Mitochondrial OXPHOS Biogenesis: Co-Regulation of Protein Synthesis, Import, and Assembly Pathways
Abstract
:1. Introduction
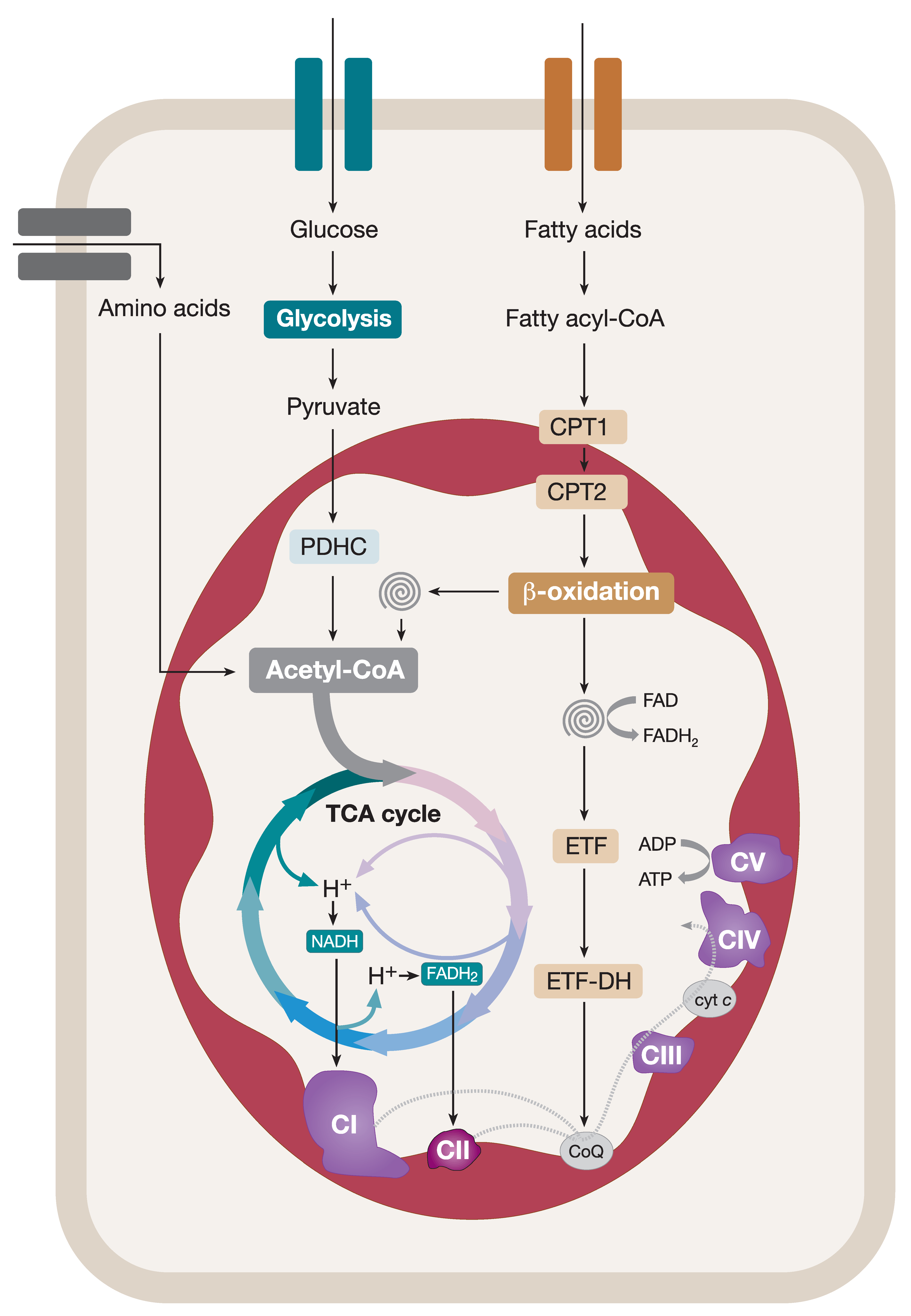
Coupling of Catabolic Pathways and the OXPHOS System
2. Co-Regulation of Mitochondrial and Nuclear Gene Expression
2.1. Mitochondrial Transcription
2.2. Mitochondrial Translation
Differences between Cytosolic and Mitochondrial Translation
2.3. Coordination of Distinct Translational Programs Facilitate Stochiometric OXPHOS Subunit Synthesis
3. Coupling of Mitochondrial Protein Import and OXPHOS Biogenesis
3.1. Nuclear-Encoded Mitochondrial Protein Import Systems
3.2. The Function of the Oxa1 Insertase in OXPHOS Assembly
3.3. Importance of Close Proximity of Import, Insertase, and Assembly Machinery
3.4. Plasticity of Mitochondrial Translation in OXPHOS Biogenesis
3.5. Mammalian Translational Activator TACO1
4. Surveillance Mechanisms Regulating Mitochondrial Protein Import
4.1. Mitochondrial Quality Control Pathways in Humans
4.2. Mitochondrial Import Stress Response Pathways in Yeast
5. The OXPHOS System and Formation of Its Early Assembly Intermediates
5.1. Complex I (NADH–Ubiquinone Oxidoreductase) Assembly
5.2. Complex II (Succinate–Ubiquinone Oxidoreductase) Assembly
5.3. Complex III (Ubiquinol–Cytochrome c Oxidoreductase) Assembly
5.4. Complex IV (Cytochrome c Oxidase) Assembly
5.5. Complex V (F1FO ATP Synthase) Assembly
6. Final Stages of OXPHOS Assembly
6.1. The LYRM Protein Family
6.2. Interactions between Mitochondrial Acyl Carrier Proteins and LYRM Proteins
6.3. The Role of mtACP and LYRM Proteins in OXPHOS Assembly
6.3.1. Complex I
6.3.2. Complex II
6.3.3. Complex III
6.3.4. Complex V
6.4. The Role of mtACP and LYRM Proteins in Fe–S Cluster Biogenesis
6.5. The Role of mtACP and LYRM Proteins in Mitoribosome Assembly
7. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Gorman, G.; Schaefer, A.; Ng, Y.; Gomez, N.; Blakely, E.; Alston, C.; Feeney, C.; Horvath, R.; Yu-Wai-Man, P.; Chinnery, P.; et al. Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann. Neurol. 2015, 77, 753–759. [Google Scholar] [CrossRef] [Green Version]
- Ghezzi, D.; Zeviani, M. Human diseases associated with defects in assembly of OXPHOS complexes. Essays Biochem. 2018, 62, 271–286. [Google Scholar] [CrossRef]
- Frazier, A.; Thorburn, D.; Compton, A. Mitochondrial energy generation disorders: Genes, mechanisms, and clues to pathology. J. Biol. Chem. 2019, 294, 5386–5395. [Google Scholar] [CrossRef] [Green Version]
- Thompson, K.; Collier, J.; Glasgow, R.; Robertson, F.; Pyle, A.; Blakely, E.; Alston, C.; Oláhová, M.; McFarland, R.; Taylor, R. Recent advances in understanding the molecular genetic basis of mitochondrial disease. J. Inherit. Metab. Dis. 2020, 43, 36–50. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Reyes, I.; Chandel, N. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef] [Green Version]
- Spinelli, J.B.; Haigis, M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef]
- Braymer, J.; Lill, R. Iron–sulfur cluster biogenesis and trafficking in mitochondria. J. Biol. Chem. 2017, 292, 12754–12763. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Youle, R. The Role of Mitochondria in Apoptosis. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef] [Green Version]
- Granatiero, V.; De Stefani, D.; Rizzuto, R. Mitochondrial Calcium Handling in Physiology and Disease. Adv. Exp. Med. Biol. 2017, 982, 25–47. [Google Scholar] [CrossRef]
- Shadel, G.; Horvath, T. Mitochondrial ROS signaling in organismal homeostasis. Cell 2015, 163, 560–569. [Google Scholar] [CrossRef] [Green Version]
- Chinnery, P.; Hudson, G. Mitochondrial genetics. Br. Med. Bull. 2013, 106, 135–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagliarini, D.; Calvo, S.; Chang, B.; Sheth, S.; Vafai, S.; Ong, S.; Walford, G.; Sugiana, C.; Boneh, A.; Chen, W.; et al. A Mitochondrial Protein Compendium Elucidates Complex I Disease Biology. Cell 2008, 134, 112–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgenstern, M.; Stiller, S.; Lubbert, P.; Peikert, C.; Dannenmaier, S.; Drepper, F.; Weill, U.; Hoss, P.; Feuerstein, R.; Gebert, M.; et al. Definition of a High-Confidence Mitochondrial Proteome at Quantitative Scale. Cell Rep. 2017, 19, 2836–2852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvo, S.; Clauser, K.; Mootha, V. MitoCarta2.0: An updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 2016, 44, D1251–D1257. [Google Scholar] [CrossRef]
- Anderson, S.; Bankier, A.; Barrell, B.; de Bruijn, M.; Coulson, A.; Drouin, J.; Eperon, I.; Nierlich, D.; Roe, B.; Sanger, F.; et al. Sequence and organization of the human mitochondrial genome. Nature 1981, 290, 457–465. [Google Scholar] [CrossRef]
- Bouda, E.; Stapon, A.; Garcia-Diaz, M. Mechanisms of mammalian mitochondrial transcription. Protein Sci. 2019, 28, 1594–1605. [Google Scholar] [CrossRef]
- Kuhl, I.; Kukat, C.; Ruzzenente, B.; Milenkovic, D.; Mourier, A.; Miranda, M.; Koolmeister, C.; Falkenberg, M.; Larsson, N. POLRMT does not transcribe nuclear genes. Nature 2014, 514, E7–E11. [Google Scholar] [CrossRef]
- Masters, B.; Stohl, L.; Clayton, D. Yeast mitochondrial RNA polymerase is homologous to those encoded by bacteriophages T3 and T7. Cell 1987, 51, 89–99. [Google Scholar] [CrossRef]
- Ringel, R.; Sologub, M.; Morozov, Y.; Litonin, D.; Cramer, P.; Temiakov, D. Structure of human mitochondrial RNA polymerase. Nature 2011, 478, 269–273. [Google Scholar] [CrossRef] [Green Version]
- Shokolenko, I.; Alexeyev, M. Mitochondrial transcription in mammalian cells. Front. Biosci. 2017, 22, 835–853. [Google Scholar] [CrossRef] [Green Version]
- Hillen, H.; Morozov, Y.; Sarfallah, A.; Temiakov, D.; Cramer, P. Structural Basis of Mitochondrial Transcription Initiation. Cell 2017, 171, 1072–1081.e1010. [Google Scholar] [CrossRef] [Green Version]
- Terzioglu, M.; Ruzzenente, B.; Harmel, J.; Mourier, A.; Jemt, E.; Lopez, M.; Kukat, C.; Stewart, J.; Wibom, R.; Meharg, C.; et al. MTERF1 binds mtDNA to prevent transcriptional interference at the light-strand promoter but is dispensable for rRNA gene transcription regulation. Cell Metab. 2013, 17, 618–626. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Posse, V.; Zhu, X.; Hyvärinen, A.; Jacobs, H.; Falkenberg, M.; Gustafsson, C. Mitochondrial transcription termination factor 1 directs polar replication fork pausing. Nucleic Acids Res. 2016, 44, 5732–5742. [Google Scholar] [CrossRef] [Green Version]
- Guja, K.; Garcia-Diaz, M. Hitting the brakes: Termination of mitochondrial transcription. Biochim. Biophys. Acta 2012, 1819, 939–947. [Google Scholar] [CrossRef] [Green Version]
- Yakubovskaya, E.; Mejia, E.; Byrnes, J.; Hambardjieva, E.; Garcia-Diaz, M. Helix unwinding and base flipping enable human MTERF1 to terminate mitochondrial transcription. Cell 2010, 141, 982–993. [Google Scholar] [CrossRef] [Green Version]
- Antonicka, H.; Shoubridge, E. Mitochondrial RNA Granules Are Centers for Posttranscriptional RNA Processing and Ribosome Biogenesis. Cell Rep. 2015, 10, 920–932. [Google Scholar] [CrossRef] [Green Version]
- Ojala, D.; Montoya, J.; Attardi, G. tRNA punctuation model of RNA processing in human mitochondria. Nature 1981, 290, 470–474. [Google Scholar] [CrossRef]
- Barchiesi, A.; Vascotto, C. Transcription, Processing, and Decay of Mitochondrial RNA in Health and Disease. Int. J. Mol. Sci. 2019, 20, 2221. [Google Scholar] [CrossRef] [Green Version]
