Molecular Regulation of α3β4 Nicotinic Acetylcholine Receptors by Lupeol in Cardiovascular System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. In Vitro Transcription
2.3. Molecular-Docking Studies
2.4. Mutagenesis
2.5. Oocyte Preparation and RNA Injection
2.6. Data Recordings Using Two-Electrode Voltage Clamp
2.7. Data Analysis
3. Results
3.1. Effect of Natural-Product Lupeol on IACh
3.2. ACh Concentration–Response Profiles and Current–Voltage Relationship
3.3. Noncompetitive Inhibition by Lupeol
3.4. Docked Modeling of Lupeol and α3β4 nACh Receptors
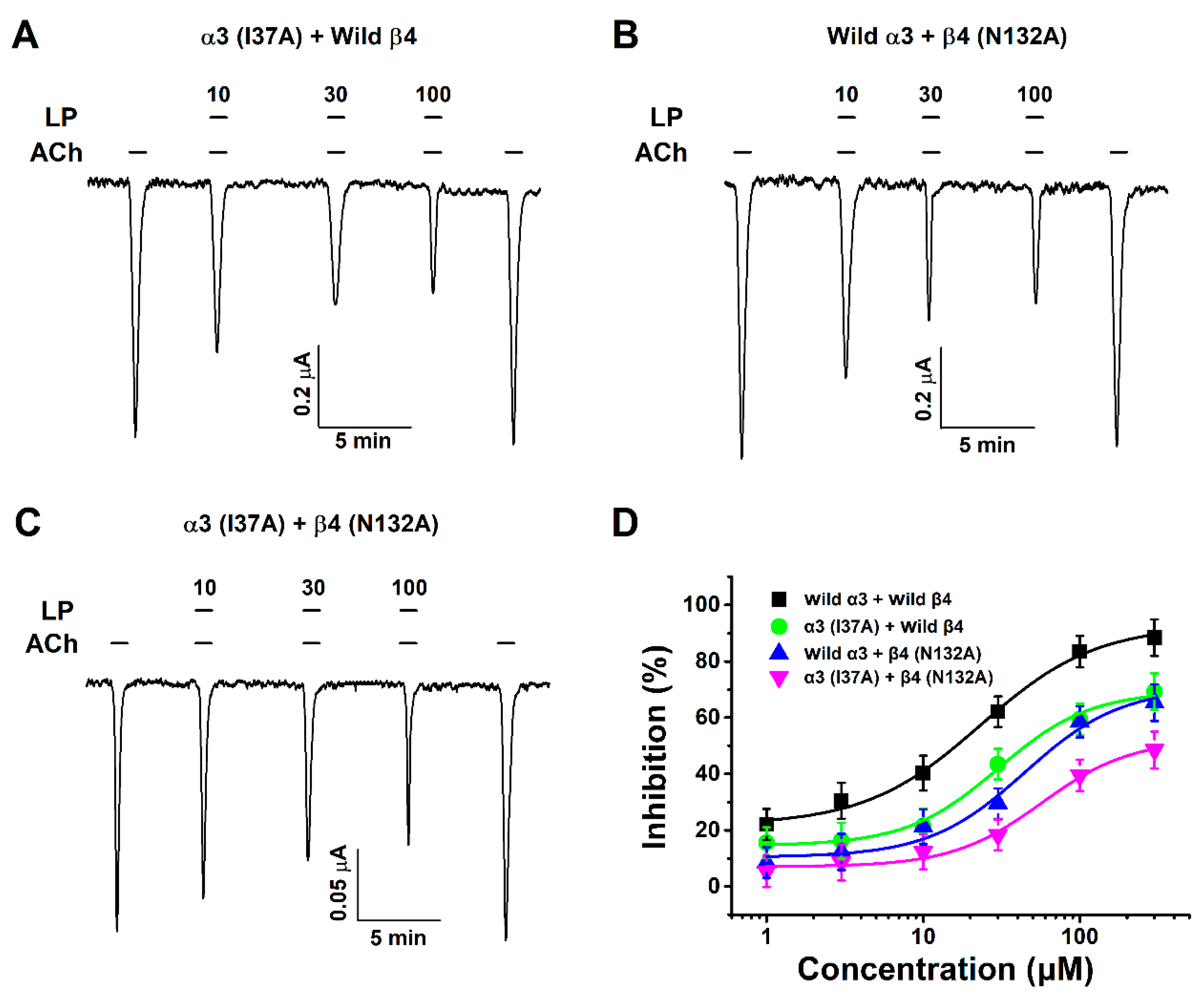
3.5. Inhibitory Effect of Lupeol on Double-Mutant α3β4 (I37A/N132A) nACh Receptors
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Kjeldsen, S.E. Hypertension and cardiovascular risk: General aspects. Pharmacol. Res. 2018, 129, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Sowers, J.R.; Epstein, M.; Frohlich, E.D. Diabetes, hypertension, and cardiovascular disease: An update. Hypertens 2001, 37, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Xi, B.; Huang, Y.; Reilly, K.H.; Li, S.; Zheng, R.; Barrio-Lopez, M.T.; Martinez-Gonzalez, M.A.; Zhou, D. Sugar-sweetened beverages and risk of hypertension and CVD: A dose–response meta-analysis. Br. J. Nutr. 2015, 113, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Vernino, S.; Hopkins, S.; Wang, Z. Autonomic ganglia, acetylcholine receptor antibodies, and autoimmune ganglionopathy. Auton. Neurosci. 2009, 146, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Kulak, J.M.; Cartier, G.E.; Jacobsen, R.B.; Yoshikami, D.; Olivera, B.M.; McIntosh, J.M. α-Conotoxin AuIB selectively blocks α3β4 nicotinic acetylcholine receptors and nicotine-evoked norepinephrine release. J. Neurosci. 1998, 18, 8571–8579. [Google Scholar] [CrossRef]
- Reitstetter, R.; Lukas, R.J.; Gruener, R. Dependence of nicotinic acetylcholine receptor recovery from desensitization on the duration of agonist exposure. J. Pharmacol. Exp. Ther. 1999, 289, 656–660. [Google Scholar]
- Sargent, P.B. The diversity of neuronal nicotinic acetylcholine receptors. Annu. Rev. Neurosci. 1993, 16, 403–443. [Google Scholar] [CrossRef]
- Valentino, R.; Dingledine, R. Presynaptic inhibitory effect of acetylcholine in the hippocampus. J. Neurosci. 1981, 1, 784–792. [Google Scholar] [CrossRef]
