The Role of Pref-1 during Adipogenic Differentiation: An Overview of Suggested Mechanisms
Abstract
:1. Introduction
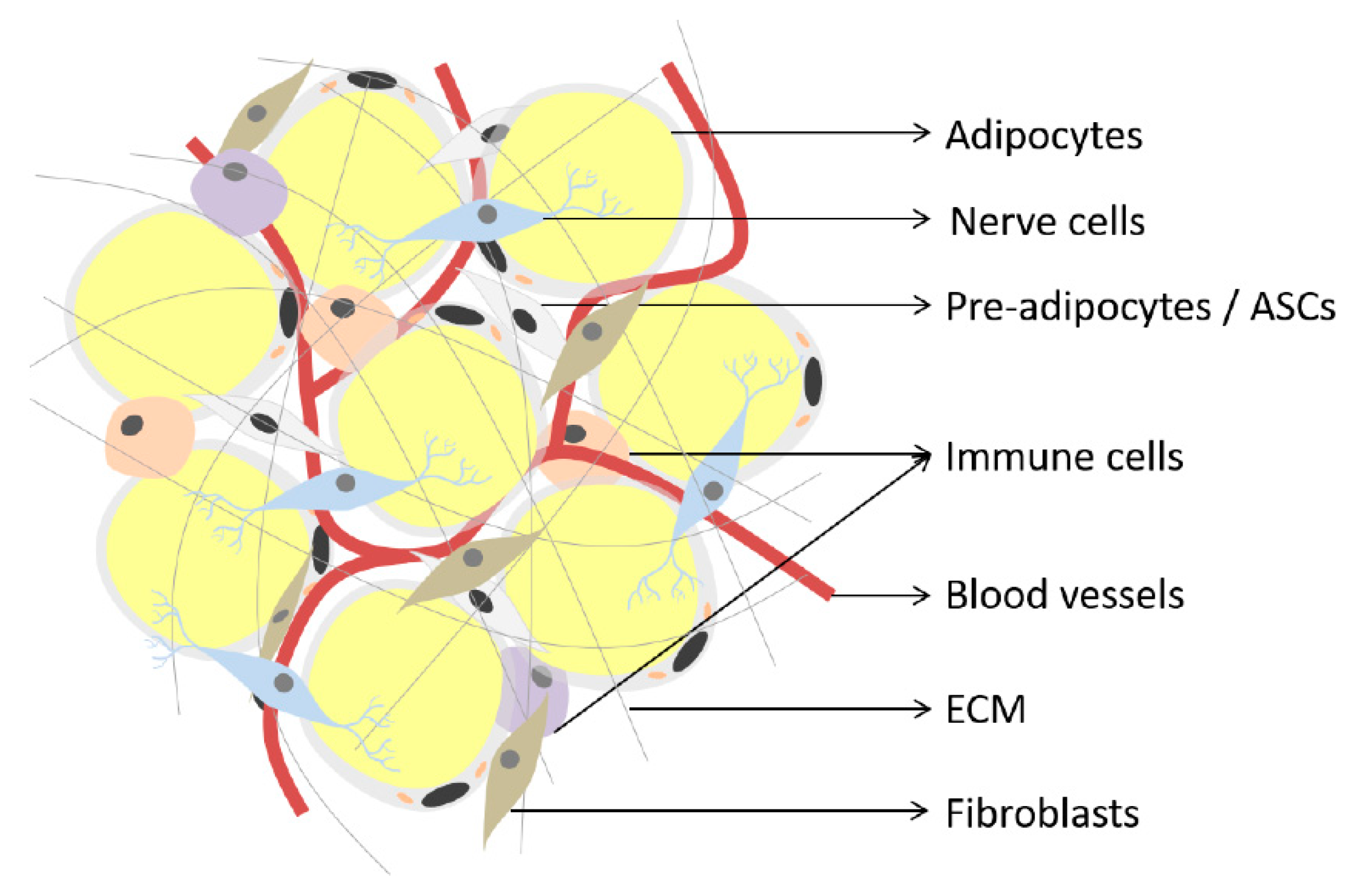
2. Adipose Tissue
3. Mesenchymal Stromal/Stem Cells
Adipogenic Differentiation Capacity of hASCs and hWJSCs
4. Adipogenesis
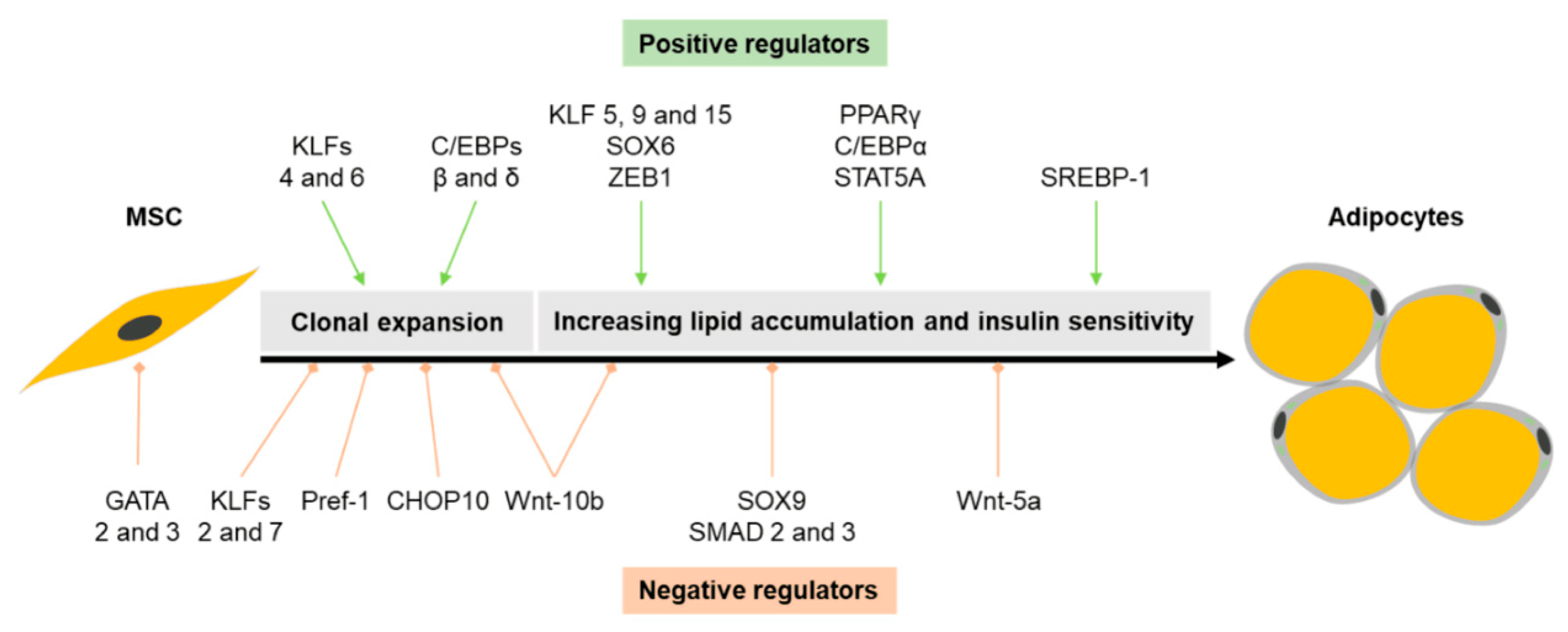
4.1. Transcriptional Regulation of White Adipogenesis
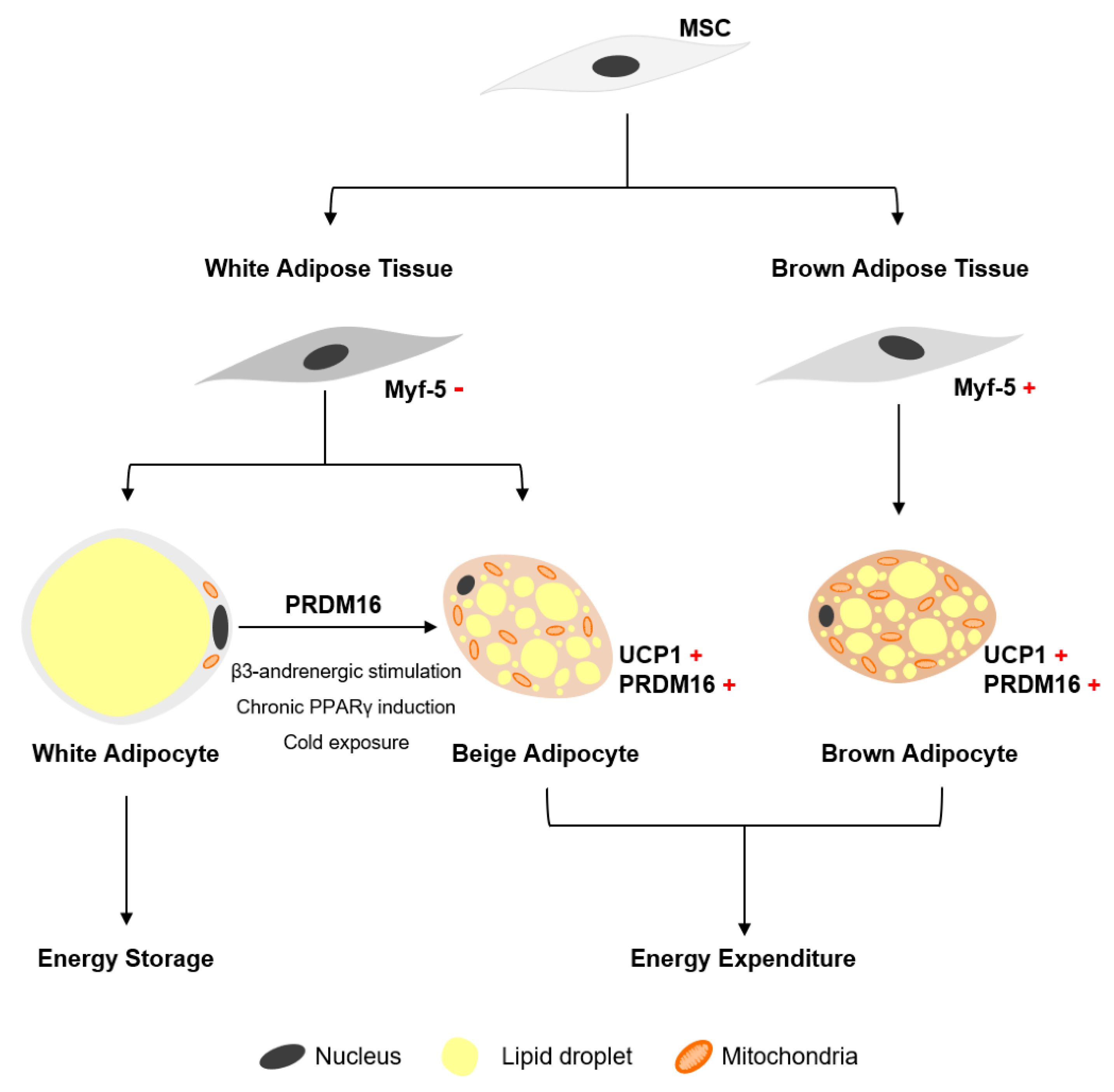
4.2. Transcriptional Regulation of Brown Adipogenesis
5. Preadipocyte Factor 1 (Pref-1)
5.1. Brief Overview on the Discovery of Pref-1
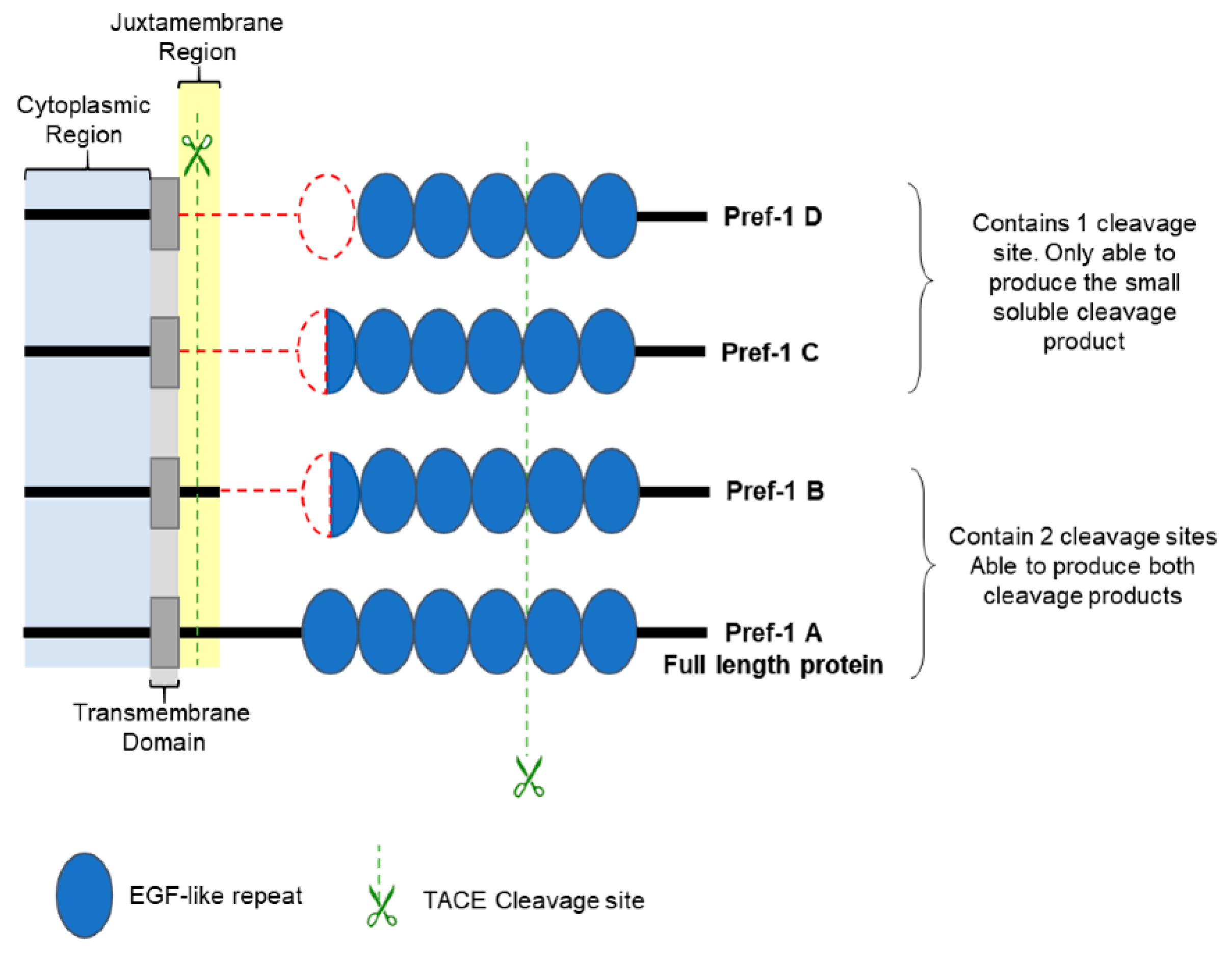
5.2. Pref-1 Structural Characteristics
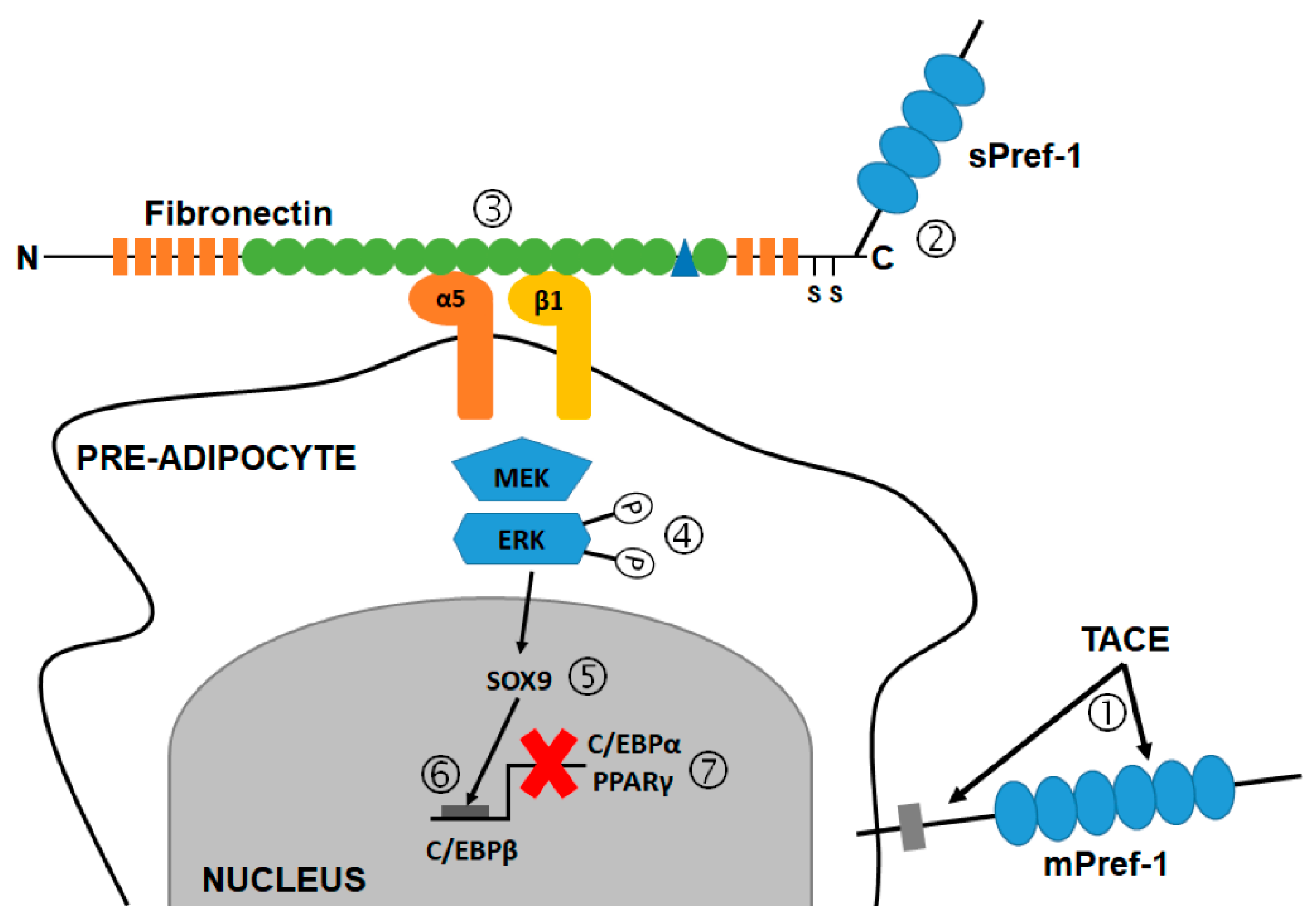
5.3. The Role of Pref-1 in Adipogenesis
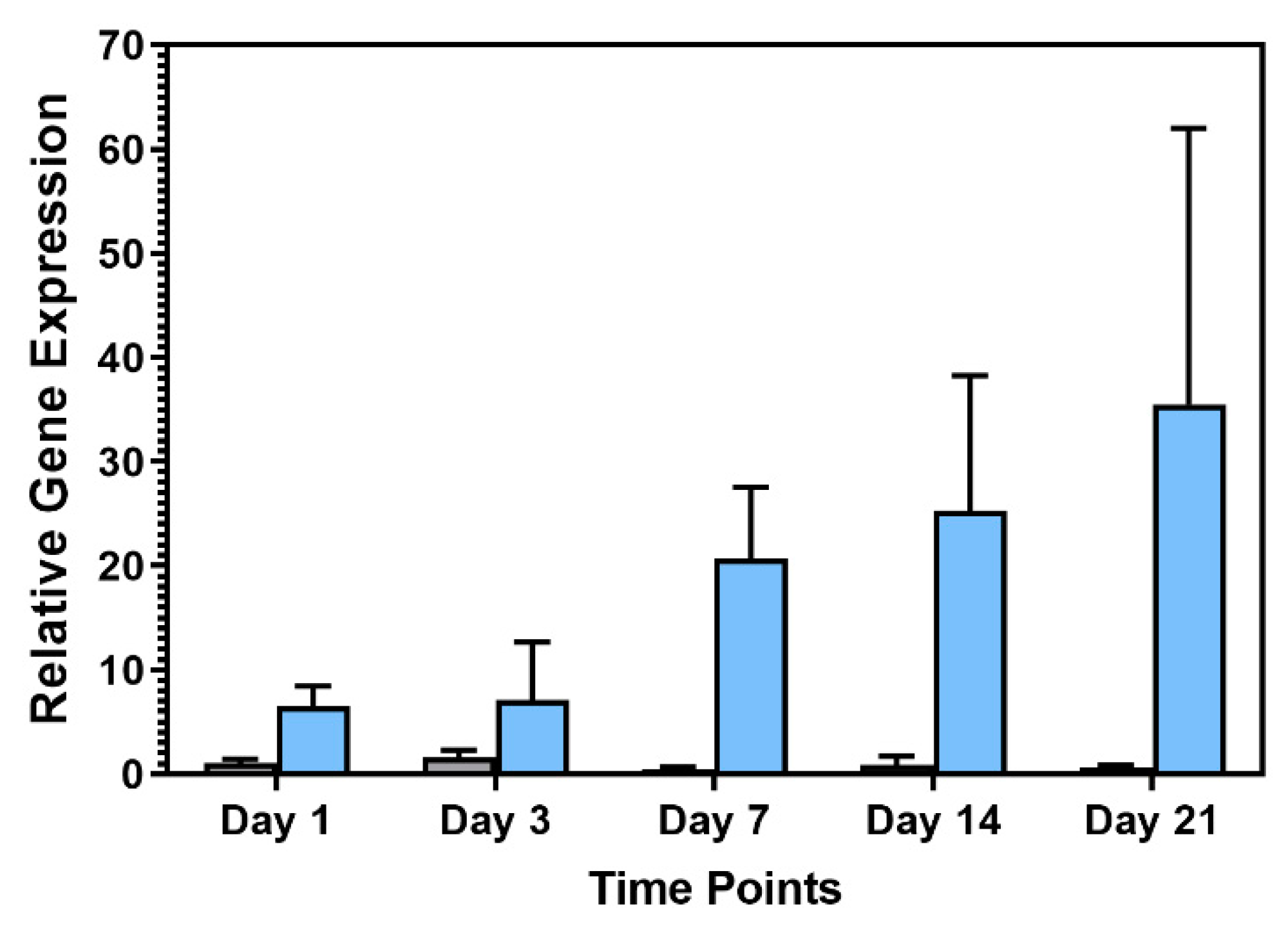
Pref-1 Expression during hASC and hWJSC Adipogenic Differentiation
6. Pref-1 Mechanism of Action
6.1. Pref-1 and the MAPK Kinase (MEK)/ERK Signaling Pathway
6.2. Pref-1 and the Notch Signaling Pathway
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | Amniotic fluid |
| AP-1 | Activating protein-1 |
| aP2 | Adipocyte protein 2 |
| ASCs | Adipose-derived stromal/stem cells |
| AT | Adipose tissue |
| BAT | Brown adipose tissue |
| BM | Bone marrow |
| BM-MSCs | Bone marrow-derived MSCs |
| BMP7 | Bone morphogenetic protein-7 |
| C/EBP | CAAT-enhancer binding proteins |
| cAMP | Cyclic Adenosine Monophosphate |
| CHOP | C/EBP homologous protein |
| cs | Cell surface |
| DLK | Delta-like |
| DLL | Delta-like ligands |
| DOS | Delta and OSM-11 |
| DP | Dental pulp |
| DSL | Delta-Serrate-LAG-2 |
| DXM | Dexamethasone |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| ERKs | Extracellular signal-regulated kinases |
| FA-1 | fetal antigen-1 |
| FABP4 | Fatty acid-binding protein 4 |
| FBS | Fetal bovine serum |
| Foxp1 | Foxhead P1 |
| hASCs | Human-derived ASCs |
| hBM-MSCs | Human-derived BM-MSCs |
| hWJSCs | Human-derived WJSCs |
| IBMX | 3-Isobutyl-1-methylxantine |
| IGF-1 | Insulin-like growth factor 1 |
| IRS-1 | Insulin receptor substrate 1 |
| JNKs | Jun amino-terminal kinases |
| kDa | kilo Dalton |
| KLFs | Krüppel-like factors |
| LDL | Low-density lipoprotein |
| MAPK | Mitogen-activated protein kinase |
| mASCs | Mouse ASCs |
| MEFs | Mouse embryo fibroblasts |
| MEK | Mitogen-activated protein kinase kinase |
| mPref-1 | Membrane bound Pref-1 |
| mRNA | Messenger ribonucleic acid |
| MSCs | Mesenchymal stromal/stem cells |
| Myf-5 | Myogenic factor 5 |
| nFS | Neonatal foreskin |
| PC | Placenta |
| PECK | Phosphoenolpyruvate carboxylase |
| PGC-1α | PPARγ co-activator-alpha |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| PRDM16 | PR domain containing 16 |
| Pref-1 | Preadipocyte factor-1 |
| PS | Periosteum |
| PV | Perivascular region |
| RT-qPCR | Reverse transcription-quantitative polymerase chain reaction |
| SAPKs | Stress-activated kinases |
| SF | Synovium fluid |
| SHED | Exfoliated deciduous teeth |
| SMAD | Mothers against decapentaplegic homolog |
| SOX | Sex determining region Y-box |
| SV | Synovium |
| sPref-1 | Soluble Pref-1 |
| SREBP-1 | Sterol regulatory element binding protein 1 |
| STAT | Signal transducer and activator of transcription |
| SVF | Stromal vascular fraction |
| TACE | Tumor necrosis factor alpha converting enzyme |
| UC | Umbilical cord |
| UCB | Umbilical cord blood |
| UCP1 | Uncoupling protein 1 |
| UTR | Untranslated region |
| WAT | White adipose tissue |
| WJSCs | Wharton’s jelly derived stromal/stem cells |
| Wnt | Wingless/integrated protein |
| ZEB1 | Zinc finger E-box binding homeobox 1 |
References
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Haider, N.; Larose, L. Harnessing adipogenesis to prevent obesity. Adipocyte 2019, 8, 98–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Sowers, J.R.; Ren, J. Targeting autophagy in obesity: From pathophysiology to management. Nat. Rev. Endocrinol. 2018, 14, 356–376. [Google Scholar] [CrossRef] [PubMed]
- Gadde, K.M.; Martin, C.K.; Berthoud, H.-R.; Heymsfield, S.B. Obesity: Pathophysiology and management. J. Am. Coll. Cardiol. 2018, 71, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Kotchen, T.A. Obesity-related hypertension: Epidemiology, pathophysiology, and clinical management. Am. J. Hypertens. 2010, 23, 1170–1178. [Google Scholar] [CrossRef]
- Baleta, A.; Mitchell, F. Country in focus: Diabetes and obesity in South Africa. Lancet Diabetes Endocrinol. 2014, 2, 687–688. [Google Scholar] [CrossRef]
- Tremmel, M.; Gerdtham, U.-G.; Nilsson, P.; Saha, S. Economic burden of obesity: A systematic literature review. Int. J. Environ. Res. Public Health 2017, 14, 435. [Google Scholar] [CrossRef]
- Rastegar, F.; Shenaq, D.; Huang, J.; Zhang, W.; Zhang, B.-Q.; He, B.-C.; Chen, L.; Zuo, G.-W.; Luo, Q.; Shi, Q.; et al. Mesenchymal stem cells: Molecular characteristics and clinical applications searched and summarized relevant literature. World J. Stem Cells 2010, 2, 67–80. [Google Scholar]
- Roobrouck, V.D.; Clavel, C.; Jacobs, S.A.; Ulloa-Montoya, F.; Crippa, S.; Sohni, A.; Roberts, S.J.; Luyten, F.P.; Van Gool, S.W.; Sampaolesi, M.; et al. Differentiation potential of human postnatal mesenchymal stem cells, mesoangioblasts, and multipotent adult progenitor cells reflected in their transcriptome and partially influenced by the culture conditions. Stem Cells 2011, 29, 871–882. [Google Scholar] [CrossRef]
- Kuri-Harcuch, W.; Velez-delValle, C.; Vazquez-Sandoval, A.; Hernández-Mosqueira, C.; Fernandez-Sanchez, V. A cellular perspective of adipogenesis transcriptional regulation. J. Cell. Physiol. 2019, 234, 1111–1129. [Google Scholar] [CrossRef]
