Identification of Alternative Mitochondrial Electron Transport Pathway Components in Chickpea Indicates a Differential Response to Salinity Stress between Cultivars
Abstract
:1. Introduction
2. Results
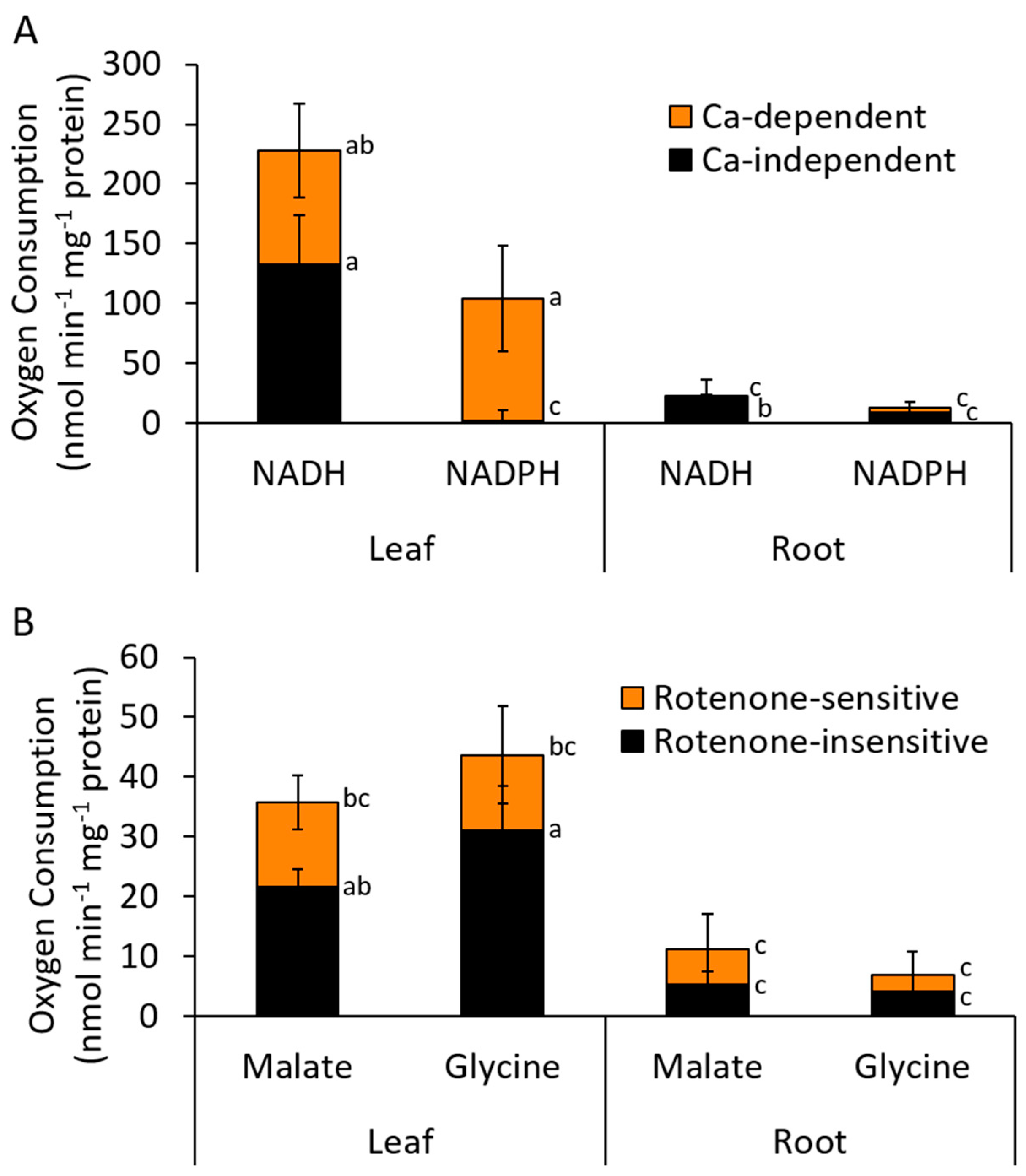
2.1. Type II NAD(P)H Dehydrogenase Activities in Chickpea
2.2. Type II NAD(P)H Dehydrogenase Genes in Chickpea
2.2.1. Gene Identification
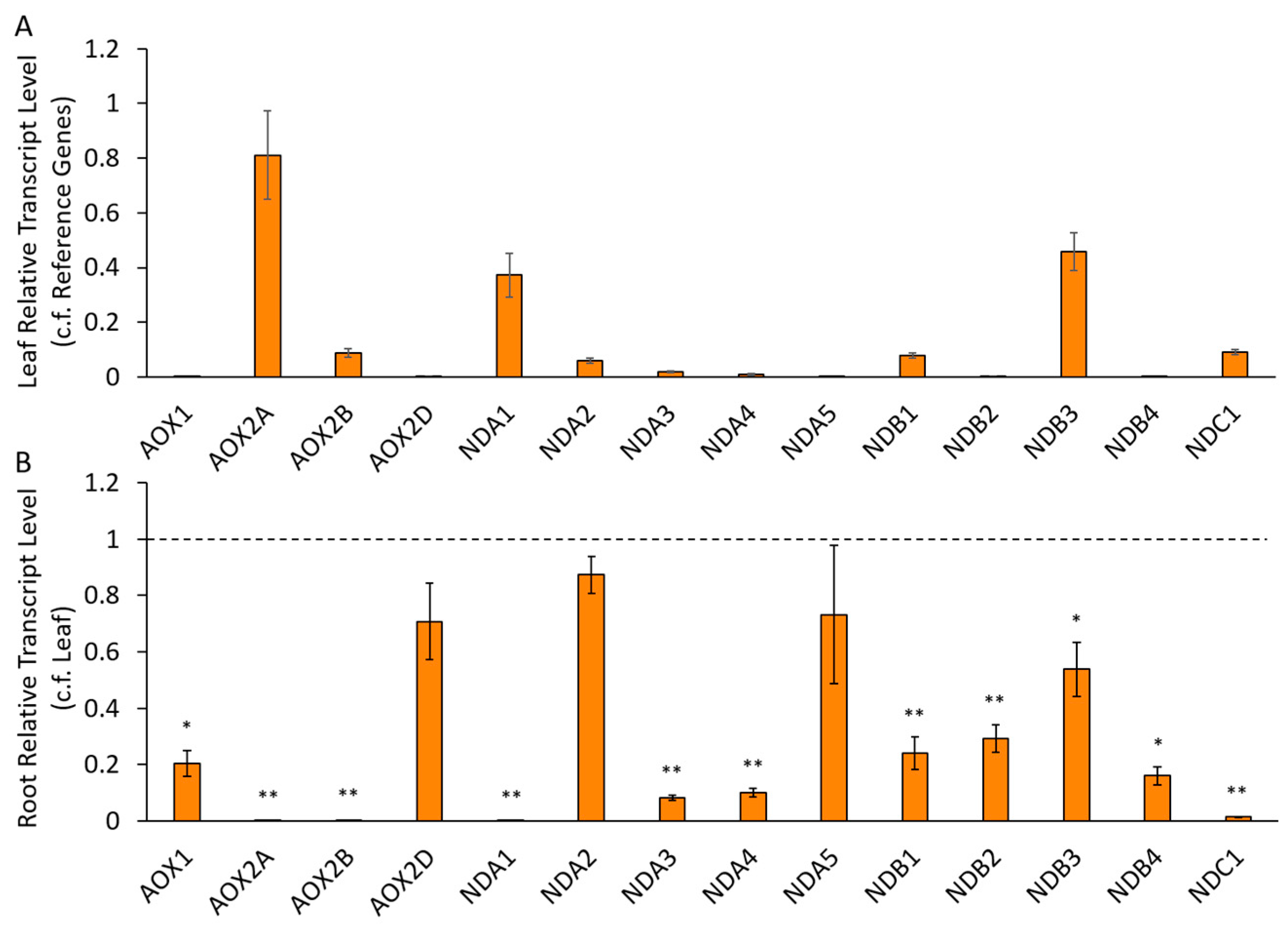
2.2.2. Differential Expression of AP Components in Chickpea Tissues
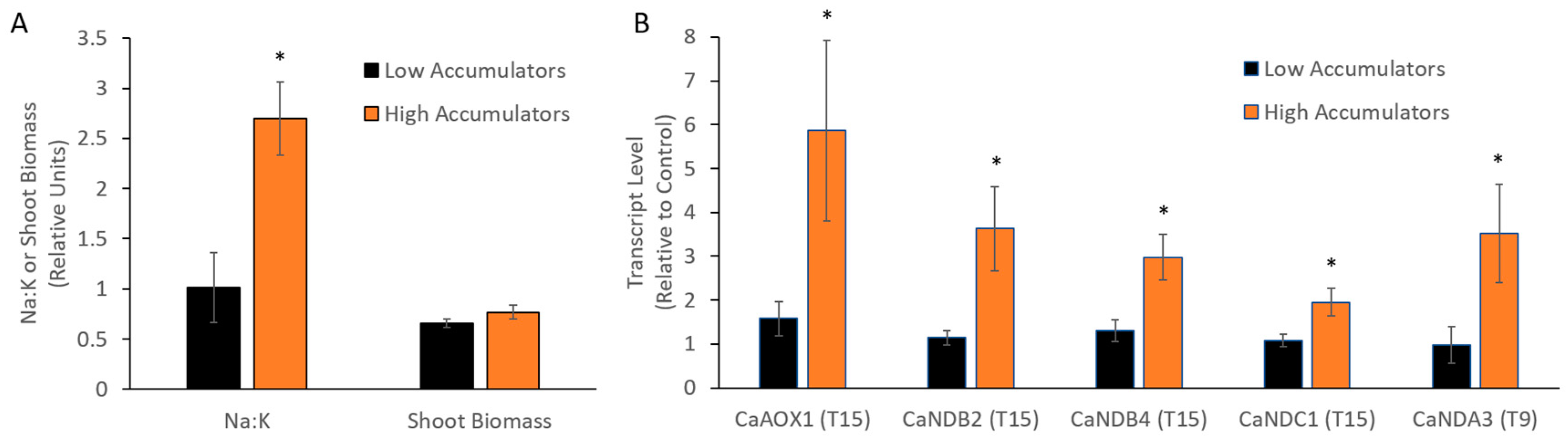
2.2.3. Expression of AP Genes in Response to Salinity Stress Varies between Cultivars and Is Related to Na Accumulation
2.3. Type II NAD(P)H Dehydrogenase Genes of Other Legumes
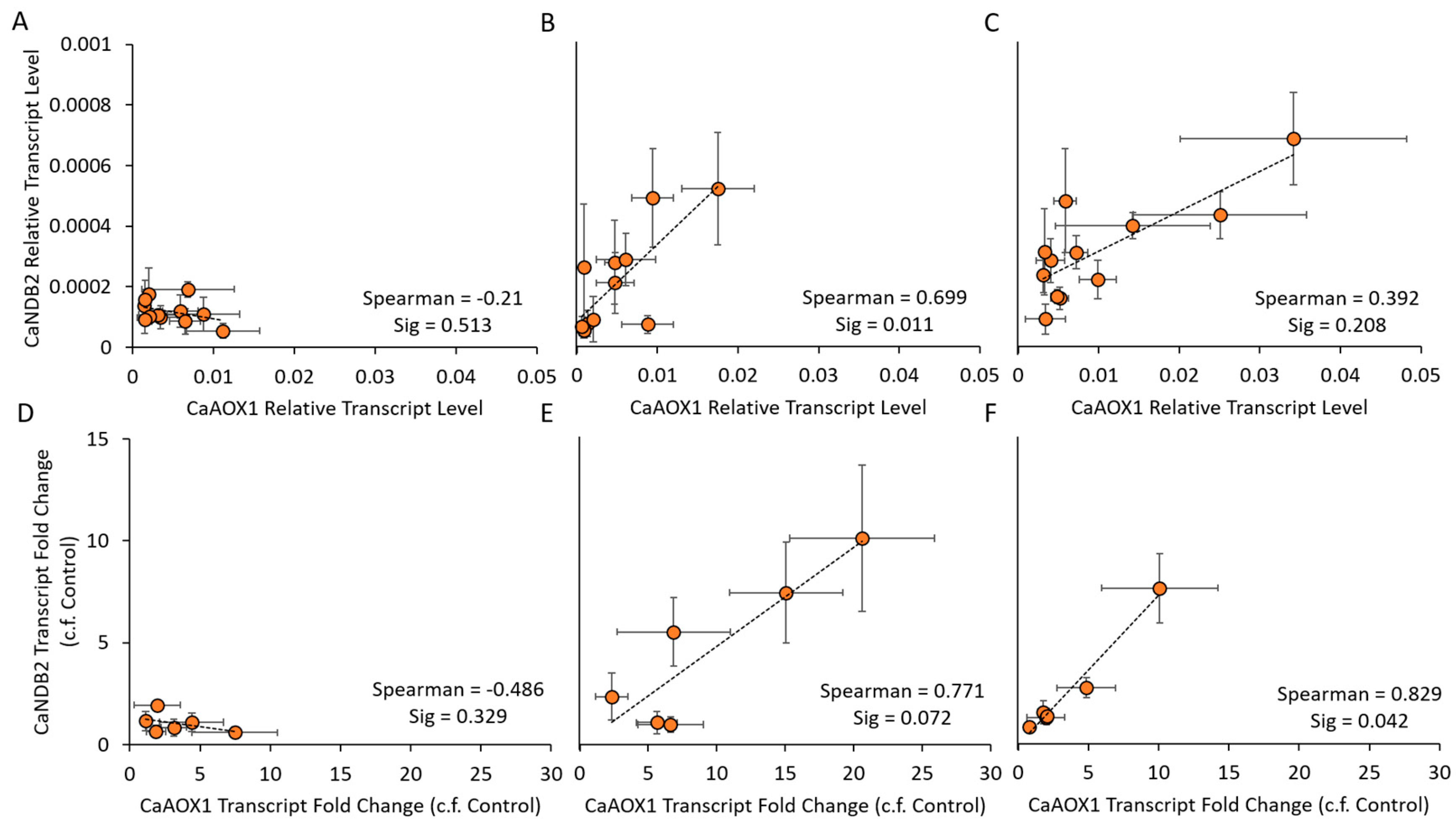
2.4. Co-Expression of AP Genes
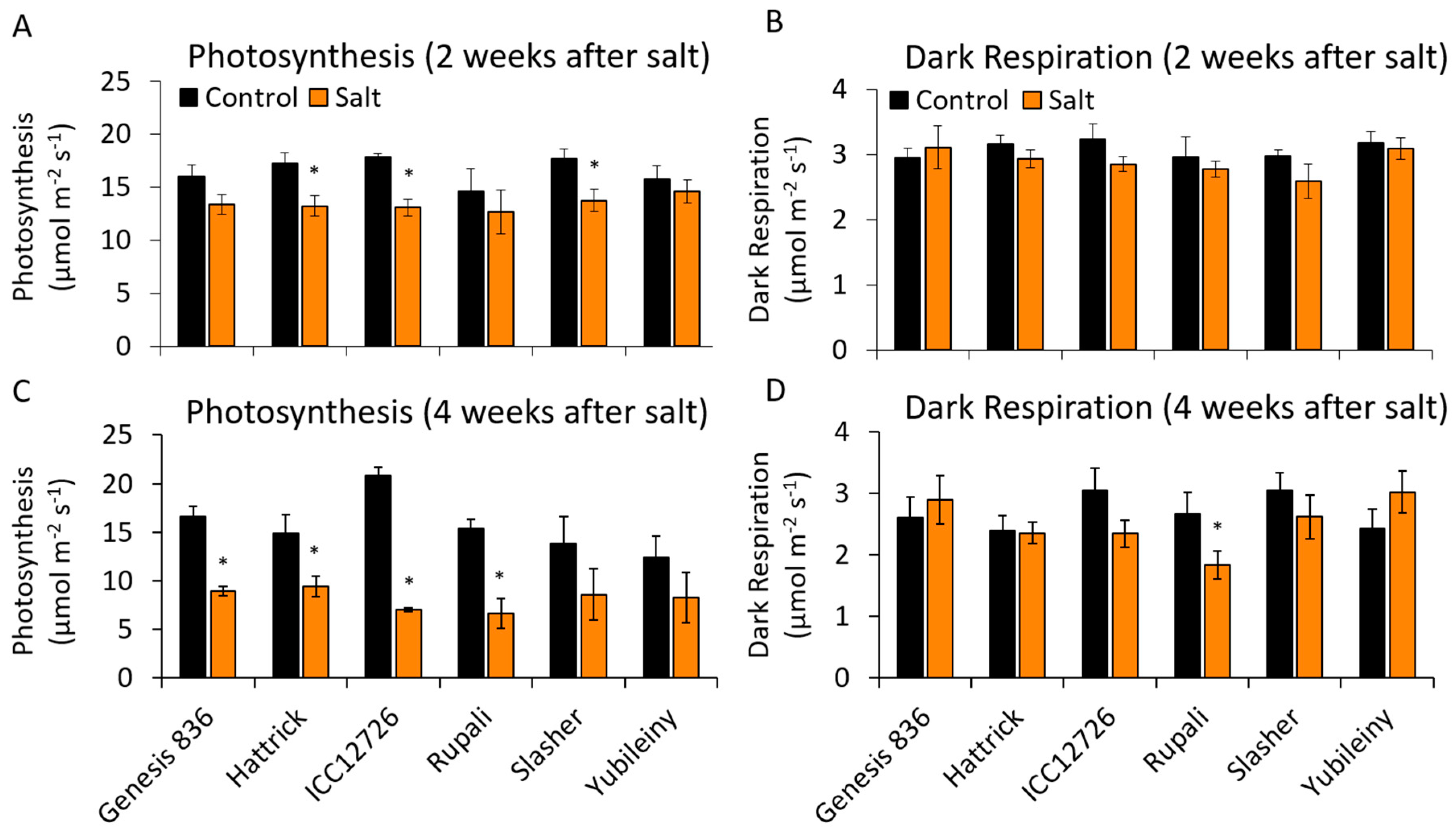
2.5. Maintaining Photosynthetic Rates during Salinity Stress
3. Discussion
3.1. NAD(P)H Oxidation by Isolated Mitochondria
3.2. Expression of AP Genes in Different Tissues
3.3. Stress-Responsive AP Genes in Chickpea
4. Conclusions
5. Materials and Methods
5.1. Plant Materials and Growth Conditions
5.2. Measurement of Type II NAD(P)H Dehydrogenase Activities in Purified Mitochondria
5.3. Identification of ND Genes in Chickpea and Other Legumes
5.4. Quantitative Real-Time Reverse-Transcriptase PCR
5.5. Salinity Experiment
5.6. Gas Exchange Measurements
5.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADP | Adenosine diphosphate |
| ANOVA | Analysis of variance |
| AOX | Alternative oxidase |
| AP | Alternative respiratory pathway |
| ATP | Adenosine triphosphate |
| DH | Dehydrogenase |
| DNA | Deoxyribonucleic acid |
| EDTA | Ethylenediaminetetraacetic acid |
| EGTA | Ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid |
| ETC | Electron transport chain |
| FAD | Flavin adenine dinucleotide |
| FPKM | Fragments per kilobase per million mapped reads |
| LED | Light emitting diode |
| MES | 2-(N-morpholino)ethanesulfonic acid |
| NAD | Nicotinamide adenine dinucleotide |
| NADH | Nicotinamide adenine dinucleotide, reduced |
| NADPH | Nicotinamide adenine dinucleotide phosphate, reduced |
| NAD(P)H | NADH and/or NADPH |
| NCBI | National Centre for Biotechnology Information |
| PAR | Photosynthetically active radiation |
| qRT-PCR | Quantitative reverse-transcriptase polymerase chain reaction |
| RNA | Ribonucleic acid |
| ROS | Reactive oxygen species |
| S.E.M. | Standard error of the mean |
| TES | 2-[(2-Hydroxy-1,1-bis(hydroxymethyl)ethyl)amino]ethanesulfonic acid |
References
- Millar, A.H.; Whelan, J.; Soole, K.L.; Day, D.A. Organization and regulation of mitochondrial respiration in plants. In Annual Review of Plant Biology; Merchant, S.S., Briggs, W.R., Ort, D., Eds.; Annual Reviews: Palo Alto, CA, USA, 2011; Volume 62, pp. 79–104. [Google Scholar]
- Affourtit, C.; Krab, K.; Moore, A.L. Control of plant mitochondrial respiration. Biochim. Biophys. Acta-Bioenerg. 2001, 1504, 58–69. [Google Scholar] [CrossRef] [Green Version]
