Human PSC-Derived Hepatocytes Express Low Levels of Viral Pathogen Recognition Receptors, but Are Capable of Mounting an Effective Innate Immune Response
Abstract
1. Introduction
2. Results
2.1. Hepatocyte Differentiation of iPSCs
2.2. Expression of Pattern Recognition Receptors (PRRs) across Different Stem Cell Lines
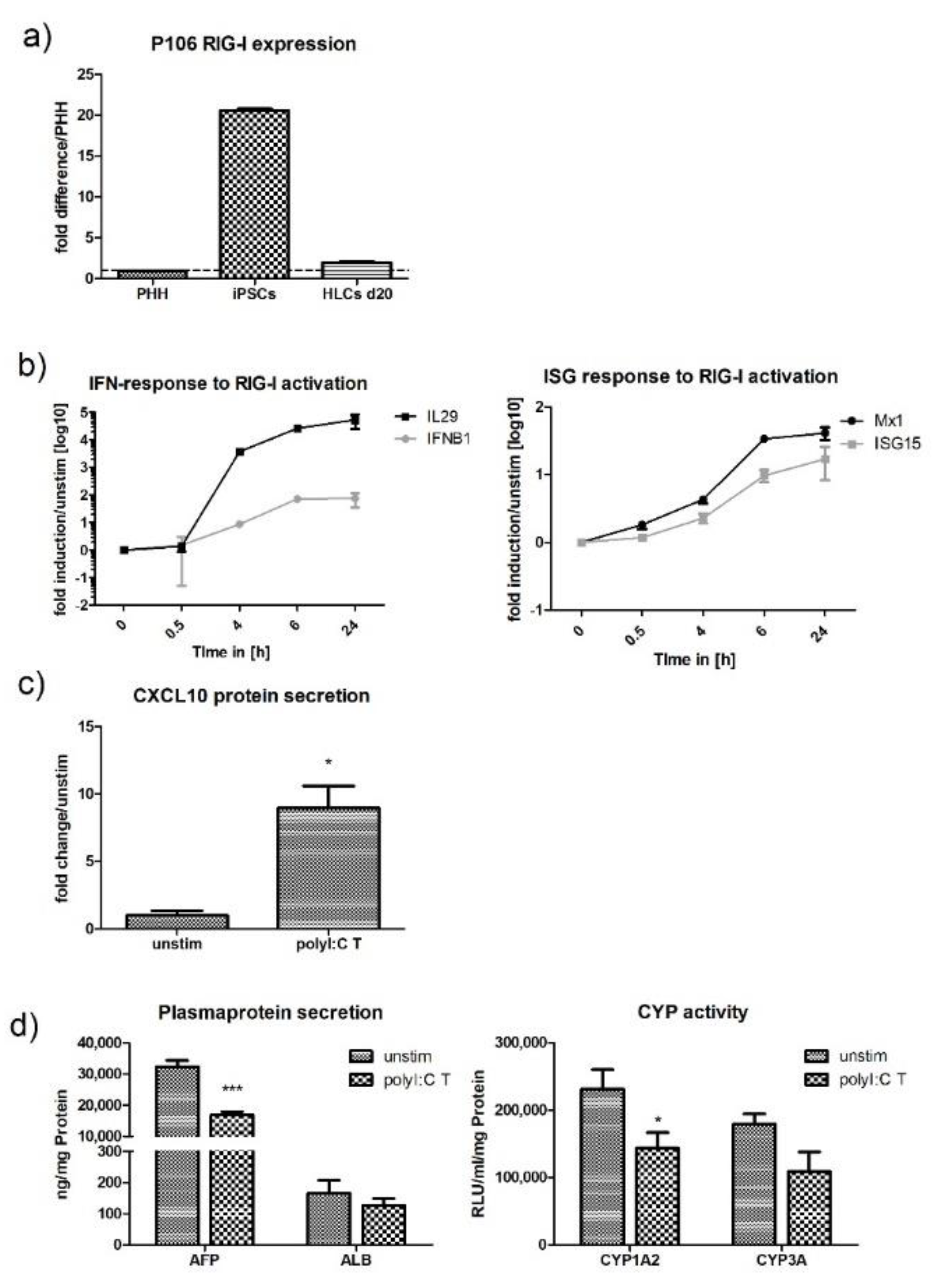
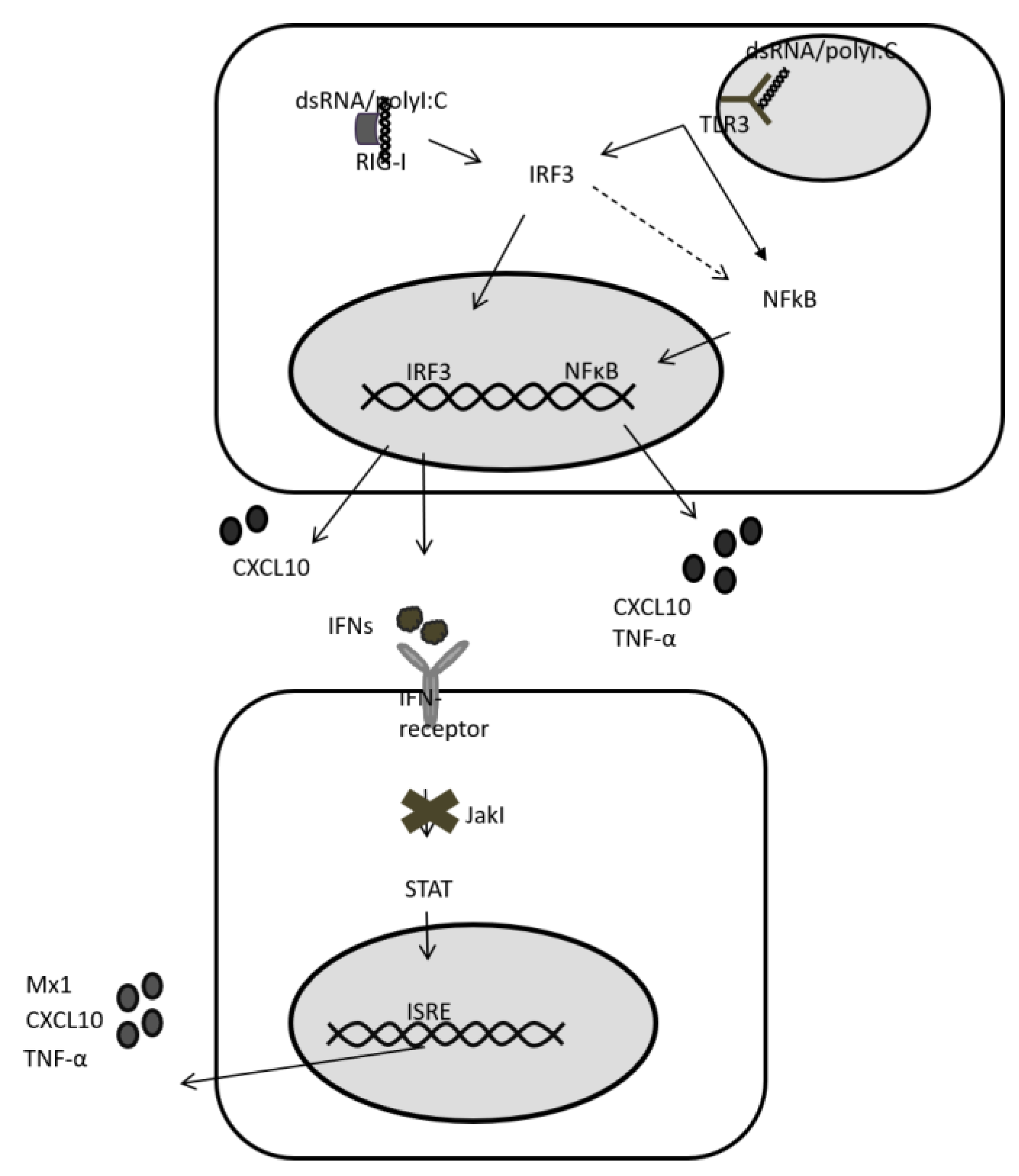
2.3. Stimulation of HLCs with PRR Ligands
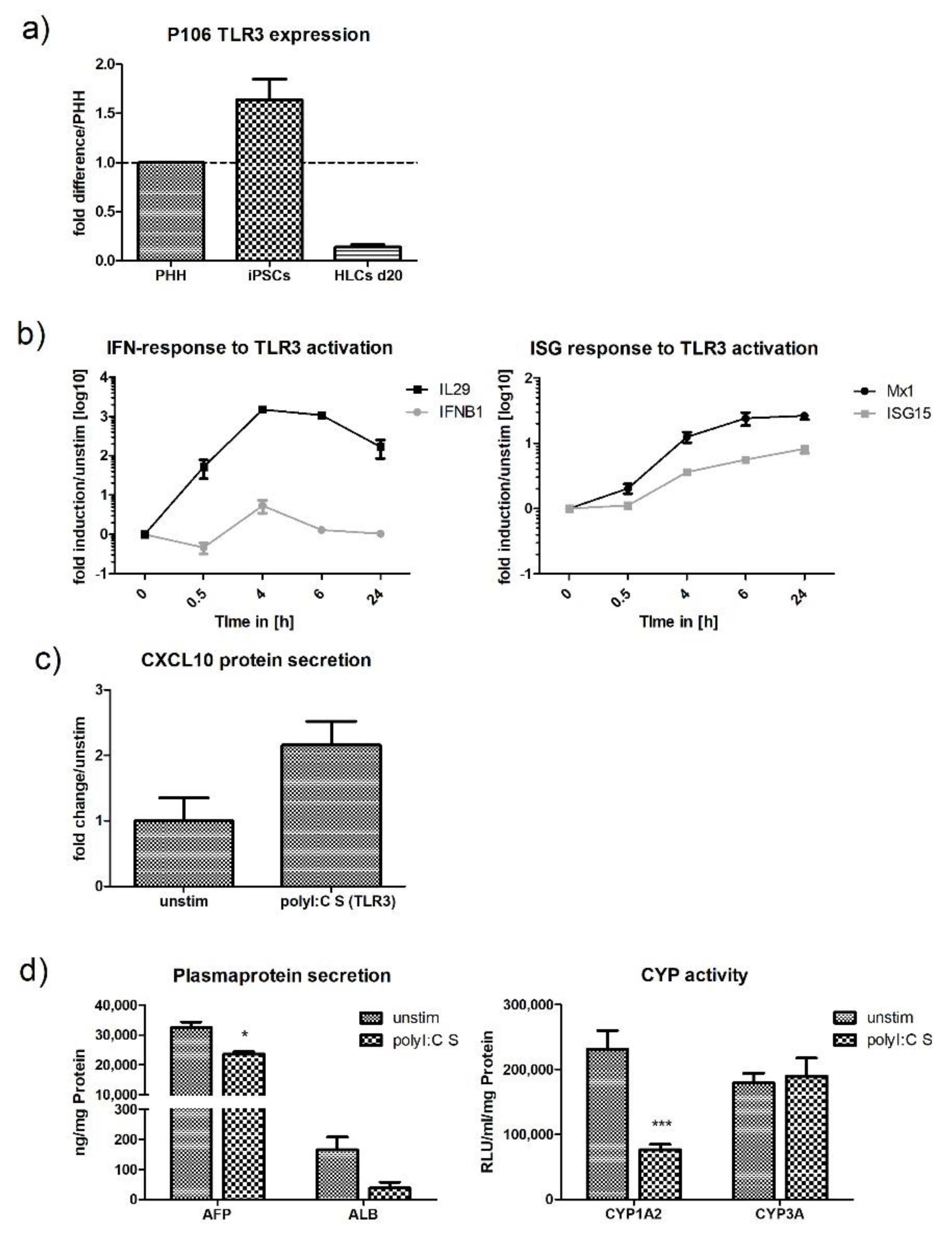
2.4. Upregulation of TLR3 mRNA and Signalling upon Stimulation
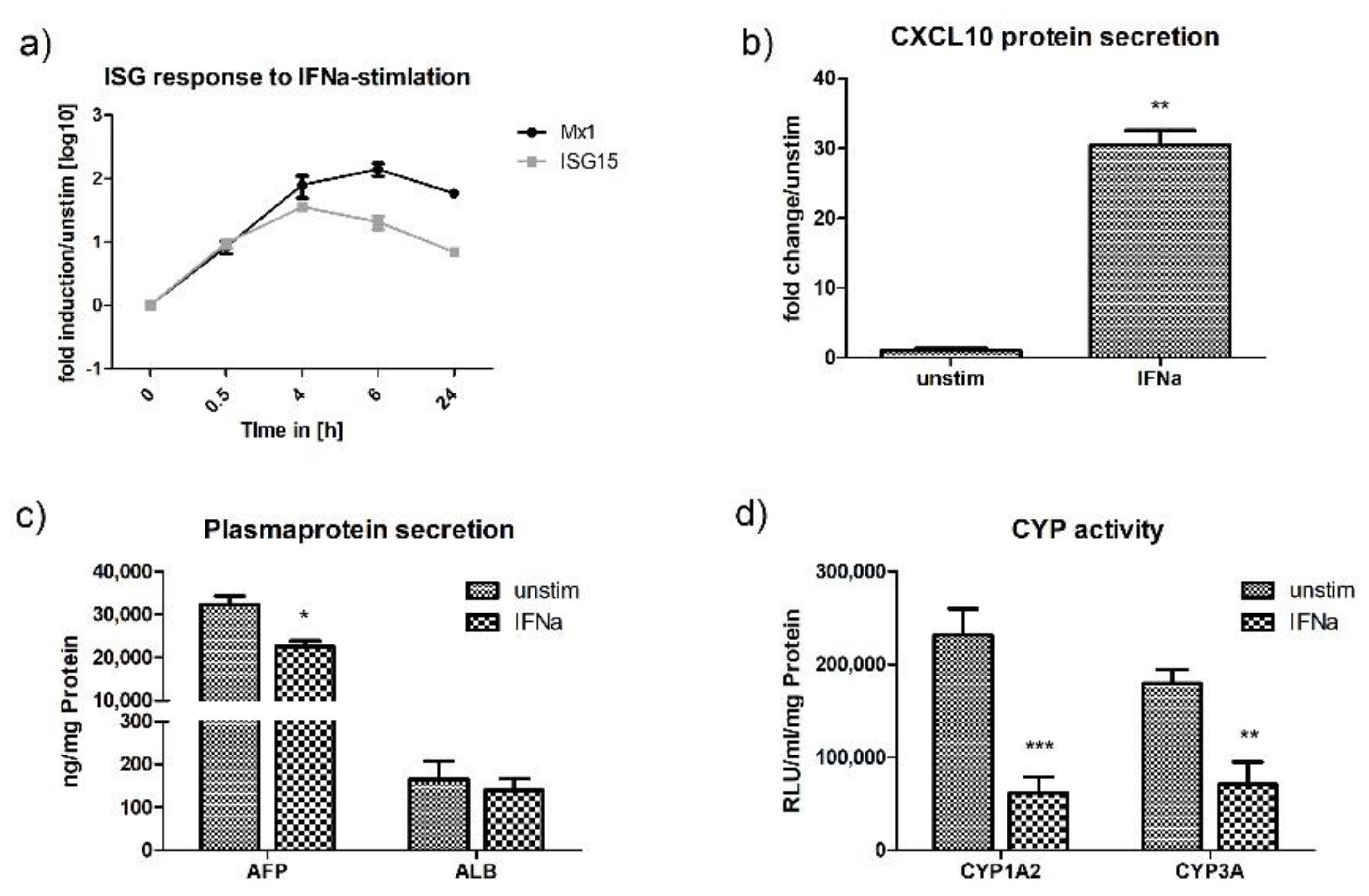
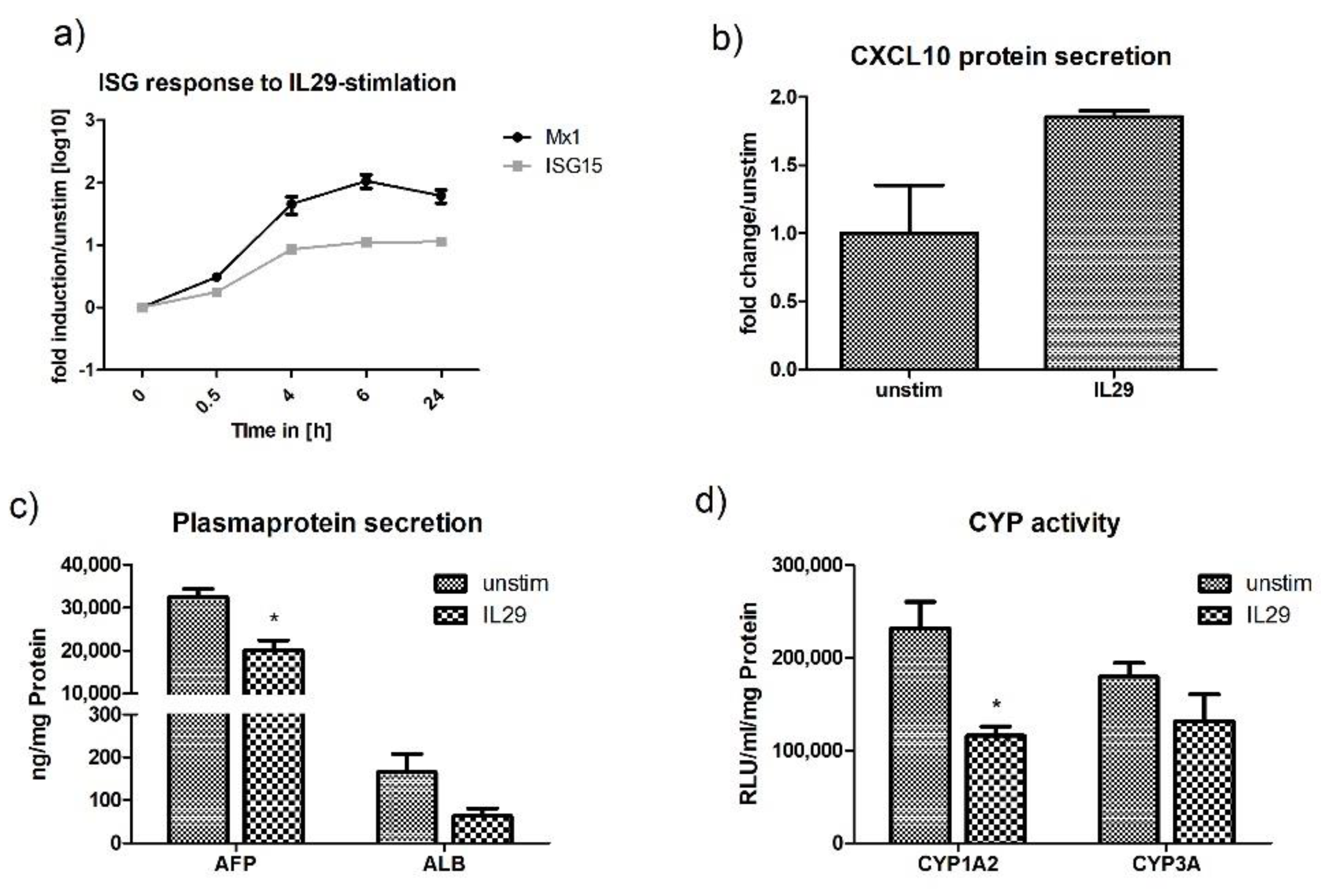
2.5. Interferon Stimulation of HLCs
2.6. TNF Alpha Stimulation of HLCs
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Hepatocyte Differentiation of iPSCs
4.3. Immunofluorescence Staining
4.4. RT-qPCR
4.5. Stimulation
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Janus Kinase/Signal Transducer and Activator of Transcription (JAK/STAT)-Pathway Inhibition
4.8. Cytochrome P450 Assays
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AFP | Alpha-fetoprotein |
| ALB | Albumin |
| CXCL10 | CXC-motif chemokine 10 |
| CYP | Cytochrome |
| DMSO | Dimethylsulfoxide |
| ELISA | Enzyme-linked immunosorbent assay |
| HBV | Hepatitis B virus |
| HCV | Hepatitis C virus |
| hESC | Human embryonic stem cell |
| HLC | Hepatocyte like cell |
| HNF4a | Hepatocyte nuclear factor 4 alpha |
| IFN | Interferon |
| IL | Interleukin |
| IRF3 | Interferon regulatory factor 3 |
| JAK/STAT | Janus kinase/signal transducer and activator of transcription |
| MDA5 | Melanoma differentiation antigen 5 |
| NFkB | Nuclear factor ‘kappa-light-chain-enhancer’ of activated B-cells |
| PAMP | Pathogen associated molecular pattern |
| PSC | Pluripotent stem cell |
| RIG-I | Retinoic acid inducible gene I |
| PRR | Pattern recognition receptor |
| TLR | Toll-like receptor |
| TNFa | Tumor necrosis factor alpha |