- Cristancho, A.G.; Lazar, M.A. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Sarjeant, K.; Stephens, J.M. Adipogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, A. Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells. World J. Stem Cells 2014, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Scheja, L.; Heeren, J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat. Rev. Endocrinol. 2019, 15, 507–524. [Google Scholar] [CrossRef]
- Ali, A.T.; Hochfeld, W.E.; Myburgh, R.; Pepper, M.S. Adipocyte and adipogenesis. Eur. J. Cell Biol. 2013, 92, 229–236. [Google Scholar] [CrossRef]
- Ikeda, K.; Maretich, P.; Kajimura, S. The common and distinct features of brown and beige adipocytes. Trends Endocrinol. Metab. 2018, 29, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Meyer, J.G.; Cai, W.; Softic, S.; Li, M.E.; Verdin, E.; Newgard, C.; Schilling, B.; Kahn, C.R. Regulation of UCP1 and mitochondrial metabolism in brown adipose tissue by reversible succinylation. Mol. Cell 2019, 74, 844–857.e7. [Google Scholar] [CrossRef]
- Fedorenko, A.; Lishko, P.V.; Kirichok, Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 2012, 151, 400–413. [Google Scholar] [CrossRef] [Green Version]
- Rosenwald, M.; Wolfrum, C. The origin and definition of brite versus white and classical brown adipocytes. Adipocyte 2014, 3, 4–9. [Google Scholar] [CrossRef]
- Shinoda, K.; Luijten, I.H.N.; Hasegawa, Y.; Hong, H.; Sonne, S.B.; Kim, M.; Xue, R.; Chondronikola, M.; Cypess, A.M.; Tseng, Y.-H.; et al. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat. Med. 2015, 21, 389–394. [Google Scholar] [CrossRef] [Green Version]
- Petrovic, N.; Walden, T.B.; Shabalina, I.G.; Timmons, J.A.; Cannon, B.; Nedergaard, J. Chronic peroxisome proliferator-activated receptor γ (PPARγ) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J. Biol. Chem. 2010, 285, 7153–7164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, T.J.; Huang, T.L.; Tran, T.T.; Zhang, H.; Townsend, K.L.; Shadrach, J.L.; Cerletti, M.; McDougall, L.E.; Giorgadze, N.; Tchkonia, T.; et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc. Natl. Acad. Sci. USA 2011, 108, 143–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cedikova, M.; Kripnerová, M.; Dvorakova, J.; Pitule, P.; Grundmanova, M.; Babuska, V.; Mullerova, D.; Kuncova, J. Mitochondria in white, brown, and beige adipocytes. Stem Cells Int. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harms, M.; Seale, P. Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.A.; Tao, C.; Gupta, R.K.; Scherer, P.E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 2013, 19, 1338–1344. [Google Scholar] [CrossRef]
- Marquez-Curtis, L.A.; Janowska-Wieczorek, A.; McGann, L.E.; Elliott, J.A.W. Mesenchymal stromal cells derived from various tissues: Biological, clinical and cryopreservation aspects. Cryobiology 2015, 71, 181–197. [Google Scholar] [CrossRef] [Green Version]
- Friedenstein, A.J.; Piatetzky-Shapiro, I.I.; Petrakova, K.V. Osteogenesis in transplants of bone marrow cells. J. Embryol. Exp. Morphol. 1966, 16, 381–390. [Google Scholar]
- Zolocinska, A. The expression of marker genes during the differentiation of mesenchymal stromal cells. Adv. Clin. Exp. Med. 2018, 27, 717–723. [Google Scholar] [CrossRef]
- Kobolak, J.; Dinnyes, A.; Memic, A.; Khademhosseini, A.; Mobasheri, A. Mesenchymal stem cells: Identification, phenotypic characterization, biological properties and potential for regenerative medicine through biomaterial micro-engineering of their niche. Methods 2016, 99, 62–68. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Sun, Y.; Wang, B.; Xiong, Y.; Lin, W.; Wei, Q.; Wang, H.; He, W.; Wang, B.; et al. Tissue source determines the differentiation potentials of mesenchymal stem cells: A comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res. Ther. 2017, 8, 275. [Google Scholar] [CrossRef] [Green Version]
- Chu, W.; Zhuang, Y.; Gan, Y.; Wang, X.; Tang, T.; Dai, K. Comparison and characterization of enriched mesenchymal stem cells obtained by the repeated filtration of autologous bone marrow through porous biomaterials. J. Transl. Med. 2019, 17, 377. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Charif, N.; Mainard, D.; Bensoussan, D.; Stoltz, J.-F.; de Isla, N. Donor’s age dependent proliferation decrease of human bone marrow mesenchymal stem cells is linked to diminished clonogenicity. Biomed. Mater. Eng. 2014, 24, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Orbay, H.; Tobita, M.; Mizuno, H. Mesenchymal stem cells isolated from adipose and other tissues: Basic biological properties and clinical applications. Stem Cells Int. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagamura-Inoue, T. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J. Stem Cells 2014, 6, 195. [Google Scholar] [CrossRef] [PubMed]
- Baer, P.C. Adipose-derived mesenchymal stromal/stem cells: An update on their phenotype in vivo and in vitro. World J. Stem Cells 2014, 6, 256. [Google Scholar] [CrossRef] [PubMed]
- Hoogduijn, M.J.; Dor, F.J.M.F. Mesenchymal stem cells: Are we ready for clinical application in transplantation and tissue regeneration? Front. Immunol. 2013, 4, 144. [Google Scholar] [CrossRef] [Green Version]
- Hassan, G.; Kasem, I.; Soukkarieh, C.; Aljamali, M. A simple method to isolate and expand human umbilical cord derived mesenchymal stem cells: Using explant method and umbilical cord blood serum. Int. J. Stem Cells 2017, 10, 184–192. [Google Scholar] [CrossRef] [Green Version]
- Davies, J.E.; Walker, J.T.; Keating, A. Concise review: Wharton’s jelly: The rich, but enigmatic, source of mesenchymal stromal cells. Stem Cells Transl. Med. 2017, 6, 1620–1630. [Google Scholar] [CrossRef]
