The Proliferation and Differentiation of Adipose-Derived Stem Cells in Neovascularization and Angiogenesis
Abstract
:1. Introduction
2. Histological and Cellular Specificity and Plasticity of Adipose Tissue
3. Molecular Mechanisms Regulating Growth and Proliferation of Adipocytes
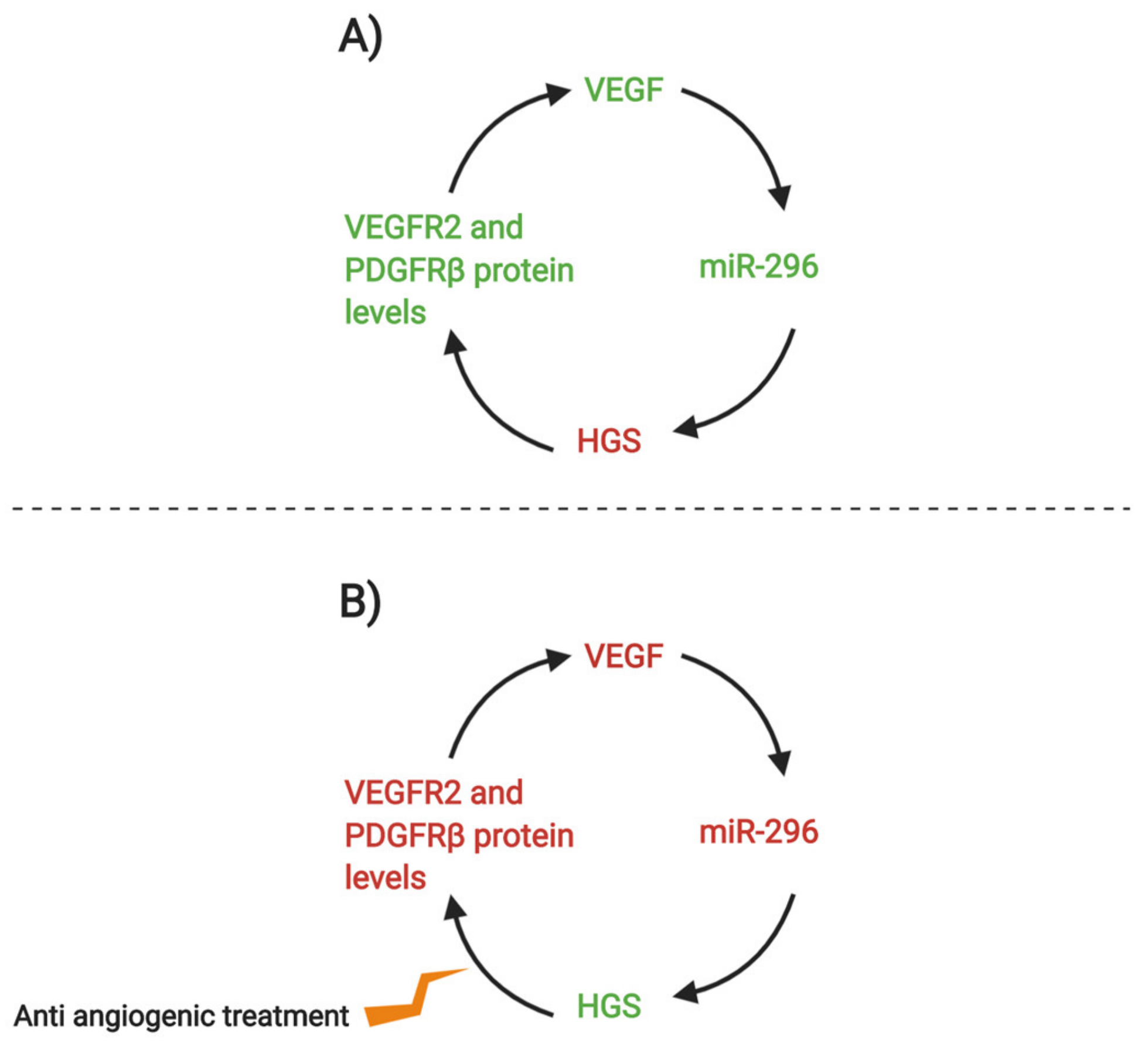
4. External Stimuli Regulating Proliferation of Adipocytes
5. Differentiation and Transdifferentiation of Adipocytes
6. External Stimuli Regulating Differentiation of Adipocytes
7. Molecular Mechanisms of Angiogenesis and Neovascularization
8. Angiogenesis
9. Neovascularization
10. Possible Relationship between Adipose-Derived Stem Cells and Neovascularization and Angiogenesis Processes—Recent Trials and Potential Clinical Applicability
11. Ischemic Heart Disease
12. Ischemic Cerebral Diseases
13. Ischemic Limb Disease
14. Allograft
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABCG2 | ATP-binding cassette subfamily G member 2 |
| ASC | Adipose-derived mesenchymal stem cell |
| BAT | Brown adipose tissue |
| CAM | Chick chorioallantoic membrane |
| EAT | Epicardial adipose tissue |
| G-CSF | Granulocyte-colony stimulating factor |
| hASC | Human adipose-derived mesenchymal stem cell |
| HGS | Hepatocyte growth factor-regulated tyrosine kinase substrate |
| HP | Heparin-Pluronic |
| IL-8 | Interleukin 8 |
| MSC | Mesenchymal stem cell |
| PPARγ | Peroxisome proliferator-activated receptor γ |
| PDGFR | Platelet-derived growth factor receptor |
| pim-1 | Serine/threonine kinases PIM-1 |
| Prh | Proline-rich homeodomain gene |
| SDF-1 | Stromal cell-derived factor 1 |
| VEGF | Vascular endothelial growth factor |
| VEGFR | Vascular endothelial growth factor receptor |
| WAT | White adipose tissue |
References
- Frese, L.; Dijkman, P.E.; Hoerstrup, S.P. Adipose Tissue-Derived Stem Cells in Regenerative Medicine. Transfus. Med. Hemother. 2016, 43, 268–274. [Google Scholar] [CrossRef]
- Ma, T.; Sun, J.; Zhao, Z.; Lei, W.; Chen, Y.; Wang, X.; Yang, J.; Shen, Z. A brief review: Adipose-derived stem cells and their therapeutic potential in cardiovascular diseases. Stem Cell Res. Ther. 2017, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Caplan, A.I. Mesenchymal stem cells: Time to change the name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef] [Green Version]
- Madonna, R.; de Caterina, R. In vitro neovasculogenic potential of resident adipose tissue precursors. Am. J. Physiol. Cell Physiol. 2008, 295, 1271–1280. [Google Scholar] [CrossRef] [Green Version]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Sarantopoulos, C.N.; Banyard, D.A.; Ziegler, M.E.; Sun, B.; Shaterian, A.; Widgerow, A.D. Elucidating the Preadipocyte and Its Role in Adipocyte Formation: A Comprehensive Review. Stem Cell Rev. Rep. 2018, 14, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, D.; Ushiyama, A.; Duda, D.G.; Xu, L.; Tam, J.; Krishna, V.; Chatterjee, K.; Garkavtsev, I.; Jain, R.K. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ Res. 2003, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Johnson, T.; Liu, D. Therapeutic angiogenesis of adipose-derived stem cells for ischemic diseases. Stem Cell Res. Ther. 2017, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.N.; Choi, J.H.; Park, J.S.; Jeon, S.Y.; Park, K.D.; Park, K.H. Differentiation of endothelial progenitor cells into endothelial cells byheparin-modified supramolecular pluronic nanogels encapsulating bFGF and complexed with VEGF165 genes. Biomaterials 2014, 35, 4716–4728. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kano, K.; Kondo, D.; Fukuda, N.; Iribe, Y.; Tanaka, N.; Matsubara, Y.; Sakuma, T.; Satomi, A.; Otaki, M.; et al. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J. Cell. Physiol. 2008, 215, 210–222. [Google Scholar] [CrossRef]
- Morigny, P.; Houssier, M.; Mouisel, E.; Langin, D. Adipocyte lipolysis and insulin resistance. Biochimie 2016, 125, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Wald, D.; Teucher, B.; Dinkel, J.; Kaaks, R.; Delorme, S.; Boeing, H.; Seidensaal, K.; Meinzer, H.P.; Heimann, T. Automatic quantification of subcutaneous and visceral adipose tissue from whole-body magnetic resonance images suitable for large cohort studies. J. Magn. Reson. Imaging 2012, 36, 1421–1434. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose tissue remodeling: Its role in energy metabolism and metabolic disorders. Front. Endocrinol. 2016, 7, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sztalryd, C.; Brasaemle, D.L. The perilipin family of lipid droplet proteins: Gatekeepers of intracellular lipolysis. Biochim. Biophys. Acta—Mol. Cell Biol. Lipids 2017, 1862, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.; Toselli, L.; Cava, E. Dietary intervention and nutritional counseling. Multidiscip. Approach Obes. 2015. [Google Scholar] [CrossRef]
- Zangi, L.; Oliveira, M.S.; Ye, L.Y.; Ma, Q.; Sultana, N.; Hadas, Y.; Chepurko, E.; Später, D.; Zhou, B.; Chew, W.L.; et al. Insulin-like growth factor 1 receptor-dependent pathway drives epicardial adipose tissue formation after myocardial injury. Circulation 2017, 135, 59–72. [Google Scholar] [CrossRef] [Green Version]
- Ansaldo, A.M.; Montecucco, F.; Sahebkar, A.; Dallegri, F.; Carbone, F. Epicardial adipose tissue and cardiovascular diseases. Int. J. Cardiol. 2019, 278, 254–260. [Google Scholar] [CrossRef]
- Cao, Y. Science in medicine Angiogenesis modulates adipogenesis and obesity. Diversity 2007, 117, 2362–2368. [Google Scholar] [CrossRef]
- Asano, A.; Morimatsu, M.; Nikami, H.; Yoshida, T.; Saito, M. Adrenergic activation of vascular endothelial growth factor mRNA expression in rat brown adipose tissue: Implication in cold-induced angiogenesis. Biochem. J. 1997, 328, 179–183. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Kitchen, C.M.; Streb, J.W.; Miano, J.M. Myocardin: A Component of a Molecular Switch for Smooth Muscle Differentiation. J. Mol. Cell. Cardiol. 2002, 34, 1345–1356. [Google Scholar] [CrossRef]
