License to Kill: When iNKT Cells Are Granted the Use of Lethal Cytotoxicity
Abstract
1. iNKT Cell Activation
2. iNKT Cell Subsets
3. Immune Cell-Mediated Cytotoxicity Mechanisms
4. iNKT Cell Cytotoxicity in Response to Infections
5. iNKT Cell Cytotoxic Activity in Other Diseases
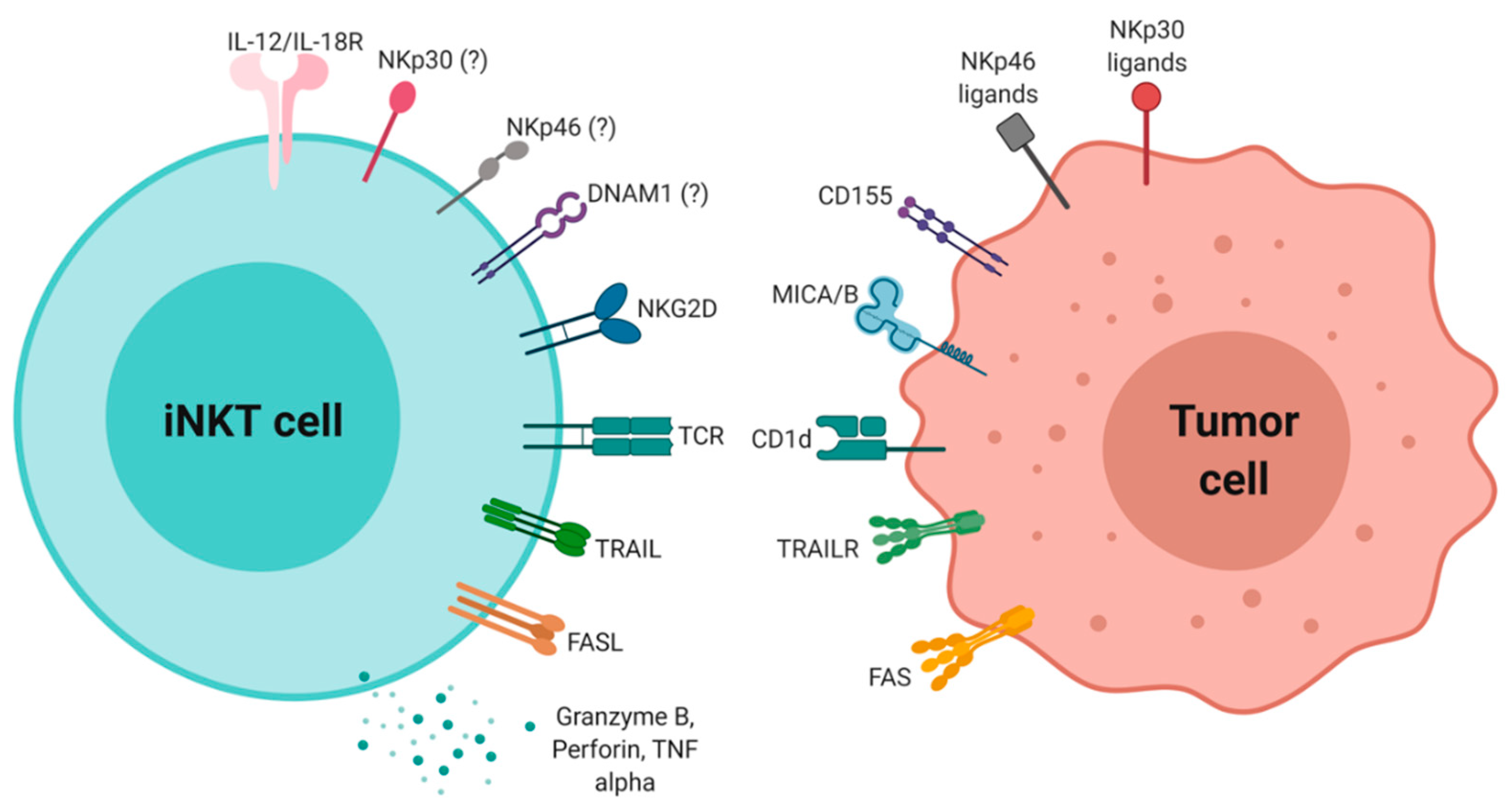
6. iNKT Cytotoxic Activity in Antitumor Immunity
7. iNKT Cell-Based Cancer Immunotherapies
8. Concluding Remarks
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| iNKT | invariant Natural Killer T cells |
| NKR | natural killer receptor |
| TCR | T cell receptor |
| NK | natural killer cells |
References
- Bendelac, A.; Savage, P.B.; Teyton, L. The Biology of NKT Cells. Annu. Rev. Microbiol. 2007, 25, 297–336. [Google Scholar] [CrossRef]
- Matsuda, J.L.; Mallevaey, T.; Scott-Browne, J.; Gapin, L. CD1d-restricted iNKT cells, the “Swiss-Army knife” of the immune system. Curr. Opin. Immunol. 2008, 20, 358–368. [Google Scholar] [CrossRef]
- Middendorp, S.; Nieuwenhuis, E.E.S. NKT cells in mucosal immunity. Mucosal Immunol. 2009, 2, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Crosby, C.M.; Kronenberg, M. specific functions of invariant natural killer T cells. Nat. Rev. Immunol. 2018, 18, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Krijgsman, D.; Hokland, M.; Kuppen, P.J.K. The role of natural killer T cells in cancer-A phenotypical and functional approach. Front. Immunol. 2018, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Gapin, L. Development of invariant natural killer T cells. Curr. Opin. Immunol. 2016, 39, 68–74. [Google Scholar] [CrossRef]
- Kinjo, Y.; Illarionov, P.; Vela, J.L.; Pei, B.; Girardi, E.; Li, X.; Li, Y.; Imamura, M.; Kaneko, Y.; Okawara, A.; et al. Invariant NKT cells recognize glycolipids from pathogenic Gram-positive bacteria. Nat. Immunol. 2012, 12, 966–974. [Google Scholar] [CrossRef]
- Kinjo, Y.; Tupin, E.; Wu, D.; Fujio, M.; Garcia-navarro, R.; Benhnia, M.R.; Zajonc, D.M.; Ben-menachem, G.; Ainge, G.D.; Painter, G.F.; et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. 2006, 7, 978–986. Nat. Immunol. 2006, 7, 978–986. [Google Scholar] [CrossRef]
- Hapil, F.Z.; Wingender, G. The interaction between invariant Natural Killer T cells and the mucosal microbiota. Immunology 2018, 155, 164–175. [Google Scholar] [CrossRef]
- Shimamura, M.; Kamijo, S.; Illarionov, P. Invariant natural killer T cells stimulated with cholesteryl glycosides modulate immune responses in allergy and delayed-type hypersensitivity. Eur. J. Immunol. 2019, 49, 348–350. [Google Scholar] [CrossRef]
- Fischer, K.; Scotet, E.; Niemeyer, M.; Koebernick, H.; Zerrahn, J.; Maillet, S.; Hurwitz, R.; Kursar, M.; Bonneville, M.; Kaufmann, S.H.E.; et al. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc. Natl. Acad. Sci. USA 2004, 101, 10685–10690. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.C.W.; Penaranda, C.; Kashyap, P.C.; Williams, B.B.; Clardy, J.; Kronenberg, M.; Sonnenburg, J.L.; Comstock, L.E.; Bluestone, J.A.; Fischbach, M.A. Production of a -Galactosylceramide by a Prominent Member of the Human Gut Microbiota. PLoS Biol. 2013, 11, e1001610. [Google Scholar]
- Liang, S.; Webb, T.; Li, Z. Probiotics Antigens Stimulate Hepatic NKT Cells. Hepatol. Res. 2013, 43, 139–146. [Google Scholar]
- Zajonc, D.M.; Girardi, E. Recognition of microbial glycolipids by Natural Killer T cells. Front. Immunol. 2015, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Facciotti, F.; Ramanjaneyulu, G.S.; Lepore, M.; Sansano, S.; Cavallari, M.; Kistowska, M.; Forss-petter, S.; Ni, G.; Colone, A.; Singhal, A.; et al. Peroxisome-derived lipids are self antigens that stimulate invariant natural killer T cells in the thymus. Nat. Immunol. 2012, 13, 474. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hogquist, K.A. How lipid-specific T cells become effectors: The differentiation of iNKT subsets. Front. Immunol. 2018, 9, 1450. [Google Scholar] [CrossRef]
- De Libero, G.; Moran, A.P.; Gober, H.J.; Rossy, E.; Shamshiev, A.; Chelnokova, O.; Mazorra, Z.; Vendetti, S.; Sacchi, A.; Prendergast, M.M.; et al. Bacterial infections promote T cell recognition of self-glycolipids. Immunity 2005, 22, 763–772. [Google Scholar] [CrossRef]
