Cadmium Pathways in Snails Follow a Complementary Strategy between Metallothionein Detoxification and Auxiliary Inactivation by Phytochelatins
Abstract
:1. Introduction
2. Results and Discussion
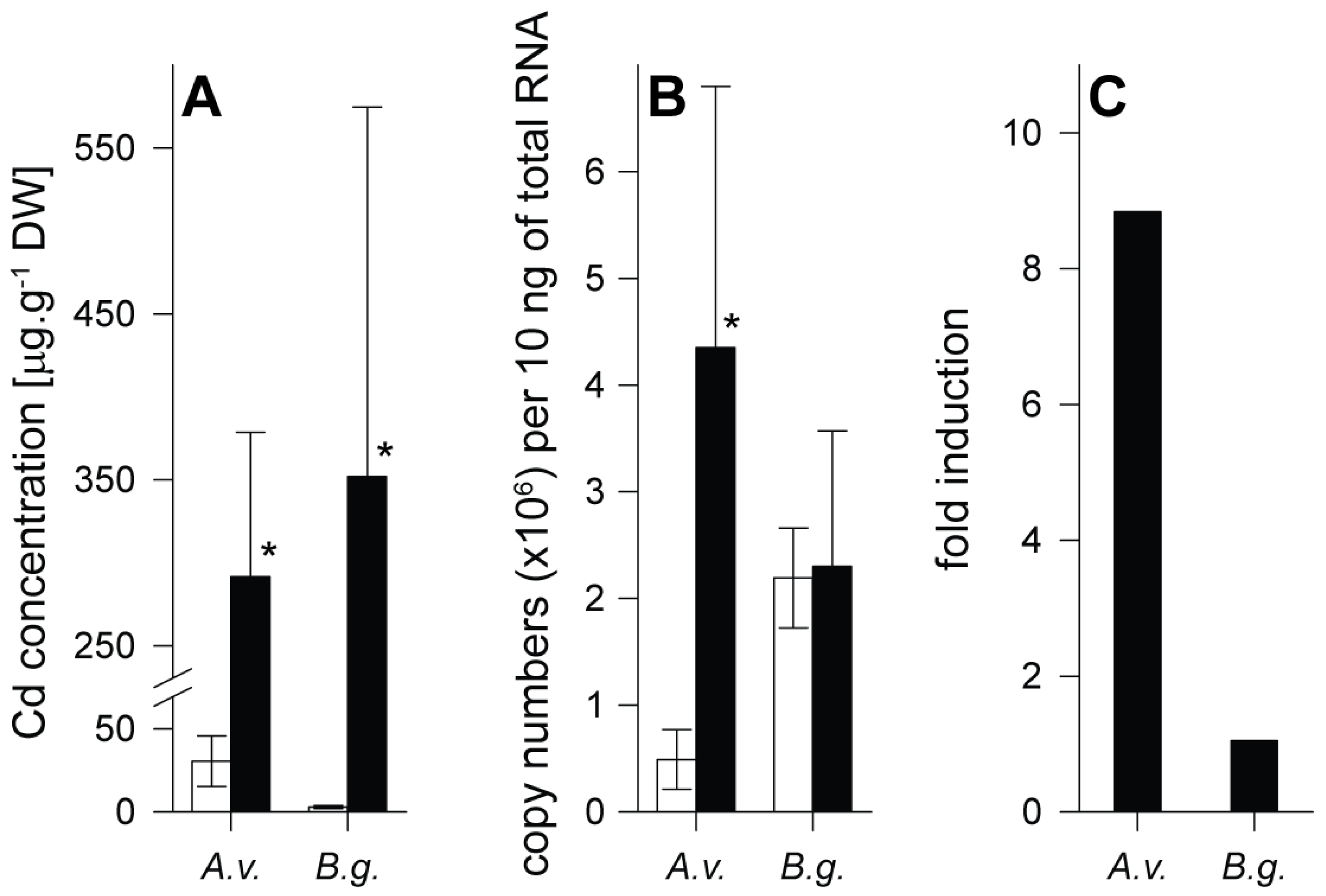
2.1. Species-Specific Differences in Cd Uptake and the Role of Metallothioneins
2.2. Transcriptomic Analyses of Gastropod Phytochelatin Synthase mRNA Transcripts
2.3. Metallothioneins versus Phytochelatins
2.4. Methodological Considerations
3. Material and Methods
3.1. Transcriptome Generation and Screening for Phytochelatins Synthase Genes
3.2. Cd Exposure and Tissue Analysis
3.3. Determination of Phytochelatins
3.4. Mass Spectroscopy
3.5. Calculation of Cd Fractions Bound to MT and to PC
3.6. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Oehlmann, J.; Schulte-Oehlmann, U. Molluscs as bioindicators. In Bioindicators & Biomonitors: Principles, Concepts, and Applications; Markert, B.A., Breure, A.M., Zechmeister, H.G., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2002; pp. 577–635. [Google Scholar]
- Dallinger, R.; Berger, B.; Triebskorn-Köhler, R.; Köhler, H.-R. Soil Biology and Ecotoxicology. In The Biology of Terrestrial Molluscs; Barker, G., Ed.; CAB International: Wallingford, UK, 2001; pp. 489–525. [Google Scholar]
- Pedrini-Martha, V.; Schnegg, R.; Baurand, P.E.; deVaufleury, A.; Dallinger, R. The physiological role and toxicological significance of the non-metal-selective cadmium/copper-metallothionein isoform differ between embryonic and adult helicid snails. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 199, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Egg, M.; Höckner, M.; Brandstätter, A.; Schuler, D.; Dallinger, R. Structural and bioinformatic analysis of the Roman snail Cd-Metallothionein gene uncovers molecular adaptation towards plasticity in coping with multifarious environmental stress. Mol. Ecol. 2009, 18, 2426–2443. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, M.; Bofill, R.; Palacios, Ò.; Atrian, S. State-of-the-art of metallothioneins at the beginning of the 21st century. Coord. Chem. Rev. 2012, 256, 46–62. [Google Scholar] [CrossRef]
- Kojima, Y. Definitions and nomenclature of metallothioneins. Methods Enzymol. 1991, 205, 8–10. [Google Scholar]
- Kägi, J.H.R.; Schäffer, A. Biochemistry of Metallothionein. Biochemistry 1988, 27, 8509–8515. [Google Scholar] [CrossRef]
- Meloni, G.; Zovo, K.; Kazantseva, J.; Palumaa, P.; Vasak, M. Organization and Assembly of Metal-Thiolate Clusters in Epithelium-specific Metallothionein-4*. J. Biol. Chem. 2006, 281, 14588–14595. [Google Scholar] [CrossRef] [Green Version]