- Moore, C.S.; Cook-Johnson, R.J.; Rudhe, C.; Whelan, J.; Day, D.A.; Wiskich, J.T.; Soole, K.L. Identification of AtNDI1, an internal non-phosphorylating NAD(P)H dehydrogenase in Arabidopsis mitochondria. Plant Physiol. 2003, 133, 1968–1978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finnegan, P.M.; Soole, K.L.; Umbach, A.L. Alternative mitochondrial electron transport proteins in higher plants. In Plant Mitochondria: From Genome to Function; Day, D.A., Millar, H., Whelan, J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 163–230. [Google Scholar]
- Michalecka, A.M.; Agius, S.C.; Møller, I.M.; Rasmusson, A.G. Identification of a mitochondrial external NADPH dehydrogenase by overexpression in transgenic Nicotiana sylvestris. Plant J. 2004, 37, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Hoefnagel, M.H.N.; Millar, A.H.; Wiskich, J.T.; Day, D.A. Cytochrome and alternative respiratory pathways compete for electrons in the presence of pyruvate in soybean mitochondria. Arch. Biochem. Biophys. 1995, 318, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Vanlerberghe, G.C. Alternative oxidase: A mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. Int. J. Mol. Sci. 2013, 14, 6805–6847. [Google Scholar] [CrossRef]
- Del-Saz, N.F.; Ribas-Carbo, M.; McDonald, A.E.; Lambers, H.; Fernie, A.R.; Florez-Sarasa, I. An in vivo perspective of the role(s) of the alternative oxidase pathway. Trends Plant Sci. 2018, 23, 206–219. [Google Scholar] [CrossRef]
- Cvetkovska, M.; Dahal, K.; Alber, N.A.; Jin, C.; Cheung, M.; Vanlerberghe, G.C. Knockdown of mitochondrial alternative oxidase induces the “stress state” of signaling molecule pools in Nicotiana tabacum, with implications for stomatal function. New Phytol. 2014, 203, 449–461. [Google Scholar] [CrossRef]
- Huang, S.B.; Van Aken, O.; Schwarzlander, M.; Belt, K.; Millar, A.H. The roles of mitochondrial reactive oxygen species in cellular signaling and stress response in plants. Plant Physiol. 2016, 171, 1551–1559. [Google Scholar] [CrossRef] [Green Version]
- Shapiguzov, A.; Vainonen, J.P.; Hunter, K.; Tossavainen, H.; Tiwari, A.; Jarvi, S.; Hellman, M.; Aarabi, F.; Alseekh, S.; Wybouw, B.; et al. Arabidopsis RCD1 coordinates chloroplast and mitochondrial functions through interaction with ANAC transcription factors. Elife 2019, 8, 35. [Google Scholar] [CrossRef]
- Wang, Y.; Berkowitz, O.; Selinski, J.; Xu, Y.; Hartmann, A.; Whelan, J. Stress responsive mitochondrial proteins in Arabidopsis thaliana. Free Radic. Biol. Med. 2018, 122, 28–39. [Google Scholar] [CrossRef]
- Wallström, S.V.; Florez-Sarasa, I.; Araujo, W.L.; Aidemark, M.; Fernandez-Fernandez, M.; Fernie, A.R.; Ribas-Carbo, M.; Rasmusson, A.G. Suppression of the external mitochondrial NADPH dehydrogenase, NDB1, in Arabidopsis thaliana affects central metabolism and vegetative growth. Mol. Plant 2014, 7, 356–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sieger, S.M.; Kristensen, B.K.; Robson, C.A.; Amirsadeghi, S.; Eng, E.W.Y.; Abdel-Mesih, A.; Moller, I.M.; Vanlerberghe, G.C. The role of alternative oxidase in modulating carbon use efficiency and growth during macronutrient stress in tobacco cells. J. Exp. Bot. 2005, 56, 1499–1515. [Google Scholar] [CrossRef] [PubMed]