- Pusztaszeri, M.P.; Seelentag, W.; Bosman, F.T. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and FLI-1 in normal human tissues. J. Histochem. Cytochem. 2006, 54, 385–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalinina, N.; Kharlampieva, D.; Loguinova, M.; Butenko, I.; Pobeguts, O.; Efimenko, A.; Ageeva, L.; Sharonov, G.; Ischenko, D.; Alekseev, D.; et al. Characterization of secretomes provides evidence for adipose-derived mesenchymal stromal cells subtypes. Stem Cell Res. Ther. 2015, 6, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartwig, S.; De Filippo, E.; Göddeke, S.; Knebel, B.; Kotzka, J.; Al-Hasani, H.; Roden, M.; Lehr, S.; Sell, H. Exosomal proteins constitute an essential part of the human adipose tissue secretome. Biochim. Biophys. Acta—Proteins Proteom. 2019, 1867, 140172. [Google Scholar] [CrossRef] [PubMed]
- Dubey, N.K.; Mishra, V.K.; Dubey, R.; Deng, Y.H.; Tsai, F.C.; Deng, W.P. Revisiting the advances in isolation, characterization and secretome of adipose-derived stromal/stem cells. Int. J. Mol. Sci. 2018, 19, 2200. [Google Scholar] [CrossRef] [Green Version]
- Sbrana, F.V.; Cortini, M.; Avnet, S.; Perut, F.; Columbaro, M.; De Milito, A.; Baldini, N. The Role of Autophagy in the Maintenance of Stemness and Differentiation of Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2016, 12, 621–633. [Google Scholar] [CrossRef]
- Yamada, Y.; Wang, X.D.; Yokoyama, S.I.; Fukuda, N.; Takakura, N. Cardiac progenitor cells in brown adipose tissue repaired damaged myocardium. Biochem. Biophys. Res. Commun. 2006, 342, 662–670. [Google Scholar] [CrossRef]
- El Sayyad, H.I.; Sobh, M.; Khalifa, S.; El-Sayyad, O.; El, S.H.I. Adipose Derived Mesenchymal Stem Cell Differentiation into Adipogenic and Osteogenic Stem Cells. Stud. Stem Cells Res. Ther. 2016, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Hosoya, Y.; Yamashita, H.; Fujita, H.; Ohsugi, M.; Tobe, K.; Kadowaki, T.; Nagai, R.; et al. Adipogenesis in Obesity Requires Close Interplay. Diabetes 2007, 56, 1517–1526. [Google Scholar] [CrossRef] [Green Version]
- Rigamonti, A.; Brennand, K.; Lau, F.; Cowan, C.A. Rapid Cellular Turnover in Adipose Tissue. PLoS ONE 2011, 6, e17637. [Google Scholar] [CrossRef] [Green Version]
- Tran, K.-V.; Gealekman, O.; Frontini, A.; Zingaretti, M.C.; Morroni, M.; Giordano, A.; Smorlesi, A.; Perugini, J.; De Matteis, R.; Sbarbati, A.; et al. The Vascular Endothelium of the Adipose Tissue Gives Rise to Both White and Brown Fat Cells. Cell Metab. 2012, 15, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Berry, D.C.; Jo, A.; Tang, W.; Arpke, R.W.; Kyba, M.; Graff, J.M. A PPARγ transcriptional cascade directs adipose progenitor cell-niche interaction and niche expansion. Nat. Commun. 2017, 8, 15926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Huang, L.; Huang, Y.; Yin, J.; Berk, A.J.; Friedman, J.M.; Wang, G. Mediator MED23 Links Insulin Signaling to the Adipogenesis Transcription Cascade. Dev. Cell. 2009, 16, 764–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.K.; Mepani, R.J.; Kleiner, S.; Lo, J.C.; Khandekar, M.J.; Cohen, P.; Frontini, A.; Bhowmick, D.C.; Ye, L.; Cinti, S.; et al. Zfp423 Expression Identifies Committed Preadipocytes and Localizes to Adipose Endothelial and Perivascular Cells. Cell Metab. 2012, 15, 230–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, L.; Liu, M.; Wang, F.; Wang, X.; Tang, Y.; Zhao, Q.; Wang, T.; Chen, Y.; Huang, C. Transcription Factor prrx1 Promotes Brown Adipose-Derived Stem Cells Differentiation to Sinus Node-Like Cells. DNA Cell Biol. 2019, 38, 1313–1322. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, X.; Zhao, B.; Li, Y.; Zhang, Y.; Li, Z.; Wang, X.; Luo, L.; Han, F.; Zhang, J.; et al. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp. Cell Res. 2018, 370, 333–342. [Google Scholar] [CrossRef]
- Fève, B. Adipogenesis: Cellular and molecular aspects. Best Pr. Res. Clin. Endocrinol. Metab. 2005, 19, 483–499. [Google Scholar] [CrossRef]
- Lefterova, M.I.; Haakonsson, A.K.; Lazar, M.A.; Mandrup, S. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol. Metab. 2014, 25, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Sarjeant, K.; Stephens, J.M. Adipogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008417. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; He, K.; Wang, L.; Hu, J.; Gu, J.; Zhou, C.; Lu, R.; Jin, Y. Stk40 represses adipogenesis through translational control of CCAAT/enhancer-binding proteins. J Cell Sci. 2015, 128, 2881–2890. [Google Scholar] [CrossRef] [Green Version]
- Gunasekar, S.K.; Xie, L.; Sah, R. SWELL signalling in adipocytes: Can fat “feel” fat? Adipocyte 2019, 8, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Steinhart, Z.; Angers, S. Wnt signaling in development and tissue homeostasis. Development 2018, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, N.; Wang, J. Wnt/β-Catenin Signaling and Obesity. Front Physiol. 2018, 9, 792. [Google Scholar] [CrossRef]
- Ishay-Ronen, D.; Diepenbruck, M.; Kalathur, R.K.R.; Sugiyama, N.; Tiede, S.; Ivanek, R.; Bantug, G.; Morini, M.F.; Wang, J.; Hess, C.; et al. Gain Fat—Lose Metastasis: Converting Invasive Breast Cancer Cells into Adipocytes Inhibits Cancer Metastasis. Cancer Cell. 2019, 35, 17–32.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Xue, T.; He, F.; Liu, Z.; Ouyang, S.; Cao, D.; Wu, J. A time-resolved proteomic analysis of transcription factors regulating adipogenesis of human adipose derived stem cells. Biochem. Biophys. Res. Commun. 2019, 511, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Suresh, V.; West, J.L. 3D Culture Facilitates VEGF-Stimulated Endothelial Differentiation of Adipose-Derived Stem Cells. Ann. Biomed. Eng. 2019, 1–11. [Google Scholar] [CrossRef]
- Hu, T.; Kitano, A.; Luu, V.; Dawson, B.; Hoegenauer, K.A.; Lee, B.H.; Nakada, D. Bmi1 Suppresses Adipogenesis in the Hematopoietic Stem Cell Niche. Stem Cell Rep. 2019, 13, 545–558. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.T.; Hochfeld, W.E.; Myburgh, R.; Pepper, M.S. Adipocyte and adipogenesis. Eur. J. Cell Biol. 2013, 92, 229–236. [Google Scholar] [CrossRef]
