Heavy Metal-Induced Cerebral Small Vessel Disease: Insights into Molecular Mechanisms and Possible Reversal Strategies
Abstract
:1. Introduction
2. Cerebral Small Vessel Disease
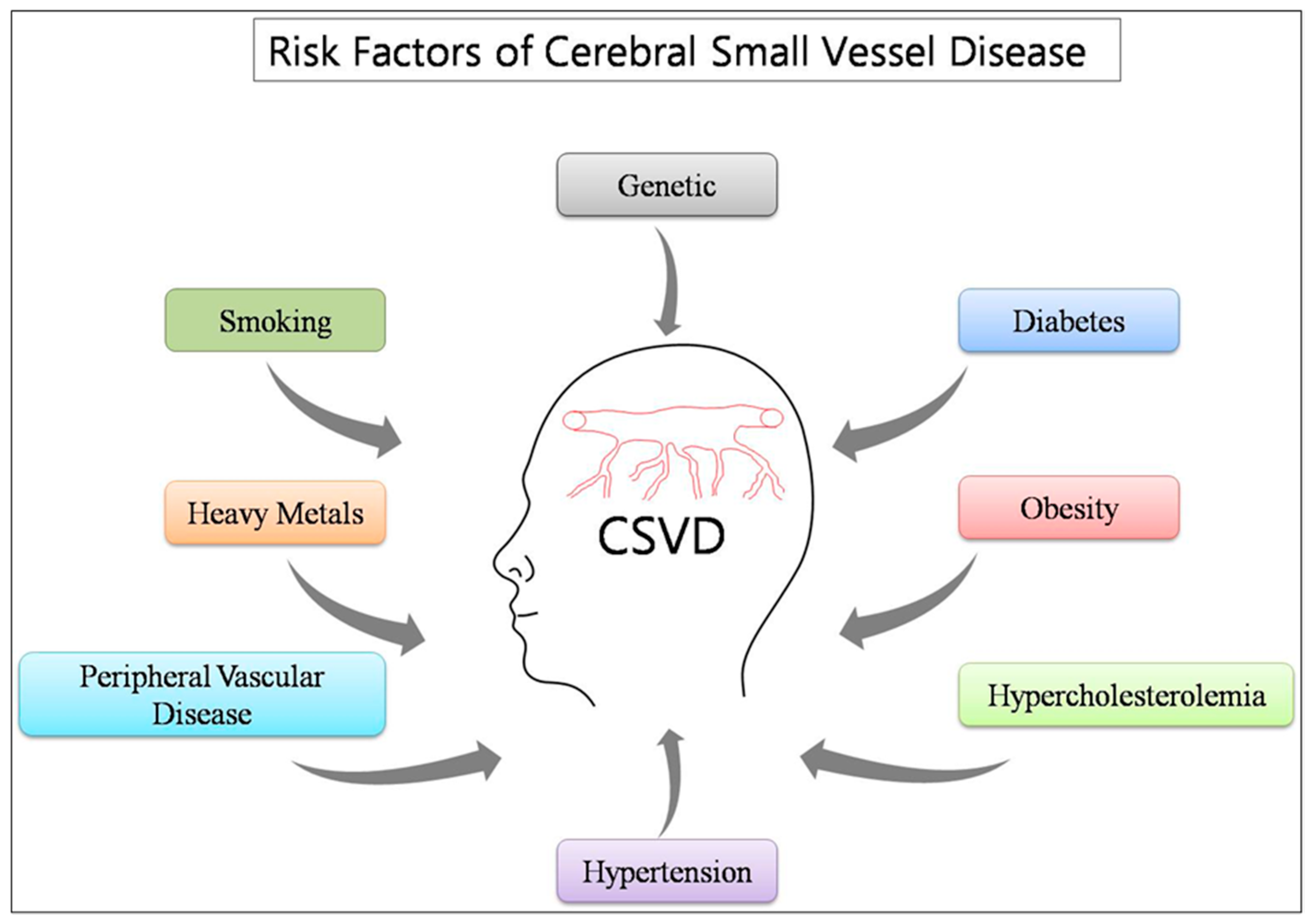
3. Risk Factors
3.1. Genetic Factors
3.2. Traditional Factors (Co-Morbid)
3.3. Environmental Factors
4. Neuroimaging Characteristics of Small Vessels Disease (SVD)
5. Role of Heavy Metals in CSVD
5.1. Lead (Pb)
5.2. Copper (Cu)
5.3. Mercury (Hg)
5.4. Arsenic (As)
5.5. Cadmium (Cd)
6. Molecular Mechanisms
6.1. Oxidative Stress
6.2. Inflammation
6.3. MMPs Expression
7. Role of Cellular Antioxidant Enzymes to Combat Metal-Induced CSVD
8. Therapeutic Strategies
9. Possible Measures to Avoid Heavy Metals Exposure
- Controls of the heavy metal level in the water and food [278];
- Alternatives to dental amalgam;
- Alternative use of heavy metal-based agrochemicals such as copper oxychloride;
- Alternative use of copper plumbing;
- Stop smoking because cigarette smoke contains cadmium that can be absorbed through the lungs [279];
- Pay attention to local fish advisories regarding mercury levels and also try to limit your consumption of larger fish because they live long and absorb more mercury from the sea [280];
- Wear masks and protective clothing to avoid occupational exposure [281].
10. Conclusions and Future Direction
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| As | Arsenic |
| ATP | Adenosine triphosphate |
| CAA | Cerebral amyloid angiopathy |
| CAT | Catalase |
| Cd | Cadmium |
| CNS | Central Nervous System |
| CSVD | Cerebral Small Vessel Disease |
| CTR1 | High-affinity copper uptake protein 1 |
| CVD | Cardiovascular disease |
| Cu | Copper |
| DMSA | 2,3-dimercaptosuccinic acid |
| DNA | Deoxyribonucleic acid |
| EC | Endothelial cells |
| EDTA | Ethylenediaminetetraacetic acid, |
| GPx | Glutathione Peroxidase |
| GSH | Glutathione |
| Hg | Mercury |
| H2O2 | Hydrogen Peroxide |
| IL | Interleukin |
| IkK | IκB kinase |
| MDA | Malondialdehyde |
| MeHg | Methyl mercury |
| MMPs | Matrix metalloproteinase |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NF-κB | Nuclear factor-kappa B3 |
| NO | Nitric oxide |
| Pb | Lead |
| ROS | Reactive oxygen species |
| SD rat | Sprague Dawley |
| SOD | Super Oxide Dismutase |
| TNF-α | Tumor necrosis factor-α |
| TTM | Tetrathiomolybdate |
| VEGF | Vascular endothelial growth factor |
| WD | Wilsons Disease |
| eNOS | Endothelial nitric oxide synthase |
| hCMEC/D3 | Human brain microvascular endothelial cells |
References
- Joutel, A.; Faraci, F.M. Cerebral small vessel disease: Insights and opportunities from mouse models of collagen IV-related small vessel disease and cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke 2014, 45, 1215–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantoni, L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010, 9, 689–701. [Google Scholar] [CrossRef]
- Das, A.S.; Regenhardt, R.W.; Vernooij, M.W.; Blacker, D.; Charidimou, A.; Viswanathan, A. Asymptomatic Cerebral Small Vessel Disease: Insights from Population-Based Studies. J. Stroke 2019, 21, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado-Godia, E.; Dwivedi, P.; Sharma, S.; Ois Santiago, A.; Roquer Gonzalez, J.; Balcells, M.; Laird, J.; Turk, M.; Suri, H.S.; Nicolaides, A.; et al. Cerebral Small Vessel Disease: A Review Focusing on Pathophysiology, Biomarkers, and Machine Learning Strategies. J. Stroke 2018, 20, 302–320. [Google Scholar] [CrossRef]
- Giau, V.V.; Bagyinszky, E.; Youn, Y.C.; An, S.S.A.; Kim, S.Y. Genetic Factors of Cerebral Small Vessel Disease and Their Potential Clinical Outcome. Int. J. Mol. Sci. 2019, 20, 4298. [Google Scholar] [CrossRef] [Green Version]
- Khan, U.; Porteous, L.; Hassan, A.; Markus, H.S. Risk factor profile of cerebral small vessel disease and its subtypes. J. Neurol. Neurosurg. Psychiatry 2007, 78, 702–706. [Google Scholar] [CrossRef] [Green Version]
- Prozialeck, W.C.; Edwards, J.R.; Nebert, D.W.; Woods, J.M.; Barchowsky, A.; Atchison, W.D. The vascular system as a target of metal toxicity. Toxicol. Sci. 2008, 102, 207–218. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, R.; Ramond, A.; O’Keeffe, L.M.; Shahzad, S.; Kunutsor, S.K.; Muka, T.; Gregson, J.; Willeit, P.; Warnakula, S.; Khan, H.; et al. Environmental toxic metal contaminants and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2018, 362, k3310. [Google Scholar] [CrossRef] [Green Version]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M.R. Heavy Metals and Human Health: Mechanistic Insight into Toxicity and Counter Defense System of Antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef] [Green Version]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. EXS 2012, 101, 133–164. [Google Scholar]
- Carvalho, C.; Moreira, P.I. Oxidative Stress: A Major Player in Cerebrovascular Alterations Associated to Neurodegenerative Events. Front. Physiol. 2018, 9, 806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flora, S.J.S.; Mittal, M.; Mehta, A. Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J. Med. Res. 2008, 128, 501. [Google Scholar] [PubMed]
- Nita, M.; Grzybowski, A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mlynek, V.; Skoczynska, A. The proinflammatory activity of cadmium. Postepy. Hig. Med. Dosw 2005, 59, 1–8. [Google Scholar]
- Li, Q.; Yang, Y.; Reis, C.; Tao, T.; Li, W.; Li, X.; Zhang, J.H. Cerebral Small Vessel Disease. Cell Transplant. 2018, 27, 1711–1722. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef] [Green Version]
- Poggesi, A.; Pasi, M.; Pescini, F.; Pantoni, L.; Inzitari, D. Circulating biologic markers of endothelial dysfunction in cerebral small vessel disease: A review. J. Cereb. Blood Flow Metab. 2016, 36, 72–94. [Google Scholar] [CrossRef] [Green Version]
- Rouhl, R.P.; van Oostenbrugge, R.J.; Damoiseaux, J.G.; Debrus-Palmans, L.L.; Theunissen, R.O.; Knottnerus, I.L.; Staals, J.E.; Delanghe, J.R.; Tervaert, J.W.; Lodder, J. Haptoglobin phenotype may alter endothelial progenitor cell cluster formation in cerebral small vessel disease. Curr. Neurovasc. Res. 2009, 6, 32–41. [Google Scholar] [CrossRef]
- Vernooij, M.W.; van der Lugt, A.; Ikram, M.A.; Wielopolski, P.A.; Niessen, W.J.; Hofman, A.; Krestin, G.P.; Breteler, M.M. Prevalence and risk factors of cerebral microbleeds: The Rotterdam Scan Study. Neurology 2008, 70, 1208–1214. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Sandercock, P.A.; Dennis, M.S.; Starr, J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke 2003, 34, 806–812. [Google Scholar] [CrossRef]
- Fassbender, K.; Bertsch, T.; Mielke, O.; Mühlhauser, F.; Hennerici, M. Adhesion molecules in cerebrovascular diseases: Evidence for an inflammatory endothelial activation in cerebral large-and small-vessel disease. Stroke 1999, 30, 1647–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lum, H.; Roebuck, K.A. Oxidant stress and endothelial cell dysfunction. Am. J. Physiol. Cell Physiol. 2001, 280, C719–C741. [Google Scholar] [CrossRef] [PubMed]
- Sena, C.M.; Louro, T.; Matafome, P.; Nunes, E.; Monteiro, P.; Seica, R. Antioxidant and vascular effects of gliclazide in type 2 diabetic rats fed high-fat diet. Physiol. Res. 2009, 58, 203–209. [Google Scholar] [PubMed]
- Sena, C.M.; Matafome, P.; Louro, T.; Nunes, E.; Fernandes, R.; Seiça, R.M. Metformin restores endothelial function in aorta of diabetic rats. Br. J. Pharmacol. 2011, 163, 424–437. [Google Scholar] [CrossRef] [Green Version]
- Norlander, A.E.; Madhur, M.S.; Harrison, D.G. The immunology of hypertension. J. Exp. Med. 2018, 215, 21–33. [Google Scholar] [CrossRef]
- Zhou, N.; Lee, J.J.; Stoll, S.; Ma, B.; Costa, K.D.; Qiu, H. Rho Kinase Regulates Aortic Vascular Smooth Muscle Cell Stiffness Via Actin/SRF/Myocardin in Hypertension. Cell Physiol. Biochem. 2017, 44, 701–715. [Google Scholar] [CrossRef]
- Rempe, R.G.; Hartz, A.M.S.; Bauer, B. Matrix metalloproteinases in the brain and blood-brain barrier: Versatile breakers and makers. J. Cereb. Blood Flow Metab. 2016, 36, 1481–1507. [Google Scholar]
- Deng, J.; Zhang, J.; Feng, C.; Xiong, L.; Zuo, Z. Critical role of matrix metalloprotease-9 in chronic high fat diet-induced cerebral vascular remodelling and increase of ischaemic brain injury in mice. Cardiovasc. Res. 2014, 103, 473–484. [Google Scholar] [CrossRef] [Green Version]
- Kelly, P.J.; Morrow, J.D.; Ning, M.; Koroshetz, W.; Lo, E.H.; Terry, E.; Milne, G.L.; Hubbard, J.; Lee, H.; Stevenson, E.; et al. Oxidative stress and matrix metalloproteinase-9 in acute ischemic stroke: The Biomarker Evaluation for Antioxidant Therapies in Stroke (BEAT-Stroke) study. Stroke 2008, 39, 100–104. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Song, Y.-N.; Liu, W.-G.; Guo, X.-L. Mmp-9, a potential target for cerebral ischemic treatment. Curr. Neuropharmacol. 2009, 7, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Haffner, C.; Malik, R.; Dichgans, M. Genetic factors in cerebral small vessel disease and their impact on stroke and dementia. J. Cereb. Blood Flow Metab. 2016, 36, 158–171. [Google Scholar] [CrossRef] [Green Version]
