The Metabolism of Epoxyeicosatrienoic Acids by Soluble Epoxide Hydrolase Is Protective against the Development of Vascular Calcification
Abstract
:1. Introduction
2. Results
2.1. High-Phosphate Conditions Promote Vascular Calcification
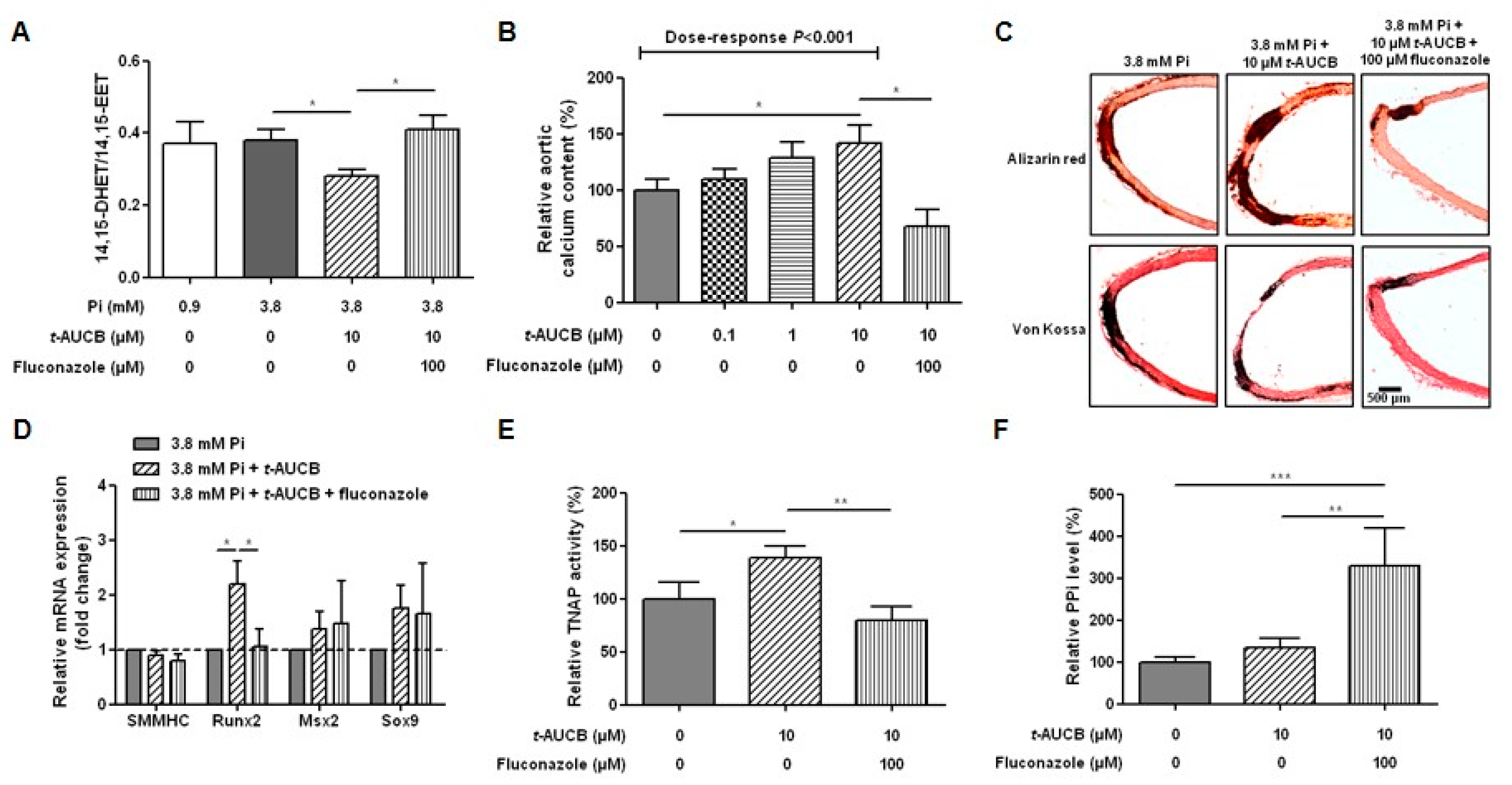
2.2. Inhibition of sEH Hydrolase Enhances Vascular Calcification
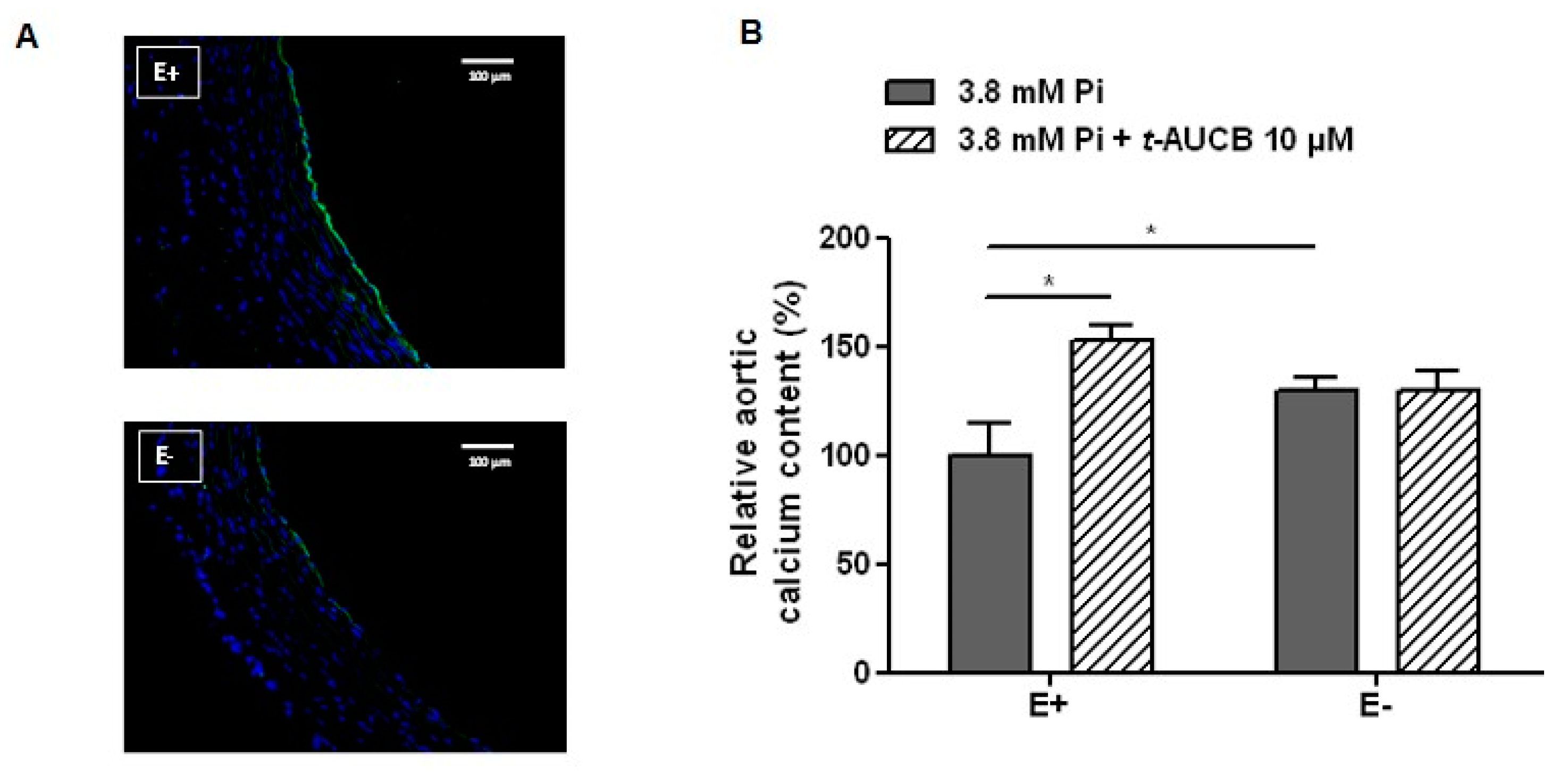
2.3. Role of the Endothelium in the Potentiation of Vascular Calcification by sEH Hydrolase Inhibition
2.4. Role of EETs in the Potentiation of Vascular Calcification Induced by sEH Hydrolase Inhibition
2.5. Relationships between the mRNA Levels of sEH and Markers of the Osteochondrogenic Differentiation in Human Carotid Tissues
3. Discussion
4. Material and Methods
4.1. Animal Experiments
4.2. Human Experiments
4.3. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shanahan, C.M.; Crouthamel, M.H.; Kapustin, A.; Giachelli, C.M. Arterial calcification in chronic kidney disease: Key roles for calcium and phosphate. Circ. Res. 2011, 109, 697–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanzer, P.; Boehm, M.; Sorribas, V.; Thiriet, M.; Janzen, J.; Zeller, T.; St Hilaire, C.; Shanahan, C. Medial vascular calcification revisited: Review and perspectives. Eur. Heart. J. 2014, 35, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A. Vascular calcification: Mechanisms of vascular smooth muscle cell calcification. Trends Cardiovasc. Med. 2015, 25, 267–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boström, K.I. Where do we stand on vascular calcification? Vascul. Pharmacol. 2016, 84, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Y.; Jumabay, M.; Ly, A.; Radparvar, M.; Cubberly, M.R.; Boström, K.I. A role for the endothelium in vascular calcification. Circ. Res. 2013, 113, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Kanno, Y.; Into, T.; Lowenstein, C.J.; Matsushita, K. Nitric oxide regulates vascular calcification by interfering with TGF-signalling. Cardiovasc. Res. 2008, 77, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Alesutan, I.; Feger, M.; Tuffaha, R.; Castor, T.; Musculus, K.; Buehling, S.S.; Heine, C.L.; Kuro-O, M.; Pieske, B.; Schmidt, K.; et al. Augmentation of phosphate-induced osteo-/chondrogenic transformation of vascular smooth muscle cells by homoarginine. Cardiovasc. Res. 2016, 110, 408–418. [Google Scholar] [CrossRef]