- Kiwaki, K.; Novak, C.M.; Hsu, D.K.; Liu, F.-T.; Levine, J.A. Galectin-3 Stimulates Preadipocyte Proliferation and Is Up-regulated in Growing Adipose Tissue*. Obesity 2007, 15, 32–39. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Ma, S.-R.; Zuo, Z.-Y.; Wu, Y.-B.; Kong, W.-J.; Wang, A.-P.; Jiang, J.-D. Berberine inhibits adipocyte differentiation, proliferation and adiposity through down-regulating galectin-3. Sci. Rep. 2019, 9, 13415. [Google Scholar] [CrossRef] [Green Version]
- Nadler, S.T.; Stoehr, J.P.; Schueler, K.L.; Tanimoto, G.; Yandell, B.S.; Attie, A.D. The expression of adipogenic genes is decreased in obesity and diabetes mellitus. Proc. Natl. Acad. Sci. USA 2000, 97, 11371–11376. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.Y.; Xia, Y.; Kim, W.-S.; Kim, M.H.; Kim, T.H.; Kim, K.J.; Park, B.-S.; Sung, J.-H. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: Increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen. 2009, 17, 540–547. [Google Scholar] [CrossRef]
- Balusamy, S.R.; Perumalsamy, H.; Ranjan, A.; Park, S.; Ramani, S. A dietary vegetable, Moringa oleifera leaves (drumstick tree) induced fat cell apoptosis by inhibiting adipogenesis in 3T3-L1 adipocytes. J. Funct. Foods 2019, 59, 251–260. [Google Scholar] [CrossRef]
- Qi, R.; Wang, J.; Wang, Q.; Qiu, X.; Yang, F.; Liu, Z.; Huang, J. MicroRNA-425 controls lipogenesis and lipolysis in adipocytes. Biochim. Biophys. Acta—Mol. Cell Biol. Lipids. 2019, 1864, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, D.; Choi, J.; Choi, H.; Ryu, J.-H.; Jeong, J.; Park, E.-J.; Kim, S.-H.; Kim, S. Dehydrodiconiferyl alcohol isolated from Cucurbita moschata shows anti-adipogenic and anti-lipogenic effects in 3T3-L1 cells and primary mouse embryonic fibroblasts. J. Biol. Chem. 2012, 287, 8839–8851. [Google Scholar] [CrossRef] [Green Version]
- Simu, S.Y.; Ahn, S.; Castro-Aceituno, V.; Singh, P.; Mathiyalagan, R.; Jiménez-Pérez, Z.E.; Hurh, J.; Oi, L.Z.; Hun, N.J.; Kim, Y.-J.; et al. Gold Nanoparticles Synthesized with Fresh Panax ginseng Leaf Extract Suppress Adipogenesis by Downregulating PPAR γ/CEBP α Signaling in 3T3-L1 Mature Adipocytes. J. Nanosci. Nanotechnol. 2019, 19, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Doğan, A.; Demirci, S.; Apdik, H.; Apdik, E.A.; Şahin, F. Mesenchymal Stem Cell Isolation from Pulp Tissue and Co-Culture with Cancer Cells to Study Their Interactions. J. Vis. Exp. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, H.; Li, J.; Shi, S.; Zhang, L.; Xiang, A.; Shi, X.; Yang, G.; Chu, G. Hhip inhibits proliferation and promotes differentiation of adipocytes through suppressing hedgehog signaling pathway. Biochem. Biophys. Res. Commun. 2019, 514, 148–156. [Google Scholar] [CrossRef] [PubMed]
- John, B.; Naczki, C.; Patel, C.; Ghoneum, A.; Qasem, S.; Salih, Z.; Said, N. Regulation of the bi-directional cross-talk between ovarian cancer cells and adipocytes by SPARC. Oncogene 2019, 38, 4366–4383. [Google Scholar] [CrossRef] [Green Version]
- Shapira, S.N.; Seale, P. Transcriptional Control of Brown and Beige Fat Development and Function. Obesity 2019, 27, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Abdalla, B.A.; Chen, J.; Nie, Q.; Zhang, X. Genomic Insights into the Multiple Factors Controlling Abdominal Fat Deposition in a Chicken Model. Front Genet. 2018, 9, 262. [Google Scholar] [CrossRef]
- Shao, M.; Wang, Q.A.; Song, A.; Vishvanath, L.; Busbuso, N.C.; Scherer, P.E.; Gupta, R.K. Cellular Origins of Beige Fat Cells Revisited. Diabetes 2019, 68, 1874–1885. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.W.; Schlein, C.; Cannon, B.; Heeren, J.; Nedergaard, J. Intact innervation is essential for diet-induced recruitment of brown adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E487–E503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.Y.; Luong, Q.; Sharma, R.; Dreyfuss, J.M.; Ussar, S.; Kahn, C.R. Developmental and functional heterogeneity of white adipocytes within a single fat depot. EMBO J. 2019, 38. [Google Scholar] [CrossRef] [PubMed]
- Westphal, S.; Gantert, T.; Kless, C.; Hüttinger, K.; Klingenspor, M.; Fromme, T. Fibroblast growth factor 8b induces uncoupling protein 1 expression in epididymal white preadipocytes. Sci. Rep. 2019, 9, 8470. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, I.; Martín-Cordero, L.; Hinchado, M.D.; Álvarez-Barrientos, A.; Ortega, E. Anti-inflammatory effect of β2 adrenergic stimulation on circulating monocytes with a pro-inflammatory state in high-fat diet-induced obesity. Brain Behav. Immun. 2019, 80, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, G.; Fang, Q.; Zhang, M.; Hui, X.; Sheng, B.; Wu, L.; Bao, Y.; Li, P.; Xu, A.; et al. Fibroblast growth factor 21 increases insulin sensitivity through specific expansion of subcutaneous fat. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, J.-W.; Yeh, S.-C.; Tsai, F.-Y.; Chen, H.-W.; Tsou, T.-C. Fibroblast growth factor 21 secretion enhances glucose uptake in mono(2-ethylhexyl)phthalate-treated adipocytes. Toxicol. In Vitro 2019, 59, 246–254. [Google Scholar] [CrossRef]
- Cuevas-Ramos, D.; Mehta, R.; Aguilar-Salinas, C.A. Fibroblast Growth Factor 21 and Browning of White Adipose Tissue. Front. Physiol. 2019, 10, 37. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Guo, Y.; Liu, Y.; Wang, Y.; Zheng, R.; Ban, Y.; Peng, L.; Yuan, Q.; Liu, W. Growth differentiation factor 11 inhibits adipogenic differentiation by activating TGF-beta/Smad signalling pathway. Cell Prolif. 2019, 52. [Google Scholar] [CrossRef]
- Smith, P.J.; Wise, L.S.; Berkowitz, R.; Wan, C.; Rubin, C.S. Insulin-like growth factor-I is an essential regulator of the differentiation of 3T3-L1 adipocytes. J. Biol. Chem. 1988, 263, 9402–9408. [Google Scholar]
- Steinbrenner, H.; Micoogullari, M.; Hoang, N.A.; Bergheim, I.; Klotz, L.-O.; Sies, H. Selenium-binding protein 1 (SELENBP1) is a marker of mature adipocytes. Redox Biol. 2019, 20, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, H.; Lou, Y.; Xie, J.; Cao, D.; Huang, X. Insulin_like growth factor I promotes adipogenesis in hemangioma stem cells from infantile hemangiomas. Mol. Med. Rep. 2019, 19, 2825–2830. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Shi, S.; Wang, H.; Liao, K. Growth arrest induces primary-cilium formation and sensitizes IGF-1-receptor signaling during differentiation induction of 3T3-L1 preadipocytes. J. Cell Sci. 2009, 122, 2760–2768. [Google Scholar] [CrossRef] [Green Version]
