenergic Agonistic Activity from Patients with Myocarditis and Dilated Cardiomyopathy. Hear. Fail. Mech. Manag. 1991, 21–29. [Google Scholar]
- Chiale, P.A.; Rosenbaum, M.B.; Elizari, M.V.; Hjalmarson, A.; Magnusson, Y.; Wallukat, G.; Hoebeke, J. High prevalence of antibodies against beta1- and beta2-adrenoceptors in patients with primary electrical cardiac abnormalities. J. Am. Coll. Cardiol. 1995, 26, 864–869. [Google Scholar] [CrossRef] [Green Version]
- Wallukat, G.; Haberland, A.; Berg, S.; Schulz, A.; Freyse, E.J.; Dahmen, C.; Kage, A.; Dandel, M.; Vetter, R.; Salzsieder, E.; et al. The First Aptamer-Apheresis Column Specifically for Clearing Blood of β1-Receptor Autoantibodies - A Successful Proof of Principle Using Autoantibody-Positive SHR Rats -. Circ. J. 2012, 76, 2449–2455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallukat, G.; Podlowski, S.; Nissen, E.; Morwinski, R.; Csonka, C.; Tosaki, A.; Blasig, I.E. Functional and structural characterization of anti-β1-adrenoceptor autoantibodies of spontaneously hypertensive rats. Mol. Cell. Biochem. 2003, 251, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Dumotier, B.M. A straightforward guide to the basic science behind arrhythmogenesis. Heart 2014, 100, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- Ferrantini, C.; Coppini, R.; Sacconi, L. Cardiomyocyte-specific Gq signaling and arrhythmias: Novel insights from DREADD technology. Cardiovasc. Res. 2019, 115, 992–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brisinda, D.; Sorbo, A.R.; Venuti, A.; Ruggieri, M.P.; Manna, R.; Fenici, P.; Wallukat, G.; Hoebeke, J.; Frustaci, A.; Fenici, R. Anti-β-adrenoceptors autoimmunity causing “idiopathic” arrhythmias and cardiomyopathy. Circ. J. 2012, 76, 1345–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tribulova, N.; Bacova, B.S.; Benova, T.E.; Knezl, V.; Barancik, M.; Slezak, J. Omega-3 index and anti-arrhythmic potential of omega-3 PUFAs. Nutrients 2017, 9, 1191. [Google Scholar] [CrossRef] [Green Version]
- Arnold, C.; Markovic, M.; Blossey, K.; Wallukat, G.; Fischer, R.; Dechend, R.; Konkel, A.; Von Schacky, C.; Luft, F.C.; Muller, D.N.; et al. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of ω-3 fatty acids. J. Biol. Chem. 2010, 285, 32720–32733. [Google Scholar] [CrossRef] [Green Version]
- Adebesin, A.M.; Wesser, T.; Vijaykumar, J.; Konkel, A.; Paudyal, M.P.; Lossie, J.; Zhu, C.; Westphal, C.; Puli, N.; Fischer, R.; et al. Development of Robust 17(R),18(S)-Epoxyeicosatetraenoic Acid (17,18-EEQ) Analogues as Potential Clinical Antiarrhythmic Agents. J. Med. Chem. 2019. [Google Scholar] [CrossRef]
- Tribulova, N.; Szeiffova Bacova, B.; Benova, T.; Viczenczova, C. Can we protect from malignant arrhythmias by modulation of cardiac cell-to-cell coupling? J. Electrocardiol. 2015, 48, 434–440. [Google Scholar] [CrossRef]
- Bačová, B.; Seč, P.; Radošinská, J.; Čertík, M.; Vachulová, A.; Tribulová, N. Lower Omega-3 index is a marker of increased propensity of hypertensive rat heart to malignant arrhythmias. Physiol. Res. 2013, 62, 201–208. [Google Scholar]
- Rodrigues, S.F.; Tran, E.D.; Fortes, Z.B.; Schmid-Schönbein, G.W. Matrix metalloproteinases cleave the β2-adrenergic receptor in spontaneously hypertensive rats. Am. J. Physiol. Hear. Circ. Physiol. 2010, 299, 25–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voit-Ostricki, L.; Lovas, S.; Watts, C.R. Conformation and domain movement analysis of human matrix metalloproteinase-2: Role of associated Zn2+ and Ca2+ ions. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friese, R.S.; Rao, F.; Khandrika, S.; Thomas, B.; Ziegler, M.G.; Schmid-Schnbein, G.W.; O’Connor, D.T. Matrix metalloproteinases: Discrete elevations in essential hypertension and hypertensive end-stage renal disease. Clin. Exp. Hypertens. 