- Bellien, J.; Joannides, R.; Richard, V.; Thuillez, C. Modulation of cytochrome-derived epoxyeicosatrienoic acids pathway: A promising pharmacological approach to prevent endothelial dysfunction in cardiovascular diseases? Pharmacol. Ther. 2011, 131, 1–17. [Google Scholar] [CrossRef]
- Morisseau, C.; Hammock, B.D. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 37–58. [Google Scholar] [CrossRef] [Green Version]
- Fornage, M.; Boerwinkle, E.; Doris, P.A.; Jacobs, D.; Liu, K.; Wong, N.D. Polymorphism of the soluble epoxide hydrolase is associated with coronary artery calcification in African-American subjects: The Coronary Artery Risk Development in Young Adults (CARDIA) study. Circulation 2004, 109, 335–339. [Google Scholar] [CrossRef] [Green Version]
- Burdon, K.P.; Lehtinen, A.B.; Langefeld, C.D.; Carr, J.J.; Rich, S.S.; Freedman, B.I.; Herrington, D.; Bowden, D.W. Genetic analysis of the soluble epoxide hydrolase gene, EPHX2, in subclinical cardiovascular disease in the Diabetes Heart Study. Diab. Vasc. Dis. Res. 2008, 5, 128–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Przybyla-Zawislak, B.D.; Srivastava, P.K.; Vazquez-Matias, J.; Mohrenweiser, H.W.; Maxwell, J.E.; Hammock, B.D.; Bradbury, J.A.; Enayetallah, A.E.; Zeldin, D.C.; Grant, D.F. Polymorphisms in human soluble epoxide hydrolase. Mol. Pharmacol. 2003, 64, 482–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Zhang, S.; Gao, J.; Lin, Y.; Shi, G.; He, W.; Touyz, R.M.; Yan, L.; Huang, H. Downregulated serum 14, 15-epoxyeicosatrienoic acid is associated with abdominal aortic calcification in patients with primary aldosteronism. Hypertension 2018, 71, 592–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lomashvili, K.A.; Cobbs, S.; Hennigar, R.A.; Hardcastle, K.I.; O’Neill, W.C. Phosphate-induced vascular calcification: Role of pyrophosphate and osteopontin. J. Am. Soc. Nephrol. 2004, 15, 1392–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Six, I.; Okazaki, H.; Gross, P.; Cagnard, J.; Boudot, C.; Maizel, J.; Drueke, T.B.; Massy, Z.A. Direct, acute effects of Klotho and FGF23 on vascular smooth muscle and endothelium. PLoS ONE 2014, 9, e93423. [Google Scholar] [CrossRef] [PubMed]