References
- Fischer, L.; Hay, D.C.; O’Farrelly, C. Innate immunity in stem cell-derived hepatocytes. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Xu, M.J.; Gao, B. Hepatocytes: A key cell type for innate immunity. Cell. Mol. Immunol. 2016, 13, 301–315. [Google Scholar] [CrossRef]
- Hay, D.C.; Zhao, D.; Ross, A.; Mandalam, R.; Lebowski, J.; Cui, W. Direct differentiation of human embryonic stem cells to hepatocyte-like cells exhibiting functional activities. Cloning Stem Cells 2007, 9, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhao, Y.; Liu, Y.; Ye, F.; Song, Z.; Qin, H.; Meng, S.; Chen, Y.; Zhou, R.; Song, X.; et al. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology 2007, 45, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Si-Tayeb, K.; Noto, F.K.; Nagaoka, M.; Li, J.; Battle, M.A.; Duris, C.; North, P.E.; Dalton, S.; Duncan, S.A. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology 2007, 51, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Cameron, K.; Lucendo-Villarin, B.; Szkolnicka, D.; Hay, D.C. Serum-Free directed differentiation of human embryonic stem cells to hepatocytes. Methods Mol. Biol. 2015, 1250, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Cameron, K.; Tan, R.; Schmidt-Heck, W.; Campos, G.; Lyall, M.J.; Wang, Y.; Lucendo-Villarin, B.; Szkolnicka, D.; Bates, N.; Kimber, S.J.; et al. Recombinant laminins drive the differentiation and self-organization of hESCderived hepatocytes. Stem Cell Rep. 2015, 5, 1250–1262. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, P.; Lucendo-Villarin, B.; Angus, A.G.N.; Szkolnicka, D.; Cameron, K.; Farnworth, L.S.; Patel, H.A.; David, C.H. Modulating innate immunity improves hepatitis C virus infection and replication in stem cell-derived hepatocytes. Stem Cell Rep. 2014, 3, 204–214. [Google Scholar] [CrossRef][Green Version]
