Graphene Oxide Scaffold Stimulates Differentiation and Proangiogenic Activities of Myogenic Progenitor Cells
Abstract
:1. Introduction
2. Results
2.1. GO Scaffold Characterisation
2.2. PMC Localise Preferentially on GO Scaffold
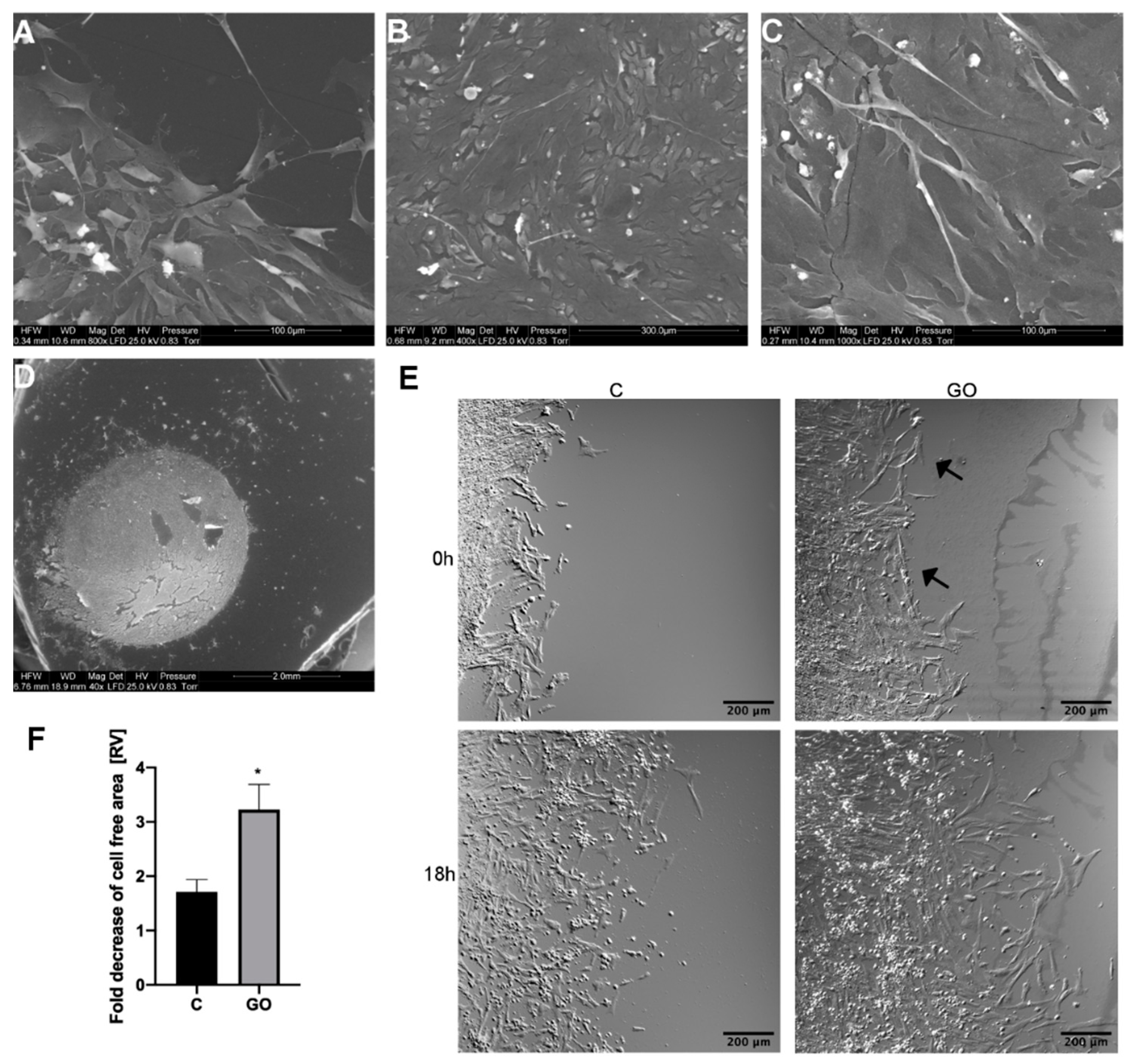
2.3. The GO Scaffold Increases Migration of PMC
2.4. The GO Scaffold Increases Expression of the MyoD1 mRNA and Enhances Synthesis of VEGF-A Proteins
3. Discussion
4. Materials and Methods
4.1. Graphene Oxide Scaffold
4.2. Cell Culture
4.3. Cell Morphology
4.4. Cell Viability
4.5. Analysis of PMC Migration on a GO Scaffold
4.6. mRNA Expression RT-PCR
4.7. Quantification of VEGF-A Protein Secretion
4.8. Angiogenesis Potential of PMC
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTB | actin beta |
| AFM | atomic force microscope |
| ATP5B | ATP synthase F1 subunit beta |
| DMEM | Dulbecco’s Modified Eagle Medium |
| ECM | extracellular matrix |
| FGF2 | fibroblast growth factor 2 |
| GO | graphene oxide |
| HH | Hamburger–Hamilton stage |
| HUVEC | human umbilical vein endothelial cells |
| MyoD1 | myogenic differentiation 1 |
| PCNA | proliferating cell nuclear antigen |
| PEC | primary eye cells |
| PHC | primary heart cells |
| PMC | primary mesenchyme cells |
| PNC | primary neuronal cells |
| PVC | primary blood vessel cells |
| SEM | scanning electron microscope |
| TEM | transmission electron microscope |
| VEGF-A | vascular endothelial growth factor A |
References
- Owen, S.C.; Shoichet, M.S. Design of three-dimensional biomimetic scaffolds. J. Biomed. Mater. Res. A 2010, 94, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Guilak, F.; Cohen, D.M.; Estes, B.T.; Gimble, J.M.; Liedtke, W.; Chen, C.S. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 2009, 5, 17–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flaim, C.J.; Teng, D.; Chien, S.; Bhatia, S.N. Combinatorial signaling microenvironments for studying stem cell fate. Stem Cells Dev. 2008. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yu, D.; Zeng, C.; Miao, Z.; Dai, L. Biocompatible Graphene Oxide-Based Glucose Biosensors. Langmuir 2010, 26, 6158–6160. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Nanda, S.; Papaefthymiou, G.; Yi, D. Functionalization of Graphene Oxide and its Biomedical Applications. Crit. Rev. Solid State Mater. Sci. 2015, 40, 1–25. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Mimeault, M.; Batra, S.K. Recent progress on tissue-resident adult stem cell biology and their therapeutic implications. Stem Cell Rev. 2008, 4, 27–49. [Google Scholar] [CrossRef] [Green Version]
- Tapscott, S.J. The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Dev. Camb. Engl. 2005, 132, 2685–2695. [Google Scholar] [CrossRef] [Green Version]
- Rudnicki, M.A.; Schnegelsberg, P.N.; Stead, R.H.; Braun, T.; Arnold, H.H.; Jaenisch, R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell 1993, 75, 1351–1359. [Google Scholar] [CrossRef]
