Various Anti-HSPA2 Antibodies Yield Different Results in Studies on Cancer-Related Functions of Heat Shock Protein A2
Abstract
:1. Introduction
2. Results
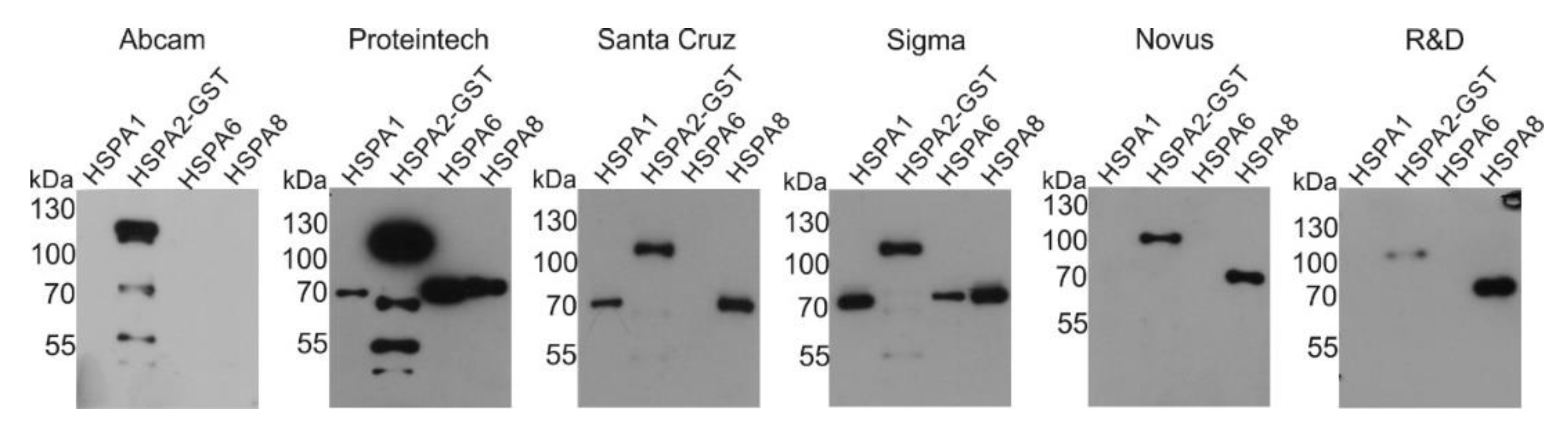
2.1. Reactivity of Commercial Anti-HSPA2 Antibodies with Highly Homologous HSPA Proteins
2.2. Detection of the Endogenous HSPA2 in Cells Cultured under Non-Stressful Conditions
2.3. Detection of the Endogenous HSPA2 in Cells under Proteotoxic Stress Conditions
2.4. The Impact of Anti-HSPA2 Antibody Used for IHC Detection on Conclusions about HSPA2 Expression in Tumors
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Experimental Conditions
4.2. Transient Transfection
4.3. Generation of Lentiviral Expression Vectors and Lentiviral Transduction
4.4. Protein Extraction and Western Blot Analysis
4.5. Immunohistochemistry (IHC)
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | Adrenocortical Carcinoma |
| AD | Adenocarcinoma |
| AML | Acute Myeloid Leukemia |
| BLC | Bladder Urothelial Carcinoma |
| BLGG | Brain Lower Grade Glioma |
| BRCA | Breast Invasive Carcinoma |
| CC | Cervical Cancer |
| DFS | Disease Free Survival |
| DLBL | Diffuse Large B-cell Lymphoma |
| FFPE | Formalin-Fixed Paraffin-Embedded |
| GBM | Glioblastoma Multiforme |
| H&N | Head and Neck |
| HCC | Hepatocellular Carcinoma |
| HSP | Heat Shock Protein |
| IHC | Immunohistochemistry |
| KICH | Kidney Chromophobe |
| KIRP | Kidney Renal Papillary Cell Carcinoma |
| mAb | Monoclonal Antibody |
| NSCLC | Non-Small Cell Lung Carcinoma |
| OS | Overall Survival |
| OV | Ovarian Serous Cystadenocarcinoma |
| pAb | Polyclonal Antibody |
| PCPG | Pheochromocytoma and Paraganglioma |
| PRECOG | PREdiction of Clinical Outcomes from Genomic Profiles |
| RFS | Relapse Free Survival |
| SARC | Sarcoma |
| SCC | Squamous Cell Carcinoma |
| SKCM | Skin Cutaneous Melanoma |
| TCGA | The Cancer Genome Atlas |
| THCA | Thyroid carcinoma |
| UCEC | Uterine Corpus Endometrial Carcinoma |
| UCS | Uterine Carcinosarcoma |
References
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2009, 14, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Boudesco, C.; Cause, S.; Jego, G.; Garrido, C. Hsp70: A Cancer Target Inside and Outside the Cell. Methods Mol. Biol. 2018, 1709, 371–396. [Google Scholar] [PubMed]
- Radons, J. The human HSP70 family of chaperones: Where do we stand? Cell Stress Chaperones 2016, 21, 379–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciocca, D.R.; Calderwood, S.K. Heat shock proteins in cancer: Diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 2005, 10, 86–103. [Google Scholar] [CrossRef] [PubMed]
- Saini, J.; Sharma, P.K. Clinical, Prognostic and Therapeutic Significance of Heat Shock Proteins in Cancer. Curr. Drug Targets 2018, 19, 1478–1490. [Google Scholar] [CrossRef]
- Klimczak, M.; Biecek, P.; Zylicz, A.; Zylicz, M. Heat shock proteins create a signature to predict the clinical outcome in breast cancer. Sci. Rep. 2019, 9, 7507. [Google Scholar] [CrossRef] [Green Version]
