Extracellular Vesicles: A Therapeutic Option for Liver Fibrosis
Abstract
:1. Introduction
2. Classification and Biogenesis of EVs
3. EVs to Treat Liver Diseases
4. EVs to Treat Liver Fibrosis
5. Advantages and Need for Future Clinical Applications of EVs
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EVs | Extracellular Vesicles |
| MVs | Microvesicles |
| ILVs | Intraluminal Vesicles |
| MVBs ESCRT | Multivesicular Bodies Endosomal Sorting Complex Required for Transport |
| HRS | Hepatocyte Growth Factor-Regulated Tyrosine Kinase Substrate |
| TSG101 | Tumor Susceptibility Gene 101 Protein |
| ALIX | Apoptosis-Linked Gene-2 Interacting Protein X |
| VPS4 | Vacuolar Protein Sorting-Associated Protein 4 |
| GTPase | Guanosine Triphosphatase |
| SNAREs | Soluble N-Ethylmaleimide-Sensitive Factor Attachment Protein Receptors |
| ARRDC1 | Arrestin Domain-Containing Protein-1 |
| ROCK | Rho-Associated Protein Kinase |
| ARF | ADP-Ribosylation Factor |
| miRNA | MicroRNA |
| HLSCs | Human Liver Stem Cells |
| MSCs | Mesenchymal Stromal Cells |
| ESC-MSCs | Embryonic Stem Cell-Derived MSCs |
| CCl4 | Carbon Tetrachloride |
| TNF-alpha | Tumor Necrosis Factor Alpha |
| IL | Interleukin |
| BM-MSCs | Bone Marrow MSCs |
| GPX1 | Glutathione Peroxidase 1 |
| ROS | Reactive Oxygen Species |
| IRI | Ischemia Reperfusion Injury |
| IFN | Interferon |
| MnSOD | Manganese Superoxide Dismutase |
| iPSC-MSCs | Induced Pluripotent Stem Cell-Derived MSCs |
| D-GalN | D-Galactosamine |
| Men-SCs | Menstrual Stem Cells |
| LPS | Lipopolysaccharide |
| ASCs | Adipose Stem Cells |
| lncRNA | Long Non-Coding RNA |
| conA | Concanavalin A |
| TGF | Transforming Growth Factor |
| HGF | Hepatocyte Growth Factor |
| TGF-beta | Trasforming Growth Factor Beta |
| AST | Aspartate Transaminase |
| ALT | Alanine Transaminase |
| EMT | Epithelial to Mesenchymal Transition |
| alpha-SMA | Alpha Smooth Muscle Actin |
| HSCs | Hepatic Stellate Cells |
| TIMP-1 | Tissue Inhibitor of Metalloproteinase-1 |
| CCNG1 | Cyclin G1 |
| IGF1R | Insulin-Like Growth Factor Receptor 1 |
| P4HA1 | Prolyl-4-Hydroxylase A1 |
| Am-MSCs | Amnion-Derived MSCs |
| PPAR | Peroxisome Proliferator-Activated Receptor |
| CCN2 | Connective Tissue Growth Factor |
| TAA | Thioacetamide |
| MMP | Matrix Metalloproteinase |
| gel-EVs | EVs Encapsulated in Polyethylene Glycol Macromeres |
| HUCPVCs | Human Umbilical Cord Perivascular Cells |
| IGF-1 | Insulin Like Growth Factor 1 |
| NASH | Non-Alcoholic Steato-Hepatitis |
| hUC-MSCs | Human Umbilical Cord MSCs |
| BDL | Bile Duct Ligation |
| TLR4 | Toll-Like Receptor 4 |
| STAT | Signa Transducer and Activation of Transcription |
| NF-kB | Nuclear Factor Kappa-light-chain-enhancer of Activated B Cells |
| TRAF | TNF Receptor Associated Factor |
| CCL | Chemokine (C-C motif) Ligand |
| SMAD | Small Mothers Against Decapentaplegic |
References
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, E.; Pap, E.; Kittel, A.; et al. Membrane Vesicles, Current State-of-the-Art: Emerging Role of Extracellular Vesicles. Cell. Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cocucci, E.; Meldolesi, J. Ectosomes and Exosomes: Shedding the Confusion between Extracellular Vesicles. Trends Cell Biol. 2015, 25, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Hirsova, P.; Ibrahim, S.H.; Verma, V.K.; Morton, L.A.; Shah, V.H.; LaRusso, N.F.; Gores, G.J.; Malhi, H. Extracellular Vesicles in Liver Pathobiology: Small Particles with Big Impact. Hepatology 2016, 64, 2219–2233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meldolesi, J. Exosomes and Ectosomes in Intercellular Communication. Curr. Biol. 2018, 28, R435–R444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hristov, M.; Erl, W.; Linder, S.; Weber, P.C. Apoptotic Bodies from Endothelial Cells Enhance the Number and Initiate the Differentiation of Human Endothelial Progenitor Cells in Vitro. Blood 2004, 104, 2761–2766. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic Comparison Defines Novel Markers to Characterize Heterogeneous Populations of Extracellular Vesicle Subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [Green Version]
- Witwer, K.W.; Buzás, E.I.; Bemis, L.T.; Bora, A.; Lässer, C.; Lötvall, J.; Nolte-’t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of Sample Collection, Isolation and Analysis Methods in Extracellular Vesicle Research. J. Extracell. Vesicles 2013, 2. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef] [Green Version]