- Marini, S.; Anderson, C.D.; Rosand, J. Genetics of cerebral small vessel disease. Stroke 2020, 51, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Staals, J.; Makin, S.D.J.; Doubal, F.N.; Dennis, M.S.; Wardlaw, J.M. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 2014, 83, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Bushara, S.O.; Noor, S.K.; Ibraheem, A.A.H.; Elmadhoun, W.M.; Ahmed, M.H. Prevalence of and risk factors for hypertension among urban communities of North Sudan: Detecting a silent killer. J. Fam. Med. Prim. Care. 2016, 5, 605–610. [Google Scholar] [PubMed]
- Vijayan, M.; Reddy, P.H. Stroke, Vascular Dementia, and Alzheimer’s Disease: Molecular Links. J. Alzheimers Dis. 2016, 54, 427–443. [Google Scholar] [CrossRef] [Green Version]
- Martini, S.R.; Williams, S.R.; Moretti, P.; Woo, D.; Worrall, B.B. A molecular/genetic approach to cerebral small-vessel disease: Beyond aging and hypertension. Brain Circ. 2015, 1, 79. [Google Scholar] [CrossRef]
- Namba, T.; Nolte, C.T.; Jackrel, J.; Grob, D. Poisoning due to organophosphate insecticides: Acute and chronic manifestations. Am. J. Med. 1971, 50, 475–492. [Google Scholar] [CrossRef]
- Lin, C.-H.; Hsu, Y.-T.; Yen, C.-C.; Chen, H.-H.; Tseng, C.-J.; Lo, Y.-K.; Chan, J.Y.H. Association between heavy metal levels and acute ischemic stroke. J. Biomed. Sci. 2018, 25, 49. [Google Scholar] [CrossRef]
- Shinkai, Y.; Kaji, T. Cellular defense mechanisms against lead toxicity in the vascular system. Biol. Pharm. Bull. 2012, 35, 1885–1891. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-H.; Byun, H.-M.; Chung, E.-C.; Chung, H.-Y.; Bae, O.-N. Loss of Integrity: Impairment of the Blood-brain Barrier in Heavy Metal-associated Ischemic Stroke. Toxicol. Res. 2013, 29, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Kumar, J.; Sathua, K.B.; Flora, S.J.S. Chronic copper exposure elicits neurotoxic responses in rat brain: Assessment of 8-hydroxy-2-deoxyguanosine activity, oxidative stress and neurobehavioral parameters. Cell. Mol. Biol. 2019, 65, 27–35. [Google Scholar] [CrossRef]
- Gattringer, T.; Eppinger, S.; Pinter, D.; Pirpamer, L.; Berghold, A.; Wunsch, G.; Ropele, S.; Wardlaw, J.M.; Enzinger, C.; Fazekas, F. Morphological MRI characteristics of recent small subcortical infarcts. Int. J. Stroke 2015, 10, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Malayeri, A.A.; El Khouli, R.H.; Zaheer, A.; Jacobs, M.A.; Corona-Villalobos, C.P.; Kamel, I.R.; Macura, K.J. Principles and applications of diffusion-weighted imaging in cancer detection, staging, and treatment follow-up. Radiographics 2011, 31, 1773–1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huisman, T.A. Diffusion-weighted imaging: Basic concepts and application in cerebral stroke and head trauma. Eur. J. Radiol. 2003, 13, 2283–2297. [Google Scholar] [CrossRef]
- Baliyan, V.; Das, C.J.; Sharma, R.; Gupta, A.K. Diffusion weighted imaging: Technique and applications. World J. Radiol. 2016, 8, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, S.; Hornberger, E.; Griebe, M.; Gass, A.; Hennerici, M.G.; Szabo, K. MRI Characteristics of the Evolution of Supratentorial Recent Small Subcortical Infarcts. Front. Neurol. 2015, 6, 118. [Google Scholar] [CrossRef] [PubMed]
- Aribisala, B.S.; Valdes Hernandez, M.C.; Royle, N.A.; Morris, Z.; Munoz Maniega, S.; Bastin, M.E.; Deary, I.J.; Wardlaw, J.M.; Bastin, M.E.; Deary, I.J.; et al. Brain atrophy associations with white matter lesions in the ageing brain: The Lothian Birth Cohort 1936. Eur. J. Radiol. 2013, 23, 1084–1092. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Valdes Hernandez, M.C.; Munoz-Maniega, S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J. Am. Heart Assoc. 2015, 4, 001140. [Google Scholar] [CrossRef] [Green Version]
- De Silva, T.M.; Miller, A.A. Cerebral Small Vessel Disease: Targeting Oxidative Stress as a Novel Therapeutic Strategy? Front. Pharmacol. 2016, 7, 61. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, R.; Grazer, A.; Enzinger, C.; Ropele, S.; Homayoon, N.; Pluta-Fuerst, A.; Schwingenschuh, P.; Katschnig, P.; Cavalieri, M.; Schmidt, H.; et al. MRI-detected white matter lesions: Do they really matter? J. Neural Transm. 2011, 118, 673–681. [Google Scholar] [CrossRef]
- Kim, H.J.; Im, K.; Kwon, H.; Lee, J.M.; Ye, B.S.; Kim, Y.J.; Cho, H.; Choe, Y.S.; Lee, K.H.; Kim, S.T.; et al. Effects of amyloid and small vessel disease on white matter network disruption. J. Alzheimers Dis. 2015, 44, 963–975. [Google Scholar] [CrossRef] [Green Version]
- Fazekas, F.; Kleinert, R.; Roob, G.; Kleinert, G.; Kapeller, P.; Schmidt, R.; Hartung, H.P. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: Evidence of microangiopathy-related microbleeds. Am. J. Neuroradiol. 1999, 20, 637–642. [Google Scholar] [PubMed]
- Patel, B.; Lawrence, A.J.; Chung, A.W.; Rich, P.; Mackinnon, A.D.; Morris, R.G.; Barrick, T.R.; Markus, H.S. Cerebral microbleeds and cognition in patients with symptomatic small vessel disease. Stroke 2013, 44, 356–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Wardlaw, J.M. Update on cerebral small vessel disease: A dynamic whole-brain disease. Stroke Vasc. Neurol. 2016, 1, 83–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordonnier, C.; Potter, G.M.; Jackson, C.A.; Doubal, F.; Keir, S.; Sudlow, C.L.; Wardlaw, J.M.; Al-Shahi Salman, R. improving interrater agreement about brain microbleeds: Development of the Brain Observer MicroBleed Scale (BOMBS). Stroke 2009, 40, 94–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Dong, H.; Seeburg, D.P.; Wojtkiewicz, G.R.; Waterman, P.; Pulli, B.; Forghani, R.; Ali, M.; Iwamoto, Y.; Swirski, F.K.; et al. Multimodal Molecular Imaging Demonstrates Myeloperoxidase Regulation of Matrix Metalloproteinase Activity in Neuroinflammation. Cell. Mol. Neurobiol. 2019, 56, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Arba, F.; Piccardi, B.; Palumbo, V.; Giusti, B.; Nencini, P.; Gori, A.M.; Sereni, A.; Nesi, M.; Pracucci, G.; Bono, G.; et al. Small Vessel Disease Is Associated with Tissue Inhibitor of Matrix Metalloproteinase-4 After Ischaemic Stroke. Transl. Stroke Res. 2019, 10, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Appelman, A.P.; van der Graaf, Y.; Vincken, K.L.; Mali, W.P.; Geerlings, M.I. Brain atrophy and cognition: Interaction with cerebrovascular pathology? Neurobiol. Aging 2011, 32, 885–893. [Google Scholar] [CrossRef]
- Berlow, Y.A.; Wells, W.M.; Ellison, J.M.; Sung, Y.H.; Renshaw, P.F.; Harper, D.G. Neuropsychiatric correlates of white matter hyperintensities in Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2010, 25, 780–788. [Google Scholar] [CrossRef]
- Pande, M.; Flora, S.J.S. Lead induced oxidative damage and its response to combined administration of α-lipoic acid and succimers in rats. Toxicology 2002, 177, 187–196. [Google Scholar] [CrossRef]
- Kalia, K.; Flora, S.J.S. Strategies for safe and effective therapeutic measures for chronic arsenic and lead poisoning. J. Occup. Health 2005, 47, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Flora, S.J.S.; Flora, G.; Saxena, G. Environmental occurrence, health effects and management of lead poisoning. In Lead; Elsevier: Amsterdam, The Netherlands, 2006; pp. 158–228. [Google Scholar]
- Flora, S.J.S.; Saxena, G.; Mehta, A. Reversal of lead-induced neuronal apoptosis by chelation treatment in rats: Role of reactive oxygen species and intracellular Ca2+. J. Pharmacol. Exp. Ther. 2007, 322, 108–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabinowitz, M.B.; Wetherill, G.W.; Kopple, J.D. Kinetic analysis of lead metabolism in healthy humans. J. Clin. Invest. 1976, 58, 260–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, L.H.; Harp, J.P.; Han, D.Y. Pb neurotoxicity: Neuropsychological effects of lead toxicity. Biomed. Res. Int. 2014, 2014, 840547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, T.; Liu, Y.; Buchner, V.; Tchounwou, P. B. Neurotoxic effects and biomarkers of lead exposure: A review. Rev. Environ. Health 2009, 24, 15–45. [Google Scholar] [CrossRef]
- .Tobwala, S.; Wang, H.-J.; Carey, J.W.; Banks, W.A.; Ercal, N. Effects of lead and cadmium on brain endothelial cell survival, monolayer permeability, and crucial oxidative stress markers in an in vitro model of the blood-brain barrier. Toxics 2014, 2, 258–275. [Google Scholar] [CrossRef] [Green Version]
- Pachauri, V.; Saxena, G.; Mehta, A.; Mishra, D.; Flora, S.J.S. Combinational chelation therapy abrogates lead-induced neurodegeneration in rats. Toxicol. Appl. Pharmacol. 2009, 240, 255–264. [Google Scholar] [CrossRef]
- Pachauri, V.; Dubey, M.; Yadav, A.; Kushwaha, P.; Flora, S. Monensin potentiates lead chelation efficacy of MiADMSA in rat brain post chronic lead exposure. Food Chem. Toxicol. 2012, 50, 4449–4460. [Google Scholar] [CrossRef]
- Kevil, C.G.; Okayama, N.; Trocha, S.D.; Kalogeris, T.J.; Coe, L.L.; Specian, R.D.; Davis, C.P.; Alexander, J.S. Expression of zonula occludens and adherens junctional proteins in human venous and arterial endothelial cells: Role of occludin in endothelial solute barriers. Microcirculation 1998, 5, 197–210. [Google Scholar] [CrossRef]
- Trocha, S.D.; Kevil, C.G.; Mancini, M.C.; Alexander, J.S. Organ preservation solutions increase endothelial permeability and promote loss of junctional proteins. Ann. Surg. 1999, 230, 105–113. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, G.; Wu, Y. Lead exposure results in hearing loss and disruption of the cochlear blood-labyrinth barrier and the protective role of iron supplement. Neurotoxicology 2013, 39, 173–181. [Google Scholar] [CrossRef]
