Can Physical Activity Support the Endocannabinoid System in the Preventive and Therapeutic Approach to Neurological Disorders?
Abstract
:1. Introduction
2. An Overview of the Endocannabinoid System—From Endo to Phytocannabinoids
Phytocannabinoids—Compounds with Dualistic Nature
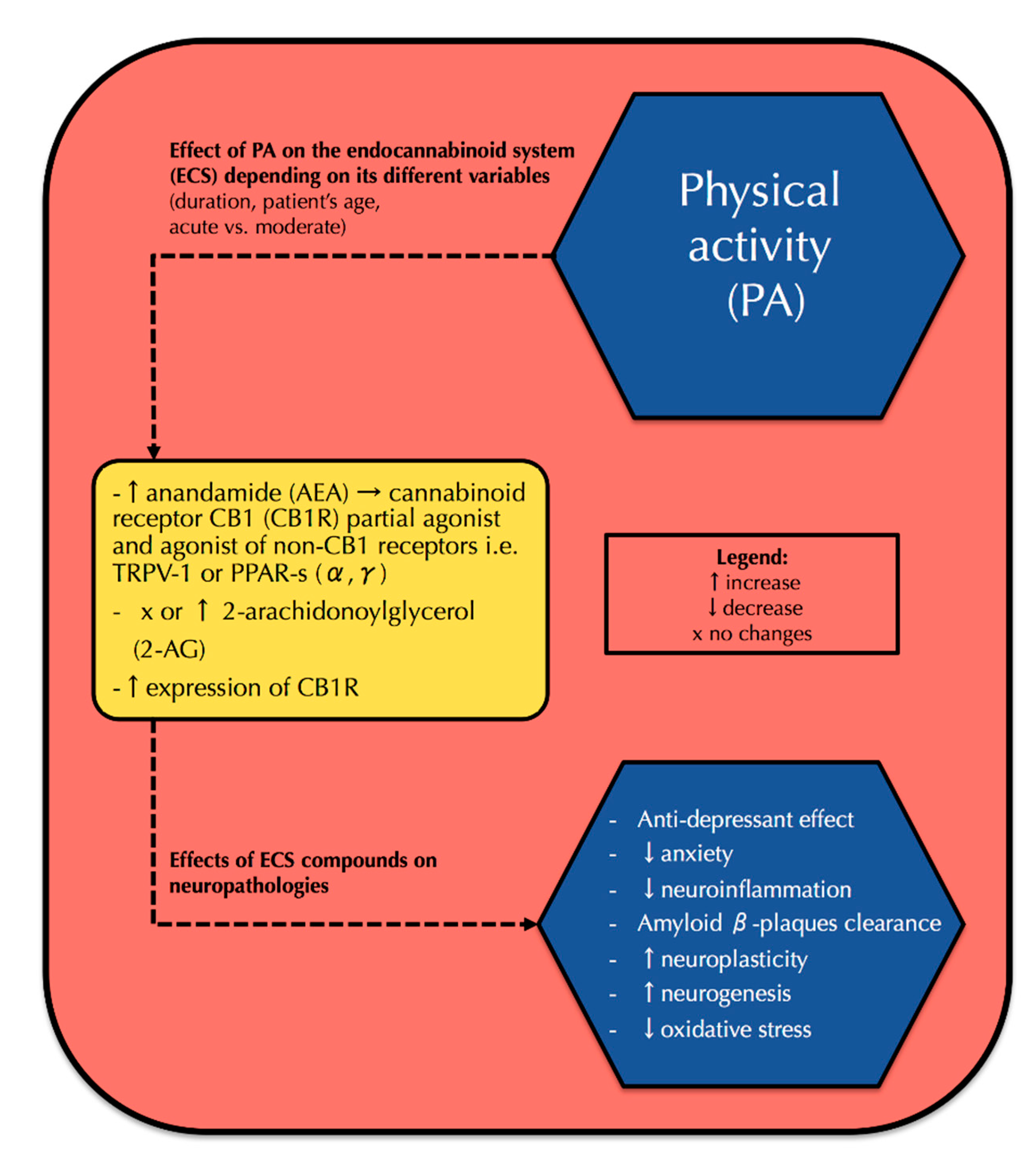
3. Physical Activity and Its Correlation with the Endocannabinoid System and Neurophysiology
3.1. PA and the Endocannabinoid System
3.2. PA and Neurophysiology—Interference with the Endocannabinoid System
4. The Endocannabinoid System and Its Correlation with Neuropathologies
4.1. Depression and Anxiety
4.2. Alzheimer’s Disease (AD)
4.3. Parkinson’s Disease (PD)
4.4. Multiple Sclerosis (MS)
4.5. Epilepsy
5. The Triad—Physical Activity, the Endocannabinoid System, and a Novel Therapeutic Approach to Neurological Pathologies—How Might All These Be Linked?