- Hanson, P.I.; Cashikar, A. Multivesicular Body Morphogenesis. Annu. Rev. Cell Dev. Biol. 2012, 28, 337–362. [Google Scholar] [CrossRef]
- Hurley, J.H.; Odorizzi, G. Get on the Exosome Bus with ALIX. Nat. Cell Biol. 2012, 14, 654–655. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Moita, C.; van Niel, G.; Kowal, J.; Vigneron, J.; Benaroch, P.; Manel, N.; Moita, L.F.; Théry, C.; Raposo, G. Analysis of ESCRT Functions in Exosome Biogenesis, Composition and Secretion Highlights the Heterogeneity of Extracellular Vesicles. J. Cell. Sci. 2013, 126, 5553–5565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamai, K.; Tanaka, N.; Nakano, T.; Kakazu, E.; Kondo, Y.; Inoue, J.; Shiina, M.; Fukushima, K.; Hoshino, T.; Sano, K.; et al. Exosome Secretion of Dendritic Cells Is Regulated by Hrs, an ESCRT-0 Protein. Biochem. Biophys. Res. Commun. 2010, 399, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Razi, M.; Futter, C.E. Distinct Roles for Tsg101 and Hrs in Multivesicular Body Formation and Inward Vesiculation. Mol. Biol. Cell 2006, 17, 3469–3483. [Google Scholar] [CrossRef] [Green Version]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan-Syntenin-ALIX Regulates the Biogenesis of Exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef]
- Babst, M.; Davies, B.A.; Katzmann, D.J. Regulation of Vps4 during MVB Sorting and Cytokinesis. Traffic 2011, 12, 1298–1305. [Google Scholar] [CrossRef]
- Stuffers, S.; Sem Wegner, C.; Stenmark, H.; Brech, A. Multivesicular Endosome Biogenesis in the Absence of ESCRTs. Traffic 2009, 10, 925–937. [Google Scholar] [CrossRef]
- Escola, J.M.; Kleijmeer, M.J.; Stoorvogel, W.; Griffith, J.M.; Yoshie, O.; Geuze, H.J. Selective Enrichment of Tetraspan Proteins on the Internal Vesicles of Multivesicular Endosomes and on Exosomes Secreted by Human B-Lymphocytes. J. Biol. Chem. 1998, 273, 20121–20127. [Google Scholar] [CrossRef] [Green Version]
- Buschow, S.I.; Nolte-’t Hoen, E.N.M.; van Niel, G.; Pols, M.S.; ten Broeke, T.; Lauwen, M.; Ossendorp, F.; Melief, C.J.M.; Raposo, G.; Wubbolts, R.; et al. MHC II in Dendritic Cells Is Targeted to Lysosomes or T Cell-Induced Exosomes via Distinct Multivesicular Body Pathways. Traffic 2009, 10, 1528–1542. [Google Scholar] [CrossRef]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in Extracellular Vesicle Formation and Function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef] [Green Version]
- Van Niel, G.; Charrin, S.; Simoes, S.; Romao, M.; Rochin, L.; Saftig, P.; Marks, M.S.; Rubinstein, E.; Raposo, G. The Tetraspanin CD63 Regulates ESCRT-Independent and—Dependent Endosomal Sorting during Melanogenesis. Dev. Cell 2011, 21, 708–721. [Google Scholar] [CrossRef] [Green Version]
- Chairoungdua, A.; Smith, D.L.; Pochard, P.; Hull, M.; Caplan, M.J. Exosome Release of β-Catenin: A Novel Mechanism That Antagonizes Wnt Signaling. J. Cell Biol. 2010, 190, 1079–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazarenko, I.; Rana, S.; Baumann, A.; McAlear, J.; Hellwig, A.; Trendelenburg, M.; Lochnit, G.; Preissner, K.T.; Zöller, M. Cell Surface Tetraspanin Tspan8 Contributes to Molecular Pathways of Exosome-Induced Endothelial Cell Activation. Cancer Res. 2010, 70, 1668–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Hernandez, D.; Gutiérrez-Vázquez, C.; Jorge, I.; López-Martín, S.; Ursa, A.; Sánchez-Madrid, F.; Vázquez, J.; Yáñez-Mó, M. The Intracellular Interactome of Tetraspanin-Enriched Microdomains Reveals Their Function as Sorting Machineries toward Exosomes. J. Biol. Chem. 2013, 288, 11649–11661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurwitz, S.N.; Conlon, M.M.; Rider, M.A.; Brownstein, N.C.; Meckes, D.G. Nanoparticle Analysis Sheds Budding Insights into Genetic Drivers of Extracellular Vesicle Biogenesis. J. Extracell. Vesicles 2016, 5, 31295. [Google Scholar] [CrossRef]
- Brügger, B.; Bankaitis, V.A. Lipids and Vesicular Transport. Biochim. Biophys. Acta 2012, 1821, 1039. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide Triggers Budding of Exosome Vesicles into Multivesicular Endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef]
- Mittelbrunn, M.; Gutiérrez-Vázquez, C.; Villarroya-Beltri, C.; González, S.; Sánchez-Cabo, F.; González, M.Á.; Bernad, A.; Sánchez-Madrid, F. Unidirectional Transfer of MicroRNA-Loaded Exosomes from T Cells to Antigen-Presenting Cells. Nat. Commun. 2011, 2, 282. [Google Scholar] [CrossRef] [Green Version]