- Paget, C.; Deng, S.; Soulard, D.; Priestman, D.A.; Speca, S.; Von Gerichten, J.; Speak, A.O.; Saroha, A.; Pewzner-Jung, Y.; Futerman, A.H.; et al. TLR9-mediated dendritic cell activation uncovers mammalian ganglioside species with specific ceramide backbones that activate invariant natural killer t cells. PLoS Biol. 2019, 17, 1–26. [Google Scholar] [CrossRef]
- Brennan, P.J.; Tatituri, R.V.V.; Brigl, M.; Kim, E.Y.; Tuli, A.; Sanderson, J.P.; Gadola, S.D.; Hsu, F.; Besra, G.S.; Brenner, M.B. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat. Immunol. 2011, 12, 1202. [Google Scholar] [CrossRef]
- Mattner, J.; Debord, K.L.; Ismail, N.; Goff, R.D.; Cantu III, C.; Dapeng, Z.; Saint-Mezard, P.; Wang, V.; Gao, Y.; Yin, N.; et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 2005, 523, 525–530. [Google Scholar] [CrossRef]
- O’Keeffe, J.; Podbielska, M.; Hogan, E.L. Invariant natural killer T cells and their ligands: Focus on multiple sclerosis. Immunology 2015, 145, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Gapin, L. INKT cell autoreactivity: What is “self” and how is it recognized? Nat. Rev. Immunol. 2010, 10, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.; Chaidos, A.; Neville, D.C.A.; Poggi, A.; Butters, T.D.; Roberts, I.A.G.; Karadimitris, A. Human Invariant NKT Cells Display Alloreactivity Instructed by Invariant TCR-CD1d Interaction and Killer Ig Receptors. J. Immunol. 2008, 181, 3268–3276. [Google Scholar] [CrossRef] [PubMed]
- López-Larrea, C.; Suárez-Alvarez, B.; López-Soto, A.; López-Vázquez, A.; Gonzalez, S. The NKG2D receptor: sensing stressed cells. Trends Mol. Med. 2008, 14, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Kuylenstierna, C.; Björkström, N.K.; Andersson, S.K.; Sahlström, P.; Bosnjak, L.; Paquin-Proulx, D.; Malmberg, K.J.; Ljunggren, H.G.; Moll, M.; Sandberg, J.K. NKG2D performs two functions in invariant NKT cells: Direct TCR-independent activation of NK-like cytolysis and co-stimulation of activation by CD1d. Eur. J. Immunol. 2011, 41, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Takeda, K.; Kaneda, H.; Matsumoto, H.; Hayakawa, Y.; Raulet, D.H.; Ikarashi, Y.; Kronenberg, M.; Yagita, H.; Kinoshita, K.; et al. NKG2A Inhibits Invariant NKT Cell Activation in Hepatic Injury. J. Immunol. 2014, 182, 250–258. [Google Scholar] [CrossRef]
- Bedard, M.; Salio, M.; Cerundolo, V. Harnessing the power of invariant natural killer T cells in cancer immunotherapy. Front. Immunol. 2017, 8, 1829. [Google Scholar] [CrossRef]
- Dao, T.; Mehal, W.Z.; Crispe, I.N. IL-18 augments perforin-dependent cytotoxicity of liver NK-T cells. J. Immunol. 1998, 161, 2217–2222. [Google Scholar]
- Getz, G.S.; Reardon, C.A. Natural killer T cells in atherosclerosis. Nat. Rev. Cardiol. 2017, 14, 304–314. [Google Scholar] [CrossRef]
- Wang, Y.; Sedimbi, S.; Löfbom, L.; Singh, A.K.; Porcelli, S.A.; Cardell, S.L. Unique invariant natural killer T cells promote intestinal polyps by suppressing TH1 immunity and promoting regulatory T cells. Mucosal Immunol. 2018, 11, 131–143. [Google Scholar] [CrossRef]
- Engel, I.; Seumois, G.; Chavez, L.; Samaniego-Castruita, D.; White, B.; Chawla, A.; Mock, D.; Vijayanand, P.; Kronenberg, M. Innate-like functions of natural killer T cell subsets result from highly divergent gene programs. Nat. Immunol. 2016, 17, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Igney, F.H.; Krammer, P.H. Death and anti-death: tumour resistance to apoptosis. Nat. Rev. Cancer 2002, 2, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Huang, B.; Shi, J. Fas ligand and lytic granule differentially control cytotoxic dynamics of natural killer cell against cancer target. Oncotarget 2016, 7, 47163. [Google Scholar] [CrossRef] [PubMed]
- Peter, M.E.; Hadji, A.; Murmann, A.E.; Brockway, S.; Putzbach, W.; Pattanayak, A.; Ceppi, P. The role of CD95 and CD95 ligand in cancer. Cell Death Differ. 2015, 22, 549–559. [Google Scholar] [CrossRef]
- Golstein, P.; Griffiths, G.M. An early history of T cell-mediated cytotoxicity. Nat. Rev. Immunol. 2018, 18, 527–535. [Google Scholar] [CrossRef]
- Metelitsa, L.S.; Weinberg, K.I.; Emanuel, P.D.; Seeger, R.C. Expression of CD1d by myelomonocytic leukemias provides a target for cytotoxic NKT cells. Leukemia 2003, 17, 1068–1077. [Google Scholar] [CrossRef]