- Wallström, S.V.; Florez-Sarasa, I.; Araujo, W.L.; Escobar, M.A.; Geisler, D.A.; Aidemark, M.; Lager, I.; Fernie, A.R.; Ribas-Carbo, M.; Rasmusson, A.G. Suppression of NDA-type alternative mitochondrial NAD(P)H dehydrogenases in Arabidopsis thaliana modifies growth and metabolism, but not high light stimulation of mitochondrial electron transport. Plant Cell Physiol. 2014, 55, 881–896. [Google Scholar] [CrossRef]
- Rasmusson, A.G.; Fernie, A.R.; van Dongen, J.T. Alternative oxidase: A defence against metabolic fluctuations? Physiol. Plant. 2009, 137, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Dahal, K.; Vanlerberghe, G.C. Alternative oxidase respiration maintains both mitochondrial and chloroplast function during drought. New Phytol. 2017, 213, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Dahal, K.; Martyn, G.D.; Vanlerberghe, G.C. Improved photosynthetic performance during severe drought in Nicotiana tabacum overexpressing a nonenergy conserving respiratory electron sink. New Phytol. 2015, 208, 382–395. [Google Scholar] [CrossRef]
- Vanlerberghe, G.C.; Martyn, G.D.; Dahal, K. Alternative oxidase: A respiratory electron transport chain pathway essential for maintaining photosynthetic performance during drought stress. Physiol. Plant. 2016, 157, 322–337. [Google Scholar] [CrossRef]
- Smith, C.A.; Melino, V.J.; Sweetman, C.; Soole, K.L. Manipulation of alternative oxidase can influence salt tolerance in Arabidopsis thaliana. Physiol. Plant. 2009, 137, 459–472. [Google Scholar] [CrossRef]
- Smith, C.; Barthet, M.; Melino, V.; Smith, P.; Day, D.; Soole, K. Alterations in the mitochondrial alternative NAD (P) H dehydrogenase NDB4 lead to changes in mitochondrial electron transport chain composition, plant growth and response to oxidative stress. Plant Cell Physiol. 2011, 52, 1222–1237. [Google Scholar] [CrossRef]
- Sweetman, C.; Waterman, C.D.; Rainbird, B.M.; Smith, P.M.C.; Jenkins, C.L.D.; Day, D.A.; Soole, K.L. AtNDB2 Is the main external NADH dehydrogenase in mitochondria and is important for tolerance to environmental stress. Plant Physiol. 2019, 181, 774–788. [Google Scholar] [CrossRef] [Green Version]
- Considine, M.J.; Holtzapffel, R.C.; Day, D.A.; Whelan, J.; Millar, A.H. Molecular distinction between alternative oxidase from monocots and dicots. Plant Physiol. 2002, 129, 949–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, J.H.; McDonald, A.E.; Arnholdt-Schmitt, B.; de Melo, D.F. A classification scheme for alternative oxidases reveals the taxonomic distribution and evolutionary history of the enzyme in angiosperms. Mitochondrion 2014, 19, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Sweetman, C.; Soole, K.L.; Jenkins, C.L.D.; Day, D.A. Genomic structure and expression of alternative oxidase genes in legumes. Plant Cell Environ. 2019, 42, 71–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmusson, A.G.; Soole, K.L.; Elthon, T.E. Alternative NAD(P)H dehydrogenases of plant mitochondria. Annu. Rev. Plant Biol. 2004, 55, 23–39. [Google Scholar] [CrossRef]
- Carrie, C.; Murcha, M.W.; Kuehn, K.; Duncan, O.; Barthet, M.; Smith, P.M.; Eubel, H.; Meyer, E.; Day, D.A.; Millar, A.H.; et al. Type II NAD(P)H dehydrogenases are targeted to mitochondria and chloroplasts or peroxisomes in Arabidopsis thaliana. FEBS Lett. 2008, 582, 3073–3079. [Google Scholar] [CrossRef] [Green Version]