- Savina, A.; Furlán, M.; Vidal, M.; Colombo, M.I. Exosome Release Is Regulated by a Calcium-Dependent Mechanism in K562 Cells. J. Biol. Chem. 2003, 278, 20083–20090. [Google Scholar] [CrossRef] [Green Version]
- Krämer-Albers, E.-M.; Bretz, N.; Tenzer, S.; Winterstein, C.; Möbius, W.; Berger, H.; Nave, K.-A.; Schild, H.; Trotter, J. Oligodendrocytes Secrete Exosomes Containing Major Myelin and Stress-Protective Proteins: Trophic Support for Axons? Proteom. Clin. Appl 2007, 1, 1446–1461. [Google Scholar] [CrossRef]
- Hoshino, D.; Kirkbride, K.C.; Costello, K.; Clark, E.S.; Sinha, S.; Grega-Larson, N.; Tyska, M.J.; Weaver, A.M. Exosome Secretion Is Enhanced by Invadopodia and Drives Invasive Behavior. Cell Rep. 2013, 5, 1159–1168. [Google Scholar] [CrossRef] [Green Version]
- Granger, E.; McNee, G.; Allan, V.; Woodman, P. The Role of the Cytoskeleton and Molecular Motors in Endosomal Dynamics. Semin. Cell Dev. Biol. 2014, 31, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Hoshino, D.; Hong, N.H.; Kirkbride, K.C.; Grega-Larson, N.E.; Seiki, M.; Tyska, M.J.; Weaver, A.M. Cortactin Promotes Exosome Secretion by Controlling Branched Actin Dynamics. J. Cell Biol. 2016, 214, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Antonyak, M.A.; Wilson, K.F.; Cerione, R.A. R(h)Oads to Microvesicles. Small GTPases 2012, 3, 219–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyenne, V.; Apaydin, A.; Rodriguez, D.; Spiegelhalter, C.; Hoff-Yoessle, S.; Diem, M.; Tak, S.; Lefebvre, O.; Schwab, Y.; Goetz, J.G.; et al. RAL-1 Controls Multivesicular Body Biogenesis and Exosome Secretion. J. Cell Biol. 2015, 211, 27–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonifacino, J.S.; Glick, B.S. The Mechanisms of Vesicle Budding and Fusion. Cell 2004, 116, 153–166. [Google Scholar] [CrossRef] [Green Version]
- Zylbersztejn, K.; Galli, T. Vesicular Traffic in Cell Navigation. FEBS J. 2011, 278, 4497–4505. [Google Scholar] [CrossRef]
- Stenmark, H. Rab GTPases as Coordinators of Vesicle Traffic. Nat. Rev. Mol. Cell Biol. 2009, 10, 513–525. [Google Scholar] [CrossRef]
- Zhen, Y.; Stenmark, H. Cellular Functions of Rab GTPases at a Glance. J. Cell. Sci. 2015, 128, 3171–3176. [Google Scholar] [CrossRef] [Green Version]
- Savina, A.; Fader, C.M.; Damiani, M.T.; Colombo, M.I. Rab11 Promotes Docking and Fusion of Multivesicular Bodies in a Calcium-Dependent Manner. Traffic 2005, 6, 131–143. [Google Scholar] [CrossRef]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b Control Different Steps of the Exosome Secretion Pathway. Nat. Cell Biol. 2010, 12, 19–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobrie, A.; Krumeich, S.; Reyal, F.; Recchi, C.; Moita, L.F.; Seabra, M.C.; Ostrowski, M.; Théry, C. Rab27a Supports Exosome-Dependent and -Independent Mechanisms That Modify the Tumor Microenvironment and Can Promote Tumor Progression. Cancer Res. 2012, 72, 4920–4930. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.; Morohashi, Y.; Yoshimura, S.-I.; Manrique-Hoyos, N.; Jung, S.; Lauterbach, M.A.; Bakhti, M.; Grønborg, M.; Möbius, W.; Rhee, J.; et al. Regulation of Exosome Secretion by Rab35 and Its GTPase-Activating Proteins TBC1D10A-C. J. Cell Biol. 2010, 189, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Nabhan, J.F.; Hu, R.; Oh, R.S.; Cohen, S.N.; Lu, Q. Formation and Release of Arrestin Domain-Containing Protein 1-Mediated Microvesicles (ARMMs) at Plasma Membrane by Recruitment of TSG101 Protein. Proc. Natl. Acad. Sci. USA 2012, 109, 4146–4151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muralidharan-Chari, V.; Clancy, J.W.; Sedgwick, A.; D’Souza-Schorey, C. Microvesicles: Mediators of Extracellular Communication during Cancer Progression. J. Cell. Sci. 2010, 123, 1603–1611. [Google Scholar] [CrossRef] [Green Version]
- McMahon, H.T.; Boucrot, E. Membrane Curvature at a Glance. J. Cell. Sci. 2015, 128, 1065–1070. [Google Scholar] [CrossRef] [Green Version]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Li, B.; Antonyak, M.A.; Zhang, J.; Cerione, R.A. RhoA Triggers a Specific Signaling Pathway That Generates Transforming Microvesicles in Cancer Cells. Oncogene 2012, 31, 4740–4749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muralidharan-Chari, V.; Clancy, J.; Plou, C.; Romao, M.; Chavrier, P.; Raposo, G.; D’Souza-Schorey, C. ARF6-Regulated Shedding of Tumor Cell-Derived Plasma Membrane Microvesicles. Curr. Biol. 2009, 19, 1875–1885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Souza-Schorey, C.; Chavrier, P. ARF Proteins: Roles in Membrane Traffic and