- Vangsness, C.T.; Sternberg, H.; Harris, L. Umbilical cord tissue offers the greatest number of harvestable mesenchymal stem cells for research and clinical application: A literature review of different harvest sites. Arthrosc. J. Arthrosc. Relat. Surg. 2015, 31, 1836–1843. [Google Scholar] [CrossRef]
- Beeravolu, N.; McKee, C.; Alamri, A.; Mikhael, S.; Brown, C.; Perez-Cruet, M.; Chaudhry, G.R. Isolation and characterization of mesenchymal stromal cells from human umbilical cord and fetal placenta. J. Vis. Exp. 2017, (122), 55224. [Google Scholar] [CrossRef] [Green Version]
- Bharti, D.; Shivakumar, S.B.; Park, J.-K.; Ullah, I.; Subbarao, R.B.; Park, J.-S.; Lee, S.-L.; Park, B.-W.; Rho, G.-J. Comparative analysis of human Wharton’s jelly mesenchymal stem cells derived from different parts of the same umbilical cord. Cell Tissue Res. 2018, 372, 51–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramanian, A.; Fong, C.-Y.; Biswas, A.; Bongso, A. Comparative characterization of cells from the various compartments of the human umbilical cord shows that the Wharton’s jelly compartment provides the best source of clinically utilizable mesenchymal stem cells. PLoS ONE 2015, 10, e0127992. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Kwon, A.; Kim, Y.; Kim, M.; Kim, J.; Choi, H.; Jekarl, D.W.; Lee, S.; Kim, J.M.; Shin, J.-C.; Park, I.Y. Tissue-specific differentiation potency of mesenchymal stromal cells from perinatal tissues. Sci. Rep. 2016, 6, 23544. [Google Scholar] [CrossRef] [PubMed]
- Ragni, E.; Viganò, M.; Parazzi, V.; Montemurro, T.; Montelatici, E.; Lavazza, C.; Budelli, S.; Vecchini, A.; Rebulla, P.; Giordano, R.; et al. Adipogenic potential in human mesenchymal stem cells strictly depends on adult or foetal tissue harvest. Int. J. Biochem. Cell Biol. 2013, 45, 2456–2466. [Google Scholar] [CrossRef] [PubMed]
- Amable, P.R.; Teixeira, M.V.T.; Carias, R.B.V.; Granjeiro, J.M.; Borojevic, R. Gene expression and protein secretion during human mesenchymal cell differentiation into adipogenic cells. BMC Cell Biol. 2014, 15, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.; Hu, J.; Zhao, J.; Liu, J.; Ouyang, W.; Yang, C.; Gong, N.; Du, L.; Khanal, A.; Chen, L. Side-by-side comparison of the biological characteristics of human umbilical cord and adipose tissue-derived mesenchymal stem cells. Biomed Res. Int. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-H.; Jo, C.H.; Kim, H.-R.; Hwang, Y. Comparison of immunological characteristics of mesenchymal stem cells from the periodontal ligament, umbilical Cord, and adipose tissue. Stem Cells Int. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Brohem, C.A.; de Carvalho, C.M.; Radoski, C.L.; Santi, F.C.; Baptista, M.C.; Swinka, B.B.; de A Urban, C.; de Araujo, L.R.R.; Graf, R.M.; Feferman, I.H.S.; et al. Comparison between fibroblasts and mesenchymal stem cells derived from dermal and adipose tissue. Int. J. Cosmet. Sci. 2013, 35, 448–457. [Google Scholar] [CrossRef]
- Li, C.; Wu, X.; Tong, J.; Yang, X.; Zhao, J.; Zheng, Q.; Zhao, G.; Ma, Z. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res. Ther. 2015, 6, 55. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.M.; Martina, M.; Hutmacher, D.W.; Hui, J.H.P.; Lee, E.H.; Lim, B. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells 2006, 25, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Manini, I.; Gulino, L.; Gava, B.; Pierantozzi, E.; Curina, C.; Rossi, D.; Brafa, A.; D’Aniello, C.; Sorrentino, V. Multi-potent progenitors in freshly isolated and cultured human mesenchymal stem cells: A comparison between adipose and dermal tissue. Cell Tissue Res. 2011, 344, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Mauney, J.R.; Nguyen, T.; Gillen, K.; Kirker-Head, C.; Gimble, J.M.; Kaplan, D.L. Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials 2007, 28, 5280–5290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed-Ahmed, S.; Fristad, I.; Lie, S.A.; Suliman, S.; Mustafa, K.; Vindenes, H.; Idris, S.B. Adipose-derived and bone marrow mesenchymal stem cells: A donor-matched comparison. Stem Cell Res. Ther. 2018, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Noël, D.; Caton, D.; Roche, S.; Bony, C.; Lehmann, S.; Casteilla, L.; Jorgensen, C.; Cousin, B. Cell specific differences between human adipose-derived and mesenchymal–stromal cells despite similar differentiation potentials. Exp. Cell Res. 2008, 314, 1575–1584. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Sekiya, I.; Yagishita, K.; Muneta, T. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheum. 2005, 52, 2521–2529. [Google Scholar] [CrossRef]
- Baksh, D.; Yao, R.; Tuan, R.S. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells 2007, 25, 1384–1392. [Google Scholar] [CrossRef] [Green Version]
- Barlow, S.; Brooke, G.; Chatterjee, K.; Price, G.; Pelekanos, R.; Rossetti, T.; Doody, M.; Venter, D.; Pain, S.; Gilshenan, K.; et al. Comparison of human placenta- and bone marrow–derived multipotent mesenchymal stem cells. Stem Cells Dev. 2008, 17, 1095–1108. [Google Scholar] [CrossRef] [Green Version]
- Batsali, A.K.; Pontikoglou, C.; Koutroulakis, D.; Pavlaki, K.I.; Damianaki, A.; Mavroudi, I.; Alpantaki, K.; Kouvidi, E.; Kontakis, G.; Papadaki, H.A. Differential expression of cell cycle and WNT pathway-related genes accounts for differences in the growth and differentiation potential of Wharton’s jelly and bone marrow-derived mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Isobe, Y.; Koyama, N.; Nakao, K.; Osawa, K.; Ikeno, M.; Yamanaka, S.; Okubo, Y.; Fujimura, K.; Bessho, K. Comparison of human mesenchymal stem cells derived from bone marrow, synovial fluid, adult dental pulp, and exfoliated deciduous tooth pulp. Int. J. Oral Maxillofac. Surg. 2016, 45, 124–131. [Google Scholar] [CrossRef]