- Bratic, A.; Clemente, P.; Calvo-Garrido, J.; Maffezzini, C.; Felser, A.; Wibom, R.; Wedell, A.; Freyer, C.; Wredenberg, A. Mitochondrial Polyadenylation is a One-Step Process Required for mRNA Integrity and tRNA Maturation. PLoS Genet. 2016, 12, e1006028. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.; Tong, L. Mitochondrial poly(A) polymerase and polyadenylation. Biochim. Biophys. Acta 2012, 1819, 992–997. [Google Scholar] [CrossRef] [Green Version]
- Betat, H.; Mörl, M. The CCA-adding enzyme: A central scrutinizer in tRNA quality control. Bioessays 2015, 37, 975–982. [Google Scholar] [CrossRef]
- Temperley, R.; Wydro, M.; Lightowlers, R.; Chrzanowska-Lightowlers, Z. Human mitochondrial mRNAs--like members of all families, similar but different. Biochim. Biophys. Acta 2010, 1797, 1081–1085. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, T. Evolution of a protein-rich mitochondrial ribosome: Implications for human genetic disease. Gene 2002, 286, 73–79. [Google Scholar] [CrossRef]
- Greber, B.; Ban, N. Structure and Function of the Mitochondrial Ribosome. Annu. Rev. Biochem. 2016, 85, 103–132. [Google Scholar] [CrossRef]
- Van der Sluis, E.; Bauerschmitt, H.; Becker, T.; Mielke, T.; Frauenfeld, J.; Berninghausen, O.; Neupert, W.; Herrmann, J.; Beckmann, R. Parallel Structural Evolution of Mitochondrial Ribosomes and OXPHOS Complexes. Genome Biol. Evol. 2015, 7, 1235–1251. [Google Scholar] [CrossRef] [Green Version]
- Amunts, A.; Brown, A.; Toots, J.; Scheres, S.; Ramakrishnan, V. The structure of the human mitochondrial ribosome. Science 2015, 348, 95–98. [Google Scholar] [CrossRef] [Green Version]
- Mai, N.; Chrzanowska-Lightowlers, Z.; Lightowlers, R. The process of mammalian mitochondrial protein synthesis. Cell Tissue Res. 2017, 367, 5–20. [Google Scholar] [CrossRef] [Green Version]
- Keckesova, Z.; Donaher, J.; De Cock, J.; Freinkman, E.; Lingrell, S.; Bachovchin, D.; Bierie, B.; Tischler, V.; Noske, A.; Okondo, M.; et al. LACTB is a tumour suppressor that modulates lipid metabolism and cell state. Nature 2017, 543, 681–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Souza, A.; Minczuk, M. Mitochondrial transcription and translation: Overview. Essays Biochem. 2018, 62, 309–320. [Google Scholar] [CrossRef] [Green Version]
- Browning, K.; Bailey-Serres, J. Mechanism of cytoplasmic mRNA translation. Arab. Book 2015, 13, e0176. [Google Scholar] [CrossRef] [Green Version]
- RajBhandary, U. Initiator transfer RNAs. J. Bacteriol. 1994, 176, 547–552. [Google Scholar] [CrossRef] [Green Version]
- Spencer, A.; Spremulli, L. Interaction of mitochondrial initiation factor 2 with mitochondrial fMet-tRNA. Nucleic Acids Res. 2004, 32, 5464–5470. [Google Scholar] [CrossRef] [Green Version]
- Soleimanpour-Lichaei, H.; Kuhl, I.; Gaisne, M.; Passos, J.; Wydro, M.; Rorbach, J.; Temperley, R.; Bonnefoy, N.; Tate, W.; Lightowlers, R.; et al. mtRF1a is a human mitochondrial translation release factor decoding the major termination codons UAA and UAG. Mol. Cell 2007, 27, 745–757. [Google Scholar] [CrossRef]
- Rorbach, J.; Richter, R.; Wessels, H.; Wydro, M.; Pekalski, M.; Farhoud, M.; Kühl, I.; Gaisne, M.; Bonnefoy, N.; Smeitink, J.; et al. The human mitochondrial ribosome recycling factor is essential for cell viability. Nucleic Acids Res. 2008, 36, 5787–5799. [Google Scholar] [CrossRef]
- Tsuboi, M.; Morita, H.; Nozaki, Y.; Akama, K.; Ueda, T.; Ito, K.; Nierhaus, K.; Takeuchi, N. EF-G2mt is an exclusive recycling factor in mammalian mitochondrial protein synthesis. Mol. Cell 2009, 35, 502–510. [Google Scholar] [CrossRef]
- Bogenhagen, D.; Ostermeyer-Fay, A.; Haley, J.; Garcia-Diaz, M. Kinetics and Mechanism of Mammalian Mitochondrial Ribosome Assembly. Cell Rep. 2018, 22, 1935–1944. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.; Rathore, S.; Kimanius, D.; Aibara, S.; Bai, X.; Rorbach, J.; Amunts, A.; Ramakrishnan, V. Structures of the human mitochondrial ribosome in native states of assembly. Nat. Struct. Mol. Biol. 2017, 24, 866–869. [Google Scholar] [CrossRef]
- Englmeier, R.; Pfeffer, S.; Forster, F. Structure of the Human Mitochondrial Ribosome Studied In Situ by Cryoelectron Tomography. Structure 2017, 25, 1574–1581.e1572. [Google Scholar] [CrossRef] [Green Version]
- Greber, B.; Boehringer, D.; Leitner, A.; Bieri, P.; Voigts-Hoffmann, F.; Erzberger, J.; Leibundgut, M.; Aebersold, R.; Ban, N. Architecture of the large subunit of the mammalian mitochondrial ribosome. Nature 2014, 505, 515–519. [Google Scholar] [CrossRef]
- Couvillion, M.; Soto, I.; Shipkovenska, G.; Churchman, L. Synchronized mitochondrial and cytosolic translation programs. Nature 2016, 533, 499–503. [Google Scholar] [CrossRef] [Green Version]
- Grevel, A.; Pfanner, N.; Becker, T. Coupling of import and assembly pathways in mitochondrial protein biogenesis. Biol. Chem. 2019, 401, 117–129. [Google Scholar] [CrossRef]
- Pfanner, N. Mitochondrial machineries for import and assembly of proteins. Biochim. Biophys. Acta 2018, 1859, e8. [Google Scholar] [CrossRef]
- Pfanner, N.; Warscheid, B.; Wiedemann, N. Mitochondrial proteins: From biogenesis to functional networks. Nat. Rev. Mol. Cell Biol. 2019, 20, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, N.; Pfanner, N. Mitochondrial Machineries for Protein Import and Assembly. Annu. Rev. Biochem. 2017, 86, 685–714. [Google Scholar] [CrossRef] [Green Version]
- Vogtle, F.; Wortelkamp, S.; Zahedi, R.; Becker, D.; Leidhold, C.; Gevaert, K.; Kellermann, J.; Voos, W.; Sickmann, A.; Pfanner, N.; et al. Global analysis of the mitochondrial N-proteome identifies a processing peptidase critical for protein stability. Cell 2009, 139, 428–439. [Google Scholar] [CrossRef]
- Hennon, S.; Soman, R.; Zhu, L.; Dalbey, R. YidC/Alb3/Oxa1 Family of Insertases. J. Biol. Chem. 2015. [Google Scholar] [CrossRef] [Green Version]
- Bonnefoy, N.; Chalvet, F.; Hamel, P.; Slonimski, P.; Dujardin, G. OXA1, a Saccharomyces cerevisiae nuclear gene whose sequence is conserved from prokaryotes to eukaryotes controls cytochrome oxidase biogenesis. J. Mol. Biol. 1994, 239, 201–212. [Google Scholar] [CrossRef]
- Altamura, N.; Capitanio, N.; Bonnefoy, N.; Papa, S.; Dujardin, G. The Saccharomyces cerevisiae OXA1 gene is required for the correct assembly of cytochrome c oxidase and oligomycin-sensitive ATP synthase. FEBS Lett. 1996, 382, 111–115. [Google Scholar] [CrossRef] [Green Version]
- Hell, K.; Neupert, W.; Stuart, R. Oxa1p acts as a general membrane insertion machinery for proteins encoded by mitochondrial DNA. EMBO J. 2001, 20, 1281–1288. [Google Scholar] [CrossRef] [Green Version]
- Hell, K.; Herrmann, J.; Pratje, E.; Neupert, W.; Stuart, R. Oxa1p, an essential component of the N-tail protein export machinery in mitochondria. Proc. Natl. Acad. Sci. USA 1998, 95, 2250–2255. [Google Scholar] [CrossRef] [Green Version]
- Hildenbeutel, M.; Theis, M.; Geier, M.; Haferkamp, I.; Neuhaus, H.; Herrmann, J.; Ott, M. The membrane insertase Oxa1 is required for efficient import of carrier proteins into mitochondria. J. Mol. Biol. 2012, 423, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Stiller, S.; Hopker, J.; Oeljeklaus, S.; Schutze, C.; Schrempp, S.; Vent-Schmidt, J.; Horvath, S.; Frazier, A.; Gebert, N.; van der Laan, M.; et al. Mitochondrial OXA Translocase Plays a Major Role in Biogenesis of Inner-Membrane Proteins. Cell Metab. 2016, 23, 901–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hell, K.; Herrmann, J.; Pratje, E.; Neupert, W.; Stuart, R. Oxa1p mediates the export of the N- and C-termini of pCoxII from the mitochondrial matrix to the intermembrane space. FEBS Lett. 1997, 418, 367–370. [Google Scholar] [CrossRef]