- Scieglinska, D.; Krawczyk, Z. Expression, function, and regulation of the testis-enriched heat shock HSPA2 gene in rodents and humans. Cell Stress Chaperones 2015, 20, 221–235. [Google Scholar] [CrossRef] [Green Version]
- Jagadish, N.; Agarwal, S.; Gupta, N.; Fatima, R.; Devi, S.; Kumar, V.; Suri, V.; Kumar, R.; Suri, V.; Sadasukhi, T.C.; et al. Heat shock protein 70-2 (HSP70-2) overexpression in breast cancer. J. Exp. Clin. Cancer Res. 2016, 35, 150. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.L.; Zhang, Y.; Li, D.D.; Zhang, F.L.; Liu, H.Y.; Liao, X.H.; Xie, H.Y.; Lu, Q.; Zhang, L.; Hong, Q.; et al. RNF144A functions as a tumor suppressor in breast cancer through ubiquitin ligase activity-dependent regulation of stability and oncogenic functions of HSPA2. Cell. Death Differ. 2020, 27, 1105–1118. [Google Scholar] [CrossRef]
- Huang, Z.; Duan, H.; Li, H. Identification of Gene Expression Pattern Related to Breast Cancer Survival Using Integrated TCGA Datasets and Genomic Tools. Biomed. Res. Int. 2015, 2015, 878546. [Google Scholar] [CrossRef] [Green Version]
- Zoppino, F.C.M.; Guerrero-Gimenez, M.E.; Castro, G.N.; Ciocca, D.R. Comprehensive transcriptomic analysis of heat shock proteins in the molecular subtypes of human breast cancer. BMC Cancer 2018, 18, 700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scieglinska, D.; Gogler-Piglowska, A.; Butkiewicz, D.; Chekan, M.; Malusecka, E.; Harasim, J.; Habryka, A.; Krawczyk, Z. HSPA2 is expressed in human tumors and correlates with clinical features in non-small cell lung carcinoma patients. Anticancer Res. 2014, 34, 2833–2840. [Google Scholar] [PubMed]
- Zhang, H.; Gao, H.; Liu, C.; Kong, Y.; Wang, C.; Zhang, H. Expression and clinical significance of HSPA2 in pancreatic ductal adenocarcinoma. Diagn. Pathol. 2015, 10, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, L.L.; Xie, Q.; Zhou, C.H.; Huang, D.W.; Tang, Z.G.; Ju, T.F. Overexpressed HSPA2 correlates with tumor angiogenesis and unfavorable prognosis in pancreatic carcinoma. Pancreatology 2017, 17, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Scieglinska, D.; Piglowski, W.; Chekan, M.; Mazurek, A.; Krawczyk, Z. Differential expression of HSPA1 and HSPA2 proteins in human tissues; tissue microarray-based immunohistochemical study. Histochem. Cell. Biol. 2011, 135, 337–350. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Chen, W.; Duan, C.J.; Zhang, C.F. Overexpression of HSPA2 is correlated with poor prognosis in esophageal squamous cell carcinoma. World J. Surg. Oncol. 2013, 11, 141. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Zhao, H.; Li, X.S.; Kang, H.R.; Ma, J.X.; Yao, F.F.; Du, N. Expression of HSPA2 in human hepatocellular carcinoma and its clinical significance. Tumour Biol. 2014, 35, 11283–11287. [Google Scholar] [CrossRef]
- Sojka, D.R.; Gogler-Pigłowska, A.; Vydra, N.; Cortez, A.J.; Filipczak, P.T.; Krawczyk, Z.; Scieglinska, D. Functional redundancy of HSPA1, HSPA2 and other HSPA proteins in non-small cell lung carcinoma (NSCLC); an implication for NSCLC treatment. Sci. Rep. 2019, 9, 14394. [Google Scholar] [CrossRef] [Green Version]