- Wanniarachchi, V.R.; Dametto, L.; Sweetman, C.; Shavrukov, Y.; Day, D.A.; Jenkins, C.L.D.; Soole, K.L. Alternative respiratory pathway component genes (AOX and ND) in rice and barley and their response to stress. Int. J. Mol. Sci. 2018, 19, 915. [Google Scholar] [CrossRef] [Green Version]
- Soole, K.L.; Dry, I.B.; Wiskich, J.T. Oxidation of NADH by plant-mitochondria - kinetics and effects of calcium-ions. Physiol. Plant. 1990, 78, 205–210. [Google Scholar] [CrossRef]
- Elhafez, D.; Murcha, M.W.; Clifton, R.; Soole, K.L.; Day, D.A.; Whelan, J. Characterization of mitochondrial alternative NAD(P)H dehydrogenases in Arabidopsis: Intraorganelle location and expression. Plant Cell Physiol. 2006, 47, 43–54. [Google Scholar] [CrossRef] [Green Version]
- Kerscher, S.J. Diversity and origin of alternative NADH: Ubiquinone oxidoreductases. Biochim. Biophys. Acta-Bioenerg. 2000, 1459, 274–283. [Google Scholar] [CrossRef] [Green Version]
- Michalecka, A.M.; Svensson, A.S.; Johansson, F.I.; Agius, S.C.; Johanson, U.; Brennicke, A.; Binder, S.; Rasmusson, A.G. Arabidopsis genes encoding mitochondrial type II NAD(P)H dehydrogenases have different evolutionary orgin and show distinct responses to light. Plant Physiol. 2003, 133, 642–652. [Google Scholar] [CrossRef] [Green Version]
- Geisler, D.A.; Broselid, C.; Hederstedt, L.; Rasmusson, A.G. Ca2+-binding and Ca2+-independent respiratory NADH and NADPH dehydrogenases of Arabidopsis thaliana. J. Biol. Chem. 2007, 282, 28455–28464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, M.S.; Rasmusson, A.G. The evolution of substrate specificity-associated residues and Ca2+-binding motifs in EF-hand-containing type II NAD(P)H dehydrogenases. Physiol. Plant. 2016, 157, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Patel, R.K.; Jhanwar, S.; Priya, P.; Bhattacharjee, A.; Yadav, G.; Bhatia, S.; Chattopadhyay, D.; Tyagi, A.K.; Jain, M. Gene discovery and tissue-specific transcriptome analysis in chickpea with massively parallel pyrosequencing and web resource development. Plant Physiol. 2011, 156, 1661–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudapa, H.; Garg, V.; Chitikineni, A.; Varshney, R.K. The RNA-Seq-based high resolution gene expression atlas of chickpea (Cicer arietinum L.) reveals dynamic spatio-temporal changes associated with growth and development. Plant Cell Environ. 2018, 41, 2209–2225. [Google Scholar] [CrossRef] [Green Version]
- Sweetman, C.; Khassanova, G.; Miller, T.K.; Booth, N.J.; Kurishbayev, A.; Jatayev, S.; Gupta, N.K.; Langridge, P.; Jenkins, C.L.D.; Soole, K.L.; et al. Salt-induced expression of intracellular vesicle trafficking genes, CaRab-GTP, and their association with Na+ accumulation in leaves of chickpea (Cicer arietinum L.). BMC Plant Biol. in Press.
- Zhang, J.Y.; de Carvalho, M.H.C.; Torres-Jerez, I.; Kang, Y.; Allen, S.N.; Huhman, D.V.; Tang, Y.H.; Murray, J.; Sumner, L.W.; Udvardi, M.K. Global reprogramming of transcription and metabolism in Medicago truncatula during progressive drought and after rewatering. Plant Cell Environ. 2014, 37, 2553–2576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.F.; Su, Z.; Dong, J.L.; Wang, T. An expression database for roots of the model legume Medicago truncatula under salt stress. BMC Genom. 2009, 10, 517. [Google Scholar] [CrossRef] [Green Version]