- Jia, L.; Dienhart, M.; Stuart, R. Oxa1 directly interacts with Atp9 and mediates its assembly into the mitochondrial F1Fo-ATP synthase complex. Mol. Biol. Cell. 2007, 18, 1897–1908. [Google Scholar] [CrossRef] [Green Version]
- Thompson, K.; Mai, N.; Olahova, M.; Scialo, F.; Formosa, L.; Stroud, D.; Garrett, M.; Lax, N.; Robertson, F.; Jou, C.; et al. OXA1L mutations cause mitochondrial encephalopathy and a combined oxidative phosphorylation defect. EMBO Mol. Med. 2018, 10. [Google Scholar] [CrossRef]
- Bonnefoy, N.; Kermorgant, M.; Groudinsky, O.; Minet, M.; Slonimski, P.; Dujardin, G. Cloning of a human gene involved in cytochrome oxidase assembly by functional complementation of an oxa1- mutation in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1994, 91, 11978–11982. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.; Dienhart, M.; Schramp, M.; McCauley, M.; Hell, K.; Stuart, R. Yeast Oxa1 interacts with mitochondrial ribosomes: The importance of the C-terminal region of Oxa1. EMBO J. 2003, 22, 6438–6447. [Google Scholar] [CrossRef]
- Kohler, R.; Boehringer, D.; Greber, B.; Bingel-Erlenmeyer, R.; Collinson, I.; Schaffitzel, C.; Ban, N. YidC and Oxa1 form dimeric insertion pores on the translating ribosome. Mol. Cell 2009, 34, 344–353. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.; Spremulli, L.; Fecko, C. Identification of protein-protein and protein-ribosome interacting regions of the C-terminal tail of human mitochondrial inner membrane protein Oxa1L. J. Biol. Chem. 2010, 285, 34991–34998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoldt, S.; Wenzel, D.; Kehrein, K.; Riedel, D.; Ott, M.; Jakobs, S. Spatial orchestration of mitochondrial translation and OXPHOS complex assembly. Nat. Cell Biol. 2018, 20, 528–534. [Google Scholar] [CrossRef] [Green Version]
- Stoldt, S.; Wenzel, D.; Hildenbeutel, M.; Wurm, C.; Herrmann, J.; Jakobs, S. The inner-mitochondrial distribution of Oxa1 depends on the growth conditions and on the availability of substrates. Mol. Biol. Cell. 2012, 23, 2292–2301. [Google Scholar] [CrossRef] [PubMed]
- Mick, D.; Dennerlein, S.; Wiese, H.; Reinhold, R.; Pacheu-Grau, D.; Lorenzi, I.; Sasarman, F.; Weraarpachai, W.; Shoubridge, E.; Warscheid, B.; et al. MITRAC Links Mitochondrial Protein Translocation to Respiratory-Chain Assembly and Translational Regulation. Cell 2012, 151, 1528–1541. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Richter-Dennerlein, R.; Pacheu-Grau, D.; Liu, F.; Zhu, Y.; Dennerlein, S.; Rehling, P. MITRAC15/COA1 promotes mitochondrial translation in a ND2 ribosome–nascent chain complex. EMBO Rep. 2020, 21, e48833. [Google Scholar] [CrossRef]
- Dennerlein, S.; Oeljeklaus, S.; Jans, D.; Hellwig, C.; Bareth, B.; Jakobs, S.; Deckers, M.; Warscheid, B.; Rehling, P. MITRAC7 acts as a COX1-specific chaperone and reveals a checkpoint during cytochrome c oxidase assembly. Cell Rep. 2015, 12, 1644–1655. [Google Scholar] [CrossRef] [Green Version]
- Richter-Dennerlein, R.; Oeljeklaus, S.; Lorenzi, I.; Ronsör, C.; Bareth, B.; Schendzielorz, A.; Wang, C.; Warscheid, B.; Rehling, P.; Dennerlein, S. Mitochondrial Protein Synthesis Adapts to Influx of Nuclear-Encoded Protein. Cell 2016, 167, 471–483.e410. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, R.; Wanschers, B.; Cuypers, T.; Esseling, J.; Riemersma, M.; van den Brand, M.; Gloerich, J.; Lasonder, E.; van den Heuvel, L.; Nijtmans, L.; et al. Iterative orthology prediction uncovers new mitochondrial proteins and identifies C12orf62 as the human ortholog of COX14, a protein involved in the assembly of cytochrome coxidase. Genome Biol. 2012, 13, R12. [Google Scholar] [CrossRef]
- Dennerlein, S.; Rehling, P. Human mitochondrial COX1 assembly into cytochrome c oxidase at a glance. J. Cell Sci. 2015. [Google Scholar] [CrossRef] [Green Version]
- Ott, M.; Amunts, A.; Brown, A. Organization and Regulation of Mitochondrial Protein Synthesis. Annu. Rev. Biochem. 2016, 85, 77–101. [Google Scholar] [CrossRef]
- Salvatori, R.; Kehrein, K.; Singh, A.; Aftab, W.; Möller-Hergt, B.; Forne, I.; Imhof, A.; Ott, M. Molecular Wiring of a Mitochondrial Translational Feedback Loop. Mol. Cell 2020, 77. [Google Scholar] [CrossRef]
- Weraarpachai, W.; Antonicka, H.; Sasarman, F.; Seeger, J.; Schrank, B.; Kolesar, J.; Lochmuller, H.; Chevrette, M.; Kaufman, B.; Horvath, R.; et al. Mutation in TACO1, encoding a translational activator of COX I, results in cytochrome c oxidase deficiency and late-onset Leigh syndrome. Nat. Genet. 2009, 41, 833–837. [Google Scholar] [CrossRef]
- Seeger, J.; Schrank, B.; Pyle, A.; Stucka, R.; Lörcher, U.; Müller-Ziermann, S.; Abicht, A.; Czermin, B.; Holinski-Feder, E.; Lochmüller, H.; et al. Clinical and neuropathological findings in patients with TACO1 mutations. Neuromuscul. Disord. 2010, 20, 720–724. [Google Scholar] [CrossRef]
- Richman, T.; Spåhr, H.; Ermer, J.; Davies, S.; Viola, H.; Bates, K.; Papadimitriou, J.; Hool, L.; Rodger, J.; Larsson, N.; et al. Loss of the RNA-binding protein TACO1 causes late-onset mitochondrial dysfunction in mice. Nat. Commun. 2016, 7, 11884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deshwal, S.; Fiedler, K.; Langer, T. Mitochondrial Proteases: Multifaceted Regulators of Mitochondrial Plasticity. Annu. Rev. Biochem. 2020. [Google Scholar] [CrossRef] [Green Version]
- Quirós, P.; Langer, T.; López-Otín, C. New roles for mitochondrial proteases in health, ageing and disease. Nat. Rev. Mol. Cell Biol. 2015, 16, 345–359. [Google Scholar] [CrossRef]
- Voos, W.; Jaworek, W.; Wilkening, A.; Bruderek, M. Protein quality control at the mitochondrion. Essays Biochem. 2016, 60, 213–225. [Google Scholar] [CrossRef]
- Brunetti, D.; Torsvik, J.; Dallabona, C.; Teixeira, P.; Sztromwasser, P.; Fernandez-Vizarra, E.; Cerutti, R.; Reyes, A.; Preziuso, C.; D’Amati, G.; et al. Defective PITRM1 mitochondrial peptidase is associated with Aβ amyloidotic neurodegeneration. EMBO Mol. Med. 2016, 8, 176–190. [Google Scholar] [CrossRef]
- Taskin, A.; Kücükköse, C.; Burger, N.; Mossmann, D.; Meisinger, C.; Vögtle, F. The novel mitochondrial matrix protease Ste23 is required for efficient presequence degradation and processing. Mol. Biol. Cell. 2017, 28, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Richter, U.; Ng, K.; Suomi, F.; Marttinen, P.; Turunen, T.; Jackson, C.; Suomalainen, A.; Vihinen, H.; Jokitalo, E.; Nyman, T.; et al. Mitochondrial stress response triggered by defects in protein synthesis quality control. Life Sci. Alliance 2019, 2, e201800219. [Google Scholar] [CrossRef] [PubMed]
- Glynn, S. Multifunctional Mitochondrial AAA Proteases. Front. Mol. Biosci. 2017, 4, 34. [Google Scholar] [CrossRef] [Green Version]
- Rugarli, E.; Langer, T. Mitochondrial quality control: A matter of life and death for neurons. EMBO J. 2012, 31, 1336–1349. [Google Scholar] [CrossRef] [Green Version]
- Ehses, S.; Raschke, I.; Mancuso, G.; Bernacchia, A.; Geimer, S.; Tondera, D.; Martinou, J.; Westermann, B.; Rugarli, E.; Langer, T. Regulation of OPA1 processing and mitochondrial fusion by m-AAA protease isoenzymes and OMA1. J. Cell Biol. 2009, 187, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- Battersby, B.; Richter, U.; Safronov, O. Mitochondrial Nascent Chain Quality Control Determines Organelle Form and Function. ACS Chem. Biol. 2019, 14, 2396–2405. [Google Scholar] [CrossRef] [PubMed]