- Vucic, D.; Dixit, V.M.; Wertz, I.E. Ubiquitylation in apoptosis: A post-translational modification at the edge of life and death. Nat. Rev. Mol. Cell Biol. 2011, 12, 439–452. [Google Scholar] [CrossRef]
- Konishi, J.; Yamazaki, K.; Yokouchi, H.; Shinagawa, N.; Iwabuchi, K.; Nishimura, M. The characteristics of human NKT cells in lung cancer—CD1d independent cytotoxicity against lung cancer cells by NKT cells and decreased human NKT cell response in lung cancer patients. Hum. Immunol. 2004, 65, 1377–1388. [Google Scholar] [CrossRef]
- Anthony, D.; Andrews, D.; Watt, S.; Trapani, J.; Smyth, M. Functional dissection of the granzyme family: Cell death and inflammation. Immunol. Rev. 2010, 235, 73–92. [Google Scholar] [CrossRef]
- Voskoboinik, I.; Whisstock, J.C.; Trapani, J.A. Perforin and granzymes: Function, dysfunction and human pathology. Nat. Rev. Immunol. 2015, 15, 388–400. [Google Scholar] [CrossRef]
- Thiery, J.; Keefe, D.; Boulant, S.; Boucrot, E.; Walch, M.; Martinvalet, D.; Goping, I.S.; Bleackley, R.C.; Kirchhausen, T.; Lieberman, J. Perforin pores in the endosomal membrane trigger the release of endocytosed granzyme B into the cytosol of target cells. Nat. Immunol. 2011, 12, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Metkar, S.S.; Marchioretto, M.; Antonini, V.; Lunelli, L.; Wang, B.; Gilbert, R.J.; Anderluh, G.; Roth, R.; Pooga, M.; Pardo, J.; et al. Perforin oligomers form arcs in cellular membranes: A locus for intracellular delivery of granzymes. Cell Death Differ. 2015, 22, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Afonina, I.S.; Cullen, S.P.; Martin, S.J. Cytotoxic and non-cytotoxic roles of the CTL/NK protease granzyme B. Immunol. Rev. 2010, 235, 105–116. [Google Scholar] [CrossRef]
- Boivin, W.A.; Cooper, D.M.; Hiebert, P.R.; Granville, D.J. Intracellular versus extracellular granzyme B in immunity and disease: Challenging the dogma. Lab. Investig. 2009, 89, 1195–1220. [Google Scholar] [CrossRef]
- Trapani, J.A.; Bird, P.I. A Renaissance in Understanding the Multiple and Diverse Functions of Granzymes? Immunity 2008, 29, 665–667. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, D.; Lieberman, J. Death by a thousand cuts: granzyme pathways of programmed cell death. Annu. Rev. Immunol. 2008, 26, 389–420. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.; Bleackley, R.C. Cytotoxic T lymphocytes: all roads lead to death. Nat. Rev. Immunol. 2002, 2, 401–409. [Google Scholar] [CrossRef]
- Sparrow, E.; Bodman-Smith, M.D. Granulysin: The attractive side of a natural born killer. Immunol. Lett. 2020, 217, 126–132. [Google Scholar] [CrossRef]
- Walch, M.; Dotiwala, F.; Mulik, S.; Thiery, J.; Kirchhausen, T.; Clayberger, C.; Krensky, A.M.; Martinvalet, D.; Lieberman, J. Cytotoxic cells kill intracellular bacteria through granulysin-mediated delivery of granzymes. Cell 2014, 157, 1309–1323. [Google Scholar] [CrossRef]
- Van Kaer, L.; Parekh, V.V.; Wu, L. The response of CD1d-restricted invariant NKT cells to microbial pathogens and their products. Front. Immunol. 2015, 6, 1–11. [Google Scholar] [CrossRef]
- Kinjo, Y.; Takatsuka, S.; Kitano, N.; Kawakubo, S.; Abe, M.; Ueno, K.; Miyazaki, Y. Functions of CD1d-restricted invariant natural killer T cells in antimicrobial immunity and potential applications for infection control. Front. Immunol. 2018, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, N.A.; Kronenberg, M. Invariant NKT Cells Amplify the Innate Immune Response to Lipopolysaccharide. J. Immunol. 2007, 178, 2706–2713. [Google Scholar] [CrossRef] [PubMed]
- Brigl, M.; Tatituri, R.V.V.; Watts, G.F.M.; Bhowruth, V.; Leadbetter, E.A.; Barton, N.; Cohen, N.R.; Hsu, F.-F.; Besra, G.S.; Brenner, M.B. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J. Exp. Med. 2011, 208, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, K.L.; Tyznik, A.J.; Kronenberg, M.; Hogquist, K.A. Antigen-Dependent versus -Independent Activation of Invariant NKT Cells during Infection. J. Immunol. 2014, 192, 5490–5498. [Google Scholar] [CrossRef]
- Dowds, C.M.; Blumberg, R.S.; Zeissig, S. Control of intestinal homeostasis through crosstalk between natural killer T cells and the intestinal microbiota. Clin. Immunol. 2014, 159, 128–133. [Google Scholar] [CrossRef]