- Kägi, J.H.R. Evolution, structure and chemical activity of class I metallothioneins: An overview. In Metallothionein III: Biological Roles and Medical Implications, Proceedings of the Metallothionein International Conference, 1992, Tsukuba, Japan; Birkhauser Verlag: Boston, MA, USA, 1993; Volume 3, pp. 29–56. [Google Scholar]
- Baumann, C.; Beil, A.; Jurt, S.; Niederwanger, M.; Palacios, O.; Capdevila, M.; Atrian, S.; Dallinger, R.; Zerbe, O. Structural Adaptation of a Protein to Increased Metal Stress: NMR Structure of a Marine Snail Metallothionein with an Additional Domain. Angew. Chem. Int. Ed. 2017, 56, 4617–4622. [Google Scholar] [CrossRef]
- Schmielau, L.; Dvorak, M.; Niederwanger, M.; Dobieszewski, N.; Pedrini-Martha, V.; Ladurner, P.; Rodríguez-Guerra Pedregal, J.; Maréchal, J.D.; Dallinger, R. Differential response to cadmium exposure by expression of a two and a three-domain metallothionein isoform in the land winkle Pomatias elegans: Valuating the marine heritage of a land snail. Sci. Total Environ. 2019, 648, 561–571. [Google Scholar] [CrossRef]
- Kägi, J.H.R. Overview of Metallothionein. Methods Enzymol. 1991, 205, 613–626. [Google Scholar]
- Roesijadi, G. Metallothionein Induction as a Measure of Response to Metal Exposure in Aquatic Animals. Environ. Health Perspect. 1994, 102, 91–95. [Google Scholar] [CrossRef] [Green Version]
- Klaassen, C.D.; Liu, J.; Diwan, B.A. Metallothionein protection of cadmium toxicity. Toxicol. Appl. Pharmacol. 2009, 238, 215–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stillman, M.J.; Zelazowski, A.J. Domain-specificity of Cd2+ and Zn2+ binding to rabbit liver metallothionein 2. Biochem. J. 1989, 262, 181–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Otvos, J.D. 111Cd NMR Studies of the Domain Specificity of Ag+ and Cu+ Binding to. Biochemistry 1996, 35, 13929–13936. [Google Scholar] [CrossRef] [PubMed]
- Tio, L.; Villarreal, L.; Atrian, S.; Capdevila, M. Functional Differentiation in the Mammalian Metallothionein. J. Biol. Chem. 2004, 279, 24403–24413. [Google Scholar]
- Kägi, J.H.R.; Kojima, Y. Chemistry and biochemistry of metallothionein. In Metallothionein II. Experientia Supplementum 52; Kägi, J.H.R., Kojima, Y., Eds.; Birkhäuser Verlag: Basel, Switzerland, 1987; Volume 52, pp. 25–61. [Google Scholar]
- Vasak, M. Standard Isolation Procedure for Metallothioneins. Methods Enzymol. 1991, 205, 41–44. [Google Scholar] [PubMed]
- Krężel, A.; Maret, W. The functions of metamorphic metallothioneins in zinc and copper metabolism. Int. J. Mol. Sci. 2017, 18, 1237. [Google Scholar] [CrossRef] [Green Version]
- Dallinger, R.; Berger, B.; Hunziker, P.; Kägi, J.H.R. Metallothionein in snail Cd and Cu metabolism. Nature 1997, 388, 237–238. [Google Scholar] [CrossRef]