- Wu, X.; Robotham, J.M.; Lee, E.; Dalton, S.; Kneteman, N.M.; Gilbert, D.M.; Tang, H. Productive hepatitis C virus infection of stem cell-derived hepatocytes reveals a critical transition to viral permissiveness during differentiation. PLoS Pathog. 2012, 8, e1002617. [Google Scholar] [CrossRef]
- Sakurai, F.; Kunito, T.; Takayama, K.; Hashimoto, R.; Tachibana, M.; Sakamoto, N.; Wakita, T.; Mizuguchi, H. Hepatitis C virus-induced innate immune responses in human iPS cell derived hepatocyte-like cells. Virus Res. 2017, 242, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Sholmai, A.; Schwartz, R.E.; Ramanan, V.; Bhatta, A.; de Jong, Y.P.; Bhatia, S.N.; Rice, C.M. Modeling host interactions with hepatitis B virus using primary and induced pluripotent stem cell-derived hepatocellular systems. Proc. Natl. Acad. Sci. USA 2014, 111, 12193–12198. [Google Scholar] [CrossRef]
- Park, H.; Serti, E.; Eke, O.; Muchmore, B.; Prokunina-Olsson, L.; Capone, S.; Folgori, A.; Rehermann, B. IL-29 is the dominant type III interferon produced by hepatocytes during acute hepatitis C virus infection. Hepatology 2012, 56, 2060–2070. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, Z.; Kato, N.; Gale, M.; Lemon, S.M. Distinct poly(I-C) and virus-activated signalling pathways leading to interferon-b production in hepatocytes. J. Biol. Chem. 2005, 280, 16739–16747. [Google Scholar] [CrossRef] [PubMed]
- Sumpter, R.; Loo, Y.-M.; Foy, E.; Li, K.; Yoneyama, M.; Fujita, T.; Lemon, S.M.; Gale, J.M. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J. Virol. 2005, 79, 2689–2699. [Google Scholar] [CrossRef] [PubMed]
- Hiet, S.; Bauhofer, O.; Zayas, M.; Roth, H.; Tanaka, Y.; Schirmacher, P.; Willemsen, J.; Grünvogel, O.; Bender, S.; Binder, M.; et al. Control of temporal activation of hepatitis C virus-induced interferon response by domain 2 of nonstructural protein 5A. J. Hepatol. 2015, 63, 829–837. [Google Scholar] [CrossRef]