- Gogler-Pigłowska, A.; Klarzyńska, K.; Sojka, D.R.; Habryka, A.; Głowala-Kosińska, M.; Herok, M.; Kryj, M.; Halczok, M.; Krawczyk, Z.; Scieglinska, D. Novel role for the testis-enriched HSPA2 protein in regulating epidermal keratinocyte differentiation. J. Cell. Physiol. 2018, 233, 2629–2644. [Google Scholar] [CrossRef]
- Scieglinska, D.; Krawczyk, Z.; Sojka, D.R.; Gogler-Pigłowska, A. Heat shock proteins in the physiology and pathophysiology of epidermal keratinocytes. Cell Stress Chaperones 2019, 24, 1027–1044. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Wang, J.; He, L.; Lin, Y.; Wu, J. Knockdown of polycomb-group RING finger 6 modulates mouse male germ cell differentiation in vitro. Cell. Physiol. Biochem. 2015, 35, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Grad, I.; Cederroth, C.R.; Walicki, J.; Grey, C.; Barluenga, S.; Winssinger, N.; De Massy, B.; Nef, S.; Picard, D. The molecular chaperone Hsp90α is required for meiotic progression of spermatocytes beyond pachytene in the mouse. PLoS ONE 2010, 5, e15770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogon, C.; Ulbricht, A.; Hesse, M.; Alberti, S.; Vijayaraj, P.; Best, D.; Adams, I.R.; Magin, T.M.; Fleischmann, B.K.; Höhfeld, J. HSP70-binding protein HSPBP1 regulates chaperone expression at a posttranslational level and is essential for spermatogenesis. Mol. Biol. Cell 2014, 25, 2260–2271. [Google Scholar] [CrossRef] [PubMed]
- Bromfield, E.G.; McLaughlin, E.A.; Aitken, R.J.; Nixon, B. Heat Shock Protein member A2 forms a stable complex with angiotensin converting enzyme and protein disulfide isomerase A6 in human spermatozoa. Mol. Hum. Reprod. 2016, 22, 93–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bromfield, E.G.; Aitken, R.J.; McLaughlin, E.A.; Nixon, B. Proteolytic degradation of heat shock protein A2 occurs in response to oxidative stress in male germ cells of the mouse. Mol. Hum. Reprod. 2017, 23, 91–105. [Google Scholar] [CrossRef] [Green Version]
- Bromfield, E.; Aitken, R.J.; Nixon, B. Novel characterization of the HSPA2-stabilizing protein BAG6 in human spermatozoa. Mol. Hum. Reprod. 2015, 21, 755–769. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Zhang, F.; Zhang, X.; Li, L.; Wang, L.; Shi, B.; Xu, J. Depression of HspA2 in human testis is associated with spermatogenic impairment and fertilization rate in ICSI treatment for azoospermic individuals. J. Assist. Reprod. Genet. 2014, 31, 1687–1693. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Yuan, X.; Bao, F.; Lv, W.; He, Z.; Tang, J.; Han, J.; Hu, J. Downregulation of HSPA2 inhibits proliferation via ERK1/2 pathway and endoplasmic reticular stress in lung adenocarcinoma. Ann. Transl. Med. 2019, 7, 540. [Google Scholar] [CrossRef]
- Rosario, M.O.; Perkins, S.L.; O'Brien, D.A.; Allen, R.L.; Eddy, E.M. Identification of the gene for the developmentally expressed 70 kDa heat-shock protein (P70) of mouse spermatogenic cells. Dev. Biol. 1992, 150, 1–11. [Google Scholar] [CrossRef]
- Scieglińska, D.; Pigłowski, W.; Mazurek, A.; Małusecka, E.; Zebracka, J.; Filipczak, P.; Krawczyk, Z. The HspA2 protein localizes in nucleoli and centrosomes of heat shocked cancer cells. J. Cell. Biochem. 2008, 104, 2193–2206. [Google Scholar] [CrossRef]