- Zhu, X.; Shi, W.; Tai, W.; Liu, F. The comparition of biological characteristics and multilineage differentiation of bone marrow and adipose derived mesenchymal stem cells. Cell Tissue Res. 2012, 350, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Durandt, C.; van Vollenstee, F.A.; Dessels, C.; Kallmeyer, K.; de Villiers, D.; Murdoch, C.; Potgieter, M.; Pepper, M.S. Novel flow cytometric approach for the detection of adipocyte subpopulations during adipogenesis. J. Lipid Res. 2016, 57, 729–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierdomenico, L. Diabetes mellitus during pregnancy interferes with the biological characteristics of Wharton’s jelly mesenchymal stem cells. Open Tissue Eng. Regen. Med. J. 2011, 4, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998, 78, 783–809. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.-Q.; Otto, T.C.; Lane, M.D. Mitotic clonal expansion: A synchronous process required for adipogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Ambele, M.A.; Dessels, C.; Durandt, C.; Pepper, M.S. Genome-wide analysis of gene expression during adipogenesis in human adipose-derived stromal cells reveals novel patterns of gene expression during adipocyte differentiation. Stem Cell Res. 2016, 16, 725–734. [Google Scholar] [CrossRef] [Green Version]
- de Sá, P.M.; Richard, A.J.; Hang, H.; Stephens, J.M. Transcriptional regulation of adipogenesis. In Comprehensive Physiology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 635–674. [Google Scholar]
- Lee, J.-E.; Schmidt, H.; Lai, B.; Ge, K. Transcriptional and epigenomic regulation of adipogenesis. Mol. Cell. Biol. 2019, 39, e00601-18. [Google Scholar] [CrossRef] [Green Version]
- Shapira, S.N.; Seale, P. Transcriptional control of brown and beige fat development and function. Obesity 2019, 27, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Chang, E.; Kim, C. Natural products and obesity: A focus on the regulation of mitotic clonal expansion during adipogenesis. Molecules 2019, 24, 1157. [Google Scholar] [CrossRef] [Green Version]
- Moon, Y.S.; Smas, C.M.; Lee, K.; Villena, J.A.; Kim, K.-H.; Yun, E.J.; Sul, H.S. Mice lacking paternally expressed Pref-1/Dlk1 display growth retardation and accelerated adiposity. Mol. Cell. Biol. 2002, 22, 5585–5592. [Google Scholar] [CrossRef] [Green Version]
- Smas, C.M.; Chen, L.; Zhao, L.; Latasa, M.-J.; Sul, H.S. Transcriptional repression of Pref-1 by glucocorticoids promotes 3T3-L1 adipocyte differentiation. J. Biol. Chem. 1999, 274, 12632–12641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smas, C.M.; Sul, H.S. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell 1993, 73, 725–734. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, K.-A.; Kim, J.-H.; Sul, H.S. Pref-1, a preadipocyte secreted factor that inhibits adipogenesis. J. Nutr. 2006, 136, 2953–2956. [Google Scholar] [CrossRef] [PubMed]
- Moseti, D.; Regassa, A.; Kim, W.-K. Molecular regulation of adipogenesis and potential anti-adipogenic bioactive molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef] [Green Version]
- Rosen, E.D.; Sarraf, P.; Troy, A.E.; Bradwin, G.; Moore, K.; Milstone, D.S.; Spiegelman, B.M.; Mortensen, R.M. PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell 1999, 4, 611–617. [Google Scholar] [CrossRef]
- Huang, H.; Lane, M.D.; Tang, Q.-Q. Effect of serum on the down-regulation of CHOP-10 during differentiation of 3T3-L1 preadipocytes. Biochem. Biophys. Res. Commun. 2005, 338, 1185–1188. [Google Scholar] [CrossRef]
- Jing, K.; Heo, J.-Y.; Song, K.-S.; Seo, K.-S.; Park, J.-H.; Kim, J.-S.; Jung, Y.-J.; Jo, D.-Y.; Kweon, G.-R.; Yoon, W.-H.; et al. Expression regulation and function of Pref-1 during adipogenesis of human mesenchymal stem cells (MSCs). Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2009, 1791, 816–826. [Google Scholar] [CrossRef]
- Rosen, E.D.; Walkey, C.J.; Puigserver, P.; Spiegelman, B.M. Transcriptional regulation of adipogenesis. Genes Dev. 2000, 14, 1293–1307. [Google Scholar]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scimè, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H.; et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008, 454, 961–967. [Google Scholar] [CrossRef] [Green Version]
- Seale, P.; Kajimura, S.; Spiegelman, B.M. Transcriptional control of brown adipocyte development and physiological function--of mice and men. Genes Dev. 2009, 23, 788–797. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Huang, S.; Ling, S.; Xu, S.; Wang, F.; Zhang, W.; Zhou, R.; He, L.; Xia, X.; Yao, Z.; et al. Foxp1 controls brown/beige adipocyte differentiation and thermogenesis through regulating β3-AR desensitization. Nat. Commun. 2019, 10, 5070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Boström, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.-H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidossis, L.S.; Porter, C.; Saraf, M.K.; Børsheim, E.; Radhakrishnan, R.S.; Chao, T.; Ali, A.; Chondronikola, M.; Mlcak, R.; Finnerty, C.C.; et al. Browning of subcutaneous white adipose tissue in humans after severe adrenergic stress. Cell Metab. 2015, 22, 219–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachman, E.S. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science 2002, 297, 843–845. [Google Scholar] [CrossRef] [Green Version]
- Ghorbani, M.; Teimourian, S.; Farzad, R.; Namvar Asl, N. Apparent histological changes of adipocytes after treatment with CL 316,243, a β-3-adrenergic receptor agonist. Drug Des. Devel. Ther. 2015, 9, 669. [Google Scholar] [CrossRef] [Green Version]
- Rajakumari, S.; Wu, J.; Ishibashi, J.; Lim, H.-W.; Giang, A.-H.; Won, K.-J.; Reed, R.R.; Seale, P. EBF2 determines and maintains brown adipocyte identity. Cell Metab. 2013, 17, 562–574. [Google Scholar] [CrossRef] [Green Version]