- Cecil, K.M.; Brubaker, C.J.; Adler, C.M.; Dietrich, K.N.; Altaye, M.; Egelhoff, J.C.; Wessel, S.; Elangovan, I.; Hornung, R.; Jarvis, K. Decreased brain volume in adults with childhood lead exposure. PLoS Med. 2008, 5, e112. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kwon, H.K.; Lee, J.M.; Cho, H.; Kim, H.J.; Park, H.K.; Jung, N.-Y.; San Lee, J.; Lee, J.; Jang, Y.K. Gray and white matter changes linking cerebral small vessel disease to gait disturbances. J. Neurol. 2016, 86, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Strużyńska, L.; Walski, M.; Gadamski, R.; Dabrowska-Bouta, B.; Rafałowska, U. Lead-induced abnormalities in blood-brain barrier permeability in experimental chronic toxicity. Mol. Chem. Neuropathol. 1997, 31, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Balbuena, P.; Li, W.; Ehrich, M. Assessments of tight junction proteins occludin, claudin 5 and scaffold proteins ZO1 and ZO2 in endothelial cells of the rat blood–brain barrier: Cellular responses to neurotoxicants malathion and lead acetate. Neurotoxicology 2011, 32, 58–67. [Google Scholar] [CrossRef]
- Shi, L.Z.; Zheng, W. Early lead exposure increases the leakage of the blood-cerebrospinal fluid barrier, in vitro. Hum. Exp. Toxicol. 2007; 26, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.A.; Russell, J.C.; Miknyoczki, S.; Ruggeri, B.; Lal, B.; Laterra, J. Vascular endothelial growth factor mediates vasogenic edema in acute lead encephalopathy. Ann. Neurol. 2004, 55, 660–667. [Google Scholar] [CrossRef]
- Song, H.; Zheng, G.; Shen, X.-F.; Liu, X.-Q.; Luo, W.-J.; Chen, J.-Y. Reduction of brain barrier tight junctional proteins by lead exposure: Role of activation of nonreceptor tyrosine kinase Src via chaperon GRP78. Toxicol. Sci. 2014, 138, 393–402. [Google Scholar] [CrossRef] [Green Version]
- Behl, M.; Zhang, Y.; Shi, Y.; Cheng, J.; Du, Y.; Zheng, W. Lead-induced accumulation of β-amyloid in the choroid plexus: Role of low density lipoprotein receptor protein-1 and protein kinase C. Neurotoxicology 2010, 31, 524–532. [Google Scholar] [CrossRef] [Green Version]
- Markovac, J.; Goldstein, G.W. Lead activates protein kinase C in immature rat brain microvessels. Toxicol. Appl. Pharmacol. 1988, 96, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Su, P.; Meng, S.; Aschner, M.; Cao, Y.; Luo, W.; Zheng, G.; Liu, M. Role of matrix metalloproteinase-2/9 (MMP2/9) in lead-induced changes in an in vitro blood-brain barrier model. Int. J. Biol. Sci. 2017, 13, 1351. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Luo, W.; Zheng, W.; Liu, Y.; Xu, H.; Zheng, G.; Dai, Z.; Zhang, W.; Chen, Y.; Chen, J. Iron supplement prevents lead-induced disruption of the blood-brain barrier during rat development. Toxicol. Appl. Pharmacol. 2007, 219, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Arnal, N.; Astiz, M.; de Alaniz, M.J.; Marra, C.A. Clinical parameters and biomarkers of oxidative stress in agricultural workers who applied copper-based pesticides. Ecotoxicol. Environ. Saf. 2011, 74, 1779–1786. [Google Scholar] [CrossRef] [PubMed]
- Arnal, N.; de Alaniz, M.J.; Marra, C.A. Alterations in copper homeostasis and oxidative stress biomarkers in women using the intrauterine device TCu380A. Toxicol. Lett. 2010, 192, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yang, W.; Che, D.; Adams, S.; Yang, L. Advances in the mechanism of high copper diets in restraining pigs growth. J. Anim. Physiol. Anim. Nutr. 2020, 104, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.D. Copper as a cofactor and regulator of copper, zinc superoxide dismutase. J. Nutr. 1992, 122, 636–640. [Google Scholar] [CrossRef]
- Choi, B.S.; Zheng, W. Copper transport to the brain by the blood-brain barrier and blood-CSF barrier. Brain Res. 2009, 1248, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Kardos, J.; Heja, L.; Simon, A.; Jablonkai, I.; Kovacs, R.; Jemnitz, K. Copper signalling: Causes and consequences. J. Cell Commun. Signal 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Van Bulck, M.; Sierra-Magro, A.; Alarcon-Gil, J.; Perez-Castillo, A.; Morales-Garcia, J.A. Novel Approaches for the Treatment of Alzheimer’s and Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 719. [Google Scholar] [CrossRef] [Green Version]
- Strozyk, D.; Launer, L.J.; Adlard, P.A.; Cherny, R.A.; Tsatsanis, A.; Volitakis, I.; Blennow, K.; Petrovitch, H.; White, L.R.; Bush, A.I. Zinc and copper modulate Alzheimer Aβ levels in human cerebrospinal fluid. Neurobiol. Aging 2009, 30, 1069–1077. [Google Scholar] [CrossRef] [Green Version]
- Bandmann, O.; Weiss, K.H.; Kaler, S.G. Wilson’s disease and other neurological copper disorders. Lancet Neurol. 2015, 14, 103–113. [Google Scholar] [CrossRef] [Green Version]
- Grover, S.; Gupta, P.; Kumar, A.; Mahajan, H. Extensive gray & white matter abnormalities in Wilson’s disease: A case report. Indian J. Radiol. Imaging 2006, 16, 91. [Google Scholar]
- Trickler, W.J.; Lantz, S.M.; Schrand, A.M.; Robinson, B.L.; Newport, G.D.; Schlager, J.J.; Paule, M.G.; Slikker, W.; Biris, A.S.; Hussain, S.M.; et al. Effects of copper nanoparticles on rat cerebral microvessel endothelial cells. Nanomedicine 2012, 7, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Quamar, S.; Kumar, J.; Mishra, A.; Flora, S. Oxidative stress and neurobehavioral changes in rats following copper exposure and their response to MiADMSA and d-pencillamine. Toxicol. Res. Appl. 2019, 3, 2397847319844782. [Google Scholar]
- Zhu, X.; Victor, T.W.; Ambi, A.; Sullivan, J.K.; Hatfield, J.; Xu, F.; Miller, L.M.; Van Nostrand, W.E. Copper accumulation and the effect of chelation treatment on cerebral amyloid angiopathy compared to parenchymal amyloid plaques. Metallomics 2020, 12, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Lamoke, F.; Mazzone, V.; Persichini, T.; Maraschi, A.; Harris, M.B.; Venema, R.C.; Colasanti, M.; Gliozzi, M.; Muscoli, C.; Bartoli, M. Amyloid β peptide-induced inhibition of endothelial nitric oxide production involves oxidative stress-mediated constitutive eNOS/HSP90 interaction and disruption of agonist-mediated Akt activation. J. Neuroinflamm. 2015, 12, 84. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, R.; Kushwaha, S.S.; Tripathi, C.; Singh, N.; Chhillar, N. Serum copper in Alzheimer’s disease and vascular dementia. Indian J. Clin. Biochem. 2008, 23, 369–374. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Yuan, Y.; Liu, Y.; Yu, Y.; Jia, N.; Zhou, L.; Wang, H.; Huang, S.; Zhang, Y.; Yang, H. Circulating Multiple Metals and Incident Stroke in Chinese Adults: The Dongfeng-Tongji Cohort. Stroke 2019, 50, 1661–1668. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Tang, Z.; Li, Y.; Hu, L.; Pan, J. The effects of copper on brain microvascular endothelial cells and claudin via apoptosis and oxidative stress. Biol. Trace Elem. Res. 2016, 174, 132–141. [Google Scholar] [CrossRef]
- Bar-Or, D.; Thomas, G.W.; Yukl, R.L.; Rael, L.T.; Shimonkevitz, R.P.; Curtis, C.G.; Winkler, J.V. Copper stimulates the synthesis and release of interleukin-8 in human endothelial cells: A possible early role in systemic inflammatory responses. Shock 2003, 20, 154–158. [Google Scholar] [CrossRef]
- Sharma, H.S.; Hussain, S.; Schlager, J.; Ali, S.F.; Sharma, A. Influence of nanoparticles on blood–brain barrier permeability and brain edema formation in rats. In Brain Edema XIV; Springer: Berlin/Heidelberg, Germany, 2010; pp. 359–364. [Google Scholar]
- Schrag, M.; Crofton, A.; Zabel, M.; Jiffry, A.; Kirsch, D.; Dickson, A.; Mao, X.W.; Vinters, H.V.; Domaille, D.W.; Chang, C.J.; et al. Effect of cerebral amyloid angiopathy on brain iron, copper, and zinc in Alzheimer’s disease. J. Alzheimers Dis. 2011, 24, 137–149. [Google Scholar] [CrossRef] [Green Version]
- Foidl, B.M.; Humpel, C. Chronic treatment with five vascular risk factors causes cerebral amyloid angiopathy but no Alzheimer pathology in C57BL6 mice. Brain Behav. Immun. 2019, 78, 52–64. [Google Scholar] [CrossRef]
- Kitazawa, M.; Cheng, D.; LaFerla, F.M. Chronic copper exposure exacerbates both amyloid and tau pathology and selectively dysregulates cdk5 in a mouse model of AD. J. Neurochem. 2009, 108, 1550–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, K.Z.; Fioresi, M.; Marques, V.B.; Vassallo, D.V. Acute copper overload induces vascular dysfunction in aortic rings due to endothelial oxidative stress and increased nitric oxide production. J. Toxicol. Environ. Health 2018, 81, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Cho, Y.-S.; Huh, Y.-D.; Park, H. Copper ion from Cu2O crystal induces AMPK-mediated autophagy via superoxide in endothelial cells. Mol. Cells 2016, 39, 195. [Google Scholar] [PubMed] [Green Version]
- Wei, H.; Zhang, W.-J.; Leboeuf, R.; Frei, B. Copper induces--and copper chelation by tetrathiomolybdate inhibits--endothelial activation in vitro. Redox. Rep. 2014, 19, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, S.; Flora, G.; Bhatnagar, P.; Flora, S.J.S. Comparative oxidative stress, metallothionein induction and organ toxicity following chronic exposure to arsenic, lead and mercury in rats. Cell Mol. Biol. 2014, 60, 13–21. [Google Scholar]
- Aschner, M.; Aschner, J.L. Mercury neurotoxicity: Mechanisms of blood-brain barrier transport. Neurosci. Biobehav. Rev. 1990, 14, 169–176. [Google Scholar] [CrossRef]