- Bibevski, S.; Zhou, Y.; McIntosh, J.M.; Zigmond, R.E.; Dunlap, M.E. Functional nicotinic acetylcholine receptors that mediate ganglionic transmission in cardiac parasympathetic neurons. J. Neurosci. 2000, 20, 5076–5082. [Google Scholar] [CrossRef]
- Cuny, H.; Yu, R.; Tae, H.S.; Kompella, S.N.; Adams, D.J. α-Conotoxins active at α3-containing nicotinic acetylcholine receptors and their molecular determinants for selective inhibition. Br. J. Pharmacol. 2018, 175, 1855–1868. [Google Scholar] [CrossRef]
- Furtado, J.; Niege, A.; Pirson, L.; Edelberg, H.; M Miranda, L.; Loira-Pastoriza, C.; Preat, V.; Larondelle, Y.; André, C.M. Pentacyclic triterpene bioavailability: An overview of in vitro and in vivo studies. Molecules 2017, 22, 400. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.S.; Khan, I.; Zia, M. Antimicrobial, cytotoxic, phytochemical and biological properties of crude extract and solid phase fractions of Monotheca buxifolia. Orient. Pharm. Exp. Med. 2019, 20, 1–8. [Google Scholar] [CrossRef]
- Alqahtani, A.; Hamid, K.; Kam, A.; Wong, K.; Abdelhak, Z.; Razmovski-Naumovski, V.; Chan, K.; Li, K.M.; Groundwater, P.W.; Li, G.Q. The pentacyclic triterpenoids in herbal medicines and their pharmacological activities in diabetes and diabetic complications. Curr. Med. Chem. 2013, 20, 908–931. [Google Scholar] [PubMed]
- Siddique, H.R.; Saleem, M. Beneficial health effects of lupeol triterpene: A review of preclinical studies. Life Sci. 2011, 88, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.B.; Sarachine, M.J. Biological activities of lupeol. Int. J. Biomed. Pharm. Sci. 2009, 3, 46–66. [Google Scholar]
- Yamaguchi, E.; Shirakawa, H.; Hata, K.; Hiwatashi, K.; Ohinata, K.; Goto, T.; Komai, M. Lupeol supplementation improves blood pressure and lipid metabolism parameters in stroke-prone spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2012, 76, 183–185. [Google Scholar]
- Guan, B.; Chen, X.; Zhang, H. Two-electrode voltage clamp. In Ion Channels; Springer: Heidelberg, Germany, 2013; pp. 79–89. [Google Scholar]
- Schreibmayer, W.; Lester, H.A.; Dascal, N. Voltage clamping of Xenopus laevis oocytes utilizing agarose-cushion electrodes. Pflügers Arch. 1994, 426, 453–458. [Google Scholar] [CrossRef]