Beyond. Nat. Rev. Mol. Cell Biol. 2006, 7, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Pellon-Cardenas, O.; Clancy, J.; Uwimpuhwe, H.; D’Souza-Schorey, C. ARF6-Regulated Endocytosis of Growth Factor Receptors Links Cadherin-Based Adhesion to Canonical Wnt Signaling in Epithelia. Mol. Cell. Biol. 2013, 33, 2963–2975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlienger, S.; Campbell, S.; Claing, A. ARF1 Regulates the Rho/MLC Pathway to Control EGF-Dependent Breast Cancer Cell Invasion. Mol. Biol. Cell 2014, 25, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; D’Asti, E.; Magnus, N.; Al-Nedawi, K.; Meehan, B.; Rak, J. Microvesicles as Mediators of Intercellular Communication in Cancer--the Emerging Science of Cellular “Debris”. Semin. Immunopathol. 2011, 33, 455–467. [Google Scholar] [CrossRef] [Green Version]
- Villarroya-Beltri, C.; Baixauli, F.; Gutiérrez-Vázquez, C.; Sánchez-Madrid, F.; Mittelbrunn, M. Sorting It out: Regulation of Exosome Loading. Semin. Cancer Biol. 2014, 28, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and Mechanisms of Extracellular Vesicle Uptake. J. Extracell. Vesicles 2014, 3. [Google Scholar] [CrossRef] [Green Version]
- Bruno, S.; Grange, C.; Deregibus, M.C.; Calogero, R.A.; Saviozzi, S.; Collino, F.; Morando, L.; Busca, A.; Falda, M.; Bussolati, B.; et al. Mesenchymal Stem Cell-Derived Microvesicles Protect against Acute Tubular Injury. J. Am. Soc. Nephrol. 2009, 20, 1053–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 Identifies Cancer Exosomes and Detects Early Pancreatic Cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Rana, S.; Yue, S.; Stadel, D.; Zöller, M. Toward Tailored Exosomes: The Exosomal Tetraspanin Web Contributes to Target Cell Selection. Int. J. Biochem. Cell Biol. 2012, 44, 1574–1584. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour Exosome Integrins Determine Organotropic Metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Barrès, C.; Blanc, L.; Bette-Bobillo, P.; André, S.; Mamoun, R.; Gabius, H.-J.; Vidal, M. Galectin-5 Is Bound onto the Surface of Rat Reticulocyte Exosomes and Modulates Vesicle Uptake by Macrophages. Blood 2010, 115, 696–705. [Google Scholar] [CrossRef] [Green Version]
- Frey, B.; Gaipl, U.S. The Immune Functions of Phosphatidylserine in Membranes of Dying Cells and Microvesicles. Semin. Immunopathol. 2011, 33, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, A.; Bandari, S.K.; Liu, J.; Mobley, J.A.; Brown, E.E.; Sanderson, R.D. Fibronectin on the Surface of Myeloma Cell-Derived Exosomes Mediates Exosome-Cell Interactions. J. Biol. Chem. 2016, 291, 1652–1663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leiss, M.; Beckmann, K.; Girós, A.; Costell, M.; Fässler, R. The Role of Integrin Binding Sites in Fibronectin Matrix Assembly in Vivo. Curr. Opin. Cell Biol. 2008, 20, 502–507. [Google Scholar] [CrossRef]
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M.Z. Embryonic Stem Cell-Derived Microvesicles Reprogram Hematopoietic Progenitors: Evidence for Horizontal Transfer of MRNA and Protein Delivery. Leukemia 2006, 20, 847–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deregibus, M.C.; Cantaluppi, V.; Calogero, R.; Lo Iacono, M.; Tetta, C.; Biancone, L.; Bruno, S.; Bussolati, B.; Camussi, G. Endothelial Progenitor Cell Derived Microvesicles Activate an Angiogenic Program in Endothelial Cells by a Horizontal Transfer of MRNA. Blood 2007, 110, 2440–2448. [Google Scholar] [CrossRef] [Green Version]
- Penfornis, P.; Vallabhaneni, K.C.; Whitt, J.; Pochampally, R. Extracellular Vesicles as Carriers of MicroRNA, Proteins and Lipids in Tumor Microenvironment. Int. J. Cancer 2016, 138, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-Mediated Transfer of MRNAs and MicroRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [Green Version]
- Skog, J.; Würdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Sena-Esteves, M.; Curry, W.T.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma Microvesicles Transport RNA and Proteins That Promote Tumour Growth and Provide Diagnostic Biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef]
- Collino, F.; Deregibus, M.C.; Bruno, S.; Sterpone, L.; Aghemo, G.; Viltono, L.; Tetta, C.; Camussi, G. Microvesicles Derived from Adult Human Bone Marrow and Tissue Specific Mesenchymal Stem Cells Shuttle Selected Pattern of MiRNAs. PLoS ONE 2010, 5, e11803. [Google Scholar] [CrossRef]