- Rhoads, R.P.; Johnson, R.M.; Rathbone, C.R.; Liu, X.; Temm-Grove, C.; Sheehan, S.M.; Hoying, J.B.; Allen, R.E. Satellite cell-mediated angiogenesis in vitro coincides with a functional hypoxia-inducible factor pathway. Am. J. Physiol.-Cell Physiol. 2009, 296, 1321–1328. [Google Scholar] [CrossRef] [Green Version]
- Latroche, C.; Weiss-Gayet, M.; Muller, L.; Gitiaux, C.; Leblanc, P.; Liot, S.; Ben-Larbi, S.; Abou-Khalil, R.; Verger, N.; Bardot, P.; et al. Coupling between Myogenesis and Angiogenesis during Skeletal Muscle Regeneration is Stimulated by Restorative Macrophages. Stem Cell Rep. 2017, 9, 2018–2033. [Google Scholar] [CrossRef] [Green Version]
- Kenry; Lee, W.C.; Loh, K.P.; Lim, C.T. When stem cells meet graphene: Opportunities and challenges in regenerative medicine. Biomaterials 2018, 155, 236–250. [Google Scholar] [CrossRef]
- Bengtson, S.; Knudsen, K.B.; Kyjovska, Z.O.; Berthing, T.; Skaug, V.; Levin, M.; Koponen, I.K.; Shivayogimath, A.; Booth, T.J.; Alonso, B.; et al. Differences in inflammation and acute phase response but similar genotoxicity in mice following pulmonary exposure to graphene oxide and reduced graphene oxide. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [Green Version]
- Sydlik, S.A.; Jhunjhunwala, S.; Webber, M.J.; Anderson, D.G.; Langer, R. In vivo compatibility of graphene oxide with differing oxidation states. ACS Nano 2015, 9, 3866–3874. [Google Scholar] [CrossRef] [Green Version]
- Kurantowicz, N.; Strojny, B.; Sawosz, E.; Jaworski, S.; Kutwin, M.; Grodzik, M.; Wierzbicki, M.; Lipińska, L.; Mitura, K.; Chwalibog, A. Biodistribution of a High Dose of Diamond, Graphite, and Graphene Oxide Nanoparticles after Multiple Intraperitoneal Injections in Rats. Nanoscale Res. Lett. 2015, 10, 398. [Google Scholar] [CrossRef] [Green Version]
- Strojny, B.; Kurantowicz, N.; Sawosz, E.; Grodzik, M.; Jaworski, S.; Kutwin, M.; Wierzbicki, M.; Hotowy, A.; Lipińska, L.; Chwalibog, A. Long term influence of carbon nanoparticles on health and liver status in rats. PLoS ONE 2015, 10, e0144821. [Google Scholar] [CrossRef] [Green Version]
- Matesanz, M.-C.; Vila, M.; Feito, M.-J.; Linares, J.; Gonçalves, G.; Vallet-Regi, M.; Marques, P.-A.A.P.; Portolés, M.-T. The effects of graphene oxide nanosheets localized on F-actin filaments on cell-cycle alterations. Biomaterials 2013, 34, 1562–1569. [Google Scholar] [CrossRef]
- Hong, S.; Lee, S.; Yi, J. Sensitive and molecular size-selective detection of proteins using a chip-based and heteroliganded gold nanoisland by localized surface plasmon resonance spectroscopy. Nanoscale Res. Lett. 2011, 6, 336. [Google Scholar] [CrossRef] [Green Version]