2009, 31, 521–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sykora, M.; Bacova, B.S.; Benova, T.E.; Barancik, M.; Zurmanova, J.; Rauchova, H.; Weismann, P.; Pavelka, S.; Kurahara, L.H.; Slezak, J.; et al. Cardiac cx43 and ECM responses to altered thyroid status are blunted in spontaneously hypertensive versus normotensive rats. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolai, E.; Sinibaldi, F.; Sannino, G.; Laganà, G.; Basoli, F.; Licoccia, S.; Cozza, P.; Santucci, R.; Piro, M.C. Omega-3 and Omega-6 Fatty Acids Act as Inhibitors of the Matrix Metalloproteinase-2 and Matrix Metalloproteinase-9 Activity. Protein J. 2017, 36, 278–285. [Google Scholar] [CrossRef]
- Kaya, Z.; Leib, C.; Katus, H.A. Autoantibodies in heart failure and cardiac dysfunction. Circ. Res. 2012, 110, 145–158. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Huang, H.X.; Liu, P.; Du, Y.H.; Wang, P.; Wang, W.; Wu, Y.; Wang, L.; Ma, C.S.; Liu, H.R. β1-Adrenoceptor autoantibodies increase the susceptibility to ventricular arrhythmias involving abnormal repolarization in guinea-pigs. Exp. Physiol. 2017, 102, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Zuo, L.; Du, Y.; Ma, J.; Wang, K.; Zhao, Y.; Bai, F.; Wu, B.; Ma, X.; Liu, H. Pro-arrhythmic action of autoantibodies against the second extracellular loop of β1-adrenoceptor and its underlying molecular mechanisms. Int. J. Cardiol. 2015, 198, 251–258. [Google Scholar] [CrossRef]
- Bildyug, N. Extracellular matrix in regulation of contractile system in cardiomyocytes. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [Green Version]
- Yanai, R.; Mulki, L.; Hasegawa, E.; Takeuchi, K.; Sweigard, H.; Suzuki, J.; Gaissert, P.; Vavvas, D.G.; Sonoda, K.H.; Rothe, M.; et al. Cytochrome P450-generated metabolites derived from ω-3 fatty acids attenuate neovascularization. Proc. Natl. Acad. Sci. USA 2014, 111, 9603–9608. [Google Scholar] [CrossRef] [Green Version]
- Campbell, A.S.; Johnstone, S.R.; Baillie, G.S.; Smith, G. β-Adrenergic modulation of myocardial conduction velocity: Connexins versus sodium current. J. Mol. Cell. Cardiol. 2014, 77, 147–154. [Google Scholar] [CrossRef] [PubMed]
- O’Quinn, M.P.; Palatinus, J.A.; Harris, B.S.; Hewett, K.W.; Gourdie, R.G. A peptide mimetic of the connexin43 carboxyl terminus reduces gap junction remodeling and induced arrhythmia following ventricular injury. Circ. Res. 2011, 108, 704–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohutova, J.; Elsnicova, B.; Holzerova, K.; Neckar, J.; Sebesta, O.; Jezkova, J.; Vecka, M.; Vebr, P.; Hornikova, D.; Bacova, B.S.; et al. Anti-arrhythmic cardiac phenotype elicited by chronic intermittent hypoxia is associated with alterations in connexin-43 expression, phosphorylation, and distribution. Front. Endocrinol. (Lausanne) 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ek-Vitorin, J.F.; King, T.J.; Heyman, N.S.; Lampe, P.D.; Burt, J.M. Selectivity of connexin 43 channels is regulated through protein kinase C-dependent phosphorylation. Circ. Res. 2006, 98, 1498–1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salameh, A.; Dhein, S. Adrenergic control of cardiac gap junction function and expression. Naunyn. Schmiedebergs. Arch. Pharmacol. 2011, 383, 331–346. [Google Scholar] [CrossRef]
- Danik, S.B.; Liu, F.; Zhang, J.; Suk, H.J.; Morley, G.E.; Fishman, G.I.; Gutstein, D.E. Modulation of cardiac gap junction expression and arrhythmic susceptibility. Circ. Res. 2004, 95, 1035–1041. [Google Scholar] [CrossRef] [Green Version]
- Dhillon, P.S.; Gray, R.; Kojodjojo, P.; Jabr, R.; Chowdhury, R.; Fry, C.H.; Peters, N.S. Relationship between gap-junctional conductance and conduction velocity in mammalian myocardium. Circ. Arrhythmia Electrophysiol. 2013, 6, 1208–1214. [Google Scholar] [CrossRef] [Green Version]