- Woldt, E.; Matz, R.L.; Terrand, J.; Mlih, M.; Gracia, C.; Foppolo, S.; Martin, S.; Bruban, V.; Ji, J.; Velot, E.; et al. Differential signaling by adaptor molecules LRP1 and ShcA regulates adipogenesis by the insulin-like growth factor-1 receptor. J. Biol. Chem. 2011, 286, 16775–16782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ando, Y.; Sato, F.; Fukunaga, H.; Iwasaki, Y.; Chiba, Y.; Tebakari, M.; Daigo, Y.; Kawashima, J.; Kamei, J. Placental extract suppresses differentiation of 3T3-L1 preadipocytes to mature adipocytes via accelerated activation of p38 MAPK during the early phase of adipogenesis. Nutr. Metab. 2019, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Young, D.A.; Choi, Y.S.; Engler, A.J.; Christman, K.L. Stimulation of adipogenesis of adult adipose-derived stem cells using substrates that mimic the stiffness of adipose tissue. Biomaterials 2013, 34, 8581–8588. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.Q.; Chen, Y.; Chai, M.; Tao, R.; Lei, Y.H.; Jia, Y.Q.; Shu, J.; Ren, J.; Li, G.; Wei, W.X.; et al. Adipose extracellular matrix promotes skin wound healing by inducing the differentiation of adipose_derived stem cells into fibroblasts. Int. J. Mol. Med. 2018, 43, 890–900. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.; Chen, R.; Wang, H.; Sun, G.; Yin, F.; Liang, B.; Yang, Y.; Sharen, G.; Wei, H.; Zhou, X.; et al. Obesity-Induced Methylation of Osteopontin Contributes to Adipogenic Differentiation of Adipose-Derived Mesenchymal Stem Cells. Stem Cells Int. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Kindler, D.; Sousa, I.S.; Schweizer, S.; Lerch, S.; Klingenspor, M.; Herzig, S.; Vegiopoulos, A. A novel growth factor-dependent thermogenic brown adipocyte cell line from defined precursor cells. BioRxiv 2019, 565168. [Google Scholar] [CrossRef]
- Tu, W.; Fu, Y.; Xie, X. RepSox, a small molecule inhibitor of the TGFβ receptor, induces brown adipogenesis and browning of white adipocytes. Acta Pharmacol. Sin. 2019, 40, 1523–1531. [Google Scholar] [CrossRef]
- Yang, K.; Guan, H.; Arany, E.; Hill, D.J.; Cao, X. Neuropeptide Y is produced in visceral adipose tissue and promotes proliferation of adipocyte precursor cells via the Y1 receptor. FASEB J. 2008, 22, 2452–2464. [Google Scholar] [CrossRef] [PubMed]
- Sabatakos, G.; Sims, N.A.; Chen, J.; Aoki, K.; Kelz, M.B.; Amling, M.; Bouali, Y.; Mukhopadhyay, K.; Ford, K.; Nestler, E.J.; et al. Overexpression of DeltaFosB transcription factor(s) increases bone formation and inhibits adipogenesis. Nat. Med. 2000, 6, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wu, Y.; Yang, H.; Hong, P.; Fang, X.; Hu, Y. H19/miR-30a/C8orf4 axis modulates the adipogenic differentiation process in human adipose tissue-derived mesenchymal stem cells. J. Cell. Physiol. 2019, 234, 20925–20934. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Khan, F.; Syeda, P.K.; Nishimura, K.; Jisaka, M.; Nagaya, T.; Shono, F.; Yokota, K. Endogenous synthesis of prostacyclin was positively regulated during the maturation phase of cultured adipocytes. Cytotechnology 2014, 66, 635–646. [Google Scholar] [CrossRef] [Green Version]
- Larian, N.; Ensor, M.; Thatcher, S.E.; English, V.; Morris, A.J.; Stromberg, A.; Cassis, L.A. Pseudomonas aeruginosa-derived pyocyanin reduces adipocyte differentiation, body weight, and fat mass as mechanisms contributing to septic cachexia. Food Chem. Toxicol. 2019, 130, 219–230. [Google Scholar] [CrossRef] [PubMed]
- McCabe, I.C.; Fedorko, A.; Myers, M.G.; Leinninger, G.; Scheller, E.; McCabe, L.R. Novel leptin receptor signaling mutants identify location and sex-dependent modulation of bone density, adiposity, and growth. J. Cell. Biochem. 2019, 120, 4398–4408. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Wang, X.; Ji, Y.; Cheng, S.; Wang, M.; Zhang, C.; Yu, X.; Zhao, R.; Zhang, W.; et al. Acetyl-coenzyme A acyltransferase 2 promote the differentiation of sheep precursor adipocytes into adipocytes. J. Cell. Biochem. 2019, 120, 8021–8031. [Google Scholar] [CrossRef]
- Goldstein, N.; Haim, Y.; Mattar, P.; Hadadi-Bechor, S.; Maixner, N.; Kovacs, P.; Blüher, M.; Rudich, A. Leptin stimulates autophagy/lysosome-related degradation of long-lived proteins in adipocytes. Adipocyte 2019, 8, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.; Li, F.; Ma, X.; Sun, J.; Jiang, R.; Tian, Y.; Han, R.; Li, G.; Wang, Y.; Li, Z.; et al. gga-miRNA-18b-3p Inhibits Intramuscular Adipocytes Differentiation in Chicken by Targeting the ACOT13 Gene. Cells 2019, 8, 556. [Google Scholar] [CrossRef] [Green Version]
- Zöller, N.; Schreiner, S.; Petry, L.; Hoffmann, S.; Steinhorst, K.; Kleemann, J.; Jäger, M.; Kaufmann, R.; Meissner, M.; Kippenberger, S. Collagen I Promotes Adipocytogenesis in Adipose-Derived Stem Cells In Vitro. Cells 2019, 8, 302. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Cao, Y. An lncRNA_miRNA_mRNA ceRNA network for adipocyte differentiation from human adipose_derived stem cells. Mol. Med. Rep. 2019, 19, 4271–4287. [Google Scholar] [CrossRef] [PubMed]
- Ahonen, M.A.; Haridas, P.A.N.; Mysore, R.; Wabitsch, M.; Fischer-Posovszky, P.; Olkkonen, V.M. miR-107 inhibits CDK6 expression, differentiation, and lipid storage in human adipocytes. Mol. Cell Endocrinol. 2019, 479, 110–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manavski, Y.; Lucas, T.; Glaser, S.F.; Dorsheimer, L.; Günther, S.; Braun, T.; Rieger, M.A.; Zeiher, A.M.; Boon, R.A.; Dimmeler, S. Clonal expansion of endothelial cells contributes to ischemia-induced neovascularization. Circ. Res. 2018, 122, 670–677. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Sang, S.; Leung, T. Methylglyoxal-Induced Retinal Angiogenesis in Zebrafish Embryo: A Potential Animal Model of Neovascular Retinopathy. J. Ophthalmol. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Trebak, M. Vascular balloon injury and intraluminal administration in rat carotid artery. J. Vis. Exp. 2014. [Google Scholar] [CrossRef] [PubMed]