- Sonou, T.; Ohya, M.; Yashiro, M.; Masumoto, A.; Nakashima, Y.; Ito, T.; Mima, T.; Negi, S.; Kimura-Suda, H.; Shigematsu, T. Mineral composition of phosphate-induced calcification in a rat aortic tissue culture model. J. Atheroscler. Thromb. 2015, 22, 1197–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lomashvili, K.A.; Garg, P.; Narisawa, S.; Millan, J.L.; O’Neill, W.C. Upregulation of alkaline phosphatase and pyrophosphate hydrolysis: Potential mechanism for uremic vascular calcification. Kidney Int. 2008, 73, 1024–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, W.C.; Lomashvili, K.A.; Malluche, H.H.; Faugere, M.C.; Riser, B.L. Treatment with pyrophosphate inhibits uremic vascular calcification. Kidney Int. 2011, 79, 512–517. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Xu, F.; Huse, L.M.; Morisseau, C.; Draper, A.J.; Newman, J.W.; Parker, C.; Graham, L.; Engler, M.M.; Hammock, B.D.; et al. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ. Res. 2000, 87, 992–998. [Google Scholar] [CrossRef] [Green Version]
- Duflot, T.; Pereira, T.; Roche, C.; Iacob, M.; Cardinael Hamza, N.E.; Thuillez, C.; Compagnon, P.; Joannidès, R.; Lamoureux, F.; Bellien, J. A sensitive LC-MS/MS method for the quantification of regioisomers of epoxyeicosatrienoic and dihydroxyeicosatrienoic acids in human plasma during endothelial stimulation. Anal. Bioanal. Chem. 2017, 409, 1845–1855. [Google Scholar] [CrossRef]
- Tsai, H.J.; Hwang, S.H.; Morisseau, C.; Yang, J.; Jones, P.D.; Kasagami, T.; Kim, I.H.; Hammock, B.D. Pharmacokinetic screening of soluble epoxide hydrolase inhibitors in dogs. Eur. J. Pharm. Sci. 2010, 40, 222–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roche, C.; Besnier, M.; Cassel, R.; Harouki, N.; Coquerel, D.; Guerrot, D.; Nicol, L.; Loizon, E.; Morisseau, C.; Remy-Jouet, I.; et al. Soluble epoxide hydrolase inhibition improves coronary endothelial function and prevents the development of cardiac alterations in obese insulin-resistant mice. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H1020–H1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louvet, L.; Büchel, J.; Steppan, S.; Passlick-Deetjen, J.; Massy, Z.A. Magnesium prevents phosphate-induced calcification in human aortic vascular smooth muscle cells. Nephrol. Dial. Transplant. 