- Salameh, A.; Krautblatter, S.; Karl, S.; Blanke, K.; Gomez, D.R.; Dhein, S.; Pfeiffer, D.; Janousek, J. The signal transduction cascade regulating the expression of the gap junction protein connexin43 by β-adrenoceptors. Br. J. Pharmacol. 2009, 158, 198–208. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, E.; Tian, Q.; Wagner, M.; Barth, M.; Xian, W.; Schröder, L.; Ruppenthal, S.; Kaestner, L.; Boehm, U.; Wartenberg, P.; et al. DREADD technology reveals major impact of Gq signaling on cardiac electrophysiology. Cardiovasc. Res. 2019, 115, 1052–1066. [Google Scholar] [CrossRef]
- Sabri, A.; Steinberg, S.F. Protein kinase C isoform-selective signals that lead to cardiac hypertrophy and the progression of heart failure. Mol. Cell. Biochem. 2003, 251, 97–101. [Google Scholar] [CrossRef]
- Rybin, V.O.; Sabri, A.; Short, J.; Braz, J.C.; Molkentin, J.D.; Steinberg, S.F. Cross-regulation of novel protein kinase C (PKC) isoform function in cardiomyocytes: Role of PKCε in activation loop phosphorylations and PKCδ in hydrophobic motif phosphorylations. J. Biol. Chem. 2003, 278, 14555–14564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palaniyandi, S.S.; Sun, L.; Ferreira, J.C.B.; Mochly-Rosen, D. Protein kinase C in heart failure: A therapeutic target? Cardiovasc. Res. 2009, 82, 229–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinberg, S.F. Distinctive activation mechanisms and functions for protein kinase Cδ. Biochem. J. 2004, 384, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Haberland, A.; Wallukat, G.; Dahmen, C.; Kage, A.; Schimke, I. Aptamer neutralization of beta1-adrenoceptor autoantibodies isolated from patients with cardiomyopathies. Circ. Res. 2011, 109, 986–992. [Google Scholar] [CrossRef] [Green Version]
- Wallukat, G.; Muñoz Saravia, S.G.; Haberland, A.; Bartel, S.; Araujo, R.; Valda, G.; Duchen, D.; Diaz Ramirez, I.; Borges, A.C.; Schimke, I. Distinct Patterns of Autoantibodies Against G-Protein-Coupled Receptors in Chagas’ Cardiomyopathy and Megacolon. Their Potential Impact for Early Risk Assessment in Asymptomatic Chagas’ Patients. J. Am. Coll. Cardiol. 2010, 55, 463–468. [Google Scholar] [CrossRef] [Green Version]
- Tribulová, N.; Bačová, B.; Radošinská, J.; Viczenczová, C.; Knezl, V.; Dosenko, V.; Benova, T.; Navarová, J.; Gonçalvesová, E.; van Rooyen, J.; et al. Up-regulation of myocardial connexin-43 in spontaneously hypertensive rats fed red palm oil is most likely implicated in its anti-arrhythmic effects. Can. J. Physiol. Pharmacol. 2012, 90, 1235–1245. [Google Scholar]
- Wallukat, G.; Wollenberger, A. Effects of the serum gamma globulin fraction of patients with allergic asthma and dilated cardiomyopathy on chronotropic beta adrenoceptor function in cultured neonatal rat heart myocytes. Biomed. Biochim. Acta 1987, 46. [Google Scholar]
- Wess, G.; Wallukat, G.; Fritscher, A.; Becker, N.P.; Wenzel, K.; Müller, J.; Schimke, I. Doberman pinschers present autoimmunity associated with functional autoantibodies: A model to study the autoimmune background of human dilated cardiomyopathy. PLoS ONE 2019, 14, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Barancik, M.; Bohacova, V.; Gibalova, L.; Sedlak, J.; Sulova, Z.; Breier, A. Potentiation of anticancer drugs: Effects of pentoxifylline on neoplastic cells. Int. J. Mol. Sci. 2012, 13, 369–382. [Google Scholar] [CrossRef] [Green Version]
- Tribulova, N.; Knezl, V.; Szeiffova Bacova, B.; Egan Benova, T.; Viczenczova, C.; Gonçalvesova, E.; Slezak, J. Disordered myocardial Ca2+ homeostasis results in substructural alterations that may promote occurrence of malignant arrhythmias. Physiol. Res. 2016, 65, S139–S148. [Google Scholar]