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 2-AG | 2-arachidonoylglycerol |
| 6-OHDA | oxidopamine |
| AA | arachidonic acid |
| ACEA | arachidonyl-2-chloroethylamide |
| ACEA | arachidonyl-2-chloroethylamide |
| ACh | acetylcholine |
| AD | Alzheimer’s disease |
| AEA | anandamide, N-arachidonoylethanolamine |
| Aβ | amyloid-β |
| BACE1 | β-secretase 1 |
| BDNF | brain-derived neurotrophic factor |
| CB1R | cannabinoid receptor type 1 |
| CB2R | cannabinoid receptor type 2 |
| CBCA | cannabichromenic acid synthase |
| CBD | cannabidiol |
| CBDA | cannabinoid acid synthase |
| CBGA | cannabigerolic acid |
| CBN | cannabinol |
| CSF | cerebrospinal fluid |
| DA | dopamine |
| DAG | diacylglycerol |
| DAGLα | diacylglycerol lipase α |
| DAGLβ | diacylglycerol lipase β |
| DHA | docosahexaenoic acid |
| EAE | autoimmune encephalomyelitis |
| eCB | endocannabinoid |
| eCBome | endocannabinoidome |
| ECS | endocannabinoid system |
| EHA | eicosapentaenoic acid |
| EPM | elevated plus maze test |
| FAAH | fatty-acid amide hydrolase |
| FST | forced swimming test |
| GABAHPA | γ-aminobutyric acidhypothalamic–pituitary–adrenal |
| HISE | high-intensity swimming exercise |
| HRmax | maximum heart rate |
| LC-PUFAs | long-chain polyunsaturated fatty acids |
| LPS | lipopolysaccharide |
| MAGL | monoacylglycerol lipase |
| MDD | major depressive disorder |
| MPTP | 1-methyl-4-phenyl-l,2,3,6-tetrahydropyridine |
| MS | multiple sclerosis |
| NAPE | N-arachidonoyl phosphatidylethanolamine |
| OEA | N-oleoylethanolamine |
| PA | physical activity |
| PD | Parkinson’s disease |
| PEA | palmitoylethanolamide |
| PPARα | peroxisome proliferator-activated receptors α |
| PPARγ | peroxisome proliferator-activated receptors γ |
| T2DM | type 2 diabetes mellitus |
| THCA | tetrahydrocannabinolic acid synthase |
| TRP | transient receptor potential |
| TRPV1 | transient receptor potential vanilloid type 1 |
| VTA | ventral tegmental area |
| Δ9-THC | Δ9-tetrahydrocannabinol |
| MVC | maximum ventilatory capacity |
| Wmax | maximal trial power output |
References
- Gitler, A.D.; Dhillon, P.; Shorter, J. Neurodegenerative disease: Models, mechanisms, and a new hope. Dis. Model. Mech. 2017, 10, 499–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erkkinen, M.G.; Kim, M.-O.; Geschwind, M.D. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2017, 10, a033118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feigin, V.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [Green Version]
- Salthouse, T. What and when of cognitive aging. Curr. Dir. Psychol. Sci. 2004, 13, 140–144. [Google Scholar] [CrossRef] [Green Version]
- Nichols, E.; Szoeke, C.E.I.; Vollset, S.E.; Abbasi, N.; Abd-Allah, F.; Abdela, J.; Aichour, M.T.E.; Akinyemi, R.O.; Alahdab, F.; Asgedom, S.W.; et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar] [CrossRef] [Green Version]
- Silvestri, C.; Di Marzo, V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013, 17, 475–490. [Google Scholar] [CrossRef] [Green Version]
- Bisogno, T.; Di Marzo, V. The role of the endocannabinoid system in Alzheimer’s disease: Facts and hypotheses. Curr. Pharm. Des. 2008, 14, 2299–2305. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, A.; McDaniel, W.F. Endocannabinoids and exercise. Br. J. Sports Med. 2004, 38, 536–541. [Google Scholar] [CrossRef]
- Feuerecker, M.; Hauer, D.; Toth, R.; Demetz, F.; Hölzl, J.; Thiel, M.; Kaufmann, I.; Schelling, G.; Choukér, A. Effects of exercise stress on the endocannabinoid system in humans under field conditions. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 112, 2777–2781. [Google Scholar] [CrossRef]
- Stone, N.L.; Millar, S.A.; Herrod, P.J.J.; Barrett, D.A.; Ortori, C.A.; Mellon, V.A.; O’Sullivan, S.E. An analysis of endocannabinoid concentrations and mood following singing and exercise in healthy volunteers. Front. Behav. Neurosci. 2018, 12. [Google Scholar] [CrossRef]
- Meyer, J.; Crombie, K.M.; Cook, D.B.; Hillard, C.J.; Koltyn, K.F. Serum endocannabinoid and mood changes after exercise in major depressive disorder. Med. Sci. Sports Exerc. 2019, 51, 1909–1917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coccaro, E.F.; Hill, M.N.; Robinson, L.; Lee, R.J. Circulating endocannabinoids and affect regulation in human subjects. Psychoneuroendocrinology 2018, 92, 66–71. [Google Scholar] [CrossRef] [PubMed]