- Zeissig, S.; Blumberg, R.S. Commensal microbial regulation of natural killer T cells at the frontiers of the mucosal immune system. FEBS Lett. 2014, 588, 4188–4194. [Google Scholar] [CrossRef]
- Amprey, J.L.; Im, J.S.; Turco, S.J.; Murray, H.W.; Illarionov, P.A.; Besra, G.S.; Porcelli, S.A.; Späth, G.F. A subset of liver NK T cells is activated during Leishmania donovani infection by CD1d-bound lipophosphoglycan. J. Exp. Med. 2004, 200, 895–904. [Google Scholar] [CrossRef]
- Robert-Gangneux, F.; Drogoul, A.S.; Rostan, O.; Piquet-Pellorce, C.; Cayon, J.; Lisbonne, M.; Herbelin, A.; Gascan, H.; Guiguen, C.; Samson, M.; et al. Invariant NKT cells drive hepatic cytokinic microenvironment favoring efficient granuloma formation and early control of Leishmania donovani infection. PLoS ONE 2012, 7, e33413. [Google Scholar] [CrossRef]
- Karmakar, S.; Bhaumik, S.K.; Paul, J.; De, T. TLR4 and NKT cell synergy in immunotherapy against visceral leishmaniasis. PLoS Pathog. 2012, 8, e1002646. [Google Scholar] [CrossRef]
- Stanley, A.C.; Zhou, Y.; Amante, F.H.; Randall, L.M.; Haque, A.; Pellicci, D.G.; Hill, G.R.; Smyth, M.J.; Godfrey, D.I.; Engwerda, C.R. Activation of invariant NKT cells exacerbates experimental visceral leishmaniasis. PLoS Pathog. 2008, 4, e1000028. [Google Scholar] [CrossRef]
- Walker, D.M.; Oghumu, S.; Gupta, G.; Mcgwire, B.S.; Drew, M.E.; Satoskar, A.R. Mechanisms of cellular invasion by intracellular parasites Mechanisms of host cell invasion in Leishmania. Cell. Mol. Life Sci. 2014, 71, 1245–1263. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Hisaeda, H.; Taniguchi, M.; Nakayama, T.; Sakai, T.; Maekawa, Y.; Nakano, Y.; Zhang, M.; Zhang, T.; Nishitani, M.; et al. CD4+ Valpha14 NKT cells play a crucial role in an early stage of protective immunity against infection with Leishmania major. Int. Immunol. 2000, 12, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Campos-martín, Y.; Colmenares, M.; López-núñez, M.; Savage, P.B.; Martínez-naves, E.; Campos-martı, Y.; Gozalbo-lo, B.; Lo, M.; Savage, P.B.; Martı, E. Immature Human Dendritic Cells Infected with Leishmania infantum Are Resistant to NK-Mediated Cytolysis but Are Efficiently Recognized by NKT Cells. J. Immunol. 2006, 176, 6172–6179. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, D.C.; Ocampo, M.; Salazar, L.M.; Patarroyo, M.A. Quantifying intracellular Mycobacterium tuberculosis: An essential issue for in vitro assays. Microbiologyopen 2018, 7, e00588. [Google Scholar] [CrossRef] [PubMed]
- Sada-Ovalle, I.; Chiba, A.; Gonzales, A.; Brenner, M.B.; Behar, S.M. Innate invariant NKT cells recognize Mycobacterium tuberculosis-infected macrophages, produce interferon-γ, and kill intracellular bacteria. PLoS Pathog. 2008, 4, e1000239. [Google Scholar] [CrossRef] [PubMed]
- Rothchild, A.C.; Jayaraman, P.; Nunes-Alves, C.; Behar, S.M. iNKT Cell Production of GM-CSF Controls Mycobacterium tuberculosis. PLoS Pathog. 2014, 10, e1003805. [Google Scholar] [CrossRef]
- Gansert, J.L.; Kiebler, V.; Engele, M.; Wittke, F.; Röllinghoff, M.; Krensky, A.M.; Porcelli, S.A.; Modlin, R.L.; Stenger, S.; Gansert, J.L.; et al. Human NKT Cells Express Granulysin and Exhibit Antimycobacterial Activity. J. Immunol. 2003, 170, 3154–3161. [Google Scholar] [CrossRef]
- Walker, N.F.; Opondo, C.; Meintjes, G.; Jhilmeet, N.; Friedland, J.S.; Elkington, P.T.; Wilkinson, R.J.; Wilkinson, K.A. Invariant Natural Killer T-cell Dynamics in Human Immunodeficiency Virus-associated Tuberculosis. Clin. Infect. Dis. 2020, 70, 1865–1874. [Google Scholar] [CrossRef]
- Bessoles, S.; Dudal, S.; Besra, G.S.; Sanchez, F.; Lafont, V. Human CD4+ invariant NKT cells are involved in antibacterial immunity against Brucella suis through CD1d-dependent but CD4-independent mechanisms. Eur. J. Immunol. 2009, 39, 1025–1035. [Google Scholar] [CrossRef]
- Xiao, W.; Li, L.; Zhou, R.; Xiao, R.; Wang, Y.; Ji, X.; Wu, M.; Wang, L.; Huang, W.; Zheng, X.; et al. EBV-Induced human CD8+ NKT cells synergise CD4+ NKT cells suppressing EBV-associated tumours upon induction of Th1-bias. Cell. Mol. Immunol. 2009, 6, 367–379. [Google Scholar] [CrossRef]