- Ekström, K.; Valadi, H.; Sjöstrand, M.; Malmhäll, C.; Bossios, A.; Eldh, M.; Lötvall, J. Characterization of MRNA and MicroRNA in Human Mast Cell-Derived Exosomes and Their Transfer to Other Mast Cells and Blood CD34 Progenitor Cells. J. Extracell. Vesicles 2012, 1. [Google Scholar] [CrossRef]
- Nolte-’t Hoen, E.N.M.; Buermans, H.P.J.; Waasdorp, M.; Stoorvogel, W.; Wauben, M.H.M.; ’t Hoen, P.A.C. Deep Sequencing of RNA from Immune Cell-Derived Vesicles Uncovers the Selective Incorporation of Small Non-Coding RNA Biotypes with Potential Regulatory Functions. Nucleic Acids Res. 2012, 40, 9272–9285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boon, R.A.; Vickers, K.C. Intercellular Transport of MicroRNAs. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 186–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiabotto, G.; Camussi, G.; Bruno, S. Role of NcRNAs in Modulation of Liver Fibrosis by Extracellular Vesicles. ExRNA 2020, 2, 7. [Google Scholar] [CrossRef]
- Herrera, M.B.; Fonsato, V.; Gatti, S.; Deregibus, M.C.; Sordi, A.; Cantarella, D.; Calogero, R.; Bussolati, B.; Tetta, C.; Camussi, G. Human Liver Stem Cell-Derived Microvesicles Accelerate Hepatic Regeneration in Hepatectomized Rats. J. Cell. Mol. Med. 2010, 14, 1605–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.Y.; Lai, R.C.; Wong, W.; Dan, Y.Y.; Lim, S.-K.; Ho, H.K. Mesenchymal Stem Cell-Derived Exosomes Promote Hepatic Regeneration in Drug-Induced Liver Injury Models. Stem Cell Res. Ther. 2014, 5, 76. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Jiang, W.; Tan, Y.; Zou, S.; Zhang, H.; Mao, F.; Gong, A.; Qian, H.; Xu, W. HucMSC Exosome-Derived GPX1 Is Required for the Recovery of Hepatic Oxidant Injury. Mol. Ther. 2017, 25, 465–479. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Tan, Y.; Cai, M.; Zhao, T.; Mao, F.; Zhang, X.; Xu, W.; Yan, Z.; Qian, H.; Yan, Y. Human Umbilical Cord MSC-Derived Exosomes Suppress the Development of CCl4-Induced Liver Injury through Antioxidant Effect. Stem. Cell. Int. 2018, 2018, 6079642. [Google Scholar] [CrossRef] [Green Version]
- Haga, H.; Yan, I.K.; Borrelli, D.A.; Matsuda, A.; Parasramka, M.; Shukla, N.; Lee, D.D.; Patel, T. Extracellular Vesicles from Bone Marrow-Derived Mesenchymal Stem Cells Protect against Murine Hepatic Ischemia/Reperfusion Injury. Liver Transpl. 2017, 23, 791–803. [Google Scholar] [CrossRef]
- Nong, K.; Wang, W.; Niu, X.; Hu, B.; Ma, C.; Bai, Y.; Wu, B.; Wang, Y.; Ai, K. Hepatoprotective Effect of Exosomes from Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells against Hepatic Ischemia-Reperfusion Injury in Rats. Cytotherapy 2016, 18, 1548–1559. [Google Scholar] [CrossRef]
- Du, Y.; Li, D.; Han, C.; Wu, H.; Xu, L.; Zhang, M.; Zhang, J.; Chen, X. Exosomes from Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells (HiPSC-MSCs) Protect Liver against Hepatic Ischemia/ Reperfusion Injury via Activating Sphingosine Kinase and Sphingosine-1-Phosphate Signaling Pathway. Cell. Physiol. Biochem. 2017, 43, 611–625. [Google Scholar] [CrossRef]
- Yao, J.; Zheng, J.; Cai, J.; Zeng, K.; Zhou, C.; Zhang, J.; Li, S.; Li, H.; Chen, L.; He, L.; et al. Extracellular Vesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Rat Hepatic Ischemia-Reperfusion Injury by Suppressing Oxidative Stress and Neutrophil Inflammatory Response. FASEB J. 2019, 33, 1695–1710. [Google Scholar] [CrossRef] [Green Version]
- Anger, F.; Camara, M.; Ellinger, E.; Germer, C.-T.; Schlegel, N.; Otto, C.; Klein, I. Human Mesenchymal Stromal Cell-Derived Extracellular Vesicles Improve Liver Regeneration After Ischemia Reperfusion Injury in Mice. Stem Cell. Dev. 2019, 28, 1451–1462. [Google Scholar] [CrossRef]
- Haga, H.; Yan, I.K.; Takahashi, K.; Matsuda, A.; Patel, T. Extracellular Vesicles from Bone Marrow-Derived Mesenchymal Stem Cells Improve Survival from Lethal Hepatic Failure in Mice. Stem Cell. Transl. Med. 2017, 6, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xiang, B.; Wang, X.; Xiang, C. Exosomes Derived from Human Menstrual Blood-Derived Stem Cells Alleviate Fulminant Hepatic Failure. Stem Cell Res. Ther. 2017, 8, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Wang, J.; Li, H.; Gao, S.; Shi, R.; Yang, D.; Wang, X.; Wang, X.; Zhu, L.; Wang, X.; et al. Extracellular Vesicles Secreted by Human Adipose-Derived Stem Cells (HASCs) Improve Survival Rate of Rats with Acute Liver Failure by Releasing LncRNA H19. EBioMedicine 2018, 34, 231–242. [Google Scholar] [CrossRef] [Green Version]