- Palacios, Ò.; Pagani, A.; Pérez-Rafael, S.; Egg, M.; Höckner, M.; Brandstätter, A.; Capdevila, M.; Atrian, S.; Dallinger, R. Shaping mechanisms of metal specificity in a family of metazoan metallothioneins: Evolutionary differentiation of mollusc metallothioneins. BMC Biol. 2011, 9, 4. [Google Scholar] [CrossRef] [Green Version]
- Benito, D.; Niederwanger, M.; Izagirre, U.; Dallinger, R.; Soto, M. Successive onset of molecular, cellular and tissue-specific responses in midgut gland of Littorina littorea exposed to sub-lethal cadmium concentrations. Int. J. Mol. Sci. 2017, 18, 1815. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, S.F.; Davies, S.K.; Bennett, M.; Raab, A.; Feldmann, J.; Kille, P.; Loureiro, S.; Spurgeon, D.J.; Bundy, J.G. Sub-lethal cadmium exposure increases phytochelatin concentrations in the aquatic snail Lymnaea stagnalis. Sci. Total Environ. 2016, 568, 1054–1058. [Google Scholar]
- Blum, R.; Beck, A.; Korte, A.; Stengel, A.; Letzel, T.; Lendzian, K.; Grill, E. Function of phytochelatin synthase in catabolism of glutathione-conjugates. Plant J. 2007, 49, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Giovanelli, J.; Mudd, S.H.; Datko, A.H. Sulfur Amino Acids in Plants. In The Biochemistry of Plants; Academic Press, Inc.: Cambridge, MA, USA, 1980; Volume 5, pp. 453–505. [Google Scholar]
- Kawakami, S.K.; Gledhill, M.; Achterberg, E.P. Determination of phytochelatins and glutathione in phytoplankton from natural waters using HPLC with fluorescence detection. Trends Anal. Chem. 2006, 25, 133–142. [Google Scholar] [CrossRef]
- Cobbett, C.S. Phytochelatins and Their Roles in Heavy Metal Detoxification. Plant Physiol. 2000, 123, 825–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bundy, J.G.; Kille, P.; Liebeke, M.; Spurgeon, D.J. Metallothioneins may not be enough—The role of phytochelatins in invertebrate metal detoxification. Environ. Sci. Technol. 2014, 48, 885–886. [Google Scholar] [CrossRef] [Green Version]
- Clemens, S.; Schroeder, J.I.; Degenkolb, T. Caenorhabditis elegans expresses a functional phytochelatin synthase. Eur. J. Biochem. 2001, 268, 3640–3643. [Google Scholar] [CrossRef]
- Vatamaniuk, O.K.; Bucher, E.A.; Ward, J.T.; Rea, P.A. A new pathway for heavy metal detoxification in animals. Phytochelatin synthase is required for cadmium tolerance in Caenorhabditis elegans. J. Biol. Chem. 2001, 276, 20817–20820. [Google Scholar] [CrossRef] [Green Version]
- Bundy, J.G.; Kille, P. Metabolites and metals in Metazoa-what role do phytochelatins play in animals? Metallomics 2014, 6, 1576–1582. [Google Scholar] [CrossRef] [Green Version]
- Dallinger, R.; Berger, B.; Gruber, C.; Hunziker, P.; Sturzenbaum, S. Metallothioneins in terrestrial invertebrates: Structural aspects, biological significance and implications for their use as biomarkers. Cell. Mol. Biol. 2000, 46, 331–346. [Google Scholar]