- Bogenhagen, D.; Haley, J. Pulse-chase SILAC–based analyses reveal selective over-synthesis and rapid turnover of mitochondrial protein components of respiratory complexes. J. Biol. Chem. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wrobel, L.; Topf, U.; Bragoszewski, P.; Wiese, S.; Sztolsztener, M.; Oeljeklaus, S.; Varabyova, A.; Lirski, M.; Chroscicki, P.; Mroczek, S.; et al. Mistargeted mitochondrial proteins activate a proteostatic response in the cytosol. Nature 2015, 524, 485–488. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X. A cytosolic network suppressing mitochondria-mediated proteostatic stress and cell death. Nature 2015, 524, 481–484. [Google Scholar] [CrossRef] [Green Version]
- Thompson, K.; Majd, H.; Dallabona, C.; Reinson, K.; King, M.; Alston, C.; He, L.; Lodi, T.; Jones, S.; Fattal-Valevski, A.; et al. Recurrent De Novo Dominant Mutations in SLC25A4 Cause Severe Early-Onset Mitochondrial Disease and Loss of Mitochondrial DNA Copy Number. Am. J. Hum. Genet. 2016, 99, 860–876. [Google Scholar] [CrossRef] [Green Version]
- Weidberg, H.; Amon, A. MitoCPR—A surveillance pathway that protects mitochondria in response to protein import stress. Science 2018, 360, eaan4146. [Google Scholar] [CrossRef] [Green Version]
- Mårtensson, C.; Priesnitz, C.; Song, J.; Ellenrieder, L.; Doan, K.; Boos, F.; Floerchinger, A.; Zufall, N.; Oeljeklaus, S.; Warscheid, B.; et al. Mitochondrial protein translocation-associated degradation. Nature 2019, 569, 679–683. [Google Scholar] [CrossRef]
- Izawa, T.; Park, S.; Zhao, L.; Hartl, F.; Neupert, W. Cytosolic Protein Vms1 Links Ribosome Quality Control to Mitochondrial and Cellular Homeostasis. Cell 2017, 171, 890–903.e818. [Google Scholar] [CrossRef] [Green Version]
- Chance, B.; Williams, G. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J. Biol. Chem. 1955, 217, 383–393. [Google Scholar]
- Keilin, D.; Hartree, E. Activity of the cytochrome system in heart muscle preparations. Biochem. J. 1947, 41, 500–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hackenbrock, C.; Chazotte, B.; Gupte, S. The random collision model and a critical assessment of diffusion and collision in mitochondrial electron transport. J. Bioenerg. Biomembr. 1986, 18, 331–368. [Google Scholar] [CrossRef] [PubMed]
- Schagger, H.; Pfeiffer, K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 2000, 19, 1777–1783. [Google Scholar] [CrossRef] [Green Version]
- Acin-Perez, R.; Enriquez, J. The function of the respiratory supercomplexes: The plasticity model. Biochim. Biophys. Acta 2014, 1837, 444–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Protasoni, M.; Pérez-Pérez, R.; Lobo-Jarne, T.; Harbour, M.; Ding, S.; Peñas, A.; Diaz, F.; Moraes, C.; Fearnley, I.; Zeviani, M.; et al. Respiratory supercomplexes act as a platform for complex III-mediated maturation of human mitochondrial complexes I and IV. EMBO J. 2020, 39, e102817. [Google Scholar] [CrossRef] [PubMed]
- Letts, J.; Sazanov, L. Clarifying the supercomplex: The higher-order organization of the mitochondrial electron transport chain. Nat. Struct. Mol. Biol. 2017, 24, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Pernas, L.; Scorrano, L. Mito-Morphosis: Mitochondrial Fusion, Fission, and Cristae Remodeling as Key Mediators of Cellular Function. Annu. Rev. Physiol. 2016, 78, 505–531. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.; Patel, J.; Khacho, M. Linking mitochondrial dynamics, cristae remodeling and supercomplex formation: How mitochondrial structure can regulate bioenergetics. Mitochondrion 2019, 49, 259–268. [Google Scholar] [CrossRef]
- Cogliati, S.; Enriquez, J.; Scorrano, L. Mitochondrial Cristae: Where Beauty Meets Functionality. Trends Biochem. Sci. 2016, 41, 261–273. [Google Scholar] [CrossRef] [Green Version]
- Signes, A.; Fernandez-Vizarra, E. Assembly of mammalian oxidative phosphorylation complexes I-V and supercomplexes. Essays Biochem. 2018, 62, 255–270. [Google Scholar] [CrossRef]
- Vinothkumar, K.; Zhu, J.; Hirst, J. Architecture of mammalian respiratory complex I. Nature 2014, 515, 80–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Vinothkumar, K.; Hirst, J. Structure of mammalian respiratory complex I. Nature 2016, 536, 354–358. [Google Scholar] [CrossRef] [Green Version]
- Clason, T.; Ruiz, T.; Schagger, H.; Peng, G.; Zickermann, V.; Brandt, U.; Michel, H.; Radermacher, M. The structure of eukaryotic and prokaryotic complex I. J. Struct. Biol. 2010, 169, 81–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirst, J. Mitochondrial complex I. Annu. Rev. Biochem. 2013, 82, 551–575. [Google Scholar] [CrossRef]
- Ripple, M.; Kim, N.; Springett, R. Mammalian complex I pumps 4 protons per 2 electrons at high and physiological proton motive force in living cells. J. Biol. Chem. 2013, 288, 5374–5380. [Google Scholar] [CrossRef] [Green Version]
- Efremov, R.; Baradaran, R.; Sazanov, L. The architecture of respiratory complex I. Nature 2010, 465, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Formosa, L.; Dibley, M.; Stroud, D.; Ryan, M. Building a complex complex: Assembly of mitochondrial respiratory chain complex I. Semin. Cell Dev. Biol. 2018, 76, 154–162. [Google Scholar] [CrossRef]
- Ogilvie, I.; Kennaway, N.; Shoubridge, E. A molecular chaperone for mitochondrial complex I assembly is mutated in a progressive encephalopathy. J. Clin. Investig. 2005, 115, 2784–2792. [Google Scholar] [CrossRef] [PubMed]
- Schlehe, J.; Journel, M.; Taylor, K.; Amodeo, K.; LaVoie, M. The mitochondrial disease associated protein Ndufaf2 is dispensable for Complex-1 assembly but critical for the regulation of oxidative stress. Neurobiol. Dis. 2013, 58, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Rhein, V.; Carroll, J.; Ding, S.; Fearnley, I.; Walker, J. NDUFAF5 hydroxylates NDUFS7 at an early stage in the assembly of human complex I. J. Biol. Chem. 2016, 291, 14851–14860. [Google Scholar] [CrossRef] [Green Version]
- Rhein, V.; Carroll, J.; Ding, S.; Fearnley, I.; Walker, J. NDUFAF7 methylates arginine 85 in the NDUFS2 subunit of human complex I. J. Biol. Chem. 2013, 288, 33016–33026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Caballero, L.; Guerrero-Castillo, S.; Nijtmans, L. Unraveling the complexity of mitochondrial complex I assembly: A dynamic process. Biochim. Biophys. Acta 2016, 1857, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Castillo, S.; Baertling, F.; Kownatzki, D.; Wessels, H.; Arnold, S.; Brandt, U.; Nijtmans, L. The Assembly Pathway of Mitochondrial Respiratory Chain Complex I. Cell Metab. 2017, 25, 128–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guarani, V.; Paulo, J.; Zhai, B.; Huttlin, E.; Gygi, S.; Harper, J. TIMMDC1/C3orf1 functions as a membrane-embedded mitochondrial complex I assembly factor through association with the MCIA complex. Mol. Cell. Biol. 2014, 34, 847–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, B.; Carroll, J.; Ding, S.; Fearnley, I.; Walker, J. Assembly factors for the membrane arm of human complex I. Proc. Natl. Acad. Sci. USA 2013, 110, 18934–18939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Formosa, L.; Muellner-Wong, L.; Reljic, B.; Sharpe, A.; Jackson, T.; Beilharz, T.; Stojanovski, D.; Lazarou, M.; Stroud, D.; Ryan, M. Dissecting the Roles of Mitochondrial Complex I Intermediate Assembly Complex Factors in the Biogenesis of Complex I. Cell Rep. 2020, 31, 107541. [Google Scholar] [CrossRef]