- Akkoyun, H.T. Effect of boric acid on some elemental levels on rat’s liver and kidney tissues during mercury chloride exposure. Cell Mol. Biol. 2018, 64, 84–88. [Google Scholar] [CrossRef]
- Cipollini, V.; Troili, F.; Giubilei, F. Emerging Biomarkers in Vascular Cognitive Impairment and Dementia: From Pathophysiological Pathways to Clinical Application. Int. J. Mol. Sci. 2019, 20, 2812. [Google Scholar] [CrossRef] [Green Version]
- Houston, M.C. Role of mercury toxicity in hypertension, cardiovascular disease, and stroke. J. Clin. Hypertens. 2011, 13, 621–627. [Google Scholar] [CrossRef]
- Siblerud, R.; Mutter, J.; Moore, E.; Naumann, J.; Walach, H. A Hypothesis and Evidence That Mercury May be an Etiological Factor in Alzheimer’s Disease. Int. J. Environ. Res. Public Health 2019, 16, 5152. [Google Scholar] [CrossRef] [Green Version]
- Ware, R.A.; Louis, W.C.; Burkholder, P.M. An ultrastructural study on the blood-brain barrier dysfunction following mercury intoxication. Acta Neuropathol. 1974, 30, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Usuki, F.; Yasutake, A.; Umehara, F.; Tokunaga, H.; Matsumoto, M.; Eto, K.; Ishiura, S.; Higuchi, I. In vivo protection of a water-soluble derivative of vitamin E, Trolox, against methylmercury-intoxication in the rat. Neurosci. Lett. 2001, 304, 199–203. [Google Scholar] [CrossRef]
- Hirooka, T.; Fujiwara, Y.; Yamamoto, C.; Yasutake, A.; Kaji, T. Methylmercury retards the repair of wounded monolayer of human brain microvascular endothelial cells by inhibiting their proliferation without nonspecific cell damage. J. Health Sci. 2007, 53, 450–456. [Google Scholar] [CrossRef] [Green Version]
- Wierzbicki, R.; Prażanowski, M.; Michalska, M.; Krajewska, U.; Mielicki, W.P. Disorders in blood coagulation in humans occupationally exposed to mercuric vapors. J. Trace Elem. Exp. Med. 2002, 15, 21–29. [Google Scholar] [CrossRef]
- Hirooka, T.; Fujiwara, Y.; Inoue, S.; Shinkai, Y.; Yamamoto, C.; Satoh, M.; Yasutake, A.; Eto, K.; Kaji, T. Suppression of fibroblast growth factor-2 expression: Possible mechanism underlying methylmercury-induced inhibition of the repair of wounded monolayers of cultured human brain microvascular endothelial cells. J. Toxicol. Sci. 2009, 34, 433–439. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Wiggers, G.A.; Furieri, L.B.; Briones, A.M.; Avendaño, M.S.; Peçanha, F.M.; Vassallo, D.V.; Salaices, M.; Alonso, M.J. Cerebrovascular endothelial dysfunction induced by mercury exposure at low concentrations. Neurotoxicology 2016, 53, 282–289. [Google Scholar] [CrossRef]
- Bjorklund, G.; Tinkov, A.A.; Dadar, M.; Rahman, M.M.; Chirumbolo, S.; Skalny, A.V.; Skalnaya, M.G.; Haley, B.E.; Ajsuvakova, O.P.; Aaseth, J. Insights into the Potential Role of Mercury in Alzheimer’s Disease. J. Mol. Neurosci. 2019, 67, 511–533. [Google Scholar] [CrossRef]
- Mutter, J.; Curth, A.; Naumann, J.; Deth, R.; Walach, H. Does inorganic mercury play a role in Alzheimer’s disease? A systematic review and an integrated molecular mechanism. J. Alzheimers Dis. 2010, 22, 357–374. [Google Scholar] [CrossRef] [Green Version]
- Mutter, J.; Naumann, J.; Schneider, R.; Walach, H. Mercury and Alzheimer’s disease. Fortschr. Neurol. Psychiatr. 2007, 75, 528–538. [Google Scholar] [CrossRef]
- Kishimoto, T.; Oguri, T.; Abe, M.; Kajitani, H.; Tada, M. Inhibitory effect of methylmercury on migration and tube formation by cultured human vascular endothelial cells. Arch. Toxicol. 1995, 69, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Fujimura, M.; Koyama, M.; Kanazawa, M.; Usuki, F.; Nishizawa, M.; Shimohata, T. Methylmercury causes blood-brain barrier damage in rats via upregulation of vascular endothelial growth factor expression. PLoS ONE 2017, 12, 0170623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, L.W.; Hartmann, H.A. Blood-brain barrier dysfunction in experimental mercury intoxication. Acta Neuropathol. 1972, 21, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, E.; Kurita, M.; Eto, K.; Kumagai, Y.; Kaji, T. Methylmercury promotes prostacyclin release from cultured human brain microvascular endothelial cells via induction of cyclooxygenase-2 through activation of the EGFR-p38 MAPK pathway by inhibiting protein tyrosine phosphatase 1B activity. J. Toxicol. 2017, 392, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Ghizoni, H.; de Souza, V.; Straliotto, M.R.; de Bem, A.F.; Farina, M.; Hort, M.A. Superoxide anion generation and oxidative stress in methylmercury-induced endothelial toxicity in vitro. Toxicol. In Vitro 2017, 38, 19–26. [Google Scholar] [CrossRef]
- Song, J.-W.; Choi, B.-S. Mercury induced the accumulation of amyloid beta (Aβ) in PC12 cells: The role of production and degradation of Aβ. Toxicol. Res. 2013, 29, 235–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, B.A.; Cassano, V.A.; Murray, C.; Exposure, A.T.F.o.A. Arsenic Exposure, Assessment, Toxicity, Diagnosis, and Management: Guidance for Occupational and Environmental Physicians. J. Occup. Environ. Med. 2018, 60, e634–e639. [Google Scholar] [CrossRef]
- Sen, P.; Biswas, T. Arsenic: The largest mass poisoning of a population in history. BMJ 2013, 346, f3625. [Google Scholar] [CrossRef]
- Majid Cheraghali, A.; Haghqoo, S.; Shalviri, G.; Shariati, Y.R.; Ghassemi, M.; Khosravi, S. Fatalities following skin exposure to arsenic. Clin. Toxicol. 2007, 45, 965–967. [Google Scholar] [CrossRef]
- Rezaei, M.; Khodayar, M.J.; Seydi, E.; Soheila, A.; Parsi, I.K. Acute, but not Chronic, Exposure to Arsenic Provokes Glucose Intolerance in Rats: Possible Roles for Oxidative Stress and the Adrenergic Pathway. Can. J. Diabetes 2017, 41, 273–280. [Google Scholar] [CrossRef]
- Huang, C.F.; Chen, Y.W.; Yang, C.Y.; Tsai, K.S.; Yang, R.S.; Liu, S.H. Arsenic and diabetes: Current perspectives. Kaohsiung J. Med. Sci. 2011, 27, 402–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aposhian, H.V.; Zakharyan, R.A.; Avram, M.D.; Sampayo-Reyes, A.; Wollenberg, M.L. A review of the enzymology of arsenic metabolism and a new potential role of hydrogen peroxide in the detoxication of the trivalent arsenic species. Toxicol. Appl. Pharmacol. 2004, 198, 327–335. [Google Scholar] [PubMed]
- Douillet, C.; Huang, M.C.; Saunders, R.J.; Dover, E.N.; Zhang, C.; Styblo, M. Knockout of arsenic (+3 oxidation state) methyltransferase is associated with adverse metabolic phenotype in mice: The role of sex and arsenic exposure. Arch. Toxicol. 2017, 91, 2617–2627. [Google Scholar] [CrossRef] [PubMed]
- Benramdane, L.; Accominotti, M.; Fanton, L.; Malicier, D.; Vallon, J.J. Arsenic speciation in human organs following fatal arsenic trioxide poisoning--a case report. Clin. Chem. 1999, 45, 301–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molin, Y.; Frisk, P.; Ilback, N.G. Arsenic trioxide affects the trace element balance in tissues in infected and healthy mice differently. Anticancer Res. 2009, 29, 83–90. [Google Scholar]
- Wang, C.H.; Jeng, J.S.; Yip, P.K.; Chen, C.L.; Hsu, L.I.; Hsueh, Y.M.; Chiou, H.Y.; Wu, M.M.; Chen, C.J. Biological gradient between long-term arsenic exposure and carotid atherosclerosis. Circulation 2002, 105, 1804–1809. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.H.; Qiu, Z.Q.; Shu, W.Q.; Zhang, Y.Y.; Zhang, L.; Chen, J.A. Effects of arsenic exposure from drinking water on spatial memory, ultra-structures and NMDAR gene expression of hippocampus in rats. Toxicol. Lett. 2009, 184, 121–125. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Bhaumik, S.; Nag Chaudhury, A.; Das Gupta, S. Arsenic induced changes in growth development and apoptosis in neonatal and adult brain cells in vivo and in tissue culture. Toxicol. Lett. 2002, 128, 73–84. [Google Scholar] [CrossRef]
- Piao, F.; Ma, N.; Hiraku, Y.; Murata, M.; Oikawa, S.; Cheng, F.; Zhong, L.; Yamauchi, T.; Kawanishi, S.; Yokoyama, K. Oxidative DNA damage in relation to neurotoxicity in the brain of mice exposed to arsenic at environmentally relevant levels. J. Occup. Health 2005, 47, 445–449. [Google Scholar] [CrossRef] [Green Version]
- Meliker, J.R.; Wahl, R.L.; Cameron, L.L.; Nriagu, J.O. Arsenic in drinking water and cerebrovascular disease, diabetes mellitus, and kidney disease in Michigan: A standardized mortality ratio analysis. J. Environ. Health 2007, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.M.; Kuo, T.L.; Hwang, Y.H.; Chen, C.J. Dose-response relation between arsenic concentration in well water and mortality from cancers and vascular diseases. Am. J. Epidemiol. 1989, 130, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Tsinovoi, C.L.; Xun, P.; McClure, L.A.; Carioni, V.M.O.; Brockman, J.D.; Cai, J.; Guallar, E.; Cushman, M.; Unverzagt, F.W.; Howard, V.J.; et al. Arsenic Exposure in Relation to Ischemic Stroke: The Reasons for Geographic and Racial Differences in Stroke Study. Stroke 2018, 49, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Mateen, F.J.; Grau-Perez, M.; Pollak, J.S.; Moon, K.A.; Howard, B.V.; Umans, J.G.; Best, L.G.; Francesconi, K.A.; Goessler, W.; Crainiceanu, C.; et al. Chronic arsenic exposure and risk of carotid artery disease: The Strong Heart Study. Environ. Res. 2017, 157, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, F.; Graziano, J.H.; Parvez, F.; Liu, M.; Paul, R.R.; Shaheen, I.; Sarwar, G.; Ahmed, A.; Islam, T.; et al. Arsenic exposure from drinking water, arsenic methylation capacity, and carotid intima-media thickness in Bangladesh. Am. J. Epidemiol. 2013, 178, 372–381. [Google Scholar] [CrossRef]