- Yuling, H.; Ruijing, X.; Li, L.; Xiang, J.; Rui, Z.; Yujuan, W.; Lijun, Z.; Chunxian, D.; Tan, X.; Xiao, W.; et al. EBV-induced human CD8+ NKT cells suppress tumorigenesis by EBV-associated malignancies. Cancer Res. 2009, 69, 7935–7944. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.K.; Tsai, K.; Allan, L.L.; Zheng, D.J.; Nie, J.C.; Biggs, C.M.; Hasan, M.R.; Kozak, F.K.; Van Den Elzen, P.; Priatel, J.J.; et al. Innate immune control of EBV-infected B cells by invariant natural killer T cells. Blood 2013, 122, 2600–2608. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Z.; Du, X.; Liu, Y.; Song, X.; Wang, T.; Tan, S.; Liang, X.; Gao, L.; Ma, C. Tim-3 blockade promotes iNKT cell function to inhibit HBV replication. J. Cell. Mol. Med. 2018, 22, 3192–3201. [Google Scholar] [CrossRef] [PubMed]
- Renneson, J.; Guabiraba, R.; Maillet, I.; Marques, R.E.; Paget, C.; Quesniaux, V.; Faveeuw, C.; Ryffel, B.; Teixeira, M.M.; Trottein, F. A Detrimental Role for Invariant Natural Killer T Cells in the Pathogenesis of Experimental Dengue Virus Infection. Am. J. Pathol. 2011, 179, 1872–1883. [Google Scholar] [CrossRef]
- Shimizu, H.; Matsuguchi, T.; Fukuda, Y.; Nakano, I.; Hayakawa, T.; Takeuchi, O.; Akira, S.; Umemura, M.; Suda, T.; Yoshikai, Y. Toll-like receptor 2 contributes to liver injury by Salmonella infection through Fas ligand expression on NKT cells in mice. Gastroenterology 2002, 123, 1265–1277. [Google Scholar] [CrossRef]
- Chen, J.; Wei, Y.; He, J.; Cui, G.; Zhu, Y.; Lu, C.; Ding, Y.; Xue, R.; Bai, L.; Uede, T.; et al. Natural killer T cells play a necessary role in modulating of immune-mediated liver injury by gut microbiota. Sci. Rep. 2014, 4, 7259. [Google Scholar] [CrossRef]
- Lee, W.Y.; Sanz, M.J.; Wong, C.H.Y.; Hardy, P.O.; Salman-Dilgimen, A.; Moriarty, T.J.; Chaconas, G.; Marquesg, A.; Krawetz, R.; Mody, C.H.; et al. Invariant natural killer T cells act as an extravascular cytotoxic barrier for joint-invading Lyme Borrelia. Proc. Natl. Acad. Sci. USA 2014, 111, 13936–13941. [Google Scholar] [CrossRef]
- Lee, W.Y.; Moriarty, T.J.; Wong, C.H.Y.; Zhou, H.; Strieter, R.M.; Van Rooijen, N.; Chaconas, G.; Kubes, P. An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nat. Immunol. 2010, 11, 295–302. [Google Scholar] [CrossRef]
- Tupin, E.; Benhnia, M.R.E.I.; Kinjo, Y.; Patsey, R.; Lena, C.J.; Haller, M.C.; Caimano, M.J.; Imamura, M.; Wong, C.H.; Crotty, S.; et al. NKT cells prevent chronic joint inflammation after infection with Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 2008, 105, 19863–19868. [Google Scholar] [CrossRef]
- Tabas, I.; Lichtman, A.H. Monocyte-Macrophages and T Cells in Atherosclerosis. Immunity 2017, 47, 621–634. [Google Scholar] [CrossRef]
- Li, Y.; To, K.; Kanellakis, P.; Hosseini, H.; Deswaerte, V.; Tipping, P.; Smyth, M.J.; Toh, B.H.; Bobik, A.; Kyaw, T. CD4+ natural killer T cells potently augment aortic root atherosclerosis by perforin-and granzyme b-dependent cytotoxicity. Circ. Res. 2015, 116, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.D.; Vanichsarn, C.; Nadeau, K.C. Increased cytotoxicity of CD4+ invariant NKT cells against CD4+CD25hiCD127lo/- regulatory T cells in allergic asthma. Eur. J. Immunol. 2008, 38, 2034–2045. [Google Scholar] [CrossRef] [PubMed]
- Umetsu, D.; DeKruyff, R. Current Perspectives: focused commentary: Key cells in asthma. J. Allergy Clin. Immunol. 2010, 125, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Zissler, U.M.; Esser-Von Bieren, J.; Jakwerth, C.A.; Chaker, A.M.; Schmidt-Weber, C.B. Current and future biomarkers in allergic asthma. Allergy Eur. J. Allergy Clin. Immunol. 2016, 71, 475–494. [Google Scholar] [CrossRef]
- Krzywinska, E.; Stockmann, C. Hypoxia, metabolism and immune cell function. Biomedicines 2018, 6, 56. [Google Scholar] [CrossRef]
- Rundqvist, H.; Velica, P.; Barbieri, L.; Gameiro, P.; Cunha, P.P.; Gojkovic, M.; Bargiela, D.; Mijwel, S.; Ahlstedt, E.; Foskolou, I.; et al. Lactate Potentiates Differentiation and Expansion of Cytotoxic T Cells. SSRN Electron. J. 2019. [Google Scholar] [CrossRef]
- Renner, K.; Singer, K.; Koehl, G.E.; Geissler, E.K.; Peter, K.; Kreutz, M. Metabolic Hallmarks of Tumor and immune Cells in the Tumor Microenvironment. Front. Immunol. 2017, 8, 248. [Google Scholar] [CrossRef]
- Webb, T.J.; Carey, G.B.; East, J.E.; Sun, W.; Bollino, D.R.; Kimball, A.S.; Brutkiewicz, R.R. Alterations in cellular metabolism modulate CD1d-mediated NKT-cell responses. Pathog. Dis. 2016, 74, ftw055. [Google Scholar] [CrossRef]