- Lemire, B. Evolution of FOXRED1, an FAD-dependent oxidoreductase necessary for NADH: Ubiquinone oxidoreductase (Complex I) assembly. Biochim. Biophys. Acta 2015, 1847, 451–457. [Google Scholar] [CrossRef] [Green Version]
- Formosa, L.; Mimaki, M.; Frazier, A.; McKenzie, M.; Stait, T.; Thorburn, D.; Stroud, D.; Ryan, M. Characterization of mitochondrial FOXRED1 in the assembly of respiratory chain complex I. Hum. Mol. Genet. 2015, 24, 2952–2965. [Google Scholar] [CrossRef] [Green Version]
- Stroud, D.; Surgenor, E.; Formosa, L.; Reljic, B.; Frazier, A.; Dibley, M.; Osellame, L.; Stait, T.; Beilharz, T.; Thorburn, D.; et al. Accessory subunits are integral for assembly and function of human mitochondrial complex I. Nature 2016, 538, 123–126. [Google Scholar] [CrossRef] [Green Version]
- Iverson, T. Catalytic mechanisms of complex II enzymes: A structural perspective. Biochim. Biophys. Acta 2013, 1827, 648–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, F.; Huo, X.; Zhai, Y.; Wang, A.; Xu, J.; Su, D.; Bartlam, M.; Rao, Z. Crystal Structure of Mitochondrial Respiratory Membrane Protein Complex II. Cell 2005, 121, 1043–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maklashina, E.; Rajagukguk, S.; Starbird, C.; McDonald, W.; Koganitsky, A.; Eisenbach, M.; Iverson, T.; Cecchini, G. Binding of the Covalent Flavin Assembly Factor to the Flavoprotein Subunit of Complex II. J. Biol. Chem. 2016, 291, 2904–2916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zafreen, L.; Walker-Kopp, N.; Huang, L.; Berry, E. In-vitro, SDH5-dependent flavinylation of immobilized human respiratory complex II flavoprotein. Arch. Biochem. Biophys. 2016, 604, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, D.; Goffrini, P.; Uziel, G.; Horvath, R.; Klopstock, T.; Lochmuller, H.; D’Adamo, P.; Gasparini, P.; Strom, T.; Prokisch, H.; et al. SDHAF1, encoding a LYR complex-II specific assembly factor, is mutated in SDH-defective infantile leukoencephalopathy. Nat. Genet. 2009, 41, 654–656. [Google Scholar] [CrossRef] [PubMed]
- Maio, N.; Ghezzi, D.; Verrigni, D.; Rizza, T.; Bertini, E.; Martinelli, D.; Zeviani, M.; Singh, A.; Carrozzo, R.; Rouault, T. Disease-Causing SDHAF1 Mutations Impair Transfer of Fe-S Clusters to SDHB. Cell Metab. 2016, 23, 292–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na, U.; Yu, W.; Cox, J.; Bricker, D.; Brockmann, K.; Rutter, J.; Thummel, C.; Winge, D. The LYR factors SDHAF1 and SDHAF3 mediate maturation of the iron-sulfur subunit of succinate dehydrogenase. Cell Metab. 2014, 20, 253–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Vranken, J.; Bricker, D.; Dephoure, N.; Gygi, S.; Cox, J.; Thummel, C.; Rutter, J. SDHAF4 promotes mitochondrial succinate dehydrogenase activity and prevents neurodegeneration. Cell Metab. 2014, 20, 241–252. [Google Scholar] [CrossRef] [Green Version]
- Belt, K.; Van Aken, O.; Murcha, M.; Millar, A.; Huang, S. An assembly factor promotes assembly of flavinated SDH1 into the succinate dehydrogenase complex. Plant Physiol. 2018, 177, 1439–1452. [Google Scholar] [CrossRef] [Green Version]
- Van Vranken, J.; Na, U.; Winge, D.; Rutter, J. Protein-mediated assembly of succinate dehydrogenase and its cofactors. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 168–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moosavi, B.; Berry, E.; Zhu, X.; Yang, W.; Yang, G. The assembly of succinate dehydrogenase: A key enzyme in bioenergetics. Cell. Mol. Life Sci. 2019, 76, 4023–4042. [Google Scholar] [CrossRef]
- Bezawork-Geleta, A.; Wen, H.; Dong, L.; Yan, B.; Vider, J.; Boukalova, S.; Krobova, L.; Vanova, K.; Zobalova, R.; Sobol, M.; et al. Alternative assembly of respiratory complex II connects energy stress to metabolic checkpoints. Nat. Commun. 2018, 9, 2221. [Google Scholar] [CrossRef] [PubMed]
- Crofts, A.; Holland, J.; Victoria, D.; Kolling, D.; Dikanov, S.; Gilbreth, R.; Lhee, S.; Kuras, R.; Kuras, M. The Q-cycle reviewed: How well does a monomeric mechanism of the bc(1) complex account for the function of a dimeric complex? Biochim. Biophys. Acta 2008, 1777, 1001–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruschke, S.; Kehrein, K.; Römpler, K.; Gröne, K.; Israel, L.; Imhof, A.; Herrmann, J.; Ott, M. Cbp3–Cbp6 interacts with the yeast mitochondrial ribosomal tunnel exit and promotes cytochrome b synthesis and assembly. J. Cell Biol. 2011, 193, 1101–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucker, E.; Wanschers, B.; Szklarczyk, R.; Mountford, H.; Wijeyeratne, X.; van den Brand, M.; Leenders, A.; Rodenburg, R.; Reljić, B.; Compton, A.; et al. Mutations in the UQCC1-interacting protein, UQCC2, cause human complex III deficiency associated with perturbed cytochrome b protein expression. PLoS Genet. 2013, 9, e1004034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanschers, B.; Szklarczyk, R.; van den Brand, M.; Jonckheere, A.; Suijskens, J.; Smeets, R.; Rodenburg, R.; Stephan, K.; Helland, I.; Elkamil, A.; et al. A mutation in the human CBP4 ortholog UQCC3 impairs complex III assembly, activity and cytochrome b stability. Hum. Mol. Genet. 2014, 23, 6356–6365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hildenbeutel, M.; Hegg, E.; Stephan, K.; Gruschke, S.; Meunier, B.; Ott, M. Assembly factors monitor sequential hemylation of cytochrome b to regulate mitochondrial translation. J. Cell Biol. 2014, 205, 511–524. [Google Scholar] [CrossRef] [Green Version]
- Ndi, M.; Marin-Buera, L.; Salvatori, R.; Singh, A.; Ott, M. Biogenesis of the bc1 Complex of the Mitochondrial Respiratory Chain. J. Mol. Biol. 2018, 430, 3892–3905. [Google Scholar] [CrossRef]
- Lill, R.; Stuart, R.; Drygas, M.; Nargang, F.; Neupert, W. Import of cytochrome c heme lyase into mitochondria: A novel pathway into the intermembrane space. EMBO J. 1992, 11, 449–456. [Google Scholar] [CrossRef]
- Smith, P.; Fox, J.; Winge, D. Biogenesis of the cytochrome bc(1) complex and role of assembly factors. Biochim. Biophys. Acta 2012, 1817, 276–286. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Vizarra, E.; Zeviani, M. Mitochondrial complex III Rieske Fe-S protein processing and assembly. Cell Cycle 2018, 17, 681–687. [Google Scholar] [CrossRef] [Green Version]
- Brandt, U.; Uribe, S.; Schägger, H.; Trumpower, B. Isolation and characterization of QCR10, the nuclear gene encoding the 8.5-kDa subunit 10 of the Saccharomyces cerevisiae cytochrome bc1 complex. J. Biol. Chem. 1994, 269, 12947–12953. [Google Scholar]
- Phillips, J.; Graham, L.; Trumpower, B. Subunit 9 of the Saccharomyces cerevisiae cytochrome bc1 complex is required for insertion of EPR-detectable iron-sulfur cluster into the Rieske iron-sulfur protein. J. Biol. Chem. 1993, 268, 11727–11736. [Google Scholar] [PubMed]
- Phillips, J.; Schmitt, M.; Brown, T.; Beckmann, J.; Trumpower, B. Isolation and characterization of QCR9, a nuclear gene encoding the 7.3-kDa subunit 9 of the Saccharomyces cerevisiae ubiquinol-cytochrome c oxidoreductase complex. An intron-containing gene with a conserved sequence occurring in the intron of COX4. J. Biol. Chem. 1990, 265, 20813–20821. [Google Scholar] [PubMed]