- Strassburg, S.; Nienhueser, H.; Björn Stark, G.; Finkenzeller, G.; Torio-Padron, N. Co-culture of adipose-derived stem cells and endothelial cells in fibrin induces angiogenesis and vasculogenesis in a chorioallantoic membrane model. J. Tissue Eng. Regen. Med. 2016, 10, 496–506. [Google Scholar] [CrossRef]
- Fantin, A.; Lampropoulou, A.; Gestri, G.; Raimondi, C.; Senatore, V.; Zachary, I.; Ruhrberg, C. NRP1 Regulates CDC42 Activation to Promote Filopodia Formation in Endothelial Tip Cells. Cell Rep. 2015, 11, 1577–1590. [Google Scholar] [CrossRef] [Green Version]
- Urbich, C.; Dimmeler, S. Endothelial progenitor cells: Characterization and role in vascular biology. Circ. Res. 2004, 95, 343–353. [Google Scholar] [CrossRef] [Green Version]
- Darvishi, B.; Majidzadeh, A.K.; Ghadirian, R.; Mosayebzadeh, M.; Farahmand, L. Recruited bone marrow derived cells, local stromal cells and IL-17 at the front line of resistance development to anti-VEGF targeted therapies. Life Sci. 2019, 217, 34–40. [Google Scholar] [CrossRef]
- Gerszten, R.E.; Garcia-Zepeda, E.A.; Lim, Y.C.; Yoshida, M.; Ding, H.A.; Gimbrone, M.A.; Luster, A.D.; Luscinskas, F.W.; Rosenzwelg, A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature 1999, 398, 718–725. [Google Scholar] [CrossRef]
- Gerhardt, H.; Golding, M.; Fruttiger, M.; Ruhrberg, C.; Lundkvist, A.; Abramsson, A.; Jeltsch, M.; Mitchell, C.; Alitalo, K.; Shima, D.; et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003, 161, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ii, M.; Kamei, N.; Alev, C.; Kwon, S.-M.; Kawamoto, A.; Akimaru, H.; Masuda, H.; Sawa, Y.; Asahara, T. CD34+ Cells Represent Highly Functional Endothelial Progenitor Cells in Murine Bone Marrow. PLoS ONE 2011, 6, e20219. [Google Scholar] [CrossRef] [PubMed]
- Maher, T.J.; Ren, Y.; Li, Q.; Braunlin, E.; Garry, M.G.; Sorrentino, B.P.; Martin, C.M. ATP-binding cassette transporter Abcg2 lineage contributes to the cardiac vasculature after oxidative stress. Am. J. Physiol. Circ. Physiol. 2014, 306, H1610–H1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akita, M.; Tanaka, K.; Matsumoto, S.; Komatsu, K.; Fujita, K. Detection of the hematopoietic stem and progenitor cell marker Cd133 during angiogenesis in three-dimensional collagen gel culture. Stem Cells Int. 2013. [Google Scholar] [CrossRef]
- Gehling, U.M.; Ergün, S.; Schumacher, U.; Wagener, C.; Pantel, K.; Otte, M.; Schuch, G.; Schafhausen, P.; Mende, T.; Kilic, N.; et al. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood 2000, 95, 3106–3112. [Google Scholar] [CrossRef]
- Park, J.S.; Yang, H.N.; Yi, S.W.; Kim, J.H.; Park, K.H. Neoangiogenesis of human mesenchymal stem cells transfected with peptide-loaded and gene-coated PLGA nanoparticles. Biomaterials 2016, 76, 226–237. [Google Scholar] [CrossRef]
- Basagiannis, D.; Zografou, S.; Murphy, C.; Fotsis, T.; Morbidelli, L.; Ziche, M.; Bleck, C.; Mercer, J.; Christoforidis, S. VEGF induces signalling and angiogenesis by directing VEGFR2 internalisation through macropinocytosis. J. Cell Sci. 2016, 129, 4091–4104. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Zhang, L.; Wang, S.; Han, Q.; Zhao, R.C. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J. Cell Sci. 2016, 129, 2182–2189. [Google Scholar] [CrossRef] [Green Version]
- Davis, P.J.; Leinung, M.; Mousa, S.A. microRNAs and Angiogenesis. Anti-Angiogenesis Strateg. Cancer Ther. 2017, 69–84. [Google Scholar] [CrossRef]
- Anand, S.; Cheresh, D.A. MicroRNA-mediated regulation of the angiogenic switch. Curr. Opin. Hematol. 2011, 18, 171–176. [Google Scholar] [CrossRef] [Green Version]
- Verdelli, C.; Avagliano, L.; Creo, P.; Guarnieri, V.; Scillitani, A.; Vicentini, L.; Steffano, G.B.; Beretta, E.; Soldati, L.; Costa, E.; et al. Tumour-associated fibroblasts contribute to neoangiogenesis in human parathyroid neoplasia. Endocr. Relat. Cancer 2015, 22, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Morbidelli, L.; Donnini, S.; Ziche, M. Therapeutic Implications of the Nitric Oxide Pathway in the Angiogenesis of Tumors and Inflammatory-Related Disorders. Ther. Appl. Nitric Oxide Cancer Inflamm. Disord. 2019, 65–91. [Google Scholar] [CrossRef]
- Würdinger, T.; Tannous, B.A.; Saydam, O.; Skog, J.; Grau, S.; Soutschek, J.; Weissleder, R.; Breakefield, X.O.; Krichevsky, A.M. miR-296 Regulates Growth Factor Receptor Overexpression in Angiogenic Endothelial Cells. Cancer Cell 2008, 14, 382–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yunus, M.; Jansson, P.J.; Kovacevic, Z.; Kalinowski, D.S.; Richardson, D.R. Tumor-induced neoangiogenesis and receptor tyrosine kinases—Mechanisms and strategies for acquired resistance. Biochim. Biophys. Acta—Gen. Subj. 2019, 1863, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Stamatopoulos, A.; Stamatopoulos, T.; Gamie, Z.; Kenanidis, E.; Ribeiro, R.D.C.; Rankin, K.S.; Gerrand, C.; Dalgarno, K.; Tsiridis, E. Mesenchymal stromal cells for bone sarcoma treatment: Roadmap to clinical practice. J. Bone Oncol. 2019, 16, 100231. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, H.; Qian, C. c-Kit-Positive Adipose Tissue-Derived Mesenchymal Stem Cells Promote the Growth and Angiogenesis of Breast Cancer. Biomed. Res. Int. 2017, 2017, 7407168. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.J.; Zeng, R.; Lu, J.H.; Lai, W.F.T.; Chen, W.H.; Liu, H.Y.; Chang, Y.T.; Deng, W.P. Adipose-derived stem cells promote tumor initiation and accelerate tumor growth by interleukin-6 production. Oncotarget 2015, 6, 7713–7726. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Lan, T.; Zhang, C.; Zeng, C.; Hou, J.; Yang, Z.; Zhang, M.; Liu, J.; Liu, B. Reciprocal activation between IL-6/STAT3 and NOX4/Akt signalings promotes proliferation and survival of non-small cell lung cancer cells. Oncotarget 2015, 6, 1031–1048. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Vickers, M.F.; Kerbel, R.S. Interleukin 6: A fibroblast-derived growth inhibitor of human melanoma cells from early but not advanced stages of tumor progression. Proc. Natl. Acad. Sci. USA 1992, 89, 9215–9219. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Kerbel, R.S. Interleukin-6 undergoes transition from paracrine growth inhibitor to autocrine stimulator during human melanoma progression. J. Cell Biol. 1993, 120, 1281–1288. [Google Scholar] [CrossRef]