- De Chiara, V.; Errico, F.; Musella, A.; Rossi, S.; Mataluni, G.; Sacchetti, L.; Siracusano, A.; Castelli, M.; Cavasinni, F.; Bernardi, G.; et al. Voluntary exercise and sucrose consumption enhance cannabinoid CB1 receptor sensitivity in the striatum. Neuropsychopharmacol. 2010, 35, 374–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.-C.; Mackie, K. an introduction to the endogenous cannabinoid system. Biol. Psychiatry 2015, 79, 516–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, S.; Kumar, U. Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef] [Green Version]
- Di Marzo, V. The endocannabinoid system in obesity and type 2 diabetes. Diabetologia 2008, 51, 1356–1367. [Google Scholar] [CrossRef] [Green Version]
- Nagarkatti, P.S.; Pandey, R.; Rieder, S.A.; Hegde, V.L.; Nagarkatti, M. Cannabinoids as novel anti-inflammatory drugs. Futur. Med. Chem. 2009, 1, 1333–1349. [Google Scholar] [CrossRef] [Green Version]
- Freitas, H.; Isaac, A.; Malcher-Lopes, R.; Diaz, B.; Trevenzoli, I.H.; Reis, R.A.D.M. Polyunsaturated fatty acids and endocannabinoids in health and disease. Nutr. Neurosci. 2017, 21, 695–714. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Harvey-White, J.; Osei-Hyiaman, D.; Razdan, R.; Gong, Q.; Chan, A.C.; Zhou, Z.; Huang, B.X.; Kim, H.-Y.; et al. A biosynthetic pathway for anandamide. Proc. Natl. Acad. Sci. USA 2006, 103, 13345–13350. [Google Scholar] [CrossRef] [Green Version]
- Fenwick, A.J.; Fowler, D.K.; Wu, S.-W.; Shaffer, F.J.; Lindberg, J.E.M.; Kinch, D.C.; Peters, J.H. Direct anandamide activation of TRPV1 produces divergent calcium and current responses. Front. Mol. Neurosci. 2017, 10. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, S.E. An update on PPAR activation by cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Marzo, V.; Silvestri, C. Lifestyle and metabolic syndrome: Contribution of the endocannabinoidome. Nutrients 2019, 11, 1956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veilleux, A.; Di Marzo, V.; Silvestri, C. The expanded endocannabinoid system/endocannabinoidome as a potential target for treating diabetes mellitus. Curr. Diabetes Rep. 2019, 19, 117. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. Cannabis therapeutics and the future of neurology. Front. Integr. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Cilio, M.R.; Cross, H.; Fernandez-Ruiz, J.; French, J.; Hill, C.; Katz, R.; Di Marzo, V.; Jutras-Aswad, D.; Notcutt, W.G.; et al. Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 2014, 55, 791–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, V.; Perucca, E. Pharmacological and therapeutic properties of cannabidiol for epilepsy. Drugs 2019, 79, 1435–1454. [Google Scholar] [CrossRef]
- Hanuš, L.O.; Meyer, S.M.; Munoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef] [Green Version]
- Degenhardt, F.; Stehle, F.; Kayser, O. The biosynthesis of cannabinoids. In Handbook of Cannabis and Related Pathologies; Academic Press: Cambridge, MA, USA, 2017; ISBN 9780128007563. [Google Scholar]
- Devinsky, O.; Marsh, E.; Friedman, D.; Thiele, E.; Laux, L.; Sullivan, J.; Miller, I.; Flamini, R.; Wilfong, A.A.; Filloux, F.; et al. Cannabidiol in patients with treatment-resistant epilepsy: An open-label interventional trial. Lancet Neurol. 2016, 15, 270–278. [Google Scholar] [CrossRef]
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 2017, 175, 133–150. [Google Scholar] [CrossRef]
- Turner, S.E.; Williams, C.M.; Iversen, L.; Whalley, B.J. Molecular pharmacology of phytocannabinoids. In Phytocannabinoids; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Du Plessis, S.S.; Agarwal, A.; Syriac, A. Marijuana, phytocannabinoids, the endocannabinoid system, and male fertility. J. Assist. Reprod. Genet. 2015, 32, 1575–1588. [Google Scholar] [CrossRef] [Green Version]
- Morales, P.; Reggio, P.H. CBD: A new hope? ACS Med. Chem. Lett. 2019, 10, 694–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zendulka, O.; Dovrtelova, G.; Nosková, K.; Turjap, M.; Sulcova, A.; Hanuš, L.; Juřica, J. Cannabinoids and cytochrome P450 interactions. Curr. Drug Metab. 2016, 17, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Tantimonaco, M.; Ceci, R.; Sabatini, S.; Catani, M.V.; Rossi, A.; Gasperi, V.; Maccarrone, M. Physical activity and the endocannabinoid system: An overview. Cell. Mol. Life Sci. 2014, 71, 2681–2698. [Google Scholar] [CrossRef] [PubMed]