- Zara, V.; Conte, L.; Trumpower, B. Evidence that the assembly of the yeast cytochrome bc1 complex involves the formation of a large core structure in the inner mitochondrial membrane. FEBS J. 2009, 276, 1900–1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, A.; Smith, P.; Fox, J.; Cui, T.; Khalimonchuk, O.; Winge, D. The LYR protein Mzm1 functions in the insertion of the Rieske Fe/S protein in yeast mitochondria. Mol. Cell. Biol. 2011, 31, 3988–3996. [Google Scholar] [CrossRef] [Green Version]
- Cui, T.; Smith, P.; Fox, J.; Khalimonchuk, O.; Winge, D. Late-stage maturation of the Rieske Fe/S protein: Mzm1 stabilizes Rip1 but does not facilitate its translocation by the AAA ATPase Bcs1. Mol. Cell. Biol. 2012, 32, 4400–4409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, E.; Lobo, T.; Fox, J.; Zeviani, M.; Winge, D.; Fernandez-Vizarra, E. LYRM7/MZM1L is a UQCRFS1 chaperone involved in the last steps of mitochondrial Complex III assembly in human cells. Biochim. Biophys. Acta 2013, 1827, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Brandt, U.; Yu, L.; Yu, C.; Trumpower, B. The mitochondrial targeting presequence of the Rieske iron-sulfur protein is processed in a single step after insertion into the cytochrome bc1 complex in mammals and retained as a subunit in the complex. J. Biol. Chem. 1993, 268, 8387–8390. [Google Scholar]
- Bottani, E.; Cerutti, R.; Harbour, M.; Ravaglia, S.; Dogan, S.; Giordano, C.; Fearnley, I.; D’Amati, G.; Viscomi, C.; Fernandez-Vizarra, E.; et al. TTC19 Plays a Husbandry Role on UQCRFS1 Turnover in the Biogenesis of Mitochondrial Respiratory Complex III. Mol. Cell 2017, 67, 96–105.e104. [Google Scholar] [CrossRef] [Green Version]
- Wikstrom, M.; Krab, K.; Sharma, V. Oxygen Activation and Energy Conservation by Cytochrome c Oxidase. Chem. Rev. 2018, 118, 2469–2490. [Google Scholar] [CrossRef] [Green Version]
- Belevich, I.; Verkhovsky, M.; Wikstrom, M. Proton-coupled electron transfer drives the proton pump of cytochrome c oxidase. Nature 2006, 440, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Muramoto, K.; Ohta, K.; Shinzawa-Itoh, K.; Kanda, K.; Taniguchi, M.; Nabekura, H.; Yamashita, E.; Tsukihara, T.; Yoshikawa, S. Bovine cytochrome c oxidase structures enable O2 reduction with minimization of reactive oxygens and provide a proton-pumping gate. Proc. Natl. Acad. Sci. USA 2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirchberg, K.; Michel, H.; Alexiev, U. Net proton uptake is preceded by multiple proton transfer steps upon electron injection into cytochrome c oxidase. J. Biol. Chem. 2012, 287, 8187–8193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Vizarra, E.; Tiranti, V.; Zeviani, M. Assembly of the oxidative phosphorylation system in humans: What we have learned by studying its defects. Biochim. Biophys. Acta 2009, 1793, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Vidoni, S.; Harbour, M.; Guerrero-Castillo, S.; Signes, A.; Ding, S.; Fearnley, I.; Taylor, R.; Tiranti, V.; Arnold, S.; Fernandez-Vizarra, E.; et al. MR-1S Interacts with PET100 and PET117 in Module-Based Assembly of Human Cytochrome c Oxidase. Cell Rep. 2017, 18, 1727–1738. [Google Scholar] [CrossRef] [Green Version]
- Strogolova, V.; Furness, A.; Robb-McGrath, M.; Garlich, J.; Stuart, R. Rcf1 and Rcf2, Members of the Hypoxia-Induced Gene 1 Protein Family, Are Critical Components of the Mitochondrial Cytochrome bc1-Cytochrome c Oxidase Supercomplex. Mol. Cell. Biol. 2012, 32, 1363–1373. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, T.; Asano, Y.; Shintani, Y.; Aoyama, H.; Kioka, H.; Tsukamoto, O.; Hikita, M.; Shinzawa-Itoh, K.; Takafuji, K.; Higo, S.; et al. Higd1a is a positive regulator of cytochrome c oxidase. Proc. Natl. Acad. Sci. USA 2015, 112, 1553–1558. [Google Scholar] [CrossRef] [Green Version]
- Clemente, P.; Peralta, S.; Cruz-Bermudez, A.; Echevarría, L.; Fontanesi, F.; Barrientos, A.; Fernandez-Moreno, M.; Garesse, R. hCOA3 Stabilizes Cytochrome c Oxidase 1 (COX1) and Promotes Cytochrome c Oxidase Assembly in Human Mitochondria. J. Biol. Chem. 2013, 288, 8321–8331. [Google Scholar] [CrossRef] [Green Version]
- Mick, D.; Vukotic, M.; Piechura, H.; Meyer, H.; Warscheid, B.; Deckers, M.; Rehling, P. Coa3 and Cox14 are essential for negative feedback regulation of COX1 translation in mitochondria. J. Cell Biol. 2010, 191, 141–154. [Google Scholar] [CrossRef] [Green Version]
- Bourens, M.; Barrientos, A. Human mitochondrial cytochrome c oxidase assembly factor COX18 acts transiently as a membrane insertase within the subunit 2 maturation module. J. Biol. Chem. 2017, 292, 7774–7783. [Google Scholar] [CrossRef] [Green Version]
- Antonicka, H.; Leary, S.; Guercin, G.; Agar, J.; Horvath, R.; Kennaway, N.; Harding, C.; Jaksch, M.; Shoubridge, E. Mutations in COX10 result in a defect in mitochondrial heme A biosynthesis and account for multiple, early-onset clinical phenotypes associated with isolated COX deficiency. Hum. Mol. Genet. 2003, 12, 2693–2702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalimonchuk, O.; Kim, H.; Watts, T.; Perez-Martinez, X.; Winge, D. Oligomerization of Heme o Synthase in Cytochrome Oxidase Biogenesis Is Mediated by Cytochrome Oxidase Assembly Factor Coa2. J. Biol. Chem. 2012, 287, 26715–26726. [Google Scholar] [CrossRef] [Green Version]
- Swenson, S.; Cannon, A.; Harris, N.; Taylor, N.; Fox, J.; Khalimonchuk, O. Analysis of oligomerization properties of heme a synthase provides insights into its function in eukaryotes. J. Biol. Chem. 2016, 291, 10411–10425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, N.; Swenson, S.; Harris, N.; Germany, E.; Fox, J.; Khalimonchuk, O. The assembly factor Pet117 couples heme a synthase activity to cytochrome oxidase assembly. J. Biol. Chem. 2017, 292, 1815–1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, D.; Gray, J.; Mitchell, L.; Antholine, W.; Hosler, J. Assembly of cytochrome-c oxidase in the absence of assembly protein Surf1p leads to loss of the active site heme. J. Biol. Chem. 2005, 280, 17652–17656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timón-Gómez, A.; Nývltová, E.; Abriata, L.; Vila, A.; Hosler, J.; Barrientos, A. Mitochondrial cytochrome c oxidase biogenesis: Recent developments. Semin. Cell Dev. Biol. 2018, 76, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Pulliam, D.; Deepa, S.; Liu, Y.; Hill, S.; Lin, A.; Bhattacharya, A.; Shi, Y.; Sloane, L.; Viscomi, C.; Zeviani, M.; et al. Complex IV-deficient Surf1−/− mice initiate mitochondrial stress responses. Biochem. J. 2014, 462, 359–371. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Yao, J.; Johns, T.; Fu, A.; Bie, I.; Macmillan, C.; Cuthbert, A.; Newbold, R.; Wang, J.; Chevrette, M.; et al. SURF1, encoding a factor involved in the biogenesis of cytochrome c oxidase, is mutated in Leigh syndrome. Nat. Genet. 1998, 20, 337–343. [Google Scholar] [CrossRef]
- Tiranti, V.; Hoertnagel, K.; Carrozzo, R.; Galimberti, C.; Munaro, M.; Granatiero, M.; Zelante, L.; Gasparini, P.; Marzella, R.; Rocchi, M.; et al. Mutations of SURF-1 in Leigh Disease Associated with Cytochrome c Oxidase Deficiency. Am. J. Human Genet. 1998, 63, 1609–1621. [Google Scholar] [CrossRef] [Green Version]
- Glerum, D.; Shtanko, A.; Tzagoloff, A. Characterization of COX17, a Yeast Gene Involved in Copper Metabolism and Assembly of Cytochrome Oxidase. J. Biol. Chem. 1996, 271, 14504–14509. [Google Scholar] [CrossRef] [Green Version]