- Puigserver, P.; Wu, Z.; Park, C.W.; Graves, R.; Wright, M.; Spiegelman, B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998, 92, 829–839. [Google Scholar] [CrossRef] [Green Version]
- Hiraike, Y.; Waki, H.; Yu, J.; Nakamura, M.; Miyake, K.; Nagano, G.; Nakaki, R.; Suzuki, K.; Kobayashi, H.; Yamamoto, S.; et al. NFIA co-localizes with PPARγ and transcriptionally controls the brown fat gene program. Nat. Cell Biol. 2017, 19, 1081–1092. [Google Scholar] [CrossRef] [Green Version]
- Kajimura, S.; Seale, P.; Kubota, K.; Lunsford, E.; Frangioni, J.V.; Gygi, S.P.; Spiegelman, B.M. Initiation of myoblast to brown fat switch by a PRDM16–C/EBP-β transcriptional complex. Nature 2009, 460, 1154–1158. [Google Scholar] [CrossRef] [Green Version]
- Helman, L.J.; Thiele, C.J.; Linehan, W.M.; Nelkin, B.D.; Baylin, S.B.; Israel, M.A. Molecular markers of neuroendocrine development and evidence of environmental regulation. Proc. Natl. Acad. Sci. USA 1987, 84, 2336–2339. [Google Scholar] [CrossRef] [Green Version]
- Helman, L.J.; Sack, N.; Plon, S.E.; Israel, M.A. The sequence of an adrenal specific human cDNA, pG2. Nucleic Acids Res. 1990, 18, 685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fay, T.N.; Jacobs, I.; Teisner, B.; Poulsen, O.; Chapman, M.G.; Stabile, I.; Bohn, H.; Westergaard, J.G.; Grudzinskas, J.G. Two fetal antigens (FA-1 and FA-2) and endometrial proteins (PP12 and PP14) isolated from amniotic fluid; preliminary observations in fetal and maternal tissues. Eur. J. Obstet. Gynecol. Reprod. Biol. 1988, 29, 73–85. [Google Scholar] [CrossRef]
- Laborda, J.; Sausville, E.A.; Hoffman, T.; Notario, V. Dlk, a putative mammalian homeotic gene differentially expressed in small cell lung carcinoma and neuroendocrine tumor cell line. J. Biol. Chem. 1993, 268, 3817–3820. [Google Scholar] [PubMed]
- Jensen, C.H.; Krogh, T.N.; Hojrup, P.; Clausen, P.P.; Skjodt, K.; Larsson, L.-I.; Enghild, J.J.; Teisner, B. Protein Structure of Fetal Antigen 1 (FA1). A novel circulating human epidermal-growth-factor-Like protein expressed in neuroendocrine tumors and its relation to the gene products of Dlk and pG2. Eur. J. Biochem. 1994, 225, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.L.; Helman, L.; Hoffman, T.; Laborda, J. Dlk, pG2 and Pref-1 mRNAs encode similar proteins belonging to the EGF-like superfamily. Identification of polymorphic variants of this RNA. Biochim. Biophys. Acta Gene Struct. Expr. 1995, 1261, 223–232. [Google Scholar]
- Wang, Y.; Zhao, L.; Smas, C.; Sul, H.S. Pref-1 interacts with fibronectin to inhibit adipocyte differentiation. Mol. Cell. Biol. 2010, 30, 3480–3492. [Google Scholar] [CrossRef] [Green Version]
- Smas, C.M.; Green, D.; Sul, H.S. Structural characterization and alternate splicing of the gene encoding the preadipocyte EGF-like protein Pref-1. Biochemistry 1994, 33, 9257–9265. [Google Scholar] [CrossRef]
- Sul, H.S. Minireview: Pref-1: Role in adipogenesis and mesenchymal cell fate. Mol. Endocrinol. 2009, 23, 1717–1725. [Google Scholar] [CrossRef] [Green Version]
- Hudak, C.S.; Sul, H.S. Pref-1, a gatekeeper of adipogenesis. Front. Endocrinol. (Lausanne). 2013, 4, 79. [Google Scholar] [CrossRef] [Green Version]
- Kopan, R.; Ilagan, M.X.G. The canonical notch signaling pathway: Unfolding the activation mechanism. Cell 2009, 137, 216–233. [Google Scholar] [CrossRef] [Green Version]
- Falix, F.A.; Aronson, D.C.; Lamers, W.H.; Gaemers, I.C. Possible roles of DLK1 in the Notch pathway during development and disease. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 988–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, S.J.; Takada, S.; Harrison, E.; Shen, S.C.; Ferguson-Smith, A.C. The atypical mammalian ligand Delta-like homologue 1 (Dlk1) can regulate Notch signalling in Drosophila. BMC Dev. Biol. 2008, 8, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smas, C.M.; Chen, L.; Sul, H.S. Cleavage of membrane-associated Pref-1 generates a soluble inhibitor of adipocyte differentiation. Mol. Cell. Biol. 1997, 17, 977–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Sul, H.S. Ectodomain shedding of preadipocyte Factor 1 (Pref-1) by tumor necrosis factor alpha converting enzyme (TACE) and inhibition of adipocyte differentiation. Mol. Cell. Biol. 2006, 26, 5421–5435. [Google Scholar] [CrossRef] [Green Version]
- Mei, B.; Zhao, L.; Chen, L.; Sul, H.S. Only the large soluble form of preadipocyte factor-1 (Pref-1), but not the small soluble and membrane forms, inhibits adipocyte differentiation: Role of alternative splicing. Biochem. J. 2002, 364, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Bauer, S.R.; Ruiz-Hidalgo, M.J.; Rudikoff, E.K.; Goldstein, J.; Laborda, J. Modulated expression of the epidermal growth factor-like homeotic protein dlk influences stromal-cell–pre-B-cell interactions, stromal cell adipogenesis, and pre-B-cell interleukin-7 requirements. Mol. Cell. Biol. 1998, 18, 5247–5255. [Google Scholar] [CrossRef] [Green Version]
- Garcés, C.; Ruiz-Hidalgo, M.J.; Bonvini, E.; Goldstein, J.; Laborda, J. Adipocyte differentiation is modulated by secreted delta-like (dlk) variants and requires the expression of membrane-associated dlk. Differentiation 1999, 64, 103–114. [Google Scholar] [CrossRef]
- Nueda, M.L.; Baladrón, V.; Sánchez-Solana, B.; Ballesteros, M.Á.; Laborda, J. The EGF-like protein dlk1 inhibits Notch signaling and potentiates adipogenesis of mesenchymal cells. J. Mol. Biol. 2007, 367, 1281–1293. [Google Scholar] [CrossRef]
- Lee, K.; Villena, J.A.; Moon, Y.S.; Kim, K.-H.; Lee, S.; Kang, C.; Sul, H.S. Inhibition of adipogenesis and development of glucose intolerance by soluble preadipocyte factor–1 (Pref-1). J. Clin. Invest. 2003, 111, 453–461. [Google Scholar] [CrossRef] [Green Version]