- Dallinger, R.; Chabicovsky, M.; Berger, B. Isoform-specific quantification of metallothionein in the terrestrial gastropod Helix pomatia I. Molecular, biochemical, and methodical background. Environ. Toxicol. Chem. 2004, 23, 890–901. [Google Scholar] [CrossRef]
- Chabicovsky, M.; Klepal, W.; Dallinger, R. Mechanisms of cadmium toxicity in terrestrial pulmonates: Programmed cell death and metallothionein overload. Environ. Toxicol. Chem. 2004, 23, 648–655. [Google Scholar] [CrossRef]
- Palacios, Ò.; Jiménez-Martí, E.; Niederwanger, M.; Gil-Moreno, S.; Zerbe, O.; Atrian, S.; Dallinger, R.; Capdevila, M. Analysis of metal-binding features of the wild type and two domain-truncated mutant variants of Littorina littorea metallothionein reveals its cd-specific character. Int. J. Mol. Sci. 2017, 18, 1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niederwanger, M.; Dvorak, M.; Schnegg, R.; Pedrini-martha, V.; Bacher, K.; Bidoli, M.; Dallinger, R. Challenging the Metallothionein (MT) Gene of Biomphalaria glabrata: Unexpected Response Patterns Due to Cadmium Exposure and Temperature Stress. Int. J. Mol. Sci. 2017, 18, 1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gnatyshyna, L.; Falfushynska, H.; Bodilovska, O.; Oleynik, O.; Golubev, A.; Stoliar, O. Metallothionein and glutathione in Lymnaea stagnalis determine the specificity of responses to the effects of ionising radiation. Radioprotection 2012, 47, 231–242. [Google Scholar] [CrossRef] [Green Version]
- Dvorak, M.; Lackner, R.; Niederwanger, M.; Rotondo, C.; Schnegg, R.; Ladurner, P.; Pedrini-Martha, V.; Salvenmoser, W.; Kremser, L.; Lindner, H.; et al. Metal binding functions of metallothioneins in the slug Arion vulgaris differ from metal-specific isoforms of terrestrial snails. Metallomics 2018, 10, 1638–1654. [Google Scholar] [CrossRef]
- Niederwanger, M.; Calatayud, S.; Zerbe, O.; Atrian, S.; Albalat, R.; Capdevila, M.; Palacios, Ò.; Dallinger, R. Biomphalaria glabrata metallothionein: Lacking metal specificity of the protein and missing gene upregulation suggest metal sequestration by exchange instead of through selective binding. Int. J. Mol. Sci. 2017, 18, 1457. [Google Scholar] [CrossRef] [Green Version]
- Romiguier, J.; Gayral, P.; Ballenghien, M.; Bernard, A.; Cahais, V.; Chenuil, A.; Chiari, Y.; Dernat, R.; Duret, L.; Faivre, N.; et al. Comparative population genomics in animals uncovers the determinants of genetic diversity. Nature 2014, 515, 261–263. [Google Scholar] [CrossRef]
- Grill, E.; Löffler, S.; Winnacker, E.-L.; Zenk, M.H. Phytochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific γ-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proc. Natl. Acad. Sci. USA 1989, 86, 6838–6842. [Google Scholar] [CrossRef] [Green Version]