- Tamura, R.; Uemoto, S.; Tabata, Y. Immunosuppressive Effect of Mesenchymal Stem Cell-Derived Exosomes on a Concanavalin A-Induced Liver Injury Model. Inflamm. Regen. 2016, 36, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damania, A.; Jaiman, D.; Teotia, A.K.; Kumar, A. Mesenchymal Stromal Cell-Derived Exosome-Rich Fractionated Secretome Confers a Hepatoprotective Effect in Liver Injury. Stem Cell Res. Ther. 2018, 9, 31. [Google Scholar] [CrossRef] [Green Version]
- Nojima, H.; Freeman, C.M.; Schuster, R.M.; Japtok, L.; Kleuser, B.; Edwards, M.J.; Gulbins, E.; Lentsch, A.B. Hepatocyte Exosomes Mediate Liver Repair and Regeneration via Sphingosine-1-Phosphate. J. Hepatol. 2016, 64, 60–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Yan, Y.; Wang, B.; Qian, H.; Zhang, X.; Shen, L.; Wang, M.; Zhou, Y.; Zhu, W.; Li, W.; et al. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Liver Fibrosis. Stem Cells Dev. 2013, 22, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Qu, Y.; Zhang, Q.; Cai, X.; Li, F.; Ma, Z.; Xu, M.; Lu, L. Exosomes Derived from MiR-181-5p-Modified Adipose-Derived Mesenchymal Stem Cells Prevent Liver Fibrosis via Autophagy Activation. J. Cell. Mol. Med. 2017, 21, 2491–2502. [Google Scholar] [CrossRef]
- Povero, D.; Pinatel, E.M.; Leszczynska, A.; Goyal, N.P.; Nishio, T.; Kim, J.; Kneiber, D.; de Araujo Horcel, L.; Eguchi, A.; Ordonez, P.M.; et al. Human Induced Pluripotent Stem Cell-Derived Extracellular Vesicles Reduce Hepatic Stellate Cell Activation and Liver Fibrosis. JCI Insight 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Ohara, M.; Ohnishi, S.; Hosono, H.; Yamamoto, K.; Yuyama, K.; Nakamura, H.; Fu, Q.; Maehara, O.; Suda, G.; Sakamoto, N. Extracellular Vesicles from Amnion-Derived Mesenchymal Stem Cells Ameliorate Hepatic Inflammation and Fibrosis in Rats. Stem Cells Int. 2018, 2018, 3212643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rong, X.; Liu, J.; Yao, X.; Jiang, T.; Wang, Y.; Xie, F. Human Bone Marrow Mesenchymal Stem Cells-Derived Exosomes Alleviate Liver Fibrosis through the Wnt/β-Catenin Pathway. Stem Cell Res. Ther. 2019, 10, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Chen, R.; Kemper, S.; Cong, M.; You, H.; Brigstock, D.R. Therapeutic Effects of Serum Extracellular Vesicles in Liver Fibrosis. J. Extracell. Vesicles 2018, 7, 1461505. [Google Scholar] [CrossRef]
- Li, X.; Chen, R.; Kemper, S.; Brigstock, D.R. Extracellular Vesicles from Hepatocytes Are Therapeutic for Toxin-Mediated Fibrosis and Gene Expression in the Liver. Front. Cell Dev. Biol 2020, 7, 368. [Google Scholar] [CrossRef]
- Mardpour, S.; Hassani, S.-N.; Mardpour, S.; Sayahpour, F.; Vosough, M.; Ai, J.; Aghdami, N.; Hamidieh, A.A.; Baharvand, H. Extracellular Vesicles Derived from Human Embryonic Stem Cell-MSCs Ameliorate Cirrhosis in Thioacetamide-Induced Chronic Liver Injury. J. Cell. Physiol. 2018, 233, 9330–9344. [Google Scholar] [CrossRef]
- Mardpour, S.; Ghanian, M.H.; Sadeghi-Abandansari, H.; Mardpour, S.; Nazari, A.; Shekari, F.; Baharvand, H. Hydrogel-Mediated Sustained Systemic Delivery of Mesenchymal Stem Cell-Derived Extracellular Vesicles Improves Hepatic Regeneration in Chronic Liver Failure. ACS Appl Mater. Interfaces 2019, 11, 37421–37433. [Google Scholar] [CrossRef]
- Fiore, E.; Domínguez, L.M.; Bayo, J.; Malvicini, M.; Atorrasagasti, C.; Rodriguez, M.; Cantero, M.J.; García, M.; Yannarelli, G.; Mazzolini, G. Human Umbilical Cord Perivascular Cells-Derived Extracellular Vesicles Mediate the Transfer of IGF-I to the Liver and Ameliorate Hepatic Fibrogenesis in Mice. Gene Ther. 2020, 27, 62–73. [Google Scholar] [CrossRef]
- Chen, L.; Lu, F.-B.; Chen, D.-Z.; Wu, J.-L.; Hu, E.; Xu, L.-M.; Zheng, M.-H.; Li, H.; Huang, Y.; Jin, X.-Y.; et al. BMSCs-Derived MiR-223-Containing Exosomes Contribute to Liver Protection in Experimental Autoimmune Hepatitis. Mol. Immunol. 2018, 93, 38–46. [Google Scholar] [CrossRef]
- Bruno, S.; Pasquino, C.; Herrera Sanchez, M.B.; Tapparo, M.; Figliolini, F.; Grange, C.; Chiabotto, G.; Cedrino, M.; Deregibus, M.C.; Tetta, C.; et al. HLSC-Derived Extracellular Vesicles Attenuate Liver Fibrosis and Inflammation in a Murine Model of Non-Alcoholic Steatohepatitis. Mol. Ther. 2020, 28, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Pu, Y.; Chen, X.; Qi, X.; Zhang, L.; Xu, L.; Li, W.; Ma, Y.; Zhou, S.; Zhu, J.; et al. HUCMSC-Extracellular Vesicles Downregulated Hepatic Stellate Cell Activation and Reduced Liver Injury in S. Japonicum-Infected Mice. Stem Cell Res. Ther. 2020, 11, 21. [Google Scholar] [CrossRef]