- Hoejberg, L.; Bastholt, L.; Schmidt, H. Interleukin-6 and melanoma. Melanoma Res. 2012, 22, 327–333. [Google Scholar] [CrossRef]
- Chang, P.H.; Pan, Y.P.; Fan, C.W.; Tseng, W.K.; Huang, J.S.; Wu, T.H.; Chou, W.C.; Wang, C.H.; Yeh, K.Y. Pretreatment serum interleukin-1β, interleukin-6, and tumor necrosis factor-α levels predict the progression of colorectal cancer. Cancer Med. 2016, 5, 426–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Singh, S.; Varney, M.L.; Kindle, S.; Singh, R.K. Modulation of CXCL-8 expression in human melanoma cells regulates tumor growth, angiogenesis, invasion, and metastasis. Cancer Med. 2012, 1, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Eble, J.A.; Niland, S. The extracellular matrix in tumor progression and metastasis. Clin. Exp. Metastasis 2019, 36, 171–198. [Google Scholar] [CrossRef]
- Preisner, F.; Leimer, U.; Sandmann, S.; Zoernig, I.; Germann, G.; Koellensperger, E. Impact of Human Adipose Tissue-Derived Stem Cells on Malignant Melanoma Cells in an In Vitro Co-culture Model. Stem Cell Rev. Rep. 2018, 14, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Nolan, D.J.; Mellick, A.S.; Bambino, K.; McDonnell, K.; Mittal, V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science 2008, 319, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, I.; Goulielmaki, M.; Devetzi, M.; Panagiotidis, M.; Koliakos, G.; Zoumpourlis, V. Mesenchymal stem cells in preclinical cancer cytotherapy: A systematic review. Stem Cell Res. Ther. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Mansour, A.G.; Huang, W.; Chrislip, L.A.; Wilkins, R.K.; Queen, N.J.; Youssef, Y.; Mao, H.C.; Caligiuri, M.A.; Cao, L. Adipocytes: A Novel Target for IL-15/IL-15Rα Cancer Gene Therapy. Mol. Ther. 2019, 27, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Ishibazawa, A.; Nagaoka, T.; Yokota, H.; Takahashi, A.; Omae, T.; Song, Y.S.; Takahashi, T.; Yoshida, A. Characteristics of retinal neovascularization in proliferative diabetic retinopathy imaged by optical coherence tomography angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6247–6255. [Google Scholar] [CrossRef] [Green Version]
- Prakash, R.; Carmichael, S.T. Blood-brain barrier breakdown and neovascularization processes after stroke and traumatic brain injury. Curr. Opin. Neurol. 2015, 28, 556–564. [Google Scholar] [CrossRef] [Green Version]
- Planat-Benard, V.; Silvestre, J.S.; Cousin, B.; André, M.; Nibbelink, M.; Tamarat, R.; Clergue, M.; Manneville, C.; Saillan-Barreau, C.; Duriez, M.; et al. Plasticity of Human Adipose Lineage Cells Toward Endothelial Cells: Physiological and Therapeutic Perspectives. Circulation 2004, 109, 656–663. [Google Scholar] [CrossRef]
- Wang, K.; Yu, L.Y.; Jiang, L.Y.; Wang, H.B.; Wang, C.Y.; Luo, Y. The paracrine effects of adipose-derived stem cells on neovascularization and biocompatibility of a macroencapsulation device. Acta Biomater. 2015, 15, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Miranville, A.; Heeschen, C.; Sengenès, C.; Curat, C.A.; Busse, R.; Bouloumié, A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation 2004, 110, 349–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of Angiogenic and Antiapoptotic Factors by Human Adipose Stromal Cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Kinnaird, T.; Stabile, E.; Burnett, M.S.; Shou, M.; Lee, C.W.; Barr, S.; Fuchs, S.; Epstein, S.E. Local Delivery of Marrow-Derived Stromal Cells Augments Collateral Perfusion Through Paracrine Mechanisms. Circulation 2004, 109, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, H.; Maeda, K.; Morishita, R.; Iguchi, S.; Nishikawa, T.; Takami, Y.; Kikuchi, Y.; Saito, Y.; Tamai, K.; Ogihara, T.; et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arter. Thromb. Vasc. Biol. 2005, 25, 2542–2547. [Google Scholar] [CrossRef]
- Donizetti-Oliveira, C.; Semedo, P.; Burgos-Silva, M.; Cenedeze, M.A.; Malheiros, D.M.A.C.; Reis, M.A.; Pacheco-Silva, A.; Câmara, N.O.S. Adipose tissue-derived stem cell treatment prevents renal disease progression. Cell Transplant. 2012, 21, 1727–1741. [Google Scholar] [CrossRef] [Green Version]
- Terlizzi, V.; Hammes, H.; Harmsen, M. Adipose-derived stromal cells contribute to microvascular stabilization in diabetic proliferative retinopathy: To be or notch to be? Diabetol. Und Stoffwechs. 2015, 10. [Google Scholar] [CrossRef]
- Ryu, H.H.; Lim, J.H.; Byeon, Y.E.; Park, J.R.; Seo, M.S.; Lee, Y.W.; Kim, W.H.; Kang, K.S.; Kweon, O.K. Functional recovery and neural differentiation after transplantation of allogenic adipose-derived stem cells in a canine model of acute spinal cord injury. J. Vet. Sci. 2009, 10, 273–284. [Google Scholar] [CrossRef] [Green Version]
- Valina, C.; Pinkernell, K.; Song, Y.H.; Bai, X.; Sadat, S.; Campeau, R.J.; Le Jemtel, T.H.; Alt, E. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur. Heart J. 2007, 28, 2667–2677. [Google Scholar] [CrossRef]
- Léobon, B.; Roncalli, J.; Joffre, C.; Mazo, M.; Boisson, M.; Barreau, C.; Calise, D.; Arnaud, E.; André, M.; Pucéat, M.; et al. Adipose-derived cardiomyogenic cells: In vitro expansion and functional improvement in a mouse model of myocardial infarction. Cardiovasc. Res. 2009, 83, 757–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Deng, J.; Tian, W.; Xiang, B.; Yang, T.; Li, G.; Wang, J.; Gruwel, M.; Kashour, T.; Rendell, J.; et al. Adipose-derived stem cells are an effective cell candidate for treatment of heart failure: An MR imaging study of rat hearts. Am. J. Physiol.—Hear Circ Physiol. 2009, 297. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Johnstone, B.H.; Cook, T.G.; Tan, J.; Fishbein, M.C.; Chen, P.-S.; March, K.L. IFATS Collection: Human Adipose Tissue-Derived Stem Cells Induce Angiogenesis and Nerve Sprouting Following Myocardial Infarction, in Conjunction with Potent Preservation of Cardiac Function. Stem Cells 2009, 27, 230–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajnoch, C.; Chachques, J.C.; Berrebi, A.; Bruneval, P.; Benoit, M.O.; Carpentier, A. Cellular therapy reverses myocardial dysfunction. J. Thorac. Cardiovasc. Surg. 2001, 121, 871–878. [Google Scholar] [CrossRef] [Green Version]