- Nueda, M.-L.; García-Ramírez, J.J.; Laborda, J.; Baladrón, V. Dlk1 specifically interacts with insulin-like growth factor binding protein 1 to modulate adipogenesis of 3T3-L1 Cells. J. Mol. Biol. 2008, 379, 428–442. [Google Scholar] [CrossRef]
- Villena, J.A.; Choi, C.S.; Wang, Y.; Kim, S.; Hwang, Y.-J.; Kim, Y.-B.; Cline, G.; Shulman, G.I.; Sul, H.S. Resistance to high-fat diet-induced obesity but exacerbated insulin resistance in mice overexpressing preadipocyte factor-1 (Pref-1): A new model of partial lipodystrophy. Diabetes 2008, 57, 3258–3266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charalambous, M.; Da Rocha, S.T.; Radford, E.J.; Medina-Gomez, G.; Curran, S.; Pinnock, S.B.; Ferrón, S.R.; Vidal-Puig, A.; Ferguson-Smith, A.C. DLK1/PREF1 regulates nutrient metabolism and protects from steatosis. Proc. Natl. Acad. Sci. USA 2014, 111, 16088–16093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armengol, J.; Villena, J.A.; Hondares, E.; Carmona, M.C.; Sul, H.S.; Iglesias, R.; Giralt, M.; Villarroya, F. Pref-1 in brown adipose tissue: Specific involvement in brown adipocyte differentiation and regulatory role of C/EBPδ. Biochem. J. 2012, 443, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Schulz, T.J.; Espinoza, D.O.; Huang, T.L.; Emanuelli, B.; Kristiansen, K.; Tseng, Y. Cross talk between insulin and bone morphogenetic protein signaling systems in brown adipogenesis. Mol. Cell. Biol. 2010, 30, 4224–4233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhee, M.; Kim, J.; Lee, M.-W.; Yoon, K.; Lee, S. Preadipocyte factor 1 regulates adipose tissue browning via TNF-α-converting enzyme-mediated cleavage. Metabolism 2019, 101, 153977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hirai, M.; Cantero, S.; Ciubotariu, R.; Dobrila, L.; Hirsh, A.; Igura, K.; Satoh, H.; Yokomi, I.; Nishimura, T.; et al. Isolation and characterization of mesenchymal stem cells from human umbilical cord blood: Reevaluation of critical factors for successful isolation and high ability to proliferate and differentiate to chondrocytes as compared to mesenchymal stem cells from bone marrow to adipose tissue. J. Cell. Biochem. 2011, 112, 1206–1218. [Google Scholar] [PubMed]
- Karagianni, M.; Brinkmann, I.; Kinzebach, S.; Grassl, M.; Weiss, C.; Bugert, P.; Bieback, K. A comparative analysis of the adipogenic potential in human mesenchymal stromal cells from cord blood and other sources. Cytotherapy 2013, 15, 76–88.e2. [Google Scholar] [CrossRef] [Green Version]
- Briana, D.D.; Papathanasiou, A.-E.; Gavrili, S.; Georgantzi, S.; Marmarinos, A.; Christou, C.; Voulgaris, K.; Gourgiotis, D.; Malamitsi-Puchner, A. Preadipocyte factor-1 in maternal, umbilical cord serum and breast milk: The impact of fetal growth. Cytokine 2019, 114, 143–148. [Google Scholar] [CrossRef]
- Lee, S.; Park, B.-J.; Kim, J.Y.; Jekarl, D.; Choi, H.Y.; Lee, S.Y.; Kim, M.; Kim, Y.; Park, M.-S. The effect of fibroblast growth factor on distinct differentiation potential of cord blood–derived unrestricted somatic stem cells and Wharton’s jelly–derived mesenchymal stem/stromal cells. Cytotherapy 2015, 17, 1723–1731. [Google Scholar] [CrossRef]
- Kluth, S.M.; Buchheiser, A.; Houben, A.P.; Geyh, S.; Krenz, T.; Radke, T.F.; Wiek, C.; Hanenberg, H.; Reinecke, P.; Wernet, P.; et al. DLK-1 as a marker to distinguish unrestricted somatic stem cells and mesenchymal stromal cells in cord blood. Stem Cells Dev. 2010, 19, 1471–1483. [Google Scholar] [CrossRef] [Green Version]
- Mitterberger, M.C.; Lechner, S.; Mattesich, M.; Kaiser, A.; Probst, D.; Wenger, N.; Pierer, G.; Zwerschke, W. DLK1(PREF1) is a negative regulator of adipogenesis in CD105+/CD90+/CD34+/CD31−/FABP4− adipose-derived stromal cells from subcutaneous abdominal fat pats of adult women. Stem Cell Res. 2012, 9, 35–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwierzina, M.E.; Ejaz, A.; Bitsche, M.; Blumer, M.J.F.; Mitterberger, M.C.; Mattesich, M.; Amann, A.; Kaiser, A.; Pechriggl, E.J.; Hörl, S.; et al. Characterization of DLK1(PREF1)+/CD34+ cells in vascular stroma of human white adipose tissue. Stem Cell Res. 2015, 15, 403–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morganstein, D.L.; Wu, P.; Mane, M.R.; Fisk, N.M.; White, R.; Parker, M.G. Human fetal mesenchymal stem cells differentiate into brown and white adipocytes: A role for ERRα in human UCP1 expression. Cell Res. 2010, 20, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Bost, F.; Aouadi, M.; Caron, L.; Binétruy, B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie 2005, 87, 51–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, D.K. MAP Kinase Pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a011254. [Google Scholar] [CrossRef]
- Gehart, H.; Kumpf, S.; Ittner, A.; Ricci, R. MAPK signalling in cellular metabolism: Stress or wellness? EMBO Rep. 2010, 11, 834–840. [Google Scholar] [CrossRef] [Green Version]
- Hu, E.; Kim, J.B.; Sarraf, P.; Spiegelman, B.M. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science 1996, 274, 2100–2103. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-A.; Kim, J.-H.; Wang, Y.; Sul, H.S. Pref-1 (Preadipocyte Factor 1) activates the MEK/extracellular signal-regulated kinase pathway to inhibit adipocyte differentiation. Mol. Cell. Biol. 2007, 27, 2294–2308. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.J.; Wise, L.S.; Berkowitz, R.; Wan, C.; Rubin, C.S. Insulin-like growth factor-I is an essential regulator of the differentiation of 3T3-L1 adipocytes. J. Biol. Chem. 1988, 263, 9402–9408. [Google Scholar]