- Lou, G.; Yang, Y.; Liu, F.; Ye, B.; Chen, Z.; Zheng, M.; Liu, Y. MiR-122 Modification Enhances the Therapeutic Efficacy of Adipose Tissue-Derived Mesenchymal Stem Cells against Liver Fibrosis. J. Cell. Mol. Med. 2017, 21, 2963–2973. [Google Scholar] [CrossRef]
- Bari, E.; Perteghella, S.; Di Silvestre, D.; Sorlini, M.; Catenacci, L.; Sorrenti, M.; Marrubini, G.; Rossi, R.; Tripodo, G.; Mauri, P.; et al. Pilot Production of Mesenchymal Stem/Stromal Freeze-Dried Secretome for Cell-Free Regenerative Nanomedicine: A Validated GMP-Compliant Process. Cells 2018, 7, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kordelas, L.; Rebmann, V.; Ludwig, A.K.; Radtke, S.; Ruesing, J.; Doeppner, T.R.; Epple, M.-; Horn, P.A.; Beelen, D.W.; Giebel, B. MSC-Derived Exosomes: A Novel Tool to Treat Therapy-Refractory Graft-Versus-Host Disease. Leukemia 2014, 28, 970–973. [Google Scholar] [CrossRef]
- Bruno, S.; Collino, F.; Deregibus, M.C.; Grange, C.; Tetta, C.; Camussi, G. Microvesicles Derived from Human Bone Marrow Mesenchymal Stem Cells Inhibit Tumor Growth. Stem Cells Dev. 2013, 22, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Fonsato, V.; Collino, F.; Herrera, M.B.; Cavallari, C.; Deregibus, M.C.; Cisterna, B.; Bruno, S.; Romagnoli, R.; Salizzoni, M.; Tetta, C.; et al. Human Liver Stem Cell-Derived Microvesicles Inhibit Hepatoma Growth in SCID Mice by Delivering Antitumor MicroRNAs. Stem Cells 2012, 30, 1985–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopatina, T.; Bruno, S.; Tetta, C.; Kalinina, N.; Porta, M.; Camussi, G. Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Commun. Signal. 2014, 12, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kholia, S.; Herrera Sanchez, M.B.; Cedrino, M.; Papadimitriou, E.; Tapparo, M.; Deregibus, M.C.; Brizzi, M.F.; Tetta, C.; Camussi, G. Human Liver Stem Cell-Derived Extracellular Vesicles Prevent Aristolochic Acid-Induced Kidney Fibrosis. Front. Immunol. 2018, 9, 1639. [Google Scholar] [CrossRef] [Green Version]
- Grange, C.; Tritta, S.; Tapparo, M.; Cedrino, M.; Tetta, C.; Camussi, G.; Brizzi, M.F. Stem Cell-Derived Extracellular Vesicles Inhibit and Revert Fibrosis Progression in a Mouse Model of Diabetic Nephropathy. Sci. Rep. 2019, 9, 4468. [Google Scholar] [CrossRef] [Green Version]
- Kholia, S.; Herrera Sanchez, M.B.; Cedrino, M.; Papadimitriou, E.; Tapparo, M.; Deregibus, M.C.; Bruno, S.; Antico, F.; Brizzi, M.F.; Quesenberry, P.J.; et al. Mesenchymal Stem Cell Derived Extracellular Vesicles Ameliorate Kidney Injury in Aristolochic Acid Nephropathy. Front. Cell Dev. Biol. 2020, 8, 188. [Google Scholar] [CrossRef]
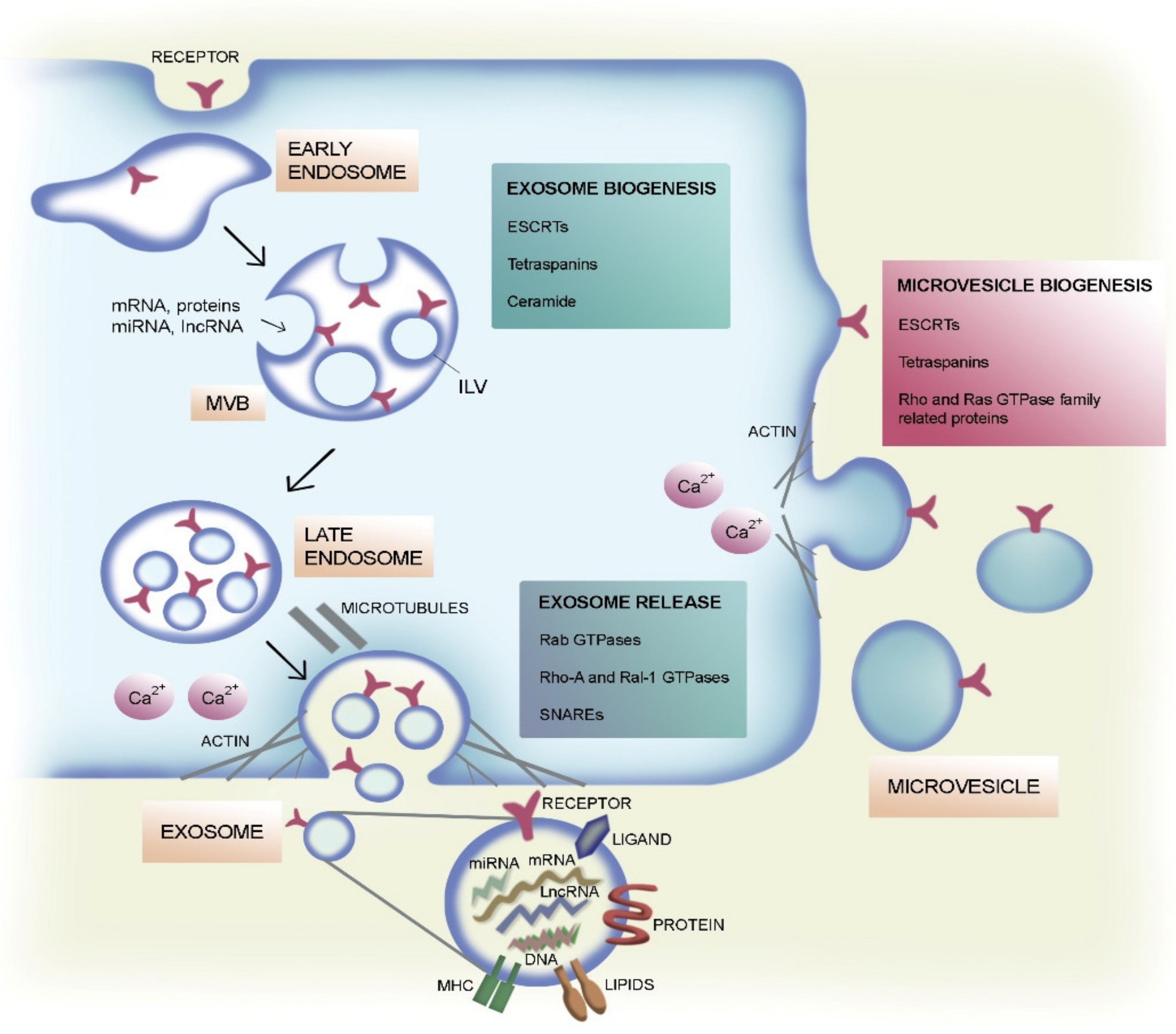
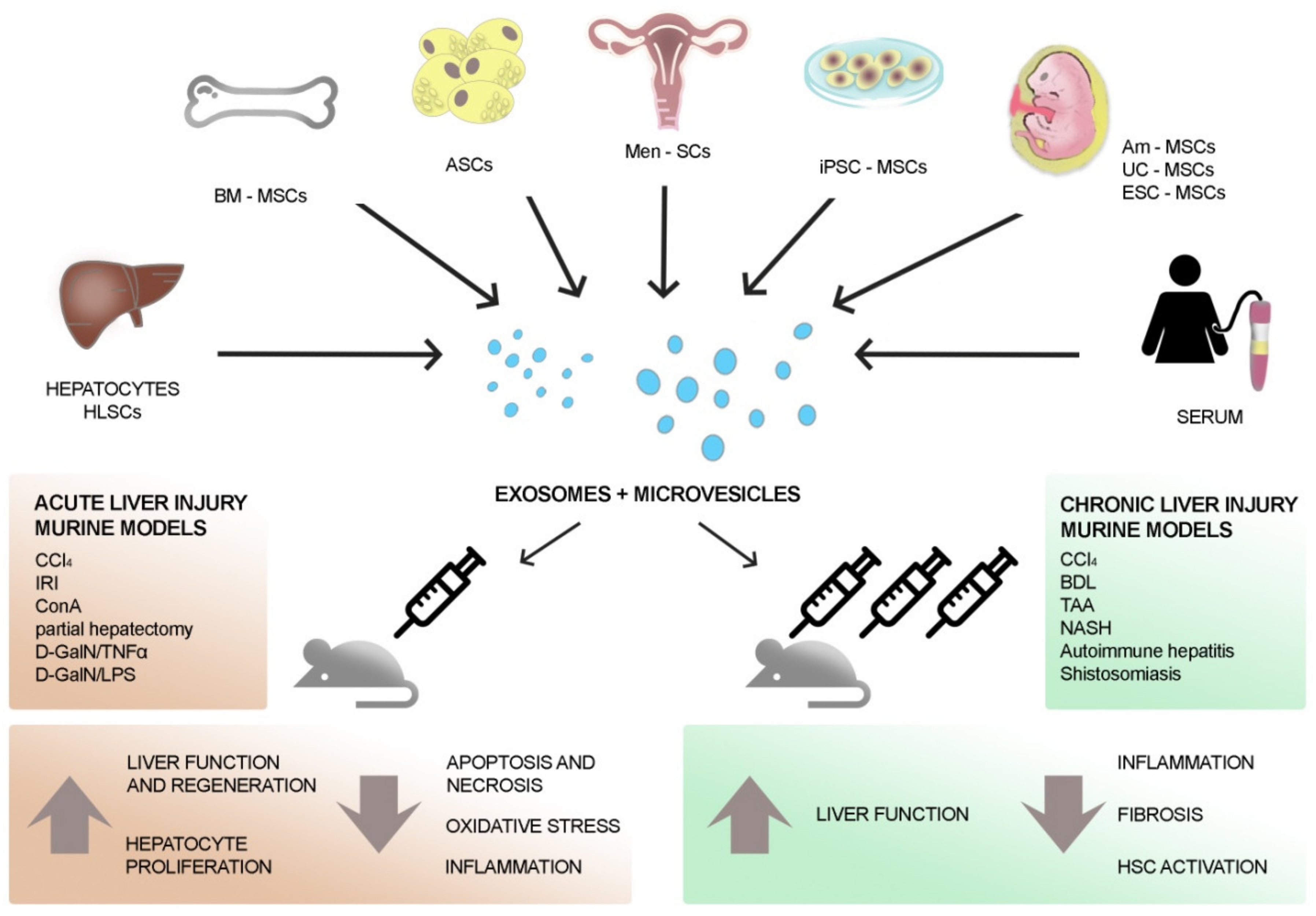
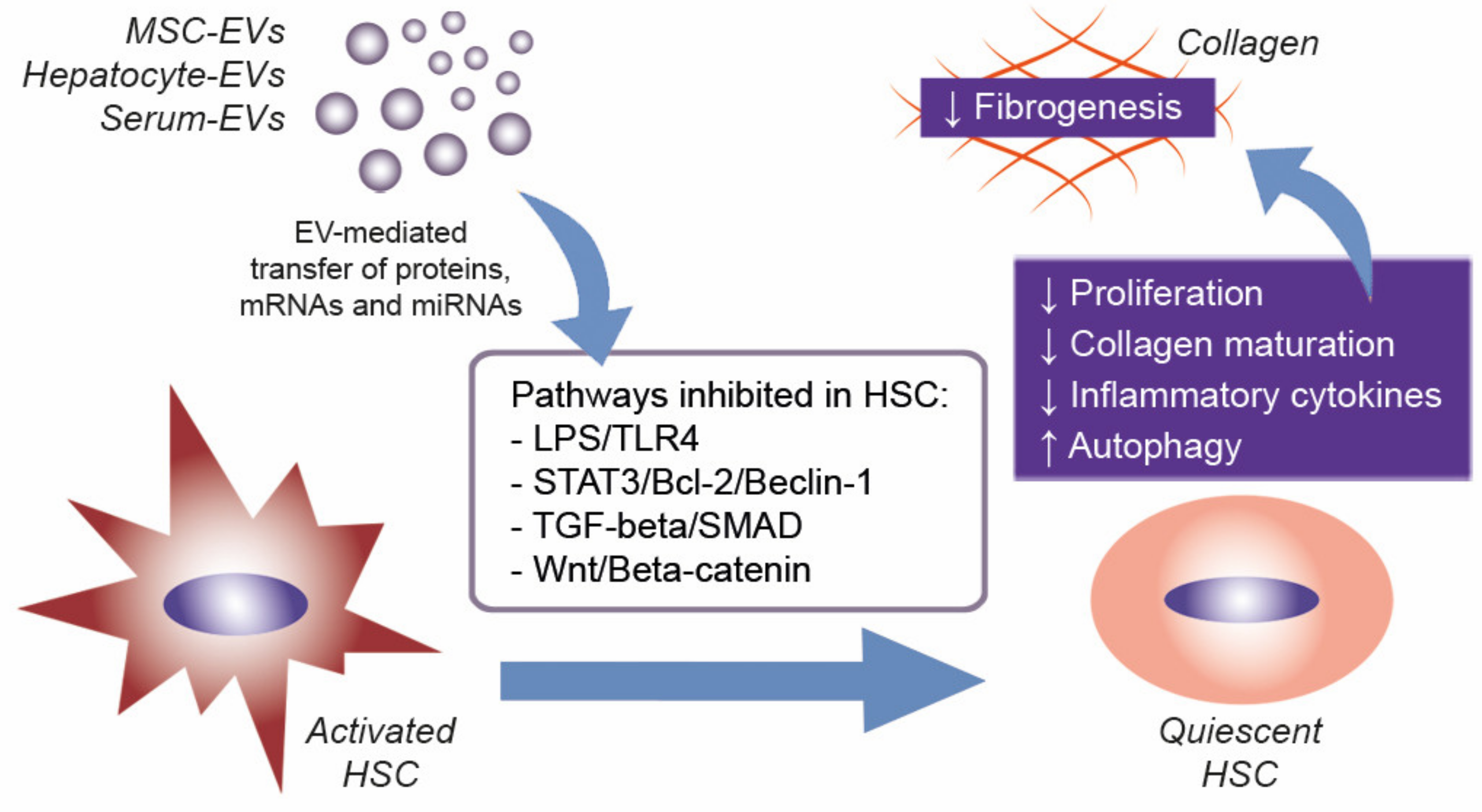
| In Vivo Models of Liver Disease | EV Sources | Route and Time of EV Administration | Effects of EV Administration | References |
|---|---|---|---|---|
| Partial hepatectomy | HLSCs | Tail vein immediately after injury | Pro-proliferative and anti-apoptotic effect on hepatocytes | [74] |
| CCl4 | ESC-MSCs | Intra-splenic injection simultaneously with the damage | Pro-proliferative effect on hepatocytes | [75] |
| CCl4 | hUC-MSCs | Tail vein or oral gavage 24 h post-CCl4 | Anti-oxidant and anti-apoptotic effects on hepatocytes | [76] |
| CCl4 | hUC-MSCs | Tail vein 24 h post-CCl4 | Inhibition of inflammation, oxidative stress, and apoptosis. Suppression of hepatic tumor development | [77] |
| IRI | Murine BM-MSCs | Tail vein 30 min before surgery | Reduction of liver necrosis and hepatocyte apoptosis by modulating inflammation. Improvement of liver function | [78] |
| IRI | hiPSC-MSCs | Inferior vena cava immediately after reperfusion | Reduce histological damage, inflammation, apoptosis, and oxidative stress, and improve hepatic function | [79,80] |
| IRI | hUC-MSCs | Tail vein immediately after surgery | Reduce apoptosis, neutrophilic infiltrates, and oxidative stress | [81] |
| IRI | Human BM-MSCs/ fibroblasts | Inferior vena cava before surgery | Reduction of liver necrosis and inflammation. Improvement of liver function and regeneration | [82] |
| D-GalN/TNF-alpha | Murine/human BM-MSCs | Tail veil or intraperitoneal injection immediately after damage | Increase in mice survival, reduction of hepatic inflammation and injury | [83] |
| D-GalN/LPS | Men-SCs | Tail vein 24 h before injury | Improvement of liver function and survival, inhibition of apoptosis | [84] |