- Kaneko, S.; Kakinuma, S.; Asahina, Y.; Kamiya, A.; Miyoshi, M.; Tsunoda, T.; Nitta, S.; Asano, Y.; Nagata, H.; Otani, S.; et al. Human induced pluripotent stem cell-derived hepatic cell lines as a new model for host interaction with hepatitis B virus. Sci. Rep. 2016, 6, 29358. [Google Scholar] [CrossRef]
- Seki, E.; Brenner, D. Toll-like receptors and adaptor molecules in liver disease: Update. Hepatology 2008, 48, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, R. Toll-like receptors in acute liver injury and regeneration. Int. Immunopharmacol. 2010, 11, 1433–1441. [Google Scholar] [CrossRef]
- Kesar, V.; Odin, J.A. Toll-like receptors and liver disease. Liver Int. 2014, 34, 184–196. [Google Scholar] [CrossRef]
- Broering, R.; Lutterbeck, M.; Trippler, M.; Kleinehr, K.; Poggenpohl, L.; Paul, A.; Gerken, G.; Schlaak, J.F. Long-term stimulation of Toll-like receptor 3 in primary human hepatocytes leads to sensitization for antiviral responses induced by poly I: C treatment. J. Viral Hepat. 2014, 21, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Aitken, A.E.; Morgan, E.T. Gene-specific effects of inflammatory cytokines on cytochrome P450 2C, 2B6 and 3A4 mRNA levels in human hepatocytes. Drug Metab. Dispos. 2007, 35, 1687–1693. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.T. Regulation of cytochrome P450 by inflammatory mediators: Why and how. Drug Metab. Dispos. 2001, 29, 207–212. [Google Scholar] [PubMed]
- Morgan, E.T. Regulation of cytochromes P450 during inflammation and infection. Drug Metab. Rev. 1997, 29, 1129–1188. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.L.; Carmichael, G.G.; Wang, R.; Hong, X.; Acharya, D.; Huang, F.; Bai, F. Attenuated innate immunity in embryonic stem cells and its implications in developmental biology and regenerative medicine. Stem Cells. 2015, 33, 3165–3173. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Alhaque, S.; Cameron, K.; Meseguer-Ripolles, J.; Lucendo-Villarin, B.; Rashidi, H.; Hay, D.C. Defined and Scalable Generation of Hepatocyte-like Cells from Human Pluripotent Stem Cells. J. Vis. Exp. 2017, 121, e55355. [Google Scholar] [CrossRef]