- Zhang, H.; Nøhr, J.; Jensen, C.H.; Petersen, R.K.; Bachmann, E.; Teisner, B.; Larsen, L.K.; Mandrup, S.; Kristiansen, K. Insulin-like growth factor-1/Insulin bypasses Pref-1/FA1-mediated inhibition of adipocyte differentiation. J. Biol. Chem. 2003, 278, 20906–20914. [Google Scholar] [CrossRef] [Green Version]
- Sale, E.M.; Atkinson, P.G.; Sale, G.J. Requirement of MAP kinase for differentiation of fibroblasts to adipocytes, for insulin activation of p90 S6 kinase and for insulin or serum stimulation of DNA synthesis. EMBO J. 1995, 14, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Boney, C.M.; Smith, R.M.; Gruppuso, P.A. Modulation of insulin-like growth factor I mitogenic signaling in 3T3- L1 preadipocyte differentiation. Endocrinology 1998, 139, 1638–1644. [Google Scholar] [CrossRef] [PubMed]
- Bost, F.; Caron, L.; Marchetti, I.; Dani, C.; Le Marchand-Brustel, Y.; Binétruy, B. Retinoic acid activation of the ERK pathway is required for embryonic stem cell commitment into the adipocyte lineage. Biochem. J. 2002, 361, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Camp, H.S.; Tafuri, S.R. Regulation of peroxisome proliferator-activated receptor γ activity by mitogen-activated protein kinase. J. Biol. Chem. 1997, 272, 10811–10816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaiswal, R.K.; Jaiswal, N.; Bruder, S.P.; Mbalaviele, G.; Marshak, D.R.; Pittenger, M.F. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J. Biol. Chem. 2000, 275, 9645–9652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Font de Mora, J.; Porras, A.; Ahn, N.; Santos, E. 3T3-L1 adipocytic differentiation. Mol. Cell. Biol. 1997, 17, 6068–6075. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Sul, H.S. Pref-1 regulates mesenchymal cell commitment and differentiation through Sox9. Cell Metab. 2009, 9, 287–302. [Google Scholar] [CrossRef] [Green Version]
- To, W.S.; Midwood, K.S. Plasma and cellular fibronectin: Distinct and independent functions during tissue repair. Fibrogenesis Tissue Repair 2011, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Pankov, R. Fibronectin at a glance. J. Cell Sci. 2002, 115, 3861–3863. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Hidalgo, M. Dlk modulates mitogen-activated protein kinase signaling to allow or prevent differentiation. Exp. Cell Res. 2002, 274, 178–188. [Google Scholar] [CrossRef]
- Kopan, R. Notch signaling. Cold Spring Harb. Perspect. Biol. 2012, 4, a011213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Souza, B.; Meloty-Kapella, L.; Weinmaster, G. Canonical and non-canonical notch ligands. Curr Top Dev Biol. 2010, 92, 73–129. [Google Scholar] [PubMed] [Green Version]
- Nueda, M.; Gómez, M.G.-; Rodríguez-ca, M.; Baladrón, V. DLK proteins modulate NOTCH signaling to influence a brown or white 3T3-L1 adipocyte fate. Sci Rep. 2018, 8, 16923. [Google Scholar] [CrossRef] [PubMed]
- Searfoss, G.H.; Jordan, W.H.; Calligaro, D.O.; Galbreath, E.J.; Schirtzinger, L.M.; Berridge, B.R.; Gao, H.; Higgins, M.A.; May, P.C.; Ryan, T.P. Adipsin, a biomarker of gastrointestinal toxicity mediated by a functional γ-secretase inhibitor. J. Biol. Chem. 2003, 278, 46107–46116. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, H.; Chao, M.Y.; Larkins-Ford, J.; Corkins, M.E.; Somers, G.A.; Tucey, T.; Dionne, H.M.; White, J.Q.; Wani, K.; Boxem, M.; et al. OSM-11 facilitates LIN-12 Notch signaling during Caenorhabditis elegans vulval development. PLoS Biol. 2008, 6, e196. [Google Scholar] [CrossRef]
- Shan, T.; Liu, J.; Wu, W.; Xu, Z.; Wang, Y. Roles of Notch signaling in adipocyte progenitor cells and mature adipocytes. J. Cell. Physiol. 2017, 232, 1258–1261. [Google Scholar] [CrossRef]
- Nichols, A.M.; Pan, Y.; Herreman, A.; Hadland, B.K.; De Strooper, B.; Kopan, R.; Huppert, S.S. Notch pathway is dispensable for adipocyte specification. Genesis 2004, 44, 40–44. [Google Scholar] [CrossRef]
- Lai, P.; Tsai, C.; Tseng, M. Active form Notch4 promotes the proliferation and differentiation of 3T3-L1 preadipocytes. Biochem. Biophys. Res. Commun. 2013, 430, 1132–1139. [Google Scholar] [CrossRef]
- Urs, S.; Turner, B.; Tang, Y.; Rostama, B.; Small, D.; Liaw, L. Effect of soluble Jagged1-mediated inhibition of Notch signaling on proliferation and differentiation of an adipocyte progenitor cell model. Adipocyte 2012, 1, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Garcés, C.; Ruiz-Hidalgo, M.J.; De Mora, J.F.; Park, C.; Miele, L.; Goldstein, J.; Bonvini, E.; Porrás, A.; Laborda, J. Notch-1 controls the expression of fatty acid-activated transcription factors and is required for adipogenesis. J. Biol. Chem. 1997, 272, 29729–29734. [Google Scholar] [CrossRef] [Green Version]
- Ross, D.A.; Rao, P.K.; Kadesch, T. Dual Roles for the Notch Target Gene Hes-1 in the Differentiation of 3T3-L1 Preadipocytes. Mol. Cell. Biol. 2004, 24, 3505–3513. [Google Scholar] [CrossRef] [Green Version]
- Ba, K.; Yang, X.; Wu, L.; Wei, X.; Fu, N.; Fu, Y.; Cai, X.; Yao, Y.; Ge, Y.; Lin, Y. Jagged-1-mediated activation of notch signalling induces adipogenesis of adipose-derived stem cells. Cell Prolif. 2012, 45, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Chen, Y.; Zheng, X.; Li, Y.; Zhang, Q.; Mo, D.; Yang, G. MicroRNA-139-5p Suppresses 3T3-L1 preadipocyte differentiation through Notch and IRS1/PI3K/Akt insulin signaling pathways. J. Cell. Biochem. 2015, 116, 1195–1204. [Google Scholar] [CrossRef]
- Vujovic, S.; Henderson, S.R.; Flanagan, A.M.; Clements, M.O. Inhibition of γ-secretases alters both proliferation and differentiation of mesenchymal stem cells. Cell Prolif. 2007, 40, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Osathanon, T.; Subbalekha, K.; Sastravaha, P.; Pavasant, P. Notch signalling inhibits the adipogenic differentiation of single-cell-derived mesenchymal stem cell clones isolated from human adipose tissue. Cell Biol. Int. 2012, 36, 1161–1170. [Google Scholar] [CrossRef]