- Sparling, P.B.; Giuffrida, A.; Piomelli, D.; Rosskopf, L.; Dietrich, A. Exercise activates the endocannabinoid system. NeuroReport 2003, 14, 1–3. [Google Scholar] [CrossRef]
- Heyman, E.; Gamelin, F.-X.; Goekint, M.; Piscitelli, F.; Roelands, B.; LeClair, E.; Di Marzo, V.; Meeusen, R. Intense exercise increases circulating endocannabinoid and BDNF levels in humans—Possible implications for reward and depression. Psychoneuroendocrinology 2012, 37, 844–851. [Google Scholar] [CrossRef]
- Heyman, E.; Gamelin, F.-X.; Aucouturier, J.; Di Marzo, V. The role of the endocannabinoid system in skeletal muscle and metabolic adaptations to exercise: Potential implications for the treatment of obesity. Obes. Rev. 2012, 13, 1110–1124. [Google Scholar] [CrossRef]
- Fuss, J.; Steinle, J.; Bindila, L.; Auer, M.K.; Kirchherr, H.; Lutz, B.; Gass, P. A runner’s high depends on cannabinoid receptors in mice. Proc. Natl. Acad. Sci. USA 2015, 112, 13105–13108. [Google Scholar] [CrossRef] [Green Version]
- Brellenthin, A.G.; Crombie, K.M.; Hillard, C.J.; Koltyn, K.F. Endocannabinoid and mood responses to exercise in adults with varying activity levels. Med. Sci. Sports Exerc. 2017, 49, 1688–1696. [Google Scholar] [CrossRef]
- Thompson, Z.; Argueta, D.; Garland, T.; DiPatrizio, N. Circulating levels of endocannabinoids respond acutely to voluntary exercise, are altered in mice selectively bred for high voluntary wheel running, and differ between the sexes. Physiol. Behav. 2016, 170, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Stensson, N.; Gerdle, B.; Ernberg, M.; Mannerkorpi, K.; Kosek, E.; Ghafouri, B. Increased anandamide and decreased pain and depression after exercise in fibromyalgia. Med. Sci. Sports Exerc. 2020. [Google Scholar] [CrossRef]
- Biedermann, S.V.; Auer, M.K.; Bindila, L.; Ende, G.; Lutz, B.; Weber-Fahr, W.; Gass, P.; Fuss, J. Restricted vs. unrestricted wheel running in mice: Effects on brain, behavior and endocannabinoids. Horm. Behav. 2016, 86, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Watkins, B. Endocannabinoids, exercise, pain, and a path to health with aging. Mol. Asp. Med. 2018, 64, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Brellenthin, A.G.; Koltyn, K.F. Exercise as an adjunctive treatment for cannabis use disorder. Am. J. Drug Alcohol Abus. 2016, 42, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Vieira, T.H.; Bastos, C.P.; Pereira, G.S.; Moreira, F.A.; Massensini, A. A role for the endocannabinoid system in exercise-induced spatial memory enhancement in mice. Hippocampus 2013, 24, 79–88. [Google Scholar] [CrossRef]
- Crombie, K.M.; Brellenthin, A.G.; Hillard, C.J.; Koltyn, K.F. Endocannabinoid and opioid system interactions in exercise-induced hypoalgesia. Pain Med. 2018, 19, 118–123. [Google Scholar] [CrossRef]
- Covey, D.P.; Mateo, Y.; Sulzer, D.; Cheer, J.F.; Lovinger, D.M. Endocannabinoid modulation of dopamine neurotransmission. Neuropharmacol. 2017, 124, 52–61. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Zou, L.; Li, H. The Endocannabinoid System as a potential mechanism through which exercise influences episodic memory function. Brain Sci. 2019, 9, 112. [Google Scholar] [CrossRef] [Green Version]
- Merrill, C.; Friend, L.N.; Newton, S.T.; Hopkins, Z.H.; Edwards, J. Ventral tegmental area dopamine and GABA neurons: Physiological properties and expression of mRNA for endocannabinoid biosynthetic elements. Sci. Rep. 2015, 5, 16176. [Google Scholar] [CrossRef] [Green Version]
- Hohmann, A.G.; Briley, E.M.; Herkenham, M. Pre- and postsynaptic distribution of cannabinoid and mu opioid receptors in rat spinal cord. Brain Res. 1999, 822, 17–25. [Google Scholar] [CrossRef]
- Oliveira, A.B.; De Mello, M.T.; Tufik, S.; Peres, M.F.P.; Belitardo, A.D.O. Weight loss and improved mood after aerobic exercise training are linked to lower plasma anandamide in healthy people. Physiol. Behav. 2019, 201, 191–197. [Google Scholar] [CrossRef]