- Garg, M.; Kanojia, D.; Saini, S.; Suri, S.; Gupta, A.; Surolia, A.; Suri, A. Germ cell-specific heat shock protein 70-2 is expressed in cervical carcinoma and is involved in the growth, migration, and invasion of cervical cells. Cancer 2010, 116, 3785–3796. [Google Scholar] [CrossRef] [PubMed]
- Malusecka, E.; Zborek, A.; Krzyzowska-Gruca, S.; Krawczyk, Z. Immunohistochemical detection of the inducible heat shock protein hsp70: A methodological study. J. Histochem. Cytochem. 2006, 54, 183–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walerych, D.; Olszewski, M.B.; Gutkowska, M.; Helwak, A.; Zylicz, M.; Zylicz, A. Hsp70 molecular chaperones are required to support p53 tumor suppressor activity under stress conditions. Oncogene 2009, 28, 4284–4294. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Tumor Type | Method | Material/Group Size | Results | Ref |
|---|---|---|---|---|
| BRCA | Analysis of gene expression data | TCGA1 | High expression of HSPA2 mRNA was associated with better OS | [10] |
| BRCA | Analysis of gene expression data | TCGA1/n = 1085 | High HSPA2 mRNA expression was associated with a good prognosis | [11] |
| BRCA | Analysis of gene expression data | TCGA1/n = 1101 KM plotter2/n = 5143 | High expression of HSPA2 mRNA was associated with better OS for patients from TCGA cohort, better OS and RFS in KM plotter cohort | [6] |
| BRCA | IHC/rabbit pAb 12797-1, Proteintech Group | primary tumor FFPE/n = 166 | High expression of HSPA2 was associated with shorter OS and RFS | [9] |
| BRCA, ACC, KICH, UCS, H&N SCC, pancreatic AD, KIRP, UCEC, rectum AD, BLGG, THCA, HCC, prostate AD | Analysis of gene expression data | TCGA1 | Favorable prognosis (survival Z-score < 0) associated with HSPA2 mRNA overexpression | [6] |
| esophageal SCC | IHC/Santa Cruz Biotechnology Inc | primary tumor FFPE/n = 120 | High HSPA2 expression was an independent negative prognostic factor for OS and DFS | [16] |
| HCC | IHC/goat pAb, Santa Cruz Biotechnology Inc. | primary tumor FFPE/n = 119 | High HSPA2 expression was an independent negative prognostic factor for OS | [17] |
| lung NSCLC | IHC/monospecific rabbit pAb (custom made) | primary tumor FFPE | High HSPA2 expression correlated with shorter OS in the stage I-II subgroup. | [12] |
| pancreatic AD | qRT-PCR | primary tumor/n = 104 | High HSPA2 mRNA level was an independent negative prognostic factor for OS | [13] |
| pancreatic carcinoma | IHC/rabbit mAb EPR4596 Abcam | primary tumor FFPE/n = 80 | High HSPA2 level was independent negative prognostic factor for RFS and OS | [14] |
| SKCM, AML, lung AD, BLC, lung SCC, OV, colon AD, CC, DLBC, PCPG, GBM, SARC | Analysis of gene expression data | TCGA1 | HSPA2 overexpression associated with poor survival | [6] |
| Catalog Number/RRID | Source | Reactivity | ||||
|---|---|---|---|---|---|---|
| A1 | A2 | A6 | A8 | |||
| HSPA1 | ADI-SPA-810-F/AB_311860 | Enzo, Life Sciences | + | - | + | - |