- Ugarte, F.; Ryser, M.; Thieme, S.; Fierro, F.A.; Navratiel, K.; Bornhäuser, M.; Brenner, S. Notch signaling enhances osteogenic differentiation while inhibiting adipogenesis in primary human bone marrow stromal cells. Exp. Hematol. 2009, 37, 867–875.e1. [Google Scholar] [CrossRef]
- Song, B.; Chi, Y.; Li, X.; Du, W.; Han, Z.-B.; Tian, J.; Li, J.; Chen, F.; Wu, H.; Han, L.; et al. Inhibition of Notch signaling promotes the adipogenic differentiation of mesenchymal stem cells through autophagy activation and PTEN-PI3K/AKT/mTOR pathway. Cell. Physiol. Biochem. 2015, 36, 1991–2002. [Google Scholar] [CrossRef]
- Lei, T.; Bi, Y.; Gao, M.J.; Gao, S.M.; Zhou, L.L.; Zheng, H.L.; Chen, X.D. HES1 inhibits adipogenesis of porcine mesenchymal stem cells via transcriptional repression of FAD24. Domest. Anim. Endocrinol. 2013, 45, 28–32. [Google Scholar] [CrossRef]
- Ross, D.A.; Hannenhalli, S.; Tobias, J.W.; Cooch, N.; Shiekhattar, R.; Kadesch, T. Functional analysis of Hes-1 in preadipocytes. Mol. Endocrinol. 2006, 20, 698–705. [Google Scholar] [CrossRef] [Green Version]
- Baladrón, V.; Ruiz-Hidalgo, M.J.; Nueda, M.L.; Díaz-Guerra, M.J.M.; García-Ramírez, J.J.; Bonvini, E.; Gubina, E.; Laborda, J. Dlk acts as a negative regulator of Notch1 activation through interactions with specific EGF-like repeats. Exp. Cell Res. 2005, 303, 343–359. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, X.; Wu, Y.; Jing, W.; Cai, X.; Tang, W.; Liu, L.; Liu, Y.; Grottkau, B.E.; Lin, Y. γ-secretase inhibitor induces adipogenesis of adipose-derived stem cells by regulation of Notch and PPAR-γ. Cell Prolif. 2010, 43, 147–156. [Google Scholar] [CrossRef] [PubMed]

| Study | Tissue Sources Compared | Reference | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amable et al., 2014 | AT | > | BM | > | WJSC | [46] | ||||||||||||
| Brohem et al., 2013 | AT | > | BM | [49] | ||||||||||||||
| Hu et al., 2013 | AT | = | WJSC | [47] | ||||||||||||||
| Li et al., 2015 | AT | = | BM | [50] | ||||||||||||||
| Liu et al., 2009 | AT | > | BM | [51] | ||||||||||||||
| Manini et al., 2011 | AT | > | BM | > | Dermis | [52] | ||||||||||||
| Maurney et al., 2007 | AT | = | BM | [53] | ||||||||||||||
| Mohamed-Ahmed et al., 2018 | AT | > | BM | > | Dermis | [54] | ||||||||||||
| Noël et al., 2007 | AT | = | BM | [55] | ||||||||||||||
| Ragni et al., 2013 | AT | > | BM | > | UCB | > | WJSC | > | AF | > | PV | [45] | ||||||
| Sakaguchi et al., 2005 | AT | = | SV | > | BM | = | PS | > | Muscle | [56] | ||||||||
| Xu et al., 2017 | AT | > | BM | [30] | ||||||||||||||
| Baksh et al., 2007 | UC | > | BM | [57] | ||||||||||||||
| Barlow et al., 2015 | BM | > | PC | [58] | ||||||||||||||
| Batsali et al., 2017 | BM | > | WJSC | [59] | ||||||||||||||
| Isobe et al., 2015 | BM | = | SF | > | SHED | > | DP | [60] | ||||||||||
| Zhu et al., 2012 | > | BM | [61] | |||||||||||||||
| Cell Type | Experimental Approach | Impact on | References | ||
|---|---|---|---|---|---|
| Notch signaling | Adipogenic Differentiation | Pref-1 Expression | |||
| 3T3-L1 | Exposure to soluble Jagged1 | Up-regulated | Decreased | Not determined | [152] |
| hes1 over-expression | Up-regulated | Decreased | Not determined | ||
| hes1 knock-down | Down-regulated | Decreased | Up-regulated | ||
| 3T3-L1 | Notch4 over-expression | Up-regulated | Increased | Down-regulated | [149] |
| 3T3-L1 | Adipogenic differentiation. No specific treatment. | Decreases over time | Increased (compared to C3H10T1/2) | Not determined | [109] |
| dlk1 knock-down | Up-regulated | Not assessed | Down-regulated | ||
| C3H10T1/2 | Adipogenic differentiation. No specific treatment. | Remained unchanged | Decreased (compared to 3T3-L1 cells) | Up-regulated | |
| dlk1 over-expression | Down-regulated | Increased | Up-regulated | ||
| dlk1 knock-down | Up-regulated | Increased | Down-regulated | ||
| Notch1 knock-down | Down-regulated | Decreased | Down-regulated | ||
| dlk1 over-expression in Notch1 knock-down cells | Down-regulated | Decreased | Up-regulated | ||
| 3T3-L1 | Transfection with miRNA (miR-139–5p mimic) | Initially increased. Gradually decreased. | Decreased | Up-regulated | [154] |
| Co-transfection with pcDNA3.1_NICD (over-expressing Notch) | Up-regulated | Increased | Not determined | ||
| Mouse BM-derived MSCs | Inhibition of γ-secretase | Decreased | Increased | Decreased | [162] |
| Human BM-derived MSCs | Adipogenic differentiation. No specific treatment. | Decreased | Increased | Decreased | [158] |
| Inhibition of γ-secretase | Decreased | Increased | Not measured | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, C.; Durandt, C.; Kallmeyer, K.; Ambele, M.A.; Pepper, M.S. The Role of Pref-1 during Adipogenic Differentiation: An Overview of Suggested Mechanisms. Int. J. Mol. Sci. 2020, 21, 4104. https://doi.org/10.3390/ijms21114104
da Silva C, Durandt C, Kallmeyer K, Ambele MA, Pepper MS. The Role of Pref-1 during Adipogenic Differentiation: An Overview of Suggested Mechanisms. International Journal of Molecular Sciences. 2020; 21(11):4104. https://doi.org/10.3390/ijms21114104
Chicago/Turabian Styleda Silva, Carina, Chrisna Durandt, Karlien Kallmeyer, Melvin A. Ambele, and Michael S. Pepper. 2020. "The Role of Pref-1 during Adipogenic Differentiation: An Overview of Suggested Mechanisms" International Journal of Molecular Sciences 21, no. 11: 4104. https://doi.org/10.3390/ijms21114104
APA Styleda Silva, C., Durandt, C., Kallmeyer, K., Ambele, M. A., & Pepper, M. S. (2020). The Role of Pref-1 during Adipogenic Differentiation: An Overview of Suggested Mechanisms. International Journal of Molecular Sciences, 21(11), 4104. https://doi.org/10.3390/ijms21114104