- Lunova, M.; Zablotskii, V.; Dempsey, N.M.; Devillers, T.; Jirsa, M.; Syková, E.; Kubinová, Š.; Lunov, O.; Dejneka, A. Modulation of collective cell behaviour by geometrical constraints. Integr. Biol. Quant. Biosci. Nano Macro 2016, 8, 1099–1110. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Yan, L.; Li, M.; Zhao, R.; Yang, X.; Ji, T.; Gu, Z.; Yin, J.-J.; Gao, X.; Nie, G. Deciphering the underlying mechanisms of oxidation-state dependent cytotoxicity of graphene oxide on mammalian cells. Toxicol. Lett. 2015, 237, 61–71. [Google Scholar] [CrossRef]
- Chng, E.L.K.; Pumera, M. The toxicity of graphene oxides: Dependence on the oxidative methods used. Chem. Eur. J. 2013, 19, 8227–8235. [Google Scholar] [CrossRef]
- Song, J.; Gao, H.; Zhu, G.; Cao, X.; Shi, X.; Wang, Y. The preparation and characterization of polycaprolactone/graphene oxide biocomposite nanofiber scaffolds and their application for directing cell behaviors. Carbon 2015, 95, 1039–1050. [Google Scholar] [CrossRef]
- Bałaban, J.; Wierzbicki, M.; Zielińska, M.; Szczepaniak, J.; Sosnowska, M.; Daniluk, K.; Cysewski, D.; Koczoń, P.; Chwalibog, A.; Sawosz, E. Effects of Graphene Oxide Nanofilm and Chicken Embryo Muscle Extract on Muscle Progenitor Cell Differentiation and Contraction. Molecules 2020, 25, 1991. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.; Nancy, D.; Krishnan, A.G.; Anjusree, G.S.; Vadukumpully, S.; Nair, S.V. Graphene oxide nanoflakes incorporated gelatin-hydroxyapatite scaffolds enhance osteogenic differentiation of human mesenchymal stem cells. Nanotechnology 2015, 26. [Google Scholar] [CrossRef]
- Kim, I.S.; Song, Y.M.; Lee, B.; Hwang, S.J. Human mesenchymal stromal cells are mechanosensitive to vibration stimuli. J. Dent. Res. 2012, 91, 1135–1140. [Google Scholar] [CrossRef]
- Chaudhuri, B.; Bhadra, D.; Moroni, L.; Pramanik, K. Myoblast differentiation of human mesenchymal stem cells on graphene oxide and electrospun graphene oxide-polymer composite fibrous meshes: Importance of graphene oxide conductivity and dielectric constant on their biocompatibility. Biofabrication 2015, 7. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Shahsavar, M. Graphene nanogrids for selective and fast osteogenic differentiation of human mesenchymal stem cells. Carbon 2013, 59, 200–211. [Google Scholar] [CrossRef]
- Haase, K.; Pelling, A.E. Investigating cell mechanics with atomic force microscopy. J. R. Soc. Interface 2015, 12. [Google Scholar] [CrossRef]
- Chaillou, T.; Lanner, J.T. Regulation of myogenesis and skeletal muscle regeneration: Effects of oxygen levels on satellite cell activity. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2016, 30, 3929–3941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, S.B.; Erdenebileg, U.; Dashnyam, K.; Jin, G.-Z.; Cha, J.-R.; El-Fiqi, A.; Knowles, J.C.; Patel, K.D.; Lee, H.-H.; Lee, J.-H.; et al. Nano-graphene oxide/polyurethane nanofibers: Mechanically flexible and myogenic stimulating matrix for skeletal tissue engineering. J. Tissue Eng. 2020, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.