- Sykora, M.; Kamocsaiova, L.; Egan Benova, T.; Frimmel, K.; Ujhazy, E.; Mach, M.; Barancik, M.; Tribulova, N.; Szeiffova Bacova, B. Alterations in myocardial connexin-43 and matrix metalloproteinase-2 signaling in response to pregnancy and oxygen deprivation of wistar rats: A pilot study. Can. J. Physiol. Pharmacol. 2019, 97, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Benova, T.; Viczenczova, C.; Radosinska, J.; Bacova, B.; Knezl, V.; Dosenko, V.; Weismann, P.; Zeman, M.; Navarova, J.; Tribulova, N. Melatonin attenuates hypertension-related proarrhythmic myocardial maladaptation of connexin-43 and propensity of the heart to lethalarrhythmias. Can. J. Physiol. Pharmacol. 2013, 91, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Podlowski, S.; Luther, H.P.; Morwinski, R.; Müller, J.; Wallukat, G. Agonistic anti-β1-adrenergic receptor autoantibodies from cardiomyopathy patients reduce the β1-adrenergic receptor expression in neonatal rat cardiomyocytes. Circulation 1998, 98, 2470–2476. [Google Scholar] [CrossRef] [Green Version]
- Szeiffová Bačova, B.; Egan Beňová, T.; Viczenczová, C.; Soukup, T.; Raučhová, H.; Pavelka, S.; Knezl, V.; Barancík, M.; Tribulová, N. Cardiac connexin-43 and PKC signaling in rats with altered thyroid status without and with omega-3 fatty acids intake. Physiol. Res. 2016, 65, S77–S90. [Google Scholar] [PubMed]







| Variables—Male | WISc | WISo3 | SHRc | SHRo3 |
| BP (mmHg) | 111.74 ± 3.52 | 95.93 ± 4.47 * | 178.77 ± 3.83 * | 165.27 ± 3.98 |
| BW (g) | 467.17 ± 17.95 | 428.17 ± 6.81 | 329.50 ± 8.37 * | 339.50 ± 6.69 |
| HW (g) | 1.18 ± 0.04 | 1.13 ± 0.02 | 1.41 ± 0.03 * | 1.35 ± 0.04 |
| LVW (g) | 0.81 ± 0.01 | 0.83 ± 0.02 | 1.16 ± 0.02 * | 1.12 ± 0.03 |
| Variables—female | WISc | WISo3 | SHRc | SHRo3 |
| BP (mmHg) | 101.18 ± 2.23 | 98.83 ± 2.91 | 187.33 ± 7.34 * | 172.83 ± 7.02 |
| BW (g) | 266.83 ± 8.18 | 295.38 ± 10.05 | 213.00 ± 5.94 * | 216.33 ± 4.97 |
| HW (g) | 0.83 ± 0.02 | 0.86 ± 0.02 | 1.31 ± 0.04 * | 1.13 ± 0.04 # |
| LVW (g) | 0.59 ± 0.01 | 0.60 ± 0.01 | 1.01 ± 0.03 * | 0.92 ± 0.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szeiffova Bacova, B.; Radosinska, J.; Wallukat, G.; Barancik, M.; Wallukat, A.; Knezl, V.; Sykora, M.; Paulis, L.; Tribulova, N. Suppression of β1-Adrenoceptor Autoantibodies is Involved in the Antiarrhythmic Effects of Omega-3 Fatty Acids in Male and Female Hypertensive Rats. Int. J. Mol. Sci. 2020, 21, 526. https://doi.org/10.3390/ijms21020526
Szeiffova Bacova B, Radosinska J, Wallukat G, Barancik M, Wallukat A, Knezl V, Sykora M, Paulis L, Tribulova N. Suppression of β1-Adrenoceptor Autoantibodies is Involved in the Antiarrhythmic Effects of Omega-3 Fatty Acids in Male and Female Hypertensive Rats. International Journal of Molecular Sciences. 2020; 21(2):526. https://doi.org/10.3390/ijms21020526
Chicago/Turabian StyleSzeiffova Bacova, Barbara, Jana Radosinska, Gerd Wallukat, Miroslav Barancik, Anne Wallukat, Vladimir Knezl, Matus Sykora, Ludovit Paulis, and Narcis Tribulova. 2020. "Suppression of β1-Adrenoceptor Autoantibodies is Involved in the Antiarrhythmic Effects of Omega-3 Fatty Acids in Male and Female Hypertensive Rats" International Journal of Molecular Sciences 21, no. 2: 526. https://doi.org/10.3390/ijms21020526
APA StyleSzeiffova Bacova, B., Radosinska, J., Wallukat, G., Barancik, M., Wallukat, A., Knezl, V., Sykora, M., Paulis, L., & Tribulova, N. (2020). Suppression of β1-Adrenoceptor Autoantibodies is Involved in the Antiarrhythmic Effects of Omega-3 Fatty Acids in Male and Female Hypertensive Rats. International Journal of Molecular Sciences, 21(2), 526. https://doi.org/10.3390/ijms21020526