- Horng, Y.; Cobine, P.; Maxfield, A.; Carr, H.; Winge, D. Specific copper transfer from the Cox17 metallochaperone to both Sco1 and Cox11 in the assembly of yeast cytochrome C oxidase. J. Biol. Chem. 2004, 279, 35334–35340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiser, L.; Di Valentin, M.; Hamer, A.; Hosler, J. Cox11p Is Required for Stable Formation of the CuB and Magnesium Centers of Cytochrome c Oxidase. J. Biol. Chem. 2000, 275, 619–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leary, S.; Sasarman, F.; Nishimura, T.; Shoubridge, E. Human SCO2 is required for the synthesis of CO II and as a thiol-disulphide oxidoreductase for SCO1. Hum. Mol. Genet. 2009, 18, 2230–2240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgada, M.; Abriata, L.; Cefaro, C.; Gajda, K.; Banci, L.; Vila, A. Loop recognition and copper-mediated disulfide reduction underpin metal site assembly of CuA in human cytochrome oxidase. Proc. Natl. Acad. Sci. USA 2015, 112, 11771–11776. [Google Scholar] [CrossRef] [Green Version]
- Elliott, L.; Saracco, S.; Fox, T. Multiple roles of the Cox20 chaperone in assembly of Saccharomyces cerevisiae cytochrome c oxidase. Genetics 2012, 190, 559–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacheu-Grau, D.; Wasilewski, M.; Oeljeklaus, S.; Gibhardt, C.; Aich, A.; Chudenkova, M.; Dennerlein, S.; Deckers, M.; Bogeski, I.; Warscheid, B.; et al. COA6 Facilitates Cytochrome c Oxidase Biogenesis as Thiol-reductase for Copper Metallochaperones in Mitochondria. J. Mol. Biol. 2020, 432, 2067–2079. [Google Scholar] [CrossRef] [PubMed]
- Stroud, D.; Maher, M.; Lindau, C.; Vögtle, F.; Frazier, A.; Surgenor, E.; Mountford, H.; Singh, A.; Bonas, M.; Oeljeklaus, S.; et al. COA6 is a mitochondrial complex IV assembly factor critical for biogenesis of mtDNA-encoded COX2. Hum. Mol. Genet. 2015, 24, 5404–5415. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A.; Pratt, A.; Soma, S.; Theriault, S.; Griffin, A.; Trivedi, P.; Gohil, V. Mitochondrial disease genes COA6, COX6B and SCO2 have overlapping roles in COX2 biogenesis. Hum. Mol. Genet. 2015, 25, 660–671. [Google Scholar] [CrossRef] [Green Version]
- Cerqua, C.; Morbidoni, V.; Desbats, M.; Doimo, M.; Frasson, C.; Sacconi, S.; Baldoin, M.; Sartori, G.; Basso, G.; Salviati, L.; et al. COX16 is required for assembly of cytochrome c oxidase in human cells and is involved in copper delivery to COX2. Biochim. Biophys. Acta 2018, 1859, 244–252. [Google Scholar] [CrossRef]
- Aich, A.; Wang, C.; Chowdhury, A.; Ronsör, C.; Pacheu-Grau, D.; Richter-Dennerlein, R.; Dennerlein, S.; Rehling, P. COX16 promotes COX2 metallation and assembly during respiratory complex IV biogenesis. Elife 2018, 7, e32572. [Google Scholar] [CrossRef]
- Pierrel, F.; Bestwick, M.; Cobine, P.; Khalimonchuk, O.; Cricco, J.; Winge, D. Coa1 links the Mss51 post-translational function to Cox1 cofactor insertion in cytochrome c oxidase assembly. EMBO J. 2007, 26, 4335–4346. [Google Scholar] [CrossRef] [PubMed]
- Balsa, E.; Marco, R.; Perales-Clemente, E.; Szklarczyk, R.; Calvo, E.; Landázuri, M.; Enríquez, J. NDUFA4 Is a Subunit of Complex IV of the Mammalian Electron Transport Chain. Cell Metab. 2012, 16, 378–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, H.; Bueler, S.; Rubinstein, J. Atomic model for the dimeric FO region of mitochondrial ATP synthase. Science 2017, 358, 936–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, B.; Klusch, N.; Langer, J.; Mills, D.; Yildiz, Ö.; Kühlbrandt, W. Rotary substates of mitochondrial ATP synthase reveal the basis of flexible F1-FO coupling. Science 2019, 364, eaaw9128. [Google Scholar] [CrossRef]
- Nijtmans, L.; Klement, P.; Houštěk, J.; van den Bogert, C. Assembly of mitochondrial ATP synthase in cultured human cells: Implications for mitochondrial diseases. Biochim. Biophys. Acta 1995, 1272, 190–198. [Google Scholar] [CrossRef] [Green Version]
- Fujikawa, M.; Sugawara, K.; Tanabe, T.; Yoshida, M. Assembly of human mitochondrial ATP synthase through two separate intermediates, F1-c-ring andb–e–g complex. FEBS Lett. 2015, 589, 2707–2712. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Ford, H.; Carroll, J.; Douglas, C.; Gonzales, E.; Ding, S.; Fearnley, I.; Walker, J. Assembly of the membrane domain of ATP synthase in human mitochondria. Proc. Natl. Acad. Sci. USA 2018, 115, 2988–2993. [Google Scholar] [CrossRef] [Green Version]
- Rühle, T.; Leister, D. Assembly of F1F0-ATP synthases. Biochim. Biophys. Acta 2015, 1847, 849–860. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; White, P.; Ackerman, S. Atp11p and Atp12p Are Assembly Factors for the F1-ATPase in Human Mitochondria. J. Biol. Chem. 2001, 276, 30773–30778. [Google Scholar] [CrossRef] [Green Version]
- Ackerman, S. Atp11p and Atp12p are chaperones for F1-ATPase biogenesis in mitochondria. Biochim. Biophys. Acta 2002, 1555, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Cizkova, A.; Stranecky, V.; Mayr, J.; Tesarova, M.; Havlickova, V.; Paul, J.; Ivanek, R.; Kuss, A.; Hansikova, H.; Kaplanova, V.; et al. TMEM70 mutations cause isolated ATP synthase deficiency and neonatal mitochondrial encephalocardiomyopathy. Nat. Genet. 2008, 40, 1288–1290. [Google Scholar] [CrossRef]
- Kovalčíková, J.; Vrbacký, M.; Pecina, P.; Tauchmannová, K.; Nůsková, H.; Kaplanová, V.; Brázdová, A.; Alán, L.; Eliáš, J.; Čunátová, K.; et al. TMEM70 facilitates biogenesis of mammalian ATP synthase by promoting subunit c incorporation into the rotor structure of the enzyme. FASEB J. 2019, 33, 14103–14117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Caballero, L.; Elurbe, D.; Baertling, F.; Guerrero-Castillo, S.; van den Brand, M.; van Strien, J.; van Dam, T.; Rodenburg, R.; Brandt, U.; Huynen, M.; et al. TMEM70 functions in the assembly of complexes I and V. Biochim. Biophys. Acta 2020, 1861, 148202. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Carroll, J.; Ding, S.; Fearnley, I.; Walker, J. Permeability transition in human mitochondria persists in the absence of peripheral stalk subunits of ATP synthase. Proc. Natl. Acad. Sci. USA 2017, 114, 9086–9091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mnatsakanyan, N.; Llaguno, M.; Yang, Y.; Yan, Y.; Weber, J.; Sigworth, F.; Jonas, E. A mitochondrial megachannel resides in monomeric F1FO ATP synthase. Nat. Commun. 2019, 10, 5823. [Google Scholar] [CrossRef] [PubMed]
- Anselmi, C.; Davies, K.; Faraldo-Gómez, J. Mitochondrial ATP synthase dimers spontaneously associate due to a long-range membrane-induced force. J. Gen. Physiol. 2018, 150, 763–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, T.; Hahn, A.; Meier, T.; Davies, K.; Kühlbrandt, W. Dimers of mitochondrial ATP synthase induce membrane curvature and self-assemble into rows. Proc. Natl. Acad. Sci. USA 2019, 116, 4250–4255. [Google Scholar] [CrossRef] [Green Version]
- Angerer, H. Eukaryotic LYR Proteins Interact with Mitochondrial Protein Complexes. Biology 2015, 4, 133–150. [Google Scholar] [CrossRef] [Green Version]
- Floyd, B.; Wilkerson, E.; Veling, M.; Minogue, C.; Xia, C.; Beebe, E.; Wrobel, R.; Cho, H.; Kremer, L.; Alston, C.; et al. Mitochondrial Protein Interaction Mapping Identifies Regulators of Respiratory Chain Function. Mol. Cell 2016, 63, 621–632. [Google Scholar] [CrossRef] [Green Version]
- Dibley, M.; Formosa, L.; Lyu, B.; Reljic, B.; McGann, D.; Muellner-Wong, L.; Kraus, F.; Sharpe, A.; Stroud, D.; Ryan, M. The mitochondrial acyl-carrier protein interaction network highlights important roles for LYRM family members in complex I and mitoribosome assembly. Mol. Cell. Proteom. 2020. [Google Scholar] [CrossRef]