- Zhang, J.; Han, C.; Dai, H.; Hou, J.; Dong, Y.; Cui, X.; Xu, L.; Zhang, M.; Xia, Q. Hypoxia-Inducible Factor-2α Limits Natural Killer T Cell Cytotoxicity in Renal Ischemia/Reperfusion Injury. J. Am. Soc. Nephrol. 2016, 27, 92–106. [Google Scholar] [CrossRef]
- Nair, S.; Dhodapkar, M.V. Natural killer T cells in cancer immunotherapy. Front. Immunol. 2017, 8, 1178. [Google Scholar] [CrossRef]
- Metelitsa, L.S. Anti-tumor potential of type-I NKT cells against CD1d-positive and CD1d-negative tumors in humans. Clin. Immunol. 2011, 140, 119–129. [Google Scholar] [CrossRef]
- Shissler, S.C.; Lee, M.S.; Webb, T.J. Mixed signals: Co-stimulation in invariant natural killer T cell-mediated cancer immunotherapy. Front. Immunol. 2017, 8, 1447. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.J.; Crowe, N.Y.; Hayakawa, Y.; Takeda, K.; Yagita, H.; Godfrey, D.I. NKT cells—conductors of tumor immunity ? Curr. Opin. Immunol. 2002, 14, 165–171. [Google Scholar] [CrossRef]
- Wolf, B.J.; Choi, J.E.; Exley, M.A. Novel approaches to exploiting invariant NKT cells in cancer immunotherapy. Front. Immunol. 2018, 9, 384. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cardell, S.L. The Yin and Yang of invariant Natural Killer T Cells in Tumor immunity — Suppression of Tumor immunity in the intestine. Front. Immunol. 2018, 8, 1945. [Google Scholar] [CrossRef] [PubMed]
- Altman, J.B.; Benavides, A.D.; Das, R.; Bassiri, H. Antitumor Responses of Invariant Natural Killer T Cells. J. Immunol. Res. 2015, 2015. [Google Scholar] [CrossRef]
- Wingender, G.; Krebs, P.; Beutler, B.; Kronenberg, M. Antigen-specific cytotoxicity by invariant NKT cells in vivo is CD95/CD178 dependent and is correlated with antigenic potency. J. Immunol. 2010, 185, 2721–2729. [Google Scholar] [CrossRef]
- Kawano, T.; Cui, J.; Koezuka, Y.; Toura, I.; Kaneko, Y.; Sato, H.; Kondo, E.; Harada, M.; Koseki, H.; Nakayama, T.; et al. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated V 14 NKT cells. Proc. Natl. Acad. Sci. USA 1998, 95, 5690–5693. [Google Scholar] [CrossRef]
- Weinkove, R.; Brooks, C.R.; Carter, J.M.; Hermans, I.F.; Ronchese, F. Functional invariant natural killer T-cell and CD1d axis in chronic lymphocytic leukemia: Implications for immunotherapy. Haematologica 2013, 98, 376–384. [Google Scholar] [CrossRef]
- Bassiri, H.; Das, R.; Guan, P.; Barrett, D.M.; Brennan, P.J.; Banerjee, P.P.; Wiener, S.J.; Orange, J.S.; Brenner, M.B.; Grupp, S.A.; et al. iNKT Cell Cytotoxic Responses Control T-Lymphoma Growth In Vitro and In Vivo. Cancer Immunol. Res. 2014, 2, 59–69. [Google Scholar] [CrossRef]
- Nicol, A.; Nieda, M.; Koezuka, Y.; Porcelli, S.; Suzuki, K.; Tadokoro, K.; Durrant, S.; Juji, T. Human invariant Vα24+ natural killer T cells activated by a-galactosylceramide (KRN7000) have cytotoxic anti-tumour activity through mechanisms distinct from T cells and natural killer cells. Immunology 2000, 99, 229–234. [Google Scholar] [CrossRef]
- Ghnewa, Y.G.; O’Reilly, V.P.; Vandenberghe, E.; Browne, P.V.; McElligott, A.M.; Doherty, D.G. Retinoic acid induction of CD1d expression primes chronic lymphocytic leukemia B cells for killing by CD8+ invariant natural killer T cells. Clin. Immunol. 2017, 183, 91–98. [Google Scholar] [CrossRef]
- Nieda, M.; Nicol, A.; Koezuka, Y.; Kikuchi, A.; Lapteva, N.; Tanaka, Y.; Tokunaga, K.; Suzuki, K.; Kayagaki, N.; Yagita, H.; et al. TRAIL expression by activated human CD4+Vα24NKT cells induces in vitro and in vivo apoptosis of human acute myeloid leukemia cells. Blood 2001, 97, 2067–2074. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T.; Nakayama, T.; Kamada, N.; Kaneko, Y.; Harada, M.; Ogura, N.; Akutsu, Y.; Motohashi, S.; Iizasa, T.; Endo, H.; et al. Antitumor Cytotoxicity Mediated by Ligand-activated Human Va24 NKT Cells. Cancer Res. 1999, 59, 5102–5105. [Google Scholar] [PubMed]
- Mattarollo, S.R.; Kenna, T.; Nieda, M.; Nicol, A.J. Chemotherapy pretreatment sensitizes solid tumor-derived cell lines to Vα24+ NKT cell-mediated cytotoxicity. Int. J. Cancer 2006, 119, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Wu, P.; Wu, X.; Zhang, T.; Zhang, T.; Wang, Z.; Zhang, S.; Qiu, F.; Huang, J. Thymosin alpha1 enhanced cytotoxicity of iNKT cells against colon cancer via upregulating CD1d expression. Cancer Lett. 2015, 356, 579–588. [Google Scholar] [CrossRef]