- Balakumar, P.; Kaur, T.; Singh, M. Potential target sites to modulate vascular endothelial dysfunction: Current perspectives and future directions. J. Toxicol. 2008, 245, 49–64. [Google Scholar] [CrossRef]
- Thompson, C.S.; Hakim, A.M. Living beyond our physiological means: Small vessel disease of the brain is an expression of a systemic failure in arteriolar function: A unifying hypothesis. Stroke 2009, 40, e322–e330. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Li, J.; Feng, C. Blood-Brain Barrier Damage as the Starting Point of Leukoaraiosis Caused by Cerebral Chronic Hypoperfusion and Its Involved Mechanisms: Effect of Agrin and Aquaporin-4. Biomed. Res. Int. 2018. [Google Scholar] [CrossRef] [Green Version]
- Pantoni, L.; Gorelick, P.B. Cerebral Small Vessel Disease; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- De Caroli, M.; Furini, A.; DalCorso, G.; Rojas, M.; Di Sansebastiano, G.P. Endomembrane Reorganization Induced by Heavy Metals. Plants 2020, 9, 482. [Google Scholar] [CrossRef] [Green Version]
- Zarazua, S.; Perez-Severiano, F.; Delgado, J.M.; Martinez, L.M.; Ortiz-Perez, D.; Jimenez-Capdeville, M.E. Decreased nitric oxide production in the rat brain after chronic arsenic exposure. Neurochem. Res. 2006, 31, 1069–1077. [Google Scholar] [CrossRef]
- Muller, S.M.; Ebert, F.; Raber, G.; Meyer, S.; Bornhorst, J.; Huwel, S.; Galla, H.J.; Francesconi, K.A.; Schwerdtle, T. Effects of arsenolipids on in vitro blood-brain barrier model. Arch. Toxicol. 2018, 92, 823–832. [Google Scholar] [CrossRef]
- Chen, S.C.; Chang, C.Y.; Lin, M.L. Vascular Hyperpermeability Response in Animals Systemically Exposed to Arsenic. Int. J. Med. Sci. 2018, 15, 425–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Mao, J.; Zhao, J.; Zhang, Y.; Li, T.; Wang, C.; Xu, L.; Hu, Q.; Wang, X.; Jiang, S.; et al. Arsenic trioxide mediates HAPI microglia inflammatory response and the secretion of inflammatory cytokine IL-6 via Akt/NF-kappaB signaling pathway. Regul. Toxicol. Pharmacol. 2016, 81, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, C.; Nie, X.; Shi, S.; Xiao, J.; Ma, X.; Dong, X.; Zhang, Y.; Han, J.; Li, T.; et al. Perfluorooctane sulfonate mediates microglial activation and secretion of TNF-alpha through Ca(2)(+)-dependent PKC-NF-small ka, CyrillicB signaling. Int. Immunopharmacol. 2015, 28, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Prakash, C.; Soni, M.; Kumar, V. Mitochondrial oxidative stress and dysfunction in arsenic neurotoxicity: A review. J. Appl. Toxicol. 2016, 36, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Kharroubi, W.; Haj Ahmed, S.; Nury, T.; Andreoletti, P.; Sakly, R.; Hammami, M.; Lizard, G. Mitochondrial dysfunction, oxidative stress and apoptotic induction in microglial BV-2 cells treated with sodium arsenate. J. Environ. Sci. 2017, 51, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Meng, D.; Chang, Q.; Pan, J.; Zhang, Z.; Chen, G.; Ke, Z.; Luo, J.; Shi, X. Arsenic inhibits neurite outgrowth by inhibiting the LKB1-AMPK signaling pathway. Environ. Health Perspect. 2010, 118, 627–634. [Google Scholar] [CrossRef] [Green Version]
- Anwar-Mohamed, A.; Elshenawy, O.H.; El-Sherbeni, A.A.; Abdelrady, M.; El-Kadi, A.O. Acute arsenic treatment alters arachidonic acid and its associated metabolite levels in the brain of C57Bl/6 mice. Can. J. Physiol. Pharmacol. 2014, 92, 693–702. [Google Scholar] [CrossRef]
- Escudero-Lourdes, C.; Uresti-Rivera, E.E.; Oliva-Gonzalez, C.; Torres-Ramos, M.A.; Aguirre-Banuelos, P.; Gandolfi, A.J. Erratum to: Cortical Astrocytes Acutely Exposed to the Monomethylarsonous Acid (MMA(III)) Show Increased Pro-inflammatory Cytokines Gene Expression that is Consistent with APP and BACE-1 Over-expression. Neurochem. Res. 2016, 41, 2573. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Ma, Z.; Yin, S.; Yan, X.; Wang, J. Arsenic and fluoride induce apoptosis, inflammation and oxidative stress in cultured human umbilical vein endothelial cells. Chemosphere 2017, 167, 454–461. [Google Scholar] [CrossRef]
- Wang, L.; Kou, M.C.; Weng, C.Y.; Hu, L.W.; Wang, Y.J.; Wu, M.J. Arsenic modulates heme oxygenase-1, interleukin-6, and vascular endothelial growth factor expression in endothelial cells: Roles of ROS, NF-kappaB, and MAPK pathways. Arch. Toxicol. 2012, 86, 879–896. [Google Scholar] [CrossRef]
- Weng, C.Y.; Chiou, S.Y.; Wang, L.; Kou, M.C.; Wang, Y.J.; Wu, M.J. Arsenic trioxide induces unfolded protein response in vascular endothelial cells. Arch. Toxicol. 2014, 88, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.; Tsai, C.-H.; Ueng, K.-C.; Tian, T.-Y.; Chen, S.-C.; Yeh, H.-I. Endothelial gap junctions are down-regulated by arsenic trioxide. Eur. J. Pharmacol. 2007, 569, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.H.; Yu, C.L.; Chang, L.W.; Yu, H.S. Low concentrations of arsenic induce vascular endothelial growth factor and nitric oxide release and stimulate angiogenesis in vitro. Chem. Res. Toxicol. 2003, 16, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Manthari, R.K.; Tikka, C.; Ommati, M.M.; Niu, R.; Sun, Z.; Wang, J.; Zhang, J.; Wang, J. Arsenic induces autophagy in developmental mouse cerebral cortex and hippocampus by inhibiting PI3K/Akt/mTOR signaling pathway: Involvement of blood-brain barrier’s tight junction proteins. Arch. Toxicol. 2018, 92, 3255–3275. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Sharma, P.M. Arsenic toxicity induced endothelial dysfunction and dementia: Pharmacological interdiction by histone deacetylase and inducible nitric oxide synthase inhibitors. Toxicol. Appl. Pharmacol. 2013, 273, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Pi, J.; Yamauchi, H.; Sun, G.; Yoshida, T.; Aikawa, H.; Fujimoto, W.; Iso, H.; Cui, R.; Waalkes, M.P.; Kumagai, Y. Vascular dysfunction in patients with chronic arsenosis can be reversed by reduction of arsenic exposure. Environ. Health Perspect. 2005, 113, 339–341. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Kang, D.; Yan, Z.; Shen, Q.; Lou, Y.; Li, Y.; Kong, A.; Pan, B.; Huang, C. Tissue distribution of tetrabromobisphenol A and cadmium in mixture inhalation exposure. Toxicol. Ind. Health 2019, 35, 165–176. [Google Scholar] [CrossRef]
- Choudhuri, S.; Liu, W.L.; Berman, N.E.; Klaassen, C.D. Cadmium accumulation and metallothionein expression in brain of mice at different stages of development. Toxicol. Lett. 1996, 84, 127–133. [Google Scholar] [CrossRef]
- Amuno, S.; Shekh, K.; Kodzhahinchev, V.; Niyogi, S. Neuropathological changes in wild muskrats (Ondatra zibethicus) and red squirrels (Tamiasciurushudsonicus) breeding in arsenic endemic areas of Yellowknife, Northwest Territories (Canada): Arsenic and cadmium accumulation in the brain and biomarkers of oxidative stress. Sci. Total Environ. 2020, 704, 135426. [Google Scholar]
- Zheng, J.L.; Yuan, S.S.; Wu, C.W.; Lv, Z.M. Acute exposure to waterborne cadmium induced oxidative stress and immunotoxicity in the brain, ovary and liver of zebrafish (Danio rerio). Aquat. Toxicol. 2016, 180, 36–44. [Google Scholar] [CrossRef]
- Monaco, A.; Capriello, T.; Grimaldi, M.C.; Schiano, V.; Ferrandino, I. Neurodegeneration in zebrafish embryos and adults after cadmium exposure. Eur. J. Histochem. 2017, 61, 2833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favorito, R.; Chiarelli, G.; Grimaldi, M.C.; De Bonis, S.; Lancieri, M.; Ferrandino, I. Bioaccumulation of cadmium and its cytotoxic effect on zebrafish brain. J. Chem. Ecol. 2011, 27, 39–46. [Google Scholar] [CrossRef]
- Xu, M.Y.; Wang, P.; Sun, Y.J.; Yang, L.; Wu, Y.J. Joint toxicity of chlorpyrifos and cadmium on the oxidative stress and mitochondrial damage in neuronal cells. Food Chem. Toxicol. 2017, 103, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Adefegha, S.A.; Omojokun, O.S.; Oboh, G.; Fasakin, O.; Ogunsuyi, O. Modulatory Effects of Ferulic Acid on Cadmium-Induced Brain Damage. J. Evid. Based Complement. Altern. Med. 2016, 21, NP56–NP61. [Google Scholar] [CrossRef]
- Shrivastava, A.N.; Triller, A.; Melki, R. Cell biology and dynamics of Neuronal Na+/K+-ATPase in health and diseases. Neuropharmacology 2018, 169, 107461. [Google Scholar] [CrossRef]
- Zou, J.; Chen, Z.; Liang, C.; Fu, Y.; Wei, X.; Lu, J.; Pan, M.; Guo, Y.; Liao, X.; Xie, H. Trefoil factor 3, cholinesterase and homocysteine: Potential predictors for Parkinson’s disease dementia and vascular parkinsonism dementia in advanced stage. Aging Dis. 2018, 9, 51. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, G.A. Matrix metalloproteinase-mediated Neuroinflammation in vascular cognitive impairment of the Binswanger type. Cell. Mol. Neurobiol. 2016, 36, 195–202. [Google Scholar] [CrossRef]
- Saleh, H.M.; El-Sayed, Y.S.; Naser, S.M.; Eltahawy, A.S.; Onoda, A.; Umezawa, M. Efficacy of alpha-lipoic acid against cadmium toxicity on metal ion and oxidative imbalance, and expression of metallothionein and antioxidant genes in rabbit brain. Environ. Sci. Pollut. Res. Int. 2017, 24, 24593–24601. [Google Scholar] [CrossRef]