- Iyer, N.J.; Tang, Y.; Mahalingam, R. Physiological, biochemical and molecular responses to a combination of drought and ozone in Medicago truncatula. Plant Cell Environ. 2013, 36, 706–720. [Google Scholar] [CrossRef]
- Hogekamp, C.; Arndt, D.; Pereira, P.A.; Becker, J.D.; Hohnjec, N.; Kuster, H. Laser microdissection unravels cell-type-specific transcription in arbuscular mycorrhizal roots, including CAAT-box transcription factor gene expression correlating with fungal contact and spread. Plant Physiol. 2011, 157, 2023–2043. [Google Scholar] [CrossRef] [Green Version]
- Diaz, P.; Betti, M.; Sanchez, D.H.; Udvardi, M.K.; Monza, J.; Marquez, A.J. Deficiency in plastidic glutamine synthetase alters proline metabolism and transcriptomic response in Lotus japonicus under drought stress. New Phytol. 2010, 188, 1001–1013. [Google Scholar] [CrossRef]
- Sanchez, D.H.; Lippold, F.; Redestig, H.; Hannah, M.A.; Erban, A.; Kramer, U.; Kopka, J.; Udvardi, M.K. Integrative functional genomics of salt acclimatization in the model legume Lotus japonicus. Plant J. 2008, 53, 973–987. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, D.H.; Pieckenstain, F.L.; Szymanski, J.; Erban, A.; Bromke, M.; Hannah, M.A.; Kraemer, U.; Kopka, J.; Udvardi, M.K. Comparative functional genomics of salt stress in related model and cultivated plants identifies and overcomes limitations to translational genomics. PLoS ONE 2011, 6, e17094. [Google Scholar] [CrossRef]
- Clifton, R.; Lister, R.; Parker, K.L.; Sappl, P.G.; Elhafez, D.; Millar, A.H.; Day, D.A.; Whelan, J. Stress-induced co-expression of alternative respiratory chain components in Arabidopsis thaliana. Plant Mol. Biol. 2005, 58, 193–212. [Google Scholar] [CrossRef] [PubMed]
- Thirkettle-Watts, D.; McCabe, T.C.; Clifton, R.; Moore, C.; Finnegan, P.M.; Day, D.A.; Whelan, J. Analysis of the alternative oxidase promoters from soybean. Plant Physiol. 2003, 133, 1158–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lescot, M.; Dehais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouze, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Ostaszewska-Bugajska, M.; Juszczuk, I.M. Changes in the OXPHOS system in leaf and root mitochondria of Arabidopsis thaliana subjected to long-term sulphur deficiency. Acta Physiol. Plant. 2016, 38, 141. [Google Scholar] [CrossRef] [Green Version]
- Day, D.A.; Dry, I.B.; Soole, K.L.; Wiskich, J.T.; Moore, A.L. Regulation of alternative pathway activity in plant-mitochondria—Deviations from Q-pool behavior during oxidation of NADH and quinols. Plant Physiol. 1991, 95, 948–953. [Google Scholar] [CrossRef] [Green Version]
- Geisler, D.A.; Johansson, F.I.; Svensson, A.S.; Rasmusson, A.G. Antimycin A treatment decreases respiratory internal rotenone-insensitive NADH oxidation capacity in potato leaves. BMC Plant Biol. 2004, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Douce, R. Mitochondria in Higher Plants; Academic Press: Orlando, FL, USA, 1985. [Google Scholar]
- Walker, J.L.; Oliver, D.J. Light-induced increases in the glycine decarboxylase multienzyme complex from pea leaf mitochondria. Arch. Biochem. Biophys. 1986, 248, 626–638. [Google Scholar] [CrossRef]