- Ha, S.; Smith, A.P.; Howden, R.; Dietrich, W.M.; Bugg, S.; O’Connell, M.J.; Goldsbrough, P.B.; Cobbett, C.S. Phytochelatin Synthase Genes from Arabidopsis and the Yeast Schizosaccharomyces pombe. Plant Cell 1999, 11, 1153–1163. [Google Scholar] [CrossRef]
- Rodrigo, M.A.M.; Anjum, N.A.; Heger, Z.; Zitka, O.; Vojtech, A.; Pereira, E.; Kizek, R. Role of Phytochelatins in Redox Caused Stress in Plants and Animals. In Agricultural and Biological Sciences “Abiotic and Biotic Stress in Plants—Recent Advances and Future Perspectives”; Shanker, A.K., Shanker, C., Eds.; IntechOpen Ltd.: London, UK, 2016. [Google Scholar]
- Dennis, K.K.; Uppal, K.; Liu, K.H.; Ma, C.; Liang, B.; Go, Y.-M.; Jones, D.P. Phytochelatin database: A resource for phytochelatin complexes of nutritional and environmental metals. Database 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Chabicovsky, M.; Niederstätter, H.; Thaler, R.; Hödl, E.; Parson, W.; Rossmanith, W.; Dallinger, R. Localization and quantification of Cd- and Cu-specific metallothionein isoform mRNA in cells and organs of the terrestrial gastropod Helix pomatia. Toxicol. Appl. Pharmacol. 2003, 190, 25–36. [Google Scholar] [CrossRef]
- Chekmeneva, E.; Prohens, R.; Díaz-Cruz, J.M.; Ariño, C.; Esteban, M. Competitive Binding of Cd and Zn with the Phytochelatin (γ-Glu-Cys)4-Gly: Comparative Study by Mass Spectrometry, Voltammetry-Multivariate Curve Resolution, and Isothermal Titration Calorimetry. Environ. Sci. Technol. 2008, 42, 2860–2866. [Google Scholar] [CrossRef] [PubMed]
- Viarengo, A.; Ponzano, E.; Dondero, F.; Fabbri, R. A Simple Spectrophotometric Method for Metallothionein Evaluation in Marine Organisms: An Application to Mediterranean and Antarctic Molluscs. Mar. Environ. Res. 1997, 44, 69–84. [Google Scholar] [CrossRef]
- Wepener, V.; van Vuren, J.H.J.; Chatiza, F.P.; Mbizi, Z.; Slabbert, L.; Masola, B. Active biomonitoring in freshwater environments: Early warning signals from biomarkers in assessing biological effects of diffuse sources of pollutants. Phys. Chem. Earth 2005, 30, 751–761. [Google Scholar] [CrossRef]
- Brown, R.J.; Galloway, T.S.; Lowe, D.; Browne, M.A.; Dissanayake, A.; Jones, M.B.; Depledge, M.H. Differential sensitivity of three marine invertebrates to copper assessed using multiple biomarkers. Aquat. Toxicol. 2004, 66, 267–278. [Google Scholar] [CrossRef]
- Ureña, R.; Bebianno, M.J.; del Ramo, J.; Torreblanca, A. Ecotoxicology and Environmental Safety Metallothionein in the freshwater gastropod Melanopsis dufouri chronically exposed to cadmium: A methodological approach. Ecotoxicol. Environ. Saf. 2010, 73, 779–787. [Google Scholar] [CrossRef]