- Hicks, S.D.; Jacob, P.; Perez, O.; Baffuto, M.; Gagnon, Z.; Middleton, F. The transcriptional signature of a runner’s high. Med. Sci. Sports Exerc. 2019, 51, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, A.; Weinstein, Y. Exercise addiction-diagnosis, bio-psychological mechanisms and treatment issues. Curr. Pharm. Des. 2014, 20, 4062–4069. [Google Scholar] [CrossRef] [PubMed]
- Liegro, D.; Schiera, G.; Proia, P.; Di Liegro, C.M.; Di Liegro, I. Physical activity and brain health. Genes 2019, 10, 720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koltyn, K.F.; Brellenthin, A.G.; Cook, D.B.; Sehgal, N.; Hillard, C. Mechanisms of exercise-induced hypoalgesia. J. Pain 2014, 15, 1294–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, R.D.S.; Galdino, G. Endogenous systems involved in exercise-induced analgesia. J. Physiol. Pharmacol. 2018, 69, 3–13. [Google Scholar]
- Hughes, L.; Patterson, S.D. The effect of blood flow restriction exercise on exercise-induced hypoalgesia and endogenous opioid and endocannabinoid mechanisms of pain modulation. J. Appl. Physiol. 2020, 128, 914–924. [Google Scholar] [CrossRef]
- Wade, B.; Loprinzi, P.D. The experimental effects of acute exercise on long-term emotional memory. J. Clin. Med. 2018, 7, 486. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Han, J. The endocannabinoid system regulates the moderate exercise-induced enhancement of learning and memory in mice. J. Sports Med. Phys. Fit. 2020, 60. [Google Scholar] [CrossRef]
- Hill, M.N.; Titterness, A.K.; Morrish, A.C.; Carrier, E.J.; Lee, T.T.-Y.; Gil-Mohapel, J.; Gorzalka, B.B.; Hillard, C.J.; Christie, B.R. Endogenous cannabinoid signaling is required for voluntary exercise-induced enhancement of progenitor cell proliferation in the hippocampus. Hippocampus 2010, 20, 513–523. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, F.F.; Ribeiro, F.; Rodrigues, R.; Sebastião, A.M.; Xapelli, S. Brain-derived neurotrophic factor (BDNF) role in cannabinoid-mediated neurogenesis. Front. Cell. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [Green Version]
- Maurus, I.; Hasan, A.; Röh, A.; Takahashi, S.; Rauchmann, B.; Keeser, D.; Malchow, B.; Schmitt, A.; Falkai, P. Neurobiological effects of aerobic exercise, with a focus on patients with schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Crombie, K.M.; Brellenthin, A.G.; Hillard, C.J.; Koltyn, K.F. Psychobiological responses to aerobic exercise in individuals with posttraumatic stress disorder. J. Trauma. Stress 2018, 31, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.B.; Ribeiro, R.T.; Mello, M.T.; Tufik, S.; Peres, M.F.P. Anandamide is related to clinical and cardiorespiratory benefits of aerobic exercise training in migraine patients: A randomized controlled clinical trial. Cannabis Cannabinoid Res. 2019, 4, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Mori, F.; Ljoka, C.; Nicoletti, C.G.; Kusayanagi, H.; Buttari, F.; Giordani, L.; Rossi, S.; Foti, C.; Centonze, D. CB1 receptor affects cortical plasticity and response to physiotherapy in multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2014, 1, e48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wade, N.; Wallace, A.L.; Swartz, A.M.; Lisdahl, K.M. Aerobic fitness level moderates the association between cannabis use and executive functioning and psychomotor speed following abstinence in adolescents and young adults. J. Int. Neuropsychol. Soc. 2018, 25, 134–145. [Google Scholar] [CrossRef]
- Sohroforouzani, A.M.; Shakerian, S.; Ghanbarzadeh, M.; Alaei, H.; Azam, M.S.; Saeed, S.; Mohsen, G.; Hojjatallah, A. Treadmill exercise improves LPS-induced memory impairments via endocannabinoid receptors and cyclooxygenase enzymes. Behav. Brain Res. 2019, 380, 112440. [Google Scholar] [CrossRef]
- Ludtke, D.D.; Siteneski, A.; Galassi, T.D.O.; Buffon, A.C.; Cidral-Filho, F.J.; Reed, W.R.; Salgado, A.S.I.; Dos Santos, A.R.; Martins, D.F. High-intensity swimming exercise reduces inflammatory pain in mice by activation of the endocannabinoid system. Scand. J. Med. Sci. Sports 2020. [Google Scholar] [CrossRef]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2019, 16, 9–29. [Google Scholar] [CrossRef]
- Ranieri, R.; Laezza, C.; Bifulco, M.; Marasco, D.; Malfitano, A.M. Endocannabinoid system in neurological disorders. Recent Patents CNS Drug Discov. 2016, 10, 90–112. [Google Scholar] [CrossRef]