- Li, R.K.; Jia, Z.Q.; Weisel, R.D.; Merante, F.; Mickle, D.A.G. Smooth muscle cell transplantation into myocardial scar tissue improves heart function. J. Mol. Cell Cardiol. 1999, 31, 513–522. [Google Scholar] [CrossRef]
- Tomita, S.; Mickle, D.A.G.; Weisel, R.D.; Jia, Z.Q.; Tumiati, L.C.; Allidina, Y.; Liu, P.; Li, R.K. Improved heart function with myogenesis and angiogenesis after autologous porcine bone marrow stromal cell transplantation. J. Thorac. Cardiovasc. Surg. 2002, 123, 1132–1140. [Google Scholar] [CrossRef] [Green Version]
- Houtgraaf, J.H.; Den Dekker, W.K.; Van Dalen, B.M.; Springeling, T.; De Jong, R.; Van Geuns, R.J.; Geleijnse, M.L.; Fernandez-Aviles, F.; Zijlsta, F.; Serruys, P.W.; et al. First experience in humans using adipose tissue-derived regenerative cells in the treatment of patients with ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2012, 59, 539–540. [Google Scholar] [CrossRef] [Green Version]
- Perin, E.C.; Sanz-Ruiz, R.; Sánchez, P.L.; Lasso, J.; Pérez-Cano, R.; Alonso-Farto, J.C.; Pérez-David, E.; Fernández-Santos, M.E.; Serruys, P.W.; Duckers, H.J.; et al. Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: The PRECISE Trial. Am. Heart J. 2014, 168. [Google Scholar] [CrossRef]
- Qayyum, A.A.; Mathiasen, A.B.; Mygind, N.D.; Kühl, J.T.; Jørgensen, E.; Helqvist, S.; Elberg, J.J.; Kofoed, K.F.; Vejlstrup, N.G.; Fischer-Nielsen, A.; et al. Adipose-Derived Stromal Cells for Treatment of Patients with Chronic Ischemic Heart Disease (MyStromalCell Trial): A Randomized Placebo-Controlled Study. Stem Cells Int. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Slevin, M.; Krupinski, J.; Slowik, A.; Kumar, P.; Szczudlik, A.; Gaffney, J. Serial measurement of vascular endothelial growth factor and transforming growth factor-β1 in serum of patients with acute ischemic stroke. Stroke 2000, 31, 1863–1870. [Google Scholar] [CrossRef]
- Leu, S.; Lin, Y.-C.; Yuen, C.-M.; Yen, C.-H.; Kao, Y.-H.; Sun, C.-K.; Yip, H.-K. Adipose-derived mesenchymal stem cells markedly attenuate brain infarct size and improve neurological function in rats. J. Transl. Med. 2010, 8, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thored, P.; Wood, J.; Arvidsson, A.; Cammenga, J.; Kokaia, Z.; Lindvall OThored, P.; Wood, J.; Arvidsson, A.; Cammenga, J.; Kokaia, Z.; et al. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke 2007, 38, 3032–3039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.J.; Song, H.S.; Bhang, S.; Lee, S.; Kang, B.G.; Lee, J.C.; An, J.; Cha, C.I.; Nam, D.H.; Kim, B.S.; et al. Therapeutic effects of human adipose stem cell-conditioned medium on stroke. J. Neurosci. Res. 2012, 90, 1794–1802. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Fernández, M.; Rodríguez-Frutos, B.; Ramos-Cejudo, J.; Teresa Vallejo-Cremades, M.; Fuentes, B.; Cerdán, S.; Díez-Tejedor, E. Effects of intravenous administration of allogenic bone marrow- and adipose tissue-derived mesenchymal stem cells on functional recovery and brain repair markers in experimental ischemic stroke. Stem Cell Res. Ther. 2013, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Liu, N.; Wang, J.-H.; Zhang, Y.-X.; Du, H.-W.; Chen, R.-H.; Huang, H.-P. The effects of adipose-derived stem cells transplantation on the expression of TGF-β1 in rat brain after cerebral ischemia. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2011, 27, 872–875. [Google Scholar] [PubMed]
- Gutierrez-Fernandez, M.; Rodríguez-Frutos, B.; Ramos-Cejudo, J.; Otero-Ortega, L.; Fuentes, B.; Vallejo-Cremades, T.T.; Sanz-Cuesta, E.E.; Díez-Tejedor, E. Comparison between xenogeneic and allogeneic adipose mesenchymal stem cells in the treatment of acute cerebral infarct: Proof of concept in rats. J. Transl. Med. 2015, 13. [Google Scholar] [CrossRef] [Green Version]
- Mi, H.M.; Sun, Y.K.; Yeon, J.K.; Su, J.K.; Jae, B.L.; Yong, C.B.; Sang, M.S.; Jin, S.J. Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell. Physiol. Biochem. 2006, 17, 279–290. [Google Scholar] [CrossRef]
- Kishimoto, S.; Inoue, K.-I.; Nakamura, S.; Hattori, H.; Ishihara, M.; Sakuma, M.; Toyoda, S.; Iwaguro, H.; Taguchi, I.; Inoue, T.; et al. Low-molecular weight heparin protamine complex augmented the potential of adipose-derived stromal cells to ameliorate limb ischemia. Atherosclerosis 2016, 249, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Shevchenko, E.K.; Makarevich, P.I.; Tsokolaeva, Z.I.; Boldyreva, M.A.; Sysoeva, V.Y.; Tkachuk, V.A.; Parfyonova, Y.V. Transplantation of modified human adipose derived stromal cells expressing VEGF165 results in more efficient angiogenic response in ischemic skeletal muscle. J. Transl. Med. 2013, 11. [Google Scholar] [CrossRef] [Green Version]
- Song, S.H.; Lee, M.O.; Lee, J.S.; Jeong, H.C.; Kim, H.G.; Kim, W.S.; Hur, M.; Cha, H.J. Genetic modification of human adipose-derived stem cells for promoting wound healing. J. Dermatol. Sci. 2012, 66, 98–107. [Google Scholar] [CrossRef]
- Yoo, J.H.; Shin, J.H.; An, M.S.; Ha, T.K.; Kim, K.H.; Bae, K.B.; Kim, T.H.; Choi, C.S.; Hong, K.H.; Kim, J.; et al. Adipose-tissue-derived stem cells enhance the healing of ischemic colonic anastomoses: An experimental study in rats. J. Korean Soc. Coloproctol. 2012, 28, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.H.; Jo, H.J.; Jung, T.D.; Ahn, M.S.; Bae, K.B.; Hong, K.H.; Kim, J.; Kim, J.T.; Kim, S.H.; Yang, Y.I. Adipose-derived stem cells on the healing of ischemic colitis: A therapeutic effect by angiogenesis. Int. J. Colorectal. Dis. 2012, 27, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shi, T.; Xu, A.; Zhang, L. 3D spheroid culture enhances survival and therapeutic capacities of MSCs injected into ischemic kidney. J. Cell. Mol. Med. 2016, 20, 1203–1213. [Google Scholar] [CrossRef] [Green Version]