2013, 28, 869–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hénaut, L.; Sanz, A.B.; Martin-Sanchez, D.; Carrasco, S.; Villa-Bellosta, R.; Aldamiz-Echevarria, G.; Massy, Z.A.; Sanchez-Nino, M.D.; Ortiz, A. TWEAK favors phosphate-induced calcification of vascular smooth muscle cells through canonical and non-canonical activation of NFκB. Cell Death. Dis. 2016, 7, e2305. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, K.M.; Deeter, C.; Krishna, U.M.; Reddy, Y.K.; Bondlela, M.; Falck, J.R.; Campbell, W.B. 14,15-epoxyeicosa-5(Z)-enoic acid: A selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ. Res. 2002, 90, 1028–1036. [Google Scholar] [CrossRef] [Green Version]
- Tintut, Y.; Parhami, F.; Boström, K.; Jackson, S.M.; Demer, L.L. cAMP stimulates osteoblast-like differentiation of calcifying vascular cells. Potential signaling pathway for vascular calcification. J. Biol. Chem. 1998, 273, 7547–7553. [Google Scholar] [CrossRef] [Green Version]
- Tintut, Y.; Patel, J.; Parhami, F.; Demer, L.L. Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation 2000, 102, 2636–2642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, M.S.; Sage, A.P.; Lu, J.; Demer, L.L.; Tintut, Y. Phosphate and pyrophosphate mediate PKA-induced vascular cell calcification. Biochem. Biophys. Res. Commun. 2008, 374, 553–558. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Sui, X.; Bradbury, J.A.; Zeldin, D.C.; Conte, M.S.; Liao, J.K. Inhibition of vascular smooth muscle cell migration by cytochrome p450 epoxygenase-derived eicosanoids. Circ. Res. 2002, 90, 1020–1027. [Google Scholar] [CrossRef] [Green Version]
- Newman, J.W.; Morisseau, C.; Harris, T.R.; Hammock, B.D. The soluble epoxide hydrolase encoded by EPXH2 is a bifunctional enzyme with novel lipid phosphate phosphatase activity. Proc. Natl. Acad. Sci. USA 2003, 100, 1558–1563. [Google Scholar] [CrossRef] [Green Version]
- Fakhry, M.; Roszkowska, M.; Briolay, A.; Bougault, C.; Guignandon, A.; Diaz-Hernandez, J.I.; Diaz-Hernandez, M.; Pikula, S.; Buchet, R.; Hamade, E.; et al. TNAP stimulates vascular smooth muscle cell trans-differentiation into chondrocytes through calcium deposition and BMP-2 activation: Possible implication in atherosclerotic plaque stability. Biochim. Biophys. Acta 2017, 1863, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Savinov, A.Y.; Salehi, M.; Yadav, M.C.; Radichev, I.; Millán, J.L.; Savinova, O.V. Transgenic overexpression of tissue-nonspecific alkaline phosphatase (TNAP) in vascular endothelium results in generalized arterial calcification. J. Am. Heart Assoc. 