-Q.; Tang, R.-Z. Biological responses to nanomaterials: Understanding nano-bio effects on cell behaviors. Drug Deliv. 2017, 24, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.S.J.; Zhang, M.; Evans, D.J.R. Changes in the proportion and number of Pax(7 + ve) and MF20(+ve) myoblasts during chick myogenesis in the head and limb. Int. J. Dev. Biol. 2004, 48, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Ishibashi, J.; Perry, R.L.; Asakura, A.; Rudnicki, M.A. MyoD induces myogenic differentiation through cooperation of its NH2- and COOH-terminal regions. J. Cell Biol. 2005, 171, 471–482. [Google Scholar] [CrossRef] [Green Version]
- Al-Musawi, S.L.; Lock, F.; Simbi, B.H.; Bayol, S.A.M.; Stickland, N.C. Muscle specific differences in the regulation of myogenic differentiation in chickens genetically selected for divergent growth rates. Differentiation 2011, 82, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Hu, P.; Geles, K.G.; Paik, J.-H.; DePinho, R.A.; Tjian, R. Codependent activators direct myoblast-specific MyoD transcription. Dev. Cell 2008, 15, 534–546. [Google Scholar] [CrossRef] [Green Version]
- Tortorella, L.L.; Milasincic, D.J.; Pilch, P.F. Critical proliferation-independent window for basic fibroblast growth factor repression of myogenesis via the p42/p44 MAPK signaling pathway. J. Biol. Chem. 2001, 276, 13709–13717. [Google Scholar] [CrossRef] [Green Version]
- Flann, K.L.; Rathbone, C.R.; Cole, L.C.; Liu, X.; Allen, R.E.; Rhoads, R.P. Hypoxia simultaneously alters satellite cell-mediated angiogenesis and hepatocyte growth factor expression. J. Cell. Physiol. 2014, 229, 572–579. [Google Scholar] [CrossRef]
- Rhoads, R.P.; Flann, K.L.; Cardinal, T.R.; Rathbone, C.R.; Liu, X.; Allen, R.E. Satellite cells isolated from aged or dystrophic muscle exhibit a reduced capacity to promote angiogenesis in vitro. Biochem. Biophys. Res. Commun. 2013, 440, 399–404. [Google Scholar] [CrossRef] [Green Version]
- Nillesen, S.T.M.; Geutjes, P.J.; Wismans, R.; Schalkwijk, J.; Daamen, W.F.; van Kuppevelt, T.H. Increased angiogenesis and blood vessel maturation in acellular collagen–heparin scaffolds containing both FGF2 and VEGF. Biomaterials 2007, 28, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Borselli, C.; Cezar, C.A.; Shvartsman, D.; Vandenburgh, H.H.; Mooney, D.J. The role of multifunctional delivery scaffold in the ability of cultured myoblasts to promote muscle regeneration. Biomaterials 2011, 32, 8905–8914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryan, B.A.; Walshe, T.E.; Mitchell, D.C.; Havumaki, J.S.; Saint-Geniez, M.; Maharaj, A.S.; Maldonado, A.E.; D’Amore, P.A. Coordinated vascular endothelial growth factor expression and signaling during skeletal myogenic differentiation. Mol. Biol. Cell 2008, 19, 994–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walthers, C.M.; Nazemi, A.; Patel, S.; Wu, B.; Dunn, J.C.Y. The