- Day, D.A.; Millar, A.H.; Wiskich, J.T.; Whelan, J. Regulation of alternative oxidase activity by pyruvate in soybean mitochondria. Plant Physiol. 1994, 106, 1421–1427. [Google Scholar] [CrossRef] [Green Version]
- Khan, H.A.; Siddique, K.H.M.; Colmer, T.D. Salt sensitivity in chickpea is determined by sodium toxicity. Planta 2016, 244, 623–637. [Google Scholar] [CrossRef]
- Vadez, V.; Krishnamurthy, L.; Serraj, R.; Gaur, P.M.; Upadhyaya, H.D.; Hoisington, D.A.; Varshney, R.K.; Turner, N.C.; Siddique, K.H.M. Large variation in salinity tolerance in chickpea is explained by differences in sensitivity at the reproductive stage. Field Crop. Res. 2007, 104, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Yamada, S.; Ozaki, H.; Noguchi, K. The mitochondrial respiratory chain maintains the photosynthetic electron flow in arabidopsis thaliana leaves under high-light stress. Plant Cell Physiol. 2020, 61, 283–295. [Google Scholar] [CrossRef]
- Jiang, Z.X.; Watanabe, C.K.A.; Miyagi, A.; Kawai-Yamada, M.; Terashima, I.; Noguchi, K. Mitochondrial AOX supports redox balance of photosynthetic electron transport, primary metabolite balance, and growth in Arabidopsis thaliana under high light. Int. J. Mol. Sci. 2019, 20, 3067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahal, K.; Wang, J.; Martyn, G.D.; Rahimy, F.; Vanlerberghe, G.C. Mitochondrial alternative oxidase maintains respiration and preserves photosynthetic capacity during moderate drought in Nicotiana tabacum. Plant Physiol. 2014, 166, 1560–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piller, L.E.; Besagni, C.; Ksas, B.; Rumeau, D.; Brehelin, C.; Glauser, G.; Kessler, F.; Havaux, M. Chloroplast lipid droplet type II NAD(P)H quinone oxidoreductase is essential for prenylquinone metabolism and vitamin K-1 accumulation. Proc. Natl. Acad. Sci. USA 2011, 108, 14354–14359. [Google Scholar] [CrossRef] [Green Version]
- Kotula, L.; Clode, P.L.; Jimenez, J.D.; Colmer, T.D. Salinity tolerance in chickpea is associated with the ability to ‘exclude’ Na from leaf mesophyll cells. J. Exp. Bot. 2019, 70, 4991–5002. [Google Scholar] [CrossRef]
- Day, D.A.; Neuburger, M.; Douce, R. Biochemical-characterization of chlorophyll-free mitochondria from pea leaves. Aust. J. Plant Physiol. 1985, 12, 219–228. [Google Scholar] [CrossRef]
- Wigge, B.; Gardeström, P. The effects of different ionic-conditions on the activity of Cytochrome C-oxidase in purified plant mitochondria. In Plant Mitochondria; Moore, A.L., Beechey, R.B., Eds.; Springer: Boston, MA, USA, 1987. [Google Scholar]
- Soole, K.L.; Smith, C.A. Analysis of Type II NAD(P)H Dehydrogenases. In Plant Mitochindria; Whelan, J., Murcha, M.W., Eds.; Humana Press: New York, NY, USA, 2015; Volume 1305. [Google Scholar]
- Varshney, R.K.; Song, C.; Saxena, R.K.; Azam, S.; Yu, S.; Sharpe, A.G.; Cannon, S.; Baek, J.; Rosen, B.D.; Tar’an, B.; et al. Draft genome sequence of chickpea (Cicer arietinum) provides a resource for trait improvement. Nat. Biotechnol. 2013, 31, 240–246. [Google Scholar] [CrossRef] [Green Version]