- Dascal, N. The use of Xenopus oocytes for the study of ion channel. Crit. Rev. Biochem. 1987, 22, 317–387. [Google Scholar] [CrossRef]
- Dutertre, S.; Lewis, R.J. Computational approaches to understand α-conotoxin interactions at neuronal nicotinic receptors. Eur. J. Biochem. 2004, 271, 2327–2334. [Google Scholar] [CrossRef]
- Dutertre, S.; Nicke, A.; Lewis, R.J. β2 subunit contribution to 4/7 α-conotoxin binding to the nicotinic acetylcholine receptor. J. Biol. Chem. 2005, 280, 30460–30468. [Google Scholar] [CrossRef]
- Rust, G.; Burgunder, J.M.; Lauterburg, T.; Cachelin, A. Expression of neuronal nicotinic acetylcholine receptor subunit genes in the rat autonomic nervous system. Eur. J. Neurosci. 1994, 6, 478–485. [Google Scholar] [CrossRef] [PubMed]




| Subunit Mutants | Imax | IC50 | nH |
|---|---|---|---|
| Wild α3 + Wild β4 | 92.8 ± 4.6 | 23.2 ± 3.7 | 1.2 ± 0.2 |
| α3 (I37A) + Wild β4 | 69.4 ± 4.0 | 30.1 ± 5.0 | 1.5 ± 0.4 |
| Wild α3 + β4 (N132A) | 70.7 ± 9.3 | 44.6 ± 14.7 | 1.4 ± 0.6 |
| α3 (I37A) + β4 (N132A) | 52.3 ± 5.2 | 57.0 ± 13.2 | 1.5 ± 0.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eom, S.; Kim, C.; Yeom, H.D.; Lee, J.; Lee, S.; Baek, Y.-B.; Na, J.; Park, S.-I.; Kim, G.-Y.; Lee, C.-M.; et al. Molecular Regulation of α3β4 Nicotinic Acetylcholine Receptors by Lupeol in Cardiovascular System. Int. J. Mol. Sci. 2020, 21, 4329. https://doi.org/10.3390/ijms21124329
Eom S, Kim C, Yeom HD, Lee J, Lee S, Baek Y-B, Na J, Park S-I, Kim G-Y, Lee C-M, et al. Molecular Regulation of α3β4 Nicotinic Acetylcholine Receptors by Lupeol in Cardiovascular System. International Journal of Molecular Sciences. 2020; 21(12):4329. https://doi.org/10.3390/ijms21124329
Chicago/Turabian StyleEom, Sanung, Chaelin Kim, Hye Duck Yeom, Jaeeun Lee, Shinhui Lee, Yeong-Bin Baek, Jinseong Na, Sang-Ik Park, Gye-Yeop Kim, Chang-Min Lee, and et al. 2020. "Molecular Regulation of α3β4 Nicotinic Acetylcholine Receptors by Lupeol in Cardiovascular System" International Journal of Molecular Sciences 21, no. 12: 4329. https://doi.org/10.3390/ijms21124329
APA StyleEom, S., Kim, C., Yeom, H. D., Lee, J., Lee, S., Baek, Y.-B., Na, J., Park, S.-I., Kim, G.-Y., Lee, C.-M., & Lee, J.-H. (2020). Molecular Regulation of α3β4 Nicotinic Acetylcholine Receptors by Lupeol in Cardiovascular System. International Journal of Molecular Sciences, 21(