- Brownell, J.; Bruckner, J.; Wagoner, J.; Thomas, E.; Loo, Y.M.; Gale, J.M.; Liang, T.J.; Polyak, S.J. Direct, interferon-independent activation of the CXCL10 promoter by NF-κB and interferon regulatory factor 3 during hepatitis C virus infection. J. Virol. 2014, 88, 1582–1590. [Google Scholar] [CrossRef]
- Ashley, C.L.; Abendroth, A.; McSharry, B.P.; Sobedman, B. Interferon-Independent upregulation of interferon-stimulated genes during human cytomegalovirus infection is dependent on IRF3 expression. Viruses 2019, 11, 246. [Google Scholar] [CrossRef]
- Asensio, V.C.; Maier, J.; Milner, R.; Boztug, K.; Kincaid, C.; Moulard, M.; Phillipson, C.; Lindsley, K.; Krucker, T.; Fox, H.S.; et al. Interferon-independent, human immunodeficiency virus type 1 gp120-mediated induction of CXCL10/IP-10 gene expression by astrocytes in vivo and in vitro. J. Virol. 2001, 75, 7067–7077. [Google Scholar] [CrossRef]
- Lee, J.; Tian, Y.; Chan, S.T.; Kim, J.Y.; Cho, C.; Ou, J.J. TNF-α Induced by hepatitis C virus via TLR7 and TLR8 in hepatocytes supports interferon signalling via an autocrine mechanism. PLoS Pathog. 2015, 11, e1004937. [Google Scholar] [CrossRef]
- Szkolnicka, D.; Farnworth, S.L.; Lucendo-Villarin, B.; Hay, D.C. Deriving functional hepatocytes from pluripotent stem cells. Curr. Protoc. Stem Cell Biol. 2014, 30, 1G.5.1–1G.5.12. [Google Scholar] [CrossRef] [PubMed]
- Meseguer-Ripolles, J.; Lucendo-Villarin, B.; Wang, Y.; Hay, D.C. Semi-automated production of hepatocyte like cells from pluripotent stem cells. J. Vis. Exp. 2018, 27, 137. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Shilagard, T.; Xiao, S.Y.; Snyder, N.; Lau, D.; Cicalese, L.; Weiss, H.; Vargas, G.; Lemon, S.M. Visualizing hepatitis C virus infections in human liver by two-photon microscopy. Gastroenterology 2009, 137, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Franchitto, A.; Onori, P.; Renzi, A.; Carpino, G.; Mancinelli, R.; Alvaro, D.; Gaudio, E. Expression of vascular endothelial growth factors and their receptors by hepatic progenitor cells in human liver diseases. Hepatobiliary Surg. Nutr. 2013, 2, 68–77. [Google Scholar] [CrossRef]
- Yan, F.; Wang, Y.; Zhang, W.; Chang, M.; He, Z.; Xu, J.; Shang, C.; Chen, T.; Liu, J.; Wang, X.; et al. Human embryonic stem cell-derived hepatoblasts are an optimal lineage stage for hepatitis C virus infection. Hepatology 2017, 66, 717–735. [Google Scholar] [CrossRef]
- Schöbel, A.; Rösch, K.; Herker, E. Functional innate immunity restricts hepatitis C Virus infection in induced pluripotent stem cell-derived hepatocytes. Sci. Rep. 2018, 8, 3893. [Google Scholar] [CrossRef]
- Reed, D.M.; Földes, G.; Gatheral, T.; Paschalaki, K.E.; Lendvai, Z.; Bagyura, Z.; Nemeth, T.; Skopal, J.; Merkely, B.; Telcian, A.G.; et al. Pathogen sensing pathways in human embryonic stem cell-derived endothelial cells: Role of NOD1 receptors. PLoS ONE 2014, 9, e91119. [Google Scholar] [CrossRef]
- El-Badawi, A.; El-Badri, N. Regulators of pluripotency and their implications in regenerative medicine. Dovepress 2015, 8, 67–80. [Google Scholar]
- Daffis, S.; Suthar, M.S.; Szretter, K.J.; Gale, M.J.; Diamond, M.S. Induction of IFN-beta and the innate antiviral response in myeloid cells occurs through an IPS-1-dependent signal that does not require IRF-3 and IRF-7. PLoS Pathog. 2009, 5, e1000607. [Google Scholar] [CrossRef]
- Hall, J.C.; Rosen, A. Type I interferons: Crucial participants in disease amplification in autoimmunity. Nat. Rev. Rheumatol. 2010, 6, 40–49. [Google Scholar] [CrossRef]