- Hiltunen, J.; Schonauer, M.; Autio, K.; Mittelmeier, T.; Kastaniotis, A.; Dieckmann, C. Mitochondrial Fatty Acid Synthesis Type II: More than Just Fatty Acids. J. Biol. Chem. 2009, 284, 9011–9015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masud, A.; Kastaniotis, A.; Rahman, M.; Autio, K.; Hiltunen, J. Mitochondrial acyl carrier protein (ACP) at the interface of metabolic state sensing and mitochondrial function. Biochim. Biophys. Acta 2019, 1866, 118540. [Google Scholar] [CrossRef] [PubMed]
- Brody, S.; Oh, C.; Hoja, U.; Schweizer, E. Mitochondrial acyl carrier protein is involved in lipoic acid synthesis in Saccharomyces cerevisiae. FEBS Lett. 1997, 408, 217–220. [Google Scholar] [CrossRef] [Green Version]
- Witkowski, A.; Joshi, A.; Smith, S. Coupling of the de Novo Fatty Acid Biosynthesis and Lipoylation Pathways in Mammalian Mitochondria. J. Biol. Chem. 2007, 282, 14178–14185. [Google Scholar] [CrossRef] [Green Version]
- Yi, X.; Maeda, N. Endogenous production of lipoic acid is essential for mouse development. Mol. Cell. Biol. 2005, 25, 8387–8392. [Google Scholar] [CrossRef] [Green Version]
- Mayr, J.; Freisinger, P.; Schlachter, K.; Rolinski, B.; Zimmermann, F.; Scheffner, T.; Haack, T.; Koch, J.; Ahting, U.; Prokisch, H.; et al. Thiamine Pyrophosphokinase Deficiency in Encephalopathic Children with Defects in the Pyruvate Oxidation Pathway. Am. J. Hum. Genet. 2011, 89, 806–812. [Google Scholar] [CrossRef] [Green Version]
- Baker, P.n.; Friederich, M.; Swanson, M.; Shaikh, T.; Bhattacharya, K.; Scharer, G.; Aicher, J.; Creadon-Swindell, G.; Geiger, E.; MacLean, K.; et al. Variant non ketotic hyperglycinemia is caused by mutations in LIAS, BOLA3 and the novel gene GLRX5. Brain 2014, 137, 366–379. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Pudas, R.; Sharma, S.; Smart, O.; Juffer, A.; Hiltunen, J.; Wierenga, R.; Haapalainen, A. Structural enzymological studies of 2-enoyl thioester reductase of the human mitochondrial FAS II pathway: New insights into its substrate recognition properties. J. Mol. Biol. 2008, 379, 830–844. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Joshi, A.; Hofmann, J.; Schweizer, E.; Smith, S. Cloning, Expression, and Characterization of the Human Mitochondrial β-Ketoacyl Synthase: COMPLEMENTATION OF THE YEAST CEM1 KNOCK-OUT STRAIN. J. Biol. Chem. 2005, 280, 12422–12429. [Google Scholar] [CrossRef] [Green Version]
- Angerer, H.; Schönborn, S.; Gorka, J.; Bahr, U.; Karas, M.; Wittig, I.; Heidler, J.; Hoffmann, J.; Morgner, N.; Zickermann, V. Acyl modification and binding of mitochondrial ACP to multiprotein complexes. Biochim. Biophys. Acta 2017, 1864, 1913–1920. [Google Scholar] [CrossRef]
- Boniecki, M.; Freibert, S.; Mühlenhoff, U.; Lill, R.; Cygler, M. Structure and functional dynamics of the mitochondrial Fe/S cluster synthesis complex. Nat. Commun. 2017, 8, 1287. [Google Scholar] [CrossRef] [Green Version]
- Van Vranken, J.; Nowinski, S.; Clowers, K.; Jeong, M.; Ouyang, Y.; Berg, J.; Gygi, J.; Gygi, S.; Winge, D.; Rutter, J. ACP Acylation Is an Acetyl-CoA-Dependent Modification Required for Electron Transport Chain Assembly. Mol. Cell 2018, 71, 567–580.e564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Runswick, M.; Fearnley, I.; Skehel, J.; Walker, J. Presence of an acyl carrier protein in NADH:ubiquinone oxidoreductase from bovine heart mitochondria. FEBS Lett. 1991, 286, 121–124. [Google Scholar] [CrossRef]
- Hou, T.; Zhang, R.; Jian, C.; Ding, W.; Wang, Y.; Ling, S.; Ma, Q.; Hu, X.; Cheng, H.; Wang, X. NDUFAB1 confers cardio-protection by enhancing mitochondrial bioenergetics through coordination of respiratory complex and supercomplex assembly. Cell Res. 2019, 29, 754–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiedorczuk, K.; Letts, J.; Degliesposti, G.; Kaszuba, K.; Skehel, M.; Sazanov, L. Atomic structure of the entire mammalian mitochondrial complex I. Nature 2016, 538, 406–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.; Gu, J.; Guo, R.; Huang, Y.; Yang, M. Structure of Mammalian Respiratory Supercomplex I1III2IV1. Cell 2016, 167, 1598–1609.e1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maio, N.; Singh, A.; Uhrigshardt, H.; Saxena, N.; Tong, W.; Rouault, T. Cochaperone binding to LYR motifs confers specificity of iron sulfur cluster delivery. Cell Metab. 2014, 19, 445–457. [Google Scholar] [CrossRef] [Green Version]
- Invernizzi, F.; Tigano, M.; Dallabona, C.; Donnini, C.; Ferrero, I.; Cremonte, M.; Ghezzi, D.; Lamperti, C.; Zeviani, M. A Homozygous Mutation in LYRM7/MZM1L Associated with Early Onset Encephalopathy, Lactic Acidosis, and Severe Reduction of Mitochondrial Complex III Activity. Hum. Mutat. 2013, 34, 1619–1622. [Google Scholar] [CrossRef] [Green Version]
- Krogan, N.; Cagney, G.; Yu, H.; Zhong, G.; Guo, X.; Ignatchenko, A.; Li, J.; Pu, S.; Datta, N.; Tikuisis, A.; et al. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature 2006, 440, 637–643. [Google Scholar] [CrossRef]
- Wachnowsky, C.; Fidai, I.; Cowan, J. Iron–sulfur cluster biosynthesis and trafficking–impact on human disease conditions. Metallomics 2018, 10, 9–29. [Google Scholar] [CrossRef]
- Lim, S.; Friemel, M.; Marum, J.; Tucker, E.; Bruno, D.; Riley, L.; Christodoulou, J.; Kirk, E.; Boneh, A.; DeGennaro, C.; et al. Mutations in LYRM4, encoding iron-sulfur cluster biogenesis factor ISD11, cause deficiency of multiple respiratory chain complexes. Hum. Mol. Genet. 2013, 22, 4460–4473. [Google Scholar] [CrossRef] [PubMed]
- Van Vranken, J.; Jeong, M.; Wei, P.; Chen, Y.; Gygi, S.; Winge, D.; Rutter, J. The mitochondrial acyl carrier protein (ACP) coordinates mitochondrial fatty acid synthesis with iron sulfur cluster biogenesis. Elife 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Fung, S.; Nishimura, T.; Sasarman, F.; Shoubridge, E. The conserved interaction of C7orf30 with MRPL14 promotes biogenesis of the mitochondrial large ribosomal subunit and mitochondrial translation. Mol. Biol. Cell. 2013, 24, 184–193. [Google Scholar] [CrossRef]
- Rorbach, J.; Gammage, P.; Minczuk, M. C7orf30 is necessary for biogenesis of the large subunit of the mitochondrial ribosome. Nucleic Acids Res. 2012, 40, 4097–4109. [Google Scholar] [CrossRef] [PubMed] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.X.; Thompson, K.; Taylor, R.W.; Oláhová, M. Mitochondrial OXPHOS Biogenesis: Co-Regulation of Protein Synthesis, Import, and Assembly Pathways. Int. J. Mol. Sci. 2020, 21, 3820. https://doi.org/10.3390/ijms21113820
Tang JX, Thompson K, Taylor RW, Oláhová M. Mitochondrial OXPHOS Biogenesis: Co-Regulation of Protein Synthesis, Import, and Assembly Pathways. International Journal of Molecular Sciences. 2020; 21(11):3820. https://doi.org/10.3390/ijms21113820
Chicago/Turabian StyleTang, Jia Xin, Kyle Thompson, Robert W. Taylor, and Monika Oláhová. 2020. "Mitochondrial OXPHOS Biogenesis: Co-Regulation of Protein Synthesis, Import, and Assembly Pathways" International Journal of Molecular Sciences 21, no. 11: 3820. https://doi.org/10.3390/ijms21113820
APA StyleTang, J. X., Thompson, K., Taylor, R. W., & Oláhová, M. (2020). Mitochondrial OXPHOS Biogenesis: Co-Regulation of Protein Synthesis, Import, and Assembly Pathways. International Journal of Molecular Sciences, 21(11), 3820. https://doi.org/10.3390/ijms21113820