- Mechoulam, R.; Parker, L.A. The endocannabinoid system and the brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef] [Green Version]
- Rubino, T.; Zamberletti, E.; Parolaro, D. Endocannabinoids and mental disorders. In Endocannabinoids; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Zhou, D.; Li, Y.; Tian, T.; Quan, W.; Wang, L.; Shao, Q.; Fu, L.Q.; Zhang, X.H.; Wang, X.Y.; Zhang, H.; et al. Role of the endocannabinoid system in the formation and development of depression. Pharmazie 2017, 72, 435–439. [Google Scholar] [PubMed]
- Neumeister, A.; Normandin, M.D.; Pietrzak, R.H.; Piomelli, D.; Zheng, M.-Q.; Gujarro-Anton, A.; Potenza, M.N.; Bailey, C.R.; Lin, S.-F.; Najafzadeh, S.; et al. Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: A positron emission tomography study. Mol. Psychiatry 2013, 18, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-J.; Chen, W.-W.; Zhang, X. Endocannabinoid system: Role in depression, reward and pain control (Review). Mol. Med. Rep. 2016, 14, 2899–2903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Muñoz, M.; Sánchez-Blázquez, P.; Callado, L.F.; Meana, J.J.; Garzón, J. Schizophrenia and depression, two poles of endocannabinoid system deregulation. Transl. Psychiatry 2017, 7, 1291. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Miao, Q.; Lu, X.; Zhang, Z.; Chen, M.; Zhang, J.; Zhai, J. The association of endocannabinoid receptor genes (CNR1 and CNR2) polymorphisms with depression: A meta-analysis. Medicine (Baltimore) 2019, 98, e17403. [Google Scholar] [CrossRef]
- Wyrofsky, R.; Mcgonigle, P.; Van Bockstaele, E.J. Drug discovery strategies that focus on the endocannabinoid signaling system in psychiatric disease. Expert Opin. Drug Discov. 2014, 10, 17–36. [Google Scholar] [CrossRef]
- Fernández-Ruiz, J.; Romero, J.; Ramos, J.A. Endocannabinoids and neurodegenerative disorders: Parkinson’s disease, Huntington’s chorea, Alzheimer’s disease, and others. In Endocannabinoids; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Basavarajappa, B.S.; Shivakumar, M.; Joshi, V.; Subbanna, S. Endocannabinoid system in neurodegenerative disorders. J. Neurochem. 2017, 142, 624–648. [Google Scholar] [CrossRef]
- De Oliveira, R.M.W.; Lino-De-Oliveira, C.; Guimarães, F.S.; Campos, A.C. Cannabinoid signalling in embryonic and adult neurogenesis: Possible implications for psychiatric and neurological disorders. Acta Neuropsychiatr. 2018, 31, 1–16. [Google Scholar] [CrossRef]
- Altamura, C.; Ventriglia, M.; Martini, M.G.; Montesano, D.; Errante, Y.; Piscitelli, F.; Scrascia, F.; Quattrocchi, C.C.; Palazzo, P.; Seccia, S.; et al. Elevation of plasma 2-arachidonoylglycerol levels in alzheimer’s disease patients as a potential protective mechanism against neurodegenerative decline. J. Alzheimer’s Dis. 2015, 46, 497–506. [Google Scholar] [CrossRef]
- Bedse, G.; Romano, A.; Lavecchia, A.M.; Cassano, T.; Gaetani, S. The role of endocannabinoid signaling in the molecular mechanisms of neurodegeneration in alzheimer’s disease. J. Alzheimer’s Dis. 2014, 43, 1115–1136. [Google Scholar] [CrossRef] [Green Version]
- Kelly, R.; Joers, V.; Tansey, M.G.; Mckernan, D.P. Microglial phenotypes and their relationship to the cannabinoid system: Therapeutic implications for Parkinson’s disease. Molecules 2020, 25, 453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Q.-W.; Yuan, Y.-H.; Chen, N.-H. The therapeutic role of cannabinoid receptors and its agonists or antagonists in Parkinson’s disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 96, 109745. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, S.; Kumar, P. Insight into the emerging role of striatal neurotransmitters in the pathophysiology of Parkinson’s disease and huntington’s disease: A review. Curr. Neuropharmacol. 2019, 17, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, F.; García-Gutiérrez, M.S.; Aracil-Fernández, A.; Lanciego, J.L.; Manzanares, J. Cannabinoid CB1 and CB2 receptors, and monoacylglycerol lipase gene expression alterations in the basal ganglia of patients with Parkinson’s disease. Neurotherapeutics 2018, 15, 459–469. [Google Scholar] [CrossRef] [Green Version]
- Ceccarini, J.; Casteels, C.; Ahmad, R.; Crabbé, M.; Van De Vliet, L. Regional changes in the type 1 cannabinoid receptor are associated with cognitive dysfunction in Parkinson ’ s disease. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2348–2357. [Google Scholar] [CrossRef]