2015, 4, e002499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morisseau, C.; Schebb, N.H.; Dong, H.; Ulu, A.; Aronov, P.A.; Hammock, B.D. Role of soluble epoxide hydrolase phosphatase activity in the metabolism of lysophosphatidic acids. Biochem. Biophys. Res. Commun. 2012, 419, 796–800. [Google Scholar] [CrossRef] [Green Version]
- Nsaibia, M.J.; Boulanger, M.C.; Bouchareb, R.; Mkannez, G.; Le Quang, K.; Hadji, F.; Argaud, D.; Dahou, A. OxLDL-derived lysophosphatidic acid promotes the progression of aortic valve stenosis through a LPAR1-RhoA-NF-κB pathway. Cardiovasc. Res. 2017, 113, 1351–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayari, H.; Bricca, G. Microarray analysis reveals overexpression of IBSP in human carotid plaques. Adv. Med. Sci. 2012, 57, 334–340. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varennes, O.; Mentaverri, R.; Duflot, T.; Kauffenstein, G.; Objois, T.; Lenglet, G.; Avondo, C.; Morisseau, C.; Brazier, M.; Kamel, S.; et al. The Metabolism of Epoxyeicosatrienoic Acids by Soluble Epoxide Hydrolase Is Protective against the Development of Vascular Calcification. Int. J. Mol. Sci. 2020, 21, 4313. https://doi.org/10.3390/ijms21124313
Varennes O, Mentaverri R, Duflot T, Kauffenstein G, Objois T, Lenglet G, Avondo C, Morisseau C, Brazier M, Kamel S, et al. The Metabolism of Epoxyeicosatrienoic Acids by Soluble Epoxide Hydrolase Is Protective against the Development of Vascular Calcification. International Journal of Molecular Sciences. 2020; 21(12):4313. https://doi.org/10.3390/ijms21124313
Chicago/Turabian StyleVarennes, Olivier, Romuald Mentaverri, Thomas Duflot, Gilles Kauffenstein, Thibaut Objois, Gaëlle Lenglet, Carine Avondo, Christophe Morisseau, Michel Brazier, Saïd Kamel, and et al. 2020. "The Metabolism of Epoxyeicosatrienoic Acids by Soluble Epoxide Hydrolase Is Protective against the Development of Vascular Calcification" International Journal of Molecular Sciences 21, no. 12: 4313. https://doi.org/10.3390/ijms21124313