| D-GalN/LPS | hASC | Iliac vein 24 h after injury | Reduction of necrosis and inflammation | [85] |
| ConA | Murine BM-MSCs | Intravenous EV injection of 20 μg/mL at 0, 8, and 16 h after injury | Reduction of hepatic necrosis, apoptosis, and inflammation | [86] |
| IRI and partial hepatectomy/ CCl4 | Rat BM-MSCs | A single injection via hepatic portal vein of 500 μg/mL of exosome-rich fractionated secretome before removing the clamp (IRI) or 24 h post-CCl4 | Improvement of hepatic regeneration and function, reduction of oxidative stress | [87] |
| IRI and partial hepatectomy | Murine hepatocytes | Intravenously EV injection 24 and 48 h after IRI, 24 h after hepatectomy | Increase of hepatocyte proliferation | [88] |
| In Vivo Model of Liver Fibrosis | EV Sources | Route and Time of EV- Administration | Effects of EV-Administration | References |
|---|---|---|---|---|
| CCl4 | hUC-MSCs | Single EV-dose directly injected into left and right hepatic lobes, 6 weeks after CCl4 treatment | Inhibition of EMT and protection of hepatocytes | [89] |
| CCl4 | miR-181-5p modified murine ASCs | Intrasplenic injection twice each week for 8 weeks concomitantly with CCl4 treatment | Anti-fibrotic effect, amelioration of liver function | [90] |
| CCl4 BDL | human-iPSCs | Tail vein three times a week for the last two weeks of the CCl4 study; tail vein daily injection for the last six days of duct ligation | Reduction of fibrosis and HSC activation | [91] |
| CCl4 NASH | hAm-MSCs | Intravenous injection at week 3 after the start of CCl4 treatment and at week 3 and 4 after starting the high fatty diet to induce NASH | Reduction of Kupffer cells, of expression levels of pro-inflammatory and pro-fibrotic cytokines, and of HSC activation | [92] |
| CCl4 | Human BM-MSCs | Single EV injection through the tail vein 8 weeks after CCl4 treatment | Improvement of liver function and reduction of fibrosis, inflammation, and HSC activation via Wnt/beta-catenin pathway | [93] |
| CCl4 TAA | Murine and human serum | Intraperitoneal administration three times per week during the last two to three weeks of CCl4 treatment; intraperitoenal administration every day during the last week of thioacetic treatment experiment | Reduction of the levels of hepatocyte death, inflammatory infiltrates, AST and ALT, pro-inflammatory cytokines, and HSC | [94] |
| CCl4 | Murine and human hepatocytes | Intraperitoneal administration three times per week during the last two weeks of the experiment | Reduction of alpha-SMA expression and of fibrosis and inflammation | [95] |
| TAA | hESC-MSCs | Intrasplenic injection | Reduction of fibrosis and immune cell infiltration, up-regulation of anti-apoptotic and anti-inflammatory genes | [96] |
| TAA | hESC-MSCs | Intraperitoneal injection of free or hydrogel-loaded EVs | Reduction of necrosis, inflammation, and fibrosis | [97] |
| TAA | HUCPVCs engineered to produce IGF-1 | On week 6 of treatment, tail vein injection every 5 days (total of three doses) | Reduction of collagen deposition and expression of fibrogenic transcripts | [98] |
| Autoimmune hepatitis | Murine BM-MSCs engineered with miR-223 | Administration of EVs at day 21, 28, and 35 | Improvement of liver structure and function and of lymphocyte infiltration | [99] |
| NASH | HLSCs | Intravenous twice a weeks starting from week 2 of diet | Improvement of liver function and reduction of fibrosis and inflammation | [100] |
| Schistosomiasis | hUCMSCs | Intravenous injection at the fourth or at the sixth week after infection | Increased mice survival, improvement of liver function, and reduction of fibrosis and inflammation | [101] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, S.; Chiabotto, G.; Camussi, G. Extracellular Vesicles: A Therapeutic Option for Liver Fibrosis. Int. J. Mol. Sci. 2020, 21, 4255. https://doi.org/10.3390/ijms21124255
Bruno S, Chiabotto G, Camussi G. Extracellular Vesicles: A Therapeutic Option for Liver Fibrosis. International Journal of Molecular Sciences. 2020; 21(12):4255. https://doi.org/10.3390/ijms21124255
Chicago/Turabian StyleBruno, Stefania, Giulia Chiabotto, and Giovanni Camussi. 2020. "Extracellular Vesicles: A Therapeutic Option for Liver Fibrosis" International Journal of Molecular Sciences 21, no. 12: 4255. https://doi.org/10.3390/ijms21124255
APA StyleBruno, S., Chiabotto, G., & Camussi, G. (2020). Extracellular Vesicles: A Therapeutic Option for Liver Fibrosis. International Journal of Molecular Sciences, 21(12), 4255. https://doi.org/10.3390/ijms21124255