- Bannai, H.; Tamada, Y.; Maruyama, O.; Nakai, K.; Miyano, S. Extensive feature detection of N-terminal protein sorting signals. Bioinformatics 2002, 18, 298–305. [Google Scholar] [CrossRef] [Green Version]
- Emanuelsson, O.; Brunak, S.; von Heijne, G.; Nielsen, H. Locating proteins in the cell using TargetP, SignalP and related tools. Nat. Protoc. 2007, 2, 953–971. [Google Scholar] [CrossRef]
- Emanuelsson, O.; Nielsen, H.; Von Heijne, G. ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Protein Sci. 1999, 8, 978–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claros, M.G.; Vincens, P. Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur. J. Biochem. 1996, 241, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Small, I.; Peeters, N.; Legeai, F.; Lurin, C. Predotar: A tool for rapidly screening proteomes for N-terminal targeting sequences. Proteomics 2004, 4, 1581–1590. [Google Scholar] [CrossRef]
- Verma, M.; Kumar, V.; Patel, R.K.; Garg, R.; Jain, M. CTDB: An integrated chickpea transcriptome database for functional and applied genomics. PLoS ONE 2015, 10, e0136880. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Campbell, J.D.; Cannon, E.K.S.; Cleary, A.M.; Huang, W.; Kalberer, S.R.; Karingula, V.; Rice, A.G.; Singh, J.; Umale, P.E.; et al. Legume information system (LegumeInfo.org): A key component of a set of federated data resources for the legume family. Nucleic Acids Res. 2016, 44, D1181–D1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mun, T.; Bachmann, A.; Gupta, V.; Stougaard, J.; Andersen, S.U. Lotus Base: An integrated information portal for the model legume Lotus japonicus. Sci. Rep. 2016, 6, 39447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdier, J.; Torres-Jerez, I.; Wang, M.Y.; Andriankaja, A.; Allen, S.N.; He, J.; Tang, Y.H.; Murray, J.D.; Udvardi, M.K. Establishment of the Lotus japonicus Gene Expression Atlas (LjGEA) and its use to explore legume seed maturation. Plant J. 2013, 74, 351–362. [Google Scholar] [CrossRef]
- Benedito, V.A.; Torres-Jerez, I.; Murray, J.D.; Andriankaja, A.; Allen, S.; Kakar, K.; Wandrey, M.; Verdier, J.; Zuber, H.; Ott, T.; et al. A gene expression atlas of the model legume Medicago truncatula. Plant J. 2008, 55, 504–513. [Google Scholar] [CrossRef]
- Li, J.; Dai, X.; Liu, T.; Zhao, P.X. LegumeIP: An interactive database for comparative genomics and transcriptomics of model legumes. Nucleic Acids Res. 2012, 40, D1221–D1229. [Google Scholar] [CrossRef] [Green Version]
- Grant, D.; Nelson, R.T.; Cannon, S.B.; Shoemaker, R.C. SoyBase, the USDA-ARS soybean genetics and genomics database. Nucleic Acids Res. 2010, 38, D843–D846. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Cannon, E.K.S.; Kalberer, S.R.; Farmer, A.D.; Cannon, S.B. PeanutBase and Other Bioinformatic Resources for Peanut. In Peanuts Genetics, Processing, and Utilization; Stalker, H.T., Wilson, R.F., Eds.; AOCS Press: Waltham, MA, USA, 2016; pp. 241–252. [Google Scholar]
- Garg, R.; Sahoo, A.; Tyagi, A.K.; Jain, M. Validation of internal control genes for quantitative gene expression studies in chickpea (Cicer arietinum L.). Biochem. Biophys. Res. Commun. 2010, 396, 283–288. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Chromosome | Genome Location | Locus Tag | mRNA Accession | Arabidopsis Ortholog (% Identity) |