- Scheuhammer, A.M.; Cherian, M.G. Quantification of Metallothionein by Silver Saturation. Methods Enzymol. 1991, 205, 78–83. [Google Scholar]
- Leung, K.M.Y.; Ibrahim, H.; Dewhurst, R.E.; Morley, N.J.; Crane, M.; Lewis, J.W. Concentrations of Metallothionein-Like Proteins and Heavy Metals in the Freshwater Snail Lymnaea stagnalis Exposed to Different Levels of Waterborne Cadmium. Bull. Environ. Contam. Toxicol. 2003, 71, 1084–1090. [Google Scholar] [CrossRef]
- Leung, K.M.Y.; Furness, R.W. Induction of Metallothionein in Dogwhelk Nucella lapillus during and after Exposure to Cadmium. Ecotoxicol. Environ. Saf. 1999, 43, 156–164. [Google Scholar] [CrossRef]
- Leung, K.M.Y.; Svavarsson, J.; Crane, M. Influence of static and fluctuating salinity on cadmium uptake and metallothionein expression by the dogwhelk Nucella lapillus (L.). J. Exp. Mar. Bio. Ecol. 2002, 274, 175–189. [Google Scholar] [CrossRef]
- Berger, B.; Dallinger, R.; Thomaser, A. Quantification of metallothionein as a biomarker for cadmium exposure in terrestrial gastropods. Environ. Toxicol. Chem. 1995, 14, 781–791. [Google Scholar] [CrossRef]
- Dallinger, R.; Lagg, B.; Egg, M.; Schipflinger, R.; Chabicovsky, M. Cd accumulation and Cd-metallothionein as a biomarker in Cepaea hortensis (Helicidae, Pulmonata) from laboratory exposure and metal-polluted habitats. Ecotoxicology 2004, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Siboni, N.; Fine, M.; Bresler, V.; Loya, Y. Coastal coal pollution increases Cd concentrations in the predatory gastropod Hexaplex trunculus and is detrimental to its health. Mar. Pollut. Bull. 2004, 49, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Downs, C.A.; Dillon, R.T.J.; Fauth, J.E.; Woodley, C.M. A molecular biomarker system for assessing the health of gastropods (Ilyanassa obsoleta) exposed to natural and anthropogenic stressors. J. Exp. Mar. Biol. Ecol. 2001, 259, 189–214. [Google Scholar] [CrossRef]
- Pedrini-Martha, V.; Niederwanger, M.; Kopp, R.; Schnegg, R.; Dallinger, R. Physiological, diurnal and stress-related variability of cadmium-metallothionein gene expression in land snails. PLoS ONE 2016, 11, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef]
- Morgulis, A.; Coulouris, G.; Raytselis, Y.; Madden, T.L.; Agarwala, R.; Schäffer, A.A. Database Indexing for Production MegaBLAST Searches. Bioinformatics 2008, 24, 1757–1764. [Google Scholar] [CrossRef]





| Id | Name | Clade | Habitat | Source | Reading Frame |
|---|---|---|---|---|---|
| M.c. | Marisa cornuarietis | Caenogastropoda | Freshwater | This study | Complete |
| P.b. | Pomacea bridgesii | Caenogastropoda | Freshwater | This study | Complete |
| P.c. | Pomacea canaliculata | Caenogastropoda | Freshwater | NCBI XP_025099563.1 | Complete |
| A.h. | Anentome helena | Caenogastropoda | Freshwater | This study | Complete |