- Dockry, É.; O’Leary, S.; Gleeson, L.E.; Lyons, J.; Keane, J.; Gray, S.G.; Doherty, D.G. Epigenetic induction of CD1d expression primes lung cancer cells for killing by invariant natural killer T cells. Oncoimmunology 2018, 7, e1428156. [Google Scholar] [CrossRef]
- Fallarini, S.; Paoletti, T.; Orsi Battaglini, N.; Lombardi, G. Invariant NKT cells increase drug-induced osteosarcoma cell death. Br. J. Pharmacol. 2012, 167, 1533–1549. [Google Scholar] [CrossRef]
- Dhodapkar, K.M.; Cirignano, B.; Chamian, F.; Zagzag, D.; Miller, D.C.; Finlay, J.L.; Steinman, R.M. Invariant natural killer T cells are preserved in patients with glioma and exhibit antitumor lytic activity following dendritic cell-mediated expansion. Int. J. Cancer 2004, 109, 893–899. [Google Scholar] [CrossRef]
- Hix, L.M.; Shi, Y.H.; Brutkiewicz, R.R.; Stein, P.L.; Wang, C.R.; Zhang, M. CD1d-expressing breast cancer cells modulate NKT cell-mediated antitumor immunity in a murine model of breast cancer metastasis. PLoS ONE 2011, 6, e20702. [Google Scholar] [CrossRef]
- Tachibana, T.; Onodera, H.; Tsuruyama, T.; Mori, A.; Nagayama, S.; Hiai, H.; Imamura, M. Increased Intratumor V A 24-Positive Natural Killer T Cells: A Prognostic Factor for Primary Colorectal Carcinomas. Clin. Cancer Res. 2005, 11, 7322–7328. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.G.; Lipson, E.J.; Brahmer, J.R. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nat. Rev. Clin. Oncol. 2014, 11, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Seeger, R.C.; Metelitsa, L.S.; Song, L.; Asgharzadeh, S.; Salo, J.; Engell, K.; Wu, H.; Sposto, R. Va24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J. Clin. Invest. 2009, 119, 1524–1536. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, F.; Delfanti, G.; Grilli, A.; Calcinotto, A.; Gorini, F.; Pucci, F.; Lucianò, R.; Grioni, M.; Recchia, A.; Benigni, F.; et al. Bimodal CD40/Fas-Dependent Crosstalk between iNKT Cells and Tumor-Associated Macrophages Impairs Prostate Cancer Progression. Cell Rep. 2018, 22, 3006–3020. [Google Scholar] [CrossRef]
- Wensveen, F.M.; Jelenčić, V.; Polić, B. NKG2D: A master regulator of immune cell responsiveness. Front. Immunol. 2018, 9, 441. [Google Scholar] [CrossRef]
- Fujii, S.I.; Shimizu, K. Immune Networks and Therapeutic Targeting of iNKT Cells in Cancer. Trends Immunol. 2019, 40, 984–997. [Google Scholar] [CrossRef]
- Joshi, S.K.; Lang, M.L. Fine tuning a well-oiled machine: Influence of NK1.1 and NKG2D on NKT cell development and function. Int. Immunopharmacol. 2013, 17, 260–266. [Google Scholar] [CrossRef][Green Version]
- Barrow, A.D.; Martin, C.J.; Colonna, M. The natural cytotoxicity receptors in health and disease. Front. Immunol. 2019, 10, 909. [Google Scholar] [CrossRef]
- Gilfillan, S.; Chan, C.J.; Cella, M.; Haynes, N.M.; Rapaport, A.S.; Boles, K.S.; Andrews, D.M.; Smyth, M.J.; Colonna, M. DNAM-1 promotes activation of cytotoxic lymphocytes by nonprofessional antigen-presenting cells and tumors. J. Exp. Med. 2008, 205, 2965–2973. [Google Scholar] [CrossRef]
- Leite-de-Moraes, M.C.; Hameg, A.; Machavoine, F.; Koezuka, Y.; Herbelin, A.; Dy, M.; Schneider, E. A Distinct IL-18-Induced Pathway to Fully Activate NK T Lymphocytes Independently from TCR Engagement. J. Immunol. 1999, 163, 5871–5876. [Google Scholar]
- Bedard, M.; Shrestha, D.; Priestman, D.A.; Wang, Y.; Schneider, F.; Matute, J.D.; Iyer, S.S.; Gileadi, U.; Prota, G.; Kandasamy, M.; et al. Sterile activation of invariant natural killer T cells by ER-stressed antigen-presenting cells. Proc. Natl. Acad. Sci. USA 2019, 116, 23671–23681. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, S.; Verheugen, E.; Venken, K.; Gaublomme, D.; Maelegheer, M.; Cloots, E.; Gysens, F.; Geest, B.G.D.; Cheng, T.; Moody, D.B.; et al. ER stress in antigen-presenting cells promotes NKT cell activation through endogenous neutral lipids. EMBO Rep. 2020. [Google Scholar] [CrossRef] [PubMed]