- Pulido, G.; Trevino, S.; Brambila, E.; Vazquez-Roque, R.; Moreno-Rodriguez, A.; Pena Rosas, U.; Moran-Perales, J.L.; Handal Silva, A.; Guevara, J.; Flores, G.; et al. The Administration of Cadmium for 2, 3 and 4 Months Causes a Loss of Recognition Memory, Promotes Neuronal Hypotrophy and Apoptosis in the Hippocampus of Rats. Neurochem. Res. 2019, 44, 485–497. [Google Scholar] [CrossRef]
- Yang, X.F.; Fan, G.Y.; Liu, D.Y.; Zhang, H.T.; Xu, Z.Y.; Ge, Y.M.; Wang, Z.L. Effect of cadmium exposure on the histopathology of cerebral cortex in juvenile mice. Biol. Trace Elem. Res. 2015, 165, 167–172. [Google Scholar] [CrossRef]
- Lopez, E.; Figueroa, S.; Oset-Gasque, M.J.; Gonzalez, M.P. Apoptosis and necrosis: Two distinct events induced by cadmium in cortical neurons in culture. Br. J. Pharmacol. 2003, 138, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Bian, J.C.; Zhong, L.X.; Zhang, Y.; Sun, Y.; Liu, Z.P. Oxidative stress and apoptotic changes of rat cerebral cortical neurons exposed to cadmium in vitro. Biomed. Environ. Sci. 2012, 25, 172–181. [Google Scholar] [PubMed]
- Zhang, Y.M.; Liu, X.Z.; Lu, H.; Mei, L.; Liu, Z.P. Lipid peroxidation and ultrastructural modifications in brain after perinatal exposure to lead and/or cadmium in rat pups. Biomed. Environ. Sci. 2009, 22, 423–429. [Google Scholar] [CrossRef]
- Ibiwoye, M.O.; Matthews, Q.; Travers, K.; Foster, J.D. Association of Acute, High-dose Cadmium Exposure with Alterations in Vascular Endothelial Barrier Antigen Expression and Astrocyte Morphology in the Developing Rat Central Nervous System. J. Comp. Pathol. 2019, 172, 37–47. [Google Scholar] [CrossRef]
- Wei, X.; Qi, Y.; Zhang, X.; Gu, X.; Cai, H.; Yang, J.; Zhang, Y. ROS act as an upstream signal to mediate cadmium-induced mitophagy in mouse brain. Neurotoxicology 2015, 46, 19–24. [Google Scholar] [CrossRef]
- Valois, A.A.; Webster, W.S. The choroid plexus and cerebral vasculature as target sites for cadmium following acute exposure in neonatal and adult mice: An autoradiographic and gamma counting study. J. Toxicol. 1987, 46, 43–55. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Abel, G.M.; Storm, D.R.; Xia, Z. Cadmium exposure impairs cognition and olfactory memory in male C57BL/6 mice. Toxicol. Sci. 2018, 161, 87–102. [Google Scholar] [CrossRef] [Green Version]
- Pm, M.M.; Shahi, M.H.; Tayyab, M.; Farheen, S.; Khanam, N.; Tabassum, S.; Ali, A. Cadmium-induced neurodegeneration and activation of noncanonical sonic hedgehog pathway in rat cerebellum. J. Biochem. Mol. Toxicol. 2019, 33, e22274. [Google Scholar]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020. [Google Scholar] [CrossRef]
- Tafuri, S.; Cocchia, N.; Landolfi, F.; Iorio, E.L.; Ciani, F. Redoxomics and oxidative stress: From the basic research to the clinical practice. Free Rad. Dis. 2016, 149–169. [Google Scholar] [CrossRef] [Green Version]
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Mechanisms of sporadic cerebral small vessel disease: Insights from neuroimaging. Lancet Neurol. 2013, 12, 483–497. [Google Scholar] [CrossRef] [Green Version]
- Valko, M.; Morris, H.; Cronin, M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahid, M.; Pourrut, B.; Dumat, C.; Nadeem, M.; Aslam, M.; Pinelli, E. Heavy-metal-induced reactive oxygen species: Phytotoxicity and physicochemical changes in plants. Rev. Environ. Contam. Toxicol. 2014, 232, 1–44. [Google Scholar] [PubMed]
- Forstermann, U. Nitric oxide and oxidative stress in vascular disease. Pflugers Arch. 2010, 459, 923–939. [Google Scholar] [CrossRef] [PubMed]
- Meza, C.A.; La Favor, J.D.; Kim, D.-H.; Hickner, R.C. Endothelial Dysfunction: Is There a Hyperglycemia-Induced Imbalance of NOX and NOS? Int. J. Mol. Sci. 2019, 20, 3775. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Ray, P.E.; Short, B.L. NF-kappaB activation plays a role in superoxide-mediated cerebral endothelial dysfunction after hypoxia/reoxygenation. Stroke 2005, 36, 1047–1052. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Cao, Y.; Ma, L.; Pei, H.; Rausch, W.D.; Li, H. Dysfunction of Cerebrovascular Endothelial Cells: Prelude to Vascular Dementia. Front. Aging Neurosci. 2018, 10, 376. [Google Scholar] [CrossRef] [Green Version]
- Gavard, J.; Gutkind, J.S. Protein kinase C-related kinase and ROCK are required for thrombin-induced endothelial cell permeability downstream from Galpha12/13 and Galpha11/q. J. Biol. Chem. 2008, 283, 29888–29896. [Google Scholar] [CrossRef] [Green Version]
- Yao, L.; Romero, M.J.; Toque, H.A.; Yang, G.; Caldwell, R.B.; Caldwell, R.W. The role of RhoA/Rho kinase pathway in endothelial dysfunction. J. Cardiovasc. Dis. Res. 2010, 1, 165–170. [Google Scholar]
- Romero, M.J.; Platt, D.H.; Tawfik, H.E.; Labazi, M.; El-Remessy, A.B.; Bartoli, M.; Caldwell, R.B.; Caldwell, R.W. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ. Res. 2008, 102, 95–102. [Google Scholar] [CrossRef]
- Brown, D.I.; Griendling, K.K. Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Circ. Res. 2015, 116, 531–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawamoto, R.; Ninomiyax, D.; Kusunoki, T.; Kasai, Y.; Ohtsuka, N.; Kumagi, T. Oxidative stress is associated with increased arterial stiffness in middle-aged and elderly community-dwelling persons. J. Clin. Gerontol. Geriatr. 2016, 7, 136–140. [Google Scholar] [CrossRef] [Green Version]
- Tejovathi, B.; Suchitra, M.M.; Suresh, V.; Reddy, V.S.; Sachan, A.; Srinivas Rao, P.V.; Bitla, A.R. Association of lipid peroxidation with endothelial dysfunction in patients with overt hypothyroidism. Exp. Clin. Endocrinol. Diabetes 2013, 121, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Houston, M.C. The role of mercury and cadmium heavy metals in vascular disease, hypertension, coronary heart disease, and myocardial infarction. Altern. Ther. Health Med. 2007, 13, S128–S133. [Google Scholar] [PubMed]
- Tang, L.; Su, J.; Liang, P. Modeling cadmium-induced endothelial toxicity using human pluripotent stem cell-derived endothelial cells. Sci. Rep. 2017, 7, 14811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sena, C.M.; Pereira, A.M.; Seica, R. Endothelial dysfunction—A major mediator of diabetic vascular disease. Biochim. Biophys. Acta 2013, 1832, 2216–2231. [Google Scholar] [CrossRef] [Green Version]
- Campbell, N.R.; Lackland, D.T.; Lisheng, L.; Niebylski, M.L.; Nilsson, P.M.; Zhang, X.H. Using the Global Burden of Disease study to assist development of nation-specific fact sheets to promote prevention and control of hypertension and reduction in dietary salt: A resource from the World Hypertension League. J. Clin. Hypertens. 2015, 17, 165–167. [Google Scholar] [CrossRef]
- Chrissobolis, S.; Miller, A.A.; Drummond, G.R.; Kemp-Harper, B.K.; Sobey, C.G. Oxidative stress and endothelial dysfunction in cerebrovascular disease. Front. Biosci. 2011, 16, 1733–1745. [Google Scholar] [CrossRef]
- Sena, C.M.; Leandro, A.; Azul, L.; Seiça, R.; Perry, G. Vascular Oxidative Stress: Impact and Therapeutic Approaches. Front. Physiol. 2018, 9, 1668. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Xia, S.; Kalionis, B.; Wan, W.; Sun, T. The role of oxidative stress and inflammation in cardiovascular aging. BioMed. Res. Int. 2014, 2014, 615312. [Google Scholar] [CrossRef]
- Laroux, F.S. Mechanisms of inflammation: The good, the bad and the ugly. Front. Biosci. 2004, 9, 3156–3162. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaser, H.; Dostert, C.; Mak, T.W.; Brenner, D. TNF and ROS Crosstalk in Inflammation. Trends Cell Biol. 2016, 26, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Barchowsky, A.; Dudek, E.J.; Treadwell, M.D.; Wetterhahn, K.E. Arsenic induces oxidant stress and NF-KB activation in cultured aortic endothelial cells. Free Radic. Biol. Med. 1996, 21, 783–790. [Google Scholar] [CrossRef]
- Kaji, T.; Suzuki, M.; Yamamoto, C.; Mishima, A.; Sakamoto, M.; Kozuka, H. Severe damage of cultured vascular endothelial cell monolayer after simultaneous exposure to cadmium and lead. Arch. Environ. Contam. Toxicol. 1995, 28, 168–172. [Google Scholar] [CrossRef]
- Tsai, S.H.; Liang, Y.C.; Chen, L.; Ho, F.M.; Hsieh, M.S.; Lin, J.K. Arsenite stimulates cyclooxygenase-2 expression through activating IkappaB kinase and nuclear factor kappaB in primary and ECV304 endothelial cells. J. Cell. Biochem. 2002, 84, 750–758. [Google Scholar] [CrossRef]
- Wei, M.; Liu, J.; Xu, M.; Rui, D.; Xu, S.; Feng, G.; Ding, Y.; Li, S.; Guo, S. Divergent Effects of Arsenic on NF-kappaB Signaling in Different Cells or Tissues: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2016, 13, 163. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Park, Y.; Wu, J.; Chen, X.p.; Lee, S.; Yang, J.; Dellsperger, K.C.; Zhang, C. Role of TNF-alpha in vascular dysfunction. Clin. Sci. 2009, 116, 219–230. [Google Scholar] [CrossRef] [Green Version]
- Shoamanesh, A.; Preis, S.R.; Beiser, A.S.; Vasan, R.S.; Benjamin, E.J.; Kase, C.S.; Wolf, P.A.; DeCarli, C.; Romero, J.R.; Seshadri, S. Inflammatory biomarkers, cerebral microbleeds, and small vessel disease: Framingham Heart Study. J. Neurol. 2015, 84, 825–832. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, G.A. Inflammation and white matter damage in vascular cognitive impairment. Stroke 2009, 40, S20–S23. [Google Scholar] [CrossRef] [Green Version]