- Bassi, M.S.; Sancesario, A.; Morace, R.; Centonze, D.; Iezzi, E. Cannabinoids in Parkinson’s disease. Cannabis Cannabinoid Res. 2017, 2, 21–29. [Google Scholar] [CrossRef]
- Mestre, L.; Carrillo-Salinas, F.J.; Mecha, M.; Feliu, A.; Guaza, C. Gut microbiota, cannabinoid system and neuroimmune interactions: New perspectives in multiple sclerosis. Biochem. Pharmacol. 2018, 157, 51–66. [Google Scholar] [CrossRef]
- Pryce, G.; Baker, D. Endocannabinoids in multiple sclerosis and amyotrophic lateral sclerosis. In Drug Delivery; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Di Marzo, V. Endocannabinoid pathways and their role in multiple sclerosis-related muscular dysfunction. Expert Rev. Neurother. 2011, 11, 9–14. [Google Scholar] [CrossRef]
- Jean-Gilles, L.; Feng, S.; Tench, C.; Chapman, V.; Kendall, D.; Barrett, D.A.; Constantinescu, C.S. Plasma endocannabinoid levels in multiple sclerosis. J. Neurol. Sci. 2009, 287, 212–215. [Google Scholar] [CrossRef]
- Rossi, S.; Bernardi, G.; Centonze, D. The endocannabinoid system in the inflammatory and neurodegenerative processes of multiple sclerosis and of amyotrophic lateral sclerosis. Exp. Neurol. 2010, 224, 92–102. [Google Scholar] [CrossRef]
- Cheung, K.A.K.; Peiris, H.N.; Wallace, G.; Holland, O.J.; Mitchell, M. The interplay between the endocannabinoid system, epilepsy and cannabinoids. Int. J. Mol. Sci. 2019, 20, 6079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Wang, Y.; Zhang, C.; Liu, C.; Yang, H.-F.; Hu, W.-H.; Zhang, J.-G.; Zhang, K. Endogenous cannabinoid system alterations and their role in epileptogenesis after brain injury in rat. Epilepsy Res. 2016, 128, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Katona, I. Cannabis and endocannabinoid signaling in epilepsy. In Drug Delivery; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Rosenberg, E.C.; Tsien, R.W.; Whalley, B.J.; Devinsky, O. Cannabinoids and epilepsy. Neurotherapeutics 2015, 12, 747–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessberger, S.; Parent, J.M. Epilepsy and adult neurogenesis. Cold Spring Harb. Perspect. Biol. 2015, 7, a020677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamelin, F.-X.; Aucouturier, J.; Iannotti, F.A.; Piscitelli, F.; Mazzarella, E.; Aveta, T.; Leriche, M.; Dupont, E.; Cieniewski-Bernard, C.; Montel, V.; et al. Effects of chronic exercise on the endocannabinoid system in Wistar rats with high-fat diet-induced obesity. J. Physiol. Biochem. 2016, 72, 183–199. [Google Scholar] [CrossRef]
- Fernández-Aranda, F.; Sauchelli, S.; Pastor, A.; González, M.L.; De La Torre, R.; Granero, R.; Jiménez-Murcia, S.; Baños, R.; Arbona, C.B.; Fernandez-Real, J.-M.; et al. Moderate-vigorous physical activity across body mass index in females: Moderating effect of endocannabinoids and temperament. PLoS ONE 2014, 9, e104534. [Google Scholar] [CrossRef] [Green Version]
| Subjects | Performed Activity | Main Outcomes | Reference |
|---|---|---|---|
| Healthy men runners (n = 8), cyclist (n = 8), controls (n = 8) | Running on a treadmill/cycling on an ergometer for 45 min (HRmax = 70%–80%) | ECS alterations: ↑AEA | [36] |
| Brain physiology and neurological alterations: Anxiolytic and analgesic effect, sense of well-being → “runner’s high” | |||
| Well trained male cyclist (n = 11) | Moderate cycling on an ergometer for 60 min (55% Wmax) followed by intense cycling for 30 min (75% Wmax) | ECS alterations: ↑AEA, PEA, OEA | [37] |
| Brain physiology and neurological alterations: Increased BDNF and cortisol levels, antidepressant and reward effect, possible promotion of neuroplasticity | |||
| Women with fibromyalgia (n = 37), controls (n = 33) | 15-week person-centered resistance exercise program | ECS alterations: ↑AEA, 2-AG | [42] |
| Brain physiology and neurological alterations: Antidepressant and analgesic effect, increased muscle strength | |||
| Patients with PTSD (n = 12), controls (n = 24) | Low/moderate 10 min warm-up (HRmax = 40%–60%) followed by 30 min of moderate walking or running on a treadmill (HRmax= 70%–75%). | ECS alterations: ↑AEA, 2-AG, OEA | [64] |