| P.e. | Pomatias elegans | Caenogastropoda | Terrestrial | This study | Missing stop codon |
| A.c. | Aplysia californica | Heterobranchia | Marine | NCBI XM_005110788.2 | Complete |
| E.c. | Elysia crispata | Heterobranchia | Marine | This study | Complete |
| B.g. | Biomphalaria glabrata | Heterobranchia Hygrophila | Freshwater | NCBI XM_013214798.1 | Complete |
| G.t. | Galba truncatula | Heterobranchia Hygrophila | Freshwater | Romiguier et al. (2014) [41] | Complete |
| L.m. | Limax maximus | Heterobranchia Stylommatophora | Terrestrial | This study | Complete |
| C.a. | Cornu aspersum | Heterobranchia Stylommatophora | Terrestrial | This study | Complete |
| H.p. | Helix pomatia | Heterobranchia Stylommatophora | Terrestrial | This study | Complete |
| A.b. | Alinda biplicata | Heterobranchia Stylommatophora | Terrestrial | This study | Complete |
| A.v. | Arion vulgaris | Heterobranchia Stylommatophora | Terrestrial | NCBI PRJEB7891 | Missing start and stop codon |
| P.v. | Patella vulgata | Patellogastropoda | Marine | This study | Complete |
| N.p. | Neritina pulligera | Neritimorpha | Freshwater | This study | Complete |
| T.l. | Titiscania limacina | Neritimorpha Cycloneritimorpha | Marine | NCBI PRJNA253054 | Missing stop codon |
| Species | Treatment | Equivalents of Cys [µmol/g Dry wt.] Available for Cd2+ Binding | |
|---|---|---|---|
| MT | PC | ||
| Arion vulgaris | Control | 0.12 | <0.01 |
| Cd-exposed | 3.81 | <0.01 | |
| Biomphalaria glabrata1 | Control | 0.04 | 0.12 |
| Cd-exposed | 2.18 | 0.55 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dvorak, M.; Schnegg, R.; Niederwanger, M.; Pedrini-Martha, V.; Ladurner, P.; Lindner, H.; Kremser, L.; Lackner, R.; Dallinger, R. Cadmium Pathways in Snails Follow a Complementary Strategy between Metallothionein Detoxification and Auxiliary Inactivation by Phytochelatins. Int. J. Mol. Sci. 2020, 21, 7. https://doi.org/10.3390/ijms21010007
Dvorak M, Schnegg R, Niederwanger M, Pedrini-Martha V, Ladurner P, Lindner H, Kremser L, Lackner R, Dallinger R. Cadmium Pathways in Snails Follow a Complementary Strategy between Metallothionein Detoxification and Auxiliary Inactivation by Phytochelatins. International Journal of Molecular Sciences. 2020; 21(1):7. https://doi.org/10.3390/ijms21010007
Chicago/Turabian StyleDvorak, Martin, Raimund Schnegg, Michael Niederwanger, Veronika Pedrini-Martha, Peter Ladurner, Herbert Lindner, Leopold Kremser, Reinhard Lackner, and Reinhard Dallinger. 2020. "Cadmium Pathways in Snails Follow a Complementary Strategy between Metallothionein Detoxification and Auxiliary Inactivation by Phytochelatins" International Journal of Molecular Sciences 21, no. 1: 7. https://doi.org/10.3390/ijms21010007