- Topakian, R.; Barrick, T.R.; Howe, F.A.; Markus, H.S. Blood-brain barrier permeability is increased in normal-appearing white matter in patients with lacunar stroke and leucoaraiosis. J. Neurol. Neurosurg. Psychiatry 2010, 81, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Candelario-Jalil, E.; Thompson, J.; Taheri, S.; Grossetete, M.; Adair, J.C.; Edmonds, E.; Prestopnik, J.; Wills, J.; Rosenberg, G.A. Matrix metalloproteinases are associated with increased blood-brain barrier opening in vascular cognitive impairment. Stroke 2011, 42, 1345–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Zhu, W.; Yun, W.; Wang, Q.; Cheng, M.; Zhang, Z.; Liu, X.; Zhou, X.; Xu, G. Correlation of matrix metalloproteinase-2 single nucleotide polymorphisms with the risk of small vessel disease (SVD). J. Neurol. Sci. 2015, 356, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Sui, G.; Zhou, Q.; Wang, C.; Lin, J.; Chai, Z.; Zhou, J. Variants in matrix metalloproteinase-9 gene are associated with hemorrhagic transformation in acute ischemic stroke patients with atherothrombosis, small artery disease, and cardioembolic stroke. Brain Behav. 2019, 9, e01294. [Google Scholar] [CrossRef] [Green Version]
- Heo, J.H.; Lucero, J.; Abumiya, T.; Koziol, J.A.; Copeland, B.R.; del Zoppo, G.J. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J. Cereb. Blood Flow Metab. 1999, 19, 624–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Estrada, E.Y.; Thompson, J.F.; Liu, W.; Rosenberg, G.A. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J. Cereb. Blood Flow Metab. 2007, 27, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Chandler, S.; Coates, R.; Gearing, A.; Lury, J.; Wells, G.; Bone, E. Matrix metalloproteinases degrade myelin basic protein. Neurosci. Lett. 1995, 201, 223–226. [Google Scholar] [CrossRef]
- Candelario-Jalil, E.; Yang, Y.; Rosenberg, G.A. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience 2009, 158, 983–994. [Google Scholar] [CrossRef] [Green Version]
- Hill, J.W.; Nemoto, E.M. Matrix-derived inflammatory mediator N-acetyl proline-glycine-proline is neurotoxic and upregulated in brain after ischemic stroke. J. Neuroinflamm. 2015, 12, 214. [Google Scholar] [CrossRef] [Green Version]
- Gerwien, H.; Hermann, S.; Zhang, X.; Korpos, E.; Song, J.; Kopka, K.; Faust, A.; Wenning, C.; Gross, C.C.; Honold, L.; et al. Imaging matrix metalloproteinase activity in multiple sclerosis as a specific marker of leukocyte penetration of the blood-brain barrier. Sci. Transl. Med. 2016, 8, 364ra152. [Google Scholar] [CrossRef]
- Boroujerdi, A.; Welser-Alves, J.V.; Milner, R. Matrix metalloproteinase-9 mediates post-hypoxic vascular pruning of cerebral blood vessels by degrading laminin and claudin-5. Angiogenesis 2015, 18, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Brilha, S.; Ong, C.W.M.; Weksler, B.; Romero, N.; Couraud, P.O.; Friedland, J.S. Matrix metalloproteinase-9 activity and a downregulated Hedgehog pathway impair blood-brain barrier function in an in vitro model of CNS tuberculosis. Sci. Rep. 2017, 7, 16031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.T.; Zhang, P.; Gao, Y.; Li, C.L.; Wang, H.J.; Chen, L.C.; Feng, Y.; Li, R.Y.; Li, Y.L.; Jiang, C.L. Early VEGF inhibition attenuates blood-brain barrier disruption in ischemic rat brains by regulating the expression of MMPs. Mol. Med. Rep. 2017, 15, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egashira, Y.; Zhao, H.; Hua, Y.; Keep, R.F.; Xi, G. White Matter Injury After Subarachnoid Hemorrhage: Role of Blood-Brain Barrier Disruption and Matrix Metalloproteinase-9. Stroke 2015, 46, 2909–2915. [Google Scholar] [CrossRef] [Green Version]
- Petty, G.W.; Brown, R.D., Jr.; Whisnant, J.P.; Sicks, J.D.; O’Fallon, W.M.; Wiebers, D.O. Ischemic stroke subtypes: A population-based study of functional outcome, survival, and recurrence. Stroke 2000, 31, 1062–1068. [Google Scholar] [CrossRef] [Green Version]
- Rempe, R.G.; Hartz, A.M.S.; Soldner, E.L.B.; Sokola, B.S.; Alluri, S.R.; Abner, E.L.; Kryscio, R.J.; Pekcec, A.; Schlichtiger, J.; Bauer, B. Matrix Metalloproteinase-Mediated Blood-Brain Barrier Dysfunction in Epilepsy. J. Neurosci. 2018, 38, 4301–4315. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.; Zhao, X.; Chen, H.; Zhong, D.; Jin, J.; Qin, Q.; Zhang, H.; Ma, S.; Li, G. beta-Dystroglycan cleavage by matrix metalloproteinase-2/-9 disturbs aquaporin-4 polarization and influences brain edema in acute cerebral ischemia. Neuroscience 2016, 326, 141–157. [Google Scholar] [CrossRef]
- Qiu, G.P.; Xu, J.; Zhuo, F.; Sun, S.Q.; Liu, H.; Yang, M.; Huang, J.; Lu, W.T.; Huang, S.Q. Loss of AQP4 polarized localization with loss of beta-dystroglycan immunoreactivity may induce brain edema following intracerebral hemorrhage. Neurosci. Lett. 2015, 588, 42–48. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, Y.; Li, P.; Jiang, A.; Sheng, X.; Jin, X.; Shi, Y.; Li, G. Matrix Metalloproteases-Mediated Cleavage on beta-Dystroglycan May Play a Key Role in the Blood-Brain Barrier After Intracerebral Hemorrhage in Rats. Med. Sci. Monit. 2019, 25, 794–800. [Google Scholar] [CrossRef]
- Kato, J.; Hayashi, M.K.; Aizu, S.; Yukutake, Y.; Takeda, J.; Yasui, M. A general anaesthetic propofol inhibits aquaporin-4 in the presence of Zn(2)(+). Biochem. J. 2013, 454, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Rao, K.V.; Jayakumar, A.R.; Reddy, P.V.; Tong, X.; Curtis, K.M.; Norenberg, M.D. Aquaporin-4 in manganese-treated cultured astrocytes. Glia 2010, 58, 1490–1499. [Google Scholar] [PubMed]
- Gunnarson, E.; Axehult, G.; Baturina, G.; Zelenin, S.; Zelenina, M.; Aperia, A. Lead induces increased water permeability in astrocytes expressing aquaporin 4. Neuroscience 2005, 136, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Vella, J.; Zammit, C.; Di Giovanni, G.; Muscat, R.; Valentino, M. The central role of aquaporins in the pathophysiology of ischemic stroke. Front. Cell Neurosci. 2015, 9, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhao, H.H.; Li, D.; Li, H.P. Neuroprotective effects of matrix metalloproteinases in cerebral ischemic rats by promoting activation and migration of astrocytes and microglia. Brain. Res. Bull. 2019, 146, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Chelluboina, B.; Klopfenstein, J.D.; Pinson, D.M.; Wang, D.Z.; Vemuganti, R.; Veeravalli, K.K. Matrix Metalloproteinase-12 Induces Blood-Brain Barrier Damage After Focal Cerebral Ischemia. Stroke 2015, 46, 3523–3531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.Y.; Choi, H.Y.; Ahn, H.J.; Ju, B.G.; Yune, T.Y. Matrix metalloproteinase-3 promotes early blood-spinal cord barrier disruption and hemorrhage and impairs long-term neurological recovery after spinal cord injury. Am. J. Pathol. 2014, 184, 2985–3000. [Google Scholar] [CrossRef]
- Rosenberg, G.A. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol. 2009, 8, 205–216. [Google Scholar] [CrossRef]
- Raffetto, J.D.; Khalil, R.A. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem. Pharmacol. 2008, 75, 346–359. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Sulaiman Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [Green Version]
- Flora, S. Role of free radicals and antioxidants in health and disease. Cell. Mol. Biol. 2007, 53, 1–2. [Google Scholar]
- Flora, S.J. Arsenic-induced oxidative stress and its reversibility. Free Radic. Biol. Med. 2011, 51, 257–281. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J. Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxid. Med. Cell Longev. 2009, 2, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Flora, S.J.S. Nutritional management can assist a significant role in alleviation of arsenicosis. J. Trace Elem. Med. Biol. 2018, 45, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Sodhi, S.; Mahajan, V. Correlation of antioxidants with lipid peroxidation and lipid profile in patients suffering from coronary artery disease. Expert Opin. Ther. Targets 2009, 13, 889–894. [Google Scholar] [CrossRef]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. Glutathione is a key player in metal-induced oxidative stress defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef] [Green Version]
- Rubino, F.M. Toxicity of Glutathione-Binding Metals: A Review of Targets and Mechanisms. Toxics 2015, 3, 20–62. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Aschner, M.; Ghersi-Egea, J.-F. Brain barrier systems: A new frontier in metal neurotoxicological research. Toxicol. Appl. Pharmacol. 2003, 192, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, H.; Kiyohara, Y.; Kato, I.; Kitazono, T.; Tanizaki, Y.; Kubo, M.; Ueno, H.; Ibayashi, S.; Fujishima, M.; Iida, M. Relationship between plasma glutathione levels and cardiovascular disease in a defined population: The Hisayama study. Stroke 2004, 35, 2072–2077. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, S.; Bhatnagar, P.; Flora, S.J.S. Changes in tissue oxidative stress, brain biogenic amines and acetylcholinesterase following co-exposure to lead, arsenic and mercury in rats. Food Chem. Toxicol. 2015, 86, 208–216. [Google Scholar] [CrossRef]
- Shukla, A.; Shukla, G.S.; Srimal, R. Cadmium-induced alterations in blood-brain barrier permeability and its possible correlation with decreased microvessel antioxidant potential in rat. Hum. Exp. Toxicol. 1996, 15, 400–405. [Google Scholar] [CrossRef]
- Chang, W.C.; Chen, S.-H.; Wu, H.-L.; Shi, G.-Y.; Murota, S.-i.; Morita, I. Cytoprotective effect of reduced glutathione in arsenical-induced endothelial cell injury. J. Toxicol. 1991, 69, 101–110. [Google Scholar] [CrossRef]