- Lee, H.; Chathuranga, K.; Lee, J. Intracellular sensing of viral genomes and viral evasion. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Lin, R.; Yang, L.; Nakhaei, P.; Sun, Q.; Sharif-Askari, E.; Julkunen, I.; Hiscott, J. Negative regulation of the retinoic acid-inducible gene I-induced antiviral state by the ubiquitin-editing protein A20. J. Biol. Chem. 2006, 281, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ye, L.; Wang, X.; Li, J.; Song, L.; Ho, W. Retinoic acid inducible gene-I (RIG-I) signalling of hepatic stellate cells inhibits hepatitis C virus replication in hepatocytes. Innate Immun. 2013, 19, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, I.B.; Nguyen, T.T.; Ringdal, M.; Tryggestad, A.M.; Bakke, O.; Lien, E.; Espevik, T.; Anthonsen, M.W. Toll-like receptor 3 associates with c-Src tyrosine kinase on endosomes to initiate antiviral signalling. EMBO J. 2006, 25, 3335–3346. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.; Pierce, S. How location governs Toll like receptor signalling. Traffic 2009, 10, 621–628. [Google Scholar] [CrossRef]
- Pelka, K.; Shibata, T.; Miyake, K.; Latz, E. Nucleic acid-sensing TLRs and autoimmunity: Novel insights from structural and cell biology. Immunol. Rev. 2016, 269, 60–75. [Google Scholar] [CrossRef]
- Diehl, A.M.; Yin, M.; Fleckenstein, J.; Yang, S.Q.; Lin, H.Z.; Brenner, D.A.; Westwick, J.; Bagby, G.; Nelson, S. Tumor necrosis factor-alpha induces c-jun during the regenerative response to liver injury. Am. J. Physiol. 1994, 267, G552–G561. [Google Scholar] [CrossRef]
- Diehl, A.M. Cytokine regulation of liver injury and repair. Immunol. Rev. 2000, 174, 160–171. [Google Scholar] [CrossRef]
- Fletcher, N.F.; Clark, A.R.; Balfe, P.; McKeating, J.A. TNF superfamily members promote hepatitis C virus entry via an NF-κB and myosin light chain kinase dependent pathway. J. Gen. Virol. 2017, 98, 405–412. [Google Scholar] [CrossRef]
- Osawa, Y.; Nagaki, M.; Banno, Y.; Brenner, D.A.; Asano, T.; Nozawa, Y.; Moriwaki, H.; Nakashima, S. Tumor necrosis factor alpha-induced interleukin-8 production via NFkB and phosphatidyl 3-kinase/Akt pathways inhibits cell apoptosis in human hepatocytes. Infect. Immun. 2002, 70, 6294–6301. [Google Scholar] [CrossRef]