| Brain physiology and neurological alterations: Antidepressant effect, analgesic effect, reduced stress, fatigue, confusion, anger, and anxiety | |||
| Patients with episodic migraine (n = 30), controls (n = 28) | 12 week aerobic exercise program—40 min of walking/running on a treadmill 3 times per week | ECS alterations: ↓AEA | [65] |
| Brain physiology and neurological alterations: Amelioration of migraine headaches, reduced frequency of migraine attacks | |||
| Women with MDD (n = 17) | 30 min of moderate cycling followed by 30 min of preferred exercise | ECS alterations: ↑AEA, OEA, ↓2-AG | [11] |
| Brain physiology and neurological alterations: Minimal antidepressant effect | |||
| Patients with relapsing-remitting MS (n = 30) | 2 weeks of therapeutic exercise program—1 h of aerobic exercise followed by 1 h of swimming in the pool. | ECS alterations: Different polymorphisms in CNR1 gene lead to various responses on physical therapy associated with altered CB1R density in motor cortex. | [66] |
| Brain physiology and neurological alterations: ↑ cortical plasticity and response to physiotherapy | |||
| Healthy men (n = 29) and women (n = 29) | Isometric handgrip exercise for 3 min (MVC = 25%) | ECS alterations: ↑AEA, 2-AG, OEA, PEA ↑CB1R | [47] |
| Brain physiology and neurological alterations: Significant analgesic effect; ECS interplays with endogenous opioid release → “exercise-induced antinociception” | |||
| Healthy women (n = 9) | 1 day—30 min of dancing 2 day—30 min of cycling on ergometer | ECS alterations: ↑OEA (only while dancing) | [10] |
| Brain physiology and neurological alterations: Reduced appetite, decreased negative emotions, “runner’s high” | |||
| Cannabis users (n = 37), controls (n = 42) | Treadmill running | ECS alterations: not described | [67] |
| Brain physiology and neurological alterations: Improved psychomotor speed, visual memory, sequencing ability among cannabis users → possible interplay between cannabinoids and physical activity | |||
| Male Sprague-Dawley rats (n = 40) | Wheel running | ECS alterations: ↑AEA, CB1R | [61] |
| Brain physiology and neurological alterations: Increased progenitor cell proliferation within dentate gyrus, promotion of neurogenesis | |||
| Male Wistar rats treated with LPS (animal model presenting signs of neuroinflammation) | Forced treadmill running for 8 weeks 5 times per week. MWT performed. | ECS alterations: ↑2-AG, CB1R | [68] |
| Brain physiology and neurological alterations: Improved memory and cognitive function, reduced inflammatory effect ↓COX-2 | |||
| Male Swiss mice (n = 72) | 5 min of treadmill running for 3 days | ECS alterations: ↑CB1R | [46] |
| Brain physiology and neurological alterations: Increased spatial memory ↑BDNF | |||
| Male Swiss mice | High-intensity swimming exercise (HISE) | ECS alterations: ↑AEA, CB1R | [69] |
| Brain physiology and neurological alterations: Significant analgesic effect → “exercise-induced antinociception”, reduced inflammation |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charytoniuk, T.; Zywno, H.; Konstantynowicz-Nowicka, K.; Berk, K.; Bzdega, W.; Chabowski, A. Can Physical Activity Support the Endocannabinoid System in the Preventive and Therapeutic Approach to Neurological Disorders? Int. J. Mol. Sci. 2020, 21, 4221. https://doi.org/10.3390/ijms21124221
Charytoniuk T, Zywno H, Konstantynowicz-Nowicka K, Berk K, Bzdega W, Chabowski A. Can Physical Activity Support the Endocannabinoid System in the Preventive and Therapeutic Approach to Neurological Disorders? International Journal of Molecular Sciences. 2020; 21(12):4221. https://doi.org/10.3390/ijms21124221
Chicago/Turabian StyleCharytoniuk, Tomasz, Hubert Zywno, Karolina Konstantynowicz-Nowicka, Klaudia Berk, Wiktor Bzdega, and Adrian Chabowski. 2020. "Can Physical Activity Support the Endocannabinoid System in the Preventive and Therapeutic Approach to Neurological Disorders?" International Journal of Molecular Sciences 21, no. 12: 4221. https://doi.org/10.3390/ijms21124221
APA StyleCharytoniuk, T., Zywno, H., Konstantynowicz-Nowicka, K., Berk, K., Bzdega, W., & Chabowski, A. (2020). Can Physical Activity Support the Endocannabinoid System in the Preventive and Therapeutic Approach to Neurological Disorders? International Journal of Molecular Sciences, 21(12), 4221. https://doi.org/10.3390/ijms21124221