- Harris, W.M.; Plastini, M.; Kappy, N.; Ortiz, T.; Chang, S.; Brown, S.; Carpenter, J.P.; Zhang, P. Endothelial differentiated adipose-derived stem cells improvement of survival and neovascularization in fat transplantation. Aesthetic Surg. J. 2019, 39, 220–232. [Google Scholar] [CrossRef]
- Mou, S.; Zhou, M.; Li, Y.; Wang, J.; Yuan, Q.; Xiao, P.; Sun, J.; Wang, Z. Extracellular Vesicles from Human Adipose-Derived Stem Cells for the Improvement of Angiogenesis and Fat-Grafting Application. Plast. Reconstr. Surg. 2019, 144, 869–880. [Google Scholar] [CrossRef]
- Naderi, N.; Griffin, M.F.; Mosahebi, A.; Butler, P.E.; Seifalian, A.M. Adipose derived stem cells and platelet rich plasma improve the tissue integration and angiogenesis of biodegradable scaffolds for soft tissue regeneration. Mol. Biol. Rep. 2020, 47, 2005–2013. [Google Scholar] [CrossRef] [Green Version]
- Bachmann, S.; Jennewein, M.; Bubel, M.; Guthörl, S.; Pohlemann, T.; Oberringer, M. Interacting adipose-derived stem cells and microvascular endothelial cells provide a beneficial milieu for soft tissue healing. Mol. Biol. Rep. 2020, 47, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Bhang, S.H.; Lee, S.; Shin, J.Y.; Lee, T.J.; Jang, H.K.; Kim, B.S. Efficacious and clinically relevant conditioned medium of human adipose-derived stem cells for therapeutic angiogenesis. Mol. Ther. 2014, 22, 862–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, M.F.; Naderi, N.; Kalaskar, D.M.; Seifalian, A.M.; Butler, P.E. Argon plasma surface modification promotes the therapeutic angiogenesis and tissue formation of tissue-engineered scaffolds in vivo by adipose-derived stem cells. Stem Cell Res. Ther. 2019, 10, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.-D.; Bai, Y.; Yan, X.-L.; Ren, J.; Zeng, Q.; Li, X.-D.; Pei, X.; Han, Y. Co-transplantation of exosomes derived from hypoxia-preconditioned adipose mesenchymal stem cells promotes neovascularization and graft survival in fat grafting. Biochem. Biophys. Res. Commun. 2018, 497, 305–312. [Google Scholar] [CrossRef]
- Manavella, D.D.; Cacciottola, L.; Payen, V.L.; Amorim, C.A.; Donnez, J.; Dolmans, M.M. Adipose tissue-derived stem cells boost vascularization in grafted ovarian tissue by growth factor secretion and differentiation into endothelial cell lineages. MHR Basic Sci. Reprod. Med. 2019, 25, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.; Cacciottola, L.; Amorim, C.A.; Manavella, D. Translational research aiming to improve survival of ovarian tissue transplants using adipose tissue-derived stem cells. Acta Obstet. Gynecol. Scand. 2019, 98, 665–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constantin, A.; Dumitrescu, M.; Mihai (Corotchi), M.C.; Jianu, D.; Simionescu, M. CO2 laser increases the regenerative capacity of human adipose-derived stem cells by a mechanism involving the redox state and enhanced secretion of pro-angiogenic molecules. Lasers Med. Sci. 2017, 32, 117–127. [Google Scholar] [CrossRef] [PubMed]


| Authors of Research | Molecules/Family of Molecules/Plant Species | Effector Function | Mechanism | References |
|---|---|---|---|---|
| Chen and Wang | β-catenin | Stimulation | Adipokine upregulation | [8] |
| Li et al. | MAFF, MXD4, BATF3 | Inhibition | Overexpression of Maff, Mxd4, Batf3 | [43] |
| Hu et al. | BMI1 | Inhibition | Repression of Pax3 | [45] |
| Ali et al. | IGF-1, prostaglandins, fatty acids | Stimulation | Extracellular signaling | [46] |
| Ali et al. | Growth hormones, cytokines, TGF-β | Inhibition | Extracellular signaling | [46] |
| Doğan et al. | Boron | Inhibition | Inhibition of PPARγ, CEBPα Regulation of β-catenin, AKT | [55] |
| Authors of Research | Molecules/Family of Molecules/Plant Species | Effector Function | Mechanism | References |
|---|---|---|---|---|
| Wang et al. | Berberine | Inhibition | Destabilization of Gal-3 mRNA, resulting in decrease of Gal-3 promoter activity | [48] |
| Lee et al. | Hypoxia | Stimulation | Upregulation of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) | [50] |
| Balusamy et al. | Moringa oleifera | Inhibition | Inhibition of PPARγ, FABP4, cEBPβ, ADIPOR1 | [51] |
| Lee et al. | dehydrodiconiferyl alcohol | Inhibition | Inhibition of C/EBPα, C/EBPβ, C/EBPδ, PPARγ | [52] |
| Simu et al. | Panax ginseng | Inhibition | Inhibition of PPARγ, CEBPα | [54] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hutchings, G.; Janowicz, K.; Moncrieff, L.; Dompe, C.; Strauss, E.; Kocherova, I.; Nawrocki, M.J.; Kruszyna, Ł.; Wąsiatycz, G.; Antosik, P.; et al. The Proliferation and Differentiation of Adipose-Derived Stem Cells in Neovascularization and Angiogenesis. Int. J. Mol. Sci. 2020, 21, 3790. https://doi.org/10.3390/ijms21113790
Hutchings G, Janowicz K, Moncrieff L, Dompe C, Strauss E, Kocherova I, Nawrocki MJ, Kruszyna Ł, Wąsiatycz G, Antosik P, et al. The Proliferation and Differentiation of Adipose-Derived Stem Cells in Neovascularization and Angiogenesis. International Journal of Molecular Sciences. 2020; 21(11):3790. https://doi.org/10.3390/ijms21113790
Chicago/Turabian StyleHutchings, Greg, Krzysztof Janowicz, Lisa Moncrieff, Claudia Dompe, Ewa Strauss, Ievgeniia Kocherova, Mariusz J. Nawrocki, Łukasz Kruszyna, Grzegorz Wąsiatycz, Paweł Antosik, and et al. 2020. "The Proliferation and Differentiation of Adipose-Derived Stem Cells in Neovascularization and Angiogenesis" International Journal of Molecular Sciences 21, no. 11: 3790. https://doi.org/10.3390/ijms21113790
APA StyleHutchings, G., Janowicz, K., Moncrieff, L., Dompe, C., Strauss, E., Kocherova, I., Nawrocki, M. J., Kruszyna, Ł., Wąsiatycz, G., Antosik, P., Shibli, J. A., Mozdziak, P., Perek, B., Krasiński, Z., Kempisty, B., & Nowicki, M. (2020). The Proliferation and Differentiation of Adipose-Derived Stem Cells in Neovascularization and Angiogenesis. International Journal of Molecular Sciences, 21(11), 3790. https://doi.org/10.3390/ijms21113790