- Song, J.; Park, J.; Oh, Y.; Lee, J.E. Glutathione Suppresses Cerebral Infarct Volume and Cell Death after Ischemic Injury: Involvement of FOXO3 Inactivation and Bcl2 Expression. Oxid. Med. Cell. Longev. 2015, 2015, 426069. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J.S.; Pachauri, V. Chelation in metal intoxication. Int. J. Environ. Res. Public Health 2010, 7, 2745–2788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergsland, N.; Tavazzi, E.; Schweser, F.; Jakimovski, D.; Hagemeier, J.; Dwyer, M.G.; Zivadinov, R. Targeting Iron Dyshomeostasis for Treatment of Neurodegenerative Disorders. CNS Drugs 2019, 33, 1073–1086. [Google Scholar] [CrossRef]
- Baldari, S.; Di Rocco, G.; Toietta, G. Current Biomedical Use of Copper Chelation Therapy. Int. J. Mol. Sci. 2020, 21, 1069. [Google Scholar] [CrossRef] [Green Version]
- Flora, S.J.S.; Bhadauria, S.; Kannan, G.; Singh, N. Arsenic induced oxidative stress and the role of antioxidant supplementation during chelation: A review. J. Environ. Biol. 2007, 28, 333. [Google Scholar]
- Sidhu, M.S.; Saour, B.M.; Boden, W.E. A TACTful reappraisal of chelation therapy in cardiovascular disease. Nat. Rev. Cardiol. 2014, 11, 180. [Google Scholar] [CrossRef]
- Anderson, T.J.; Hubacek, J.; Wyse, D.G.; Knudtson, M.L. Effect of chelation therapy on endothelial function in patients with coronary artery disease: PATCH substudy. J. Am. Coll. Cardiol. 2003, 41, 420–425. [Google Scholar] [CrossRef] [Green Version]
- Seely, D.M.R.; Wu, P.; Mills, E.J. EDTA chelation therapy for cardiovascular disease: A systematic review. BMC Cardiovasc Disord. 2005, 5, 32. [Google Scholar] [CrossRef] [Green Version]
- Lamas, G.A.; Ergui, I. Chelation therapy to treat atherosclerosis, particularly in diabetes: Is it time to reconsider? Expert Rev. Cardiovasc. Ther. 2016, 14, 927–938. [Google Scholar] [CrossRef] [Green Version]
- Solenkova, N.V.; Newman, J.D.; Berger, J.S.; Thurston, G.; Hochman, J.S.; Lamas, G.A. Metal pollutants and cardiovascular disease: Mechanisms and consequences of exposure. Am. Heart J. 2014, 168, 812–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sompamit, K.; Kukongviriyapan, U.; Donpunha, W.; Nakmareong, S.; Kukongviriyapan, V. Reversal of cadmium-induced vascular dysfunction and oxidative stress by meso-2,3-dimercaptosuccinic acid in mice. Toxicol. Lett. 2010, 198, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J.S. Preventive and Therapeutic Strategies for Acute and Chronic Human Arsenic Exposure. In Arsenic in Drinking Water and Food; Springer: Berlin/Heidelberg, Germany, 2020; pp. 341–370. [Google Scholar]
- Satarug, S.; Moore, M.R. Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ. Health Persp. 2004, 112, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Blanchemanche, S.; Marette, S.; Roosen, J.; Verger, P. ‘Do not eat fish more than twice a week’. Rational choice regulation and risk communication: Uncertainty transfer from risk assessment to public. Health Risk Soc. 2010, 12, 271–292. [Google Scholar] [CrossRef]
- Remor, A.P.; Totti, C.C.; Moreira, D.A.; Dutra, G.P.; Heuser, V.D.; Boeira, J.M. Occupational exposure of farm workers to pesticides: Biochemical parameters and evaluation of genotoxicity. Environ. Internat. 2009, 35, 273–278. [Google Scholar] [CrossRef] [PubMed]



| System | Concentration | Exposure Duration | Toxicity | Ref. |
|---|---|---|---|---|
| In-vitro (hCMEC/D3 Cell line | 25–200 µM Pb | 48 h |
| [67] |
| In-vivo male Wistar rats | 200 mg/L lead acetate Drinking water | 3 Months |
| [75] |
| In-vitro RBE4 cell line | 10−5 M and 10−6 M lead acetate at | 2 h, 4 h, 8 h, 16 h, and 24 h |
| [76] |
| In-vitro epithelial Z310 cells | 5 and 10 μM Pb | Pre and post exposure |
| [77] |
| In-vivo Sprague-Dawley dams | 4% lead carbonate via feed | 5, 10, 15 days |
| [78] |
| In-vivo Male SD rats | 100, 200, 300 PPM/mL Drinking water | eight weeks |
| [79] |
| In-vivo Male SD rats | 50 mg/kg Pb acetate i.p., injection | 24 h |
| [80] |
| Primary culture brain microvessels isolated from 6-day-old rat pups | 1 μM lead | 0–60 min |
| [81] |
| In vitro C6 glia cells and ECV304 | 2.5, 5, 10 μM Pb | 6, 12, 24, 48 h |
| [82] |
| In- vivo Male Sprague–Dawley rats | 342 μg Pb/mL as Pb acetate | Once every other day 6 weeks |
| [83] |
| System | Concentration | Exposure Duration | Toxicity | Ref. |
|---|---|---|---|---|
| In-vitro rBMECs cells | 1.5–50 μg/mL (Cu nanoparticles) | 0–8 h |
| [94] |
| Primary culture (SPF Wistar neonate rats) Brain microvascular endothelial cells (BMECS) | 30–300 μM (Cucl2) | 12 h |
| [100] |
| In-vitro HUVEC, HMEC-L, and HIAEC cells | 10 to 50 μM (Cucl2) | 12 h |
| [101] |
| In-vivo Sprague Dawley rats | IP 50 mg/kg IV 30 mg/kg Cortical superfusion (20 µg/10 µL) (Cu nanoparticles) | 24 h |
| [102] |
| AD patients | - | - |
| [103] |
| In-vivo C57BL6 mice | 1 mg/L (Cucl2 + cholesterol As a risk factor) Drinking water | 4 weeks |
| [104] |
| In-vivo 3xTg-AD | 250 ppm Cu sulfate (CuSO4) Drinking water | 3 or 9 months |
| [105] |
| In-vivo Male Wistar rats | 10 µg/mL (Cucl2) | 1 h |
| [106] |
| In- vitro Bovine aortic endothelial cells (BAECs) | 0–500 μg/mL Cu2O | 12 h |
| [107] |
| In- vitro human aortic endothelial cells (HAECs) | 100 μM Cupric sulfate | 0–16 h |
| [108] |
| System | Concentration | Exposure Duration | Toxicity | Ref. |
|---|---|---|---|---|
| In-vivo normotensive Wistar rats | HgCl2 (first dose 4.6 μg/kg, subsequent dose 0.07 μg/kg/day, im to cover daily loss) | 30 days |
| [115] |
| In-vitro HUVECs | (1.0–5.0 microM) MeHg | 24 h |
| [125] |
| In-vitro Human brain micro-vascular cells | (1, 2, 3 µM MeHg) (2 µM HgCl2) | 24 h |
| [117] |
| In-vivo male Wistar rats | 20-ppm MeHg Drinking water | 4 weeks |
| [126] |
| In-vivo Sprague-Dawley rats | 1.0 mg/kg Mercuric bichloride Subcutaneous | 30 min, 1 h, 6 h, 12 h, 24 h, and 1 week after the mercury administration |
| [127] |
| In-vitro Human brain microvascular endothelial cells | (1, 2, 3, and 5 µM) MeHg | 24 h |
| [128] |
| In- vitro Bovine aortic endothelial cells (BAECs) | 1 μM MeHg | 1, 3 or 6 h, 24 h |
| [129] |
| In-vitro Human brain microvascular endothelial cells | 1, 2, 3 µM MeHg | 24 h |
| [119] |
| In-vitro PC12 cells | 0, 10, 100, 1000 nM HgCl2 | 48 h |
| [130] |
| System | Concentration | Exposure Duration | Toxicity | Ref. |
|---|---|---|---|---|
| Invitro HUVECs | 5 µM arsenic trioxide | 24 h |
| [164] |
| In vitro SVEC4-10 | 7.5 µM arsenic trioxide | 4–6 h |
| [165] |
| In vitro SVEC4-10 | 5 and 7.5 µM arsenic trioxide | 4–6 h |
| [166] |
| In vitro HAEC | 1, 10, 100, and 1000 ng/mL Arsenic trioxide | 5–72 h |
| [167] |
| In vitro HUVECs | 1–5 µM Sodium arsenite | 24 h |
| [168] |
| Invivo Kunming mice | 0.15 mg 1.5 mg 15 mg arsenic trioxide/L Drinking water | whole lactation period (postnatal day 42) |
| [169] |
| Invivo Wistar rats | 100 ppm Sodiumarsenite Drinking water | 60 days |
| [170] |
| Invivo Wistar rats | 4–5 mg/kg/ day arsenite Drinking water | Gestation, lactation and until 4 months of age |
| [154] |
| 54 arsenicosis patients | - | - |
| [171] |
| System | Concentration | ExposureDuration | Toxicity | Ref. |
|---|---|---|---|---|
| Zebrafish | 1 mg/L | 24 h and 96 h |
| [175] |
| Zebrafish embryos | 9 µM 1.0 mg/L | 24 h 7 days |
| [176] [177] |
| Mice | 3 mg/L Drinking water | 20 weeks |
| [192] |
| Rats | 3 mg/kg Orally | 28 days |
| [193] |
| Rats | 5 mg/kg bodyweight Orally | 21 days |
| [179] |
| Rabbits | CdCl2 3 mg/kg × bw Orally | 30 days |
| [183] |
| Rats | 32.5 ppm Drinking water | 2, 3- and 4-month |
| [184] |
| Juvenile mice | 3.74 mg/kg Orally | 10 days |
| [185] |
| Rats | 4 mg/kg bw i.p. Route | Single-dose |
| [189] |
| Compound Name | Abbreviation | Molecular Formula | Structure |
|---|---|---|---|
| Calcium Disodium Ethylenediamine Tetra acetic Acid | CaNa2EDTA | C10H12CaN2Na2O8 |  |
| British Anti-Lewisite or 2,3- Dimercaprol | BAL | C3H8OS2 |  |
| Tetrathiomolybdate | TM | MoS42- |  |
| D-Pencillamine | DPA | C5H11NO2S |  |
| meso-2,3-dimercaptosuccinic acid | DMSA | C4H6O4S2 |  |
| Sodium 2,3 Dimercaptopropane-l-Sulphonate | DMPS | C3H7NaO3S3 |  |
| Monoisoamyldimercaptosuccinic acid | MiADMSA | C9H16O4S2 |  |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patwa, J.; Flora, S.J.S. Heavy Metal-Induced Cerebral Small Vessel Disease: Insights into Molecular Mechanisms and Possible Reversal Strategies. Int. J. Mol. Sci. 2020, 21, 3862. https://doi.org/10.3390/ijms21113862
Patwa J, Flora SJS. Heavy Metal-Induced Cerebral Small Vessel Disease: Insights into Molecular Mechanisms and Possible Reversal Strategies. International Journal of Molecular Sciences. 2020; 21(11):3862. https://doi.org/10.3390/ijms21113862
Chicago/Turabian StylePatwa, Jayant, and Swaran Jeet Singh Flora. 2020. "Heavy Metal-Induced Cerebral Small Vessel Disease: Insights into Molecular Mechanisms and Possible Reversal Strategies" International Journal of Molecular Sciences 21, no. 11: 3862. https://doi.org/10.3390/ijms21113862
APA StylePatwa, J., & Flora, S. J. S. (2020). Heavy Metal-Induced Cerebral Small Vessel Disease: Insights into Molecular Mechanisms and Possible Reversal Strategies. International Journal of Molecular Sciences, 21(11), 3862. https://doi.org/10.3390/ijms21113862