- Aitken, A.E.; Richardson, T.A.; Morgan, E.T. Regulation of drug-metabolizing enzymes and transporters in inflammation. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 123–149. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razzak, Z.; Loyer, P.; Fautrel, A.; Gautier, J.C.; Corcos, L.; Turlin, B.; Beaune, P.; Guillouzo, A. Cytokines down-regulate expression of major cytochrome P-450 enzymes in adult human hepatocytes in primary culture. Mol. Pharmacol. 1993, 44, 707–715. [Google Scholar] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, L.; Lucendo-Villarin, B.; Hay, D.C.; O’Farrelly, C. Human PSC-Derived Hepatocytes Express Low Levels of Viral Pathogen Recognition Receptors, but Are Capable of Mounting an Effective Innate Immune Response. Int. J. Mol. Sci. 2020, 21, 3831. https://doi.org/10.3390/ijms21113831
Fischer L, Lucendo-Villarin B, Hay DC, O’Farrelly C. Human PSC-Derived Hepatocytes Express Low Levels of Viral Pathogen Recognition Receptors, but Are Capable of Mounting an Effective Innate Immune Response. International Journal of Molecular Sciences. 2020; 21(11):3831. https://doi.org/10.3390/ijms21113831
Chicago/Turabian StyleFischer, Lena, Baltasar Lucendo-Villarin, David C. Hay, and Cliona O’Farrelly. 2020. "Human PSC-Derived Hepatocytes Express Low Levels of Viral Pathogen Recognition Receptors, but Are Capable of Mounting an Effective Innate Immune Response" International Journal of Molecular Sciences 21, no. 11: 3831. https://doi.org/10.3390/ijms21113831
APA StyleFischer, L., Lucendo-Villarin, B., Hay, D. C., & O’Farrelly, C. (2020). Human PSC-Derived Hepatocytes Express Low Levels of Viral Pathogen Recognition Receptors, but Are Capable of Mounting an Effective Innate Immune Response. International Journal of Molecular Sciences, 21(11), 3831. https://doi.org/10.3390/ijms21113831