Effect of Scaffold Macroporosity on Angiogenesis and Cell Survival in Tissue-Engineered Smooth Muscle. Biomaterials 2014, 35, 5129–5137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sosnowska, M.; Kutwin, M.; Jaworski, S.; Strojny, B.; Wierzbicki, M.; Szczepaniak, J.; Łojkowski, M.; Święszkowski, W.; Bałaban, J.; Chwalibog, A.; et al. Mechano-signalling, induced by fullerene C60 nanofilms, arrests the cell cycle in the G2/M phase and decreases proliferation of liver cancer cells. Int. J. Nanomed. 2019, 14, 6197–6215. [Google Scholar] [CrossRef] [Green Version]
- Heckman, C.; Kanagasundaram, S.; Cayer, M.; Paige, J. Preparation of cultured cells for scanning electron microscope. Protoc. Exch. 2007. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Carpentier, G.; Martinelli, M.; Courty, J.; Cascone, I. Angiogenesis Analyzer for ImageJ. 4th ImageJ User and Developer Conference Proceedings; Mondorf-les-Bains: Luxembourg, 2012; pp. 198–201. [Google Scholar]




| Target | Forward Primer | Reverse Primer |
|---|---|---|
| FGF2 | GGCACTGAAATGTGCAACAG | TCCAGGTCCAGTTTTTGGTC |
| VEGF-A | TGAGGGCCTAGAATGTGTCC | TCTTTTGACCCTTCCCCTTT |
| PCNA | TGCACGCATTTGTAGAGACC | AGTCAGCTGGACTGGCTCAT |
| MyoD1 | CGGCGGCTCAGCAAGGTCAAC | CGGCCCGCTGTACTCCATCATG |
| ATP5B | GTTATTCGGTGTTCGCTGGT | GTAGACCAGAGCGACCTTGG |
| ACTB | GTCCACCTTCCAGCAGATGT | ATAAAGCCATGCCAATCTCG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wierzbicki, M.; Hotowy, A.; Kutwin, M.; Jaworski, S.; Bałaban, J.; Sosnowska, M.; Wójcik, B.; Wędzińska, A.; Chwalibog, A.; Sawosz, E. Graphene Oxide Scaffold Stimulates Differentiation and Proangiogenic Activities of Myogenic Progenitor Cells. Int. J. Mol. Sci. 2020, 21, 4173. https://doi.org/10.3390/ijms21114173
Wierzbicki M, Hotowy A, Kutwin M, Jaworski S, Bałaban J, Sosnowska M, Wójcik B, Wędzińska A, Chwalibog A, Sawosz E. Graphene Oxide Scaffold Stimulates Differentiation and Proangiogenic Activities of Myogenic Progenitor Cells. International Journal of Molecular Sciences. 2020; 21(11):4173. https://doi.org/10.3390/ijms21114173
Chicago/Turabian StyleWierzbicki, Mateusz, Anna Hotowy, Marta Kutwin, Sławomir Jaworski, Jaśmina Bałaban, Malwina Sosnowska, Barbara Wójcik, Aleksandra Wędzińska, André Chwalibog, and Ewa Sawosz. 2020. "Graphene Oxide Scaffold Stimulates Differentiation and Proangiogenic Activities of Myogenic Progenitor Cells" International Journal of Molecular Sciences 21, no. 11: 4173. https://doi.org/10.3390/ijms21114173
APA StyleWierzbicki, M., Hotowy, A., Kutwin, M., Jaworski, S., Bałaban, J., Sosnowska, M., Wójcik, B., Wędzińska, A., Chwalibog, A., & Sawosz, E. (2020). Graphene Oxide Scaffold Stimulates Differentiation and Proangiogenic Activities of Myogenic Progenitor Cells. International Journal of Molecular Sciences, 21(11), 4173. https://doi.org/10.3390/ijms21114173