- McEwen-Smith, R.M.; Salio, M.; Cerundolo, V. The regulatory role of invariant NKT Cells in tumor immunity. Cancer Immunol. Res. 2015, 3, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.A.; Seo, H.; Kim, I.K.; Jeon, I.; Kang, C.Y. Roles of NKT cells in cancer immunotherapy. Arch. Pharm. Res. 2019, 42, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; He, K.; Tian, C.; Sun, H.; Zhu, C.; Bai, S.; Liu, J.; Wu, Q.; Xie, D.; Yue, T.; et al. Impaired lipid biosynthesis hinders anti-tumor efficacy of intratumoral iNKT cells. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, B.; Botticelli, A.; Pierelli, L.; Nuti, M.; Alimandi, M. CAR-T with license to kill solid tumors in search of a winning strategy. Int. J. Mol. Sci. 2019, 20, 1903. [Google Scholar] [CrossRef]
- Martinez, M.; Moon, E.K. CAR T cells for solid tumors: New strategies for finding, infiltrating, and surviving in the tumor microenvironment. Front. Immunol. 2019, 10, 128. [Google Scholar] [CrossRef]


| Microorganism | Pathogenicity | Antigen |
|---|---|---|
| Arthrobacter | Commensal, opportunist | M-AcM-MAG |
| Aspergillus fumigatus and Aspergillus niger | Opportunists | Asperamide B |
| Bacteroides fragilis | Commensal, opportunist | α-GalCer (Bf) |
| Bacteroides vulgatus | Commensal, opportunist | α-GalCer |
| Borrelia burgdorferi | Pathogen | BbGL-II (1,2-di-O-acyl- 3-O-a Dgalactopyranosyl-sn-glycerol, 6) |
| Candida albicans | Commensal, opportunist | ChAcMan |
| Ehrlichia muris | Pathogen in rodents only | Not defined |
| Entamoeba histolytica | Opportunist | Lipopeptidophosphoglycan (EhLPPG) |
| Helicobacter pylori | Commensal, opportunist | Cholestoryl-a-glucosides, especially monoacyl a-CPG |
| Lactobacillus casei | Commensal | Glc-DAG |
| Leishmania donovani | Opportunist | Lipophosphoglycan (LPG) |
| Mycobacterium tuberculosis | Pathogen | Phosphatidylinositol mannoside (PIM) |
| Prevotella copri | Commensal | α-GalCer |
| Rothia dentocariosa | Commensal, opportunist | M-AcM-MAG |
| Saccharopolyspora | Environmental, opportunist | M-AcM-MAG |
| Sphingomonas paucimobilis | Commensal, opportunist | a-glucuronosyl ceramide (GSL-1/ aGlcUCer) |
| Sphingomonas yanoikuyae | Environmental, commensal, opportunist | a-galacturonosyl-ceramides |
| Sphingomonas wittichi | No pathogenicity reported | a-galacturonosyl-ceramides |
| Streptococcus pneumoniae and Group B Streptococcus | Commensal, opportunists | SPN-Glc-DAG, SPN-Gal-Glc-DAG |
| Disease | Role of iNKT Cell Cytotoxicity | Killing Mechanism | References |
|---|---|---|---|
| Leishmania infantum infection | Protective | Not addressed | [63] |
| Brucella suis infection | Protective | Fas ligand upregulation | [69] |
| Epstein-Barr virus infection | Protective | Infected cell killing by IFN gamma and TNF alpha production | [70,71,72] |
| Borrelia burgdoferi infection | Protective | Bacteria death by Granzyme B release | [77] |
| Mycobacterium tuberculosis infection | Protective | Infected cell and bacteria elimination by granulysin release | [67] |
| Hepatitis B virus infection | Protective | Elimination of infected cells by IFN gamma, TNF alpha production and cytotoxic granule release | [73] |
| Atherosclerosis | Pathogenic | Granzyme B and perforin release | [81] |
| Allergic asthma | Pathogenic | Increase in granzyme B and perforin. Killing of Tregs in vitro | [82] |
| Liver injury | Pathogenic | Hepatocyte cell death by Fas ligand upregulation, perforin and granzyme B release | [26,74,75,76] |
| Renal ischemia/reperfusion injury | Pathogenic | Fas ligand upregulation | [89] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Basabe, A.; Strati, F.; Facciotti, F. License to Kill: When iNKT Cells Are Granted the Use of Lethal Cytotoxicity. Int. J. Mol. Sci. 2020, 21, 3909. https://doi.org/10.3390/ijms21113909
Díaz-Basabe A, Strati F, Facciotti F. License to Kill: When iNKT Cells Are Granted the Use of Lethal Cytotoxicity. International Journal of Molecular Sciences. 2020; 21(11):3909. https://doi.org/10.3390/ijms21113909
Chicago/Turabian StyleDíaz-Basabe, Angélica, Francesco Strati, and Federica Facciotti. 2020. "License to Kill: When iNKT Cells Are Granted the Use of Lethal Cytotoxicity" International Journal of Molecular Sciences 21, no. 11: 3909. https://doi.org/10.3390/ijms21113909
APA StyleDíaz-Basabe, A., Strati, F., & Facciotti, F. (2020). License to Kill: When iNKT Cells Are Granted the Use of Lethal Cytotoxicity. International Journal of Molecular Sciences, 21(11), 3909. https://doi.org/10.3390/ijms21113909