| 10995-1-AP/AB_2264230 | Proteintech Group | + | - | + | +/- | |
| HSPA2 | Ab108416/AB_10862351 | Abcam | - | + | - | - |
| 12797-1-AP/AB_2119687 | Proteintech Group | + | + | + | + | |
| sc-1600010/n.d. | Santa Cruz Biotechnology Inc. | + | + | - | + | |
| HPA000798/AB_1079090 | Sigma-Aldrich | + | + | + | + | |
| MAB6010/AB_1964604 | Novus Biologicals LLC | - | + | - | + | |
| MAB6010/AB_1964604 | R&D Systems Inc. | - | + | - | + | |
| HSPA6 | ADI-SPA-754/AB_10615942 | Enzo, Life Sciences | - | - | + | - |
| HSPA8 | sc-7298/AB_627761 | Santa Cruz Biotechnology Inc. | - | - | - | + |
| Host/Clonality | Clone | Catalog Numer/RRID | Source | Dilution WB/IHC | Immunogen | |
|---|---|---|---|---|---|---|
| HSPA1 | Mo/mAb | C92F3A-5 | ADI-SPA-810-F/AB_311860 | Enzo, Life Sciences, Famingdale, NY, USA | 1:5000/n.d. | Native human HSPA1 protein |
| Ra/pAb | 10995-1-AP/AB_2264230 | Proteintech Group, Rosemont, IL, USA | 1:25000/n.d. | HSP70 fusion protein (Ag1446) | ||
| HSPA2 | Ra/mAb | EPR4596 | Ab108416/AB_10862351 | Abcam, Cambridge, UK | 1:5000/1:5000 | Recombinant fragment matching to human HSPA2 aa 450-650 |
| Ra/pAb | 12797-1-AP/AB_2119687 | Proteintech Group, Rosemont, IL, USA | 1:3000/1:50 | HSPA2 Fusion Protein (Ag3539) | ||
| G/pAb | sc-1600010 (K12)/nd | Santa Cruz Biotechnology Inc., Dallas, TX, USA | 1:2000/1:200 | Peptide matching to internal region of human HSPA2 | ||
| Ra/pAb | HPA000798/AB_1079090 | Sigma Aldrich, St. Louis, MO, USA | 1:2000/n.d. | Recombinant Protein Epitope Signature Tag (PrEST) | ||
| Mo/mAb | 520608 | MAB6010/AB_1964604 | Novus Biologicals LLC, CO, USA | 1:5000/1:200 | Recombinant human HSPA2 Lys529-Gly616 | |
| Mo/mAb | 520608 | MAB6010/AB_1964604 | R&D Systems Inc., Minneapolis, MN, USA | 1:2000/n.d. | Recombinant human HSPA2 Lys529-Gly616 | |
| HSPA6 | Mo/mAb | 165f | ADI-SPA-754/AB_10615942 or AB_2039272 | Enzo, Life Sciences, Famingdale, NY, USA | 1:3000/n.d. | Synthetic peptide mapping near the C-terminus of human HSPA6 |
| HSPA8 | Mo/mAb | B-6 | sc-7298/AB_627761 | Santa Cruz Biotechnology Inc., Dallas, TX, USA | 1:7500/n.d. | Epitope mapping 583-601 at the C-terminus of human HSPA8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scieglinska, D.; Sojka, D.R.; Gogler-Pigłowska, A.; Chumak, V.; Krawczyk, Z. Various Anti-HSPA2 Antibodies Yield Different Results in Studies on Cancer-Related Functions of Heat Shock Protein A2. Int. J. Mol. Sci. 2020, 21, 4296. https://doi.org/10.3390/ijms21124296
Scieglinska D, Sojka DR, Gogler-Pigłowska A, Chumak V, Krawczyk Z. Various Anti-HSPA2 Antibodies Yield Different Results in Studies on Cancer-Related Functions of Heat Shock Protein A2. International Journal of Molecular Sciences. 2020; 21(12):4296. https://doi.org/10.3390/ijms21124296
Chicago/Turabian StyleScieglinska, Dorota, Damian Robert Sojka, Agnieszka Gogler-Pigłowska, Vira Chumak, and Zdzisław Krawczyk. 2020. "Various Anti-HSPA2 Antibodies Yield Different Results in Studies on Cancer-Related Functions of Heat Shock Protein A2" International Journal of Molecular Sciences 21, no. 12: 4296. https://doi.org/10.3390/ijms21124296