|---|---|---|---|---|---|
| CaNDA1 | Ca5 | 25473001..25476933 complement | LOC101503077 | XM_004500217 | AtNDA1 and 2 (72.9 and 73.7) |
| CaNDA2 [CaNDA5] | Ca2 | 25573447..25583926 complement | LOC101505730 | XM_004490552 [XM_004490553] | AtNDA1 and 2 (72.4 and 73.0) [AtNDA1 and 2 (70.5)] |
| CaNDA3 | Ca6 | 16203497..16207730 | LOC101510826 | XM_004504931 | AtNDA1 and 2 (65.7 and 66.1) |
| CaNDA4 | Ca4 | 31223913..31228505 | LOC101502969 | XM_004497885 | AtNDA1 and 2 (65.9 and 65.8) |
| CaNDB1 | Ca6 | 55894321..55900219 | LOC101508266 | XM_004507164 | AtNDB1 (69.5) |
| CaNDB2 | Ca1 | 1865091..1871364 complement | LOC101508897 | XM_012713291 | AtNDB2 and 3 (71.6 and 70.6) |
| CaNDB3 | Ca6 | 15947443..15953377 complement | LOC101502575 | XM_012717034 | AtNDB2 and 3 (71.3 and 71.0) |
| CaNDB4 | Ca6 | 55911093..55914789 | LOC101508589 | XM_004507165 | AtNDB2-4 (66.3–67.5) |
| CaNDC1 | Ca6 | 6799526..6805435 complement | LOC101503757 | XM_004503838 | AtNDC1 (67.8) |
| Gene Name | Garg et al. [35], FPKM | Kudapa et al. [36], FPKM | ||||||
|---|---|---|---|---|---|---|---|---|
| 15 d.o. Shoot | 15 d.o. Root | Veg. Leaf | Veg. Root | Rep. Leaf | Rep. Root | Sen. Leaf | Sen. Root | |
| CaNDA1 | 81 | 0 | 54 | 0.4 | 69 | 0.3 | 73 | 0 |
| CaNDA2 | 13 | 24 | 16 | 39 | 17 | 26 | 22 | 25 |
| CaNDA3 | - | - | - | - | - | - | - | - |
| CaNDA4 | 0 | 0 | 14 | 2 | 8 | 6 | 9 | 2 |
| CaNDB1 | 63 | 0 | 20 | 9 | 38 | 17 | 49 | 26 |
| CaNDB2 | - | - | - | - | - | - | - | - |
| CaNDB3 | 125 | 260 | 77 | 105 | 56 | 59 | 65 | 47 |
| CaNDB4 | - | - | - | - | - | - | - | - |
| CaNDC1 | 47 | 0 | 71 | 5 | 49 | 6 | 45 | 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sweetman, C.; Miller, T.K.; Booth, N.J.; Shavrukov, Y.; Jenkins, C.L.D.; Soole, K.L.; Day, D.A. Identification of Alternative Mitochondrial Electron Transport Pathway Components in Chickpea Indicates a Differential Response to Salinity Stress between Cultivars. Int. J. Mol. Sci. 2020, 21, 3844. https://doi.org/10.3390/ijms21113844
Sweetman C, Miller TK, Booth NJ, Shavrukov Y, Jenkins CLD, Soole KL, Day DA. Identification of Alternative Mitochondrial Electron Transport Pathway Components in Chickpea Indicates a Differential Response to Salinity Stress between Cultivars. International Journal of Molecular Sciences. 2020; 21(11):3844. https://doi.org/10.3390/ijms21113844
Chicago/Turabian StyleSweetman, Crystal, Troy K. Miller, Nicholas J. Booth, Yuri Shavrukov, Colin L.D. Jenkins, Kathleen L. Soole, and David A. Day. 2020. "Identification of Alternative Mitochondrial Electron Transport Pathway Components in Chickpea Indicates a Differential Response to Salinity Stress between Cultivars" International Journal of Molecular Sciences 21, no. 11: 3844. https://doi.org/10.3390/ijms21113844
APA StyleSweetman, C., Miller, T. K., Booth, N. J., Shavrukov, Y., Jenkins, C. L. D., Soole, K. L., & Day, D. A. (2020). Identification of Alternative Mitochondrial Electron Transport Pathway Components in Chickpea Indicates a Differential Response to Salinity Stress between Cultivars. International Journal of Molecular Sciences, 21(11), 3844. https://doi.org/10.3390/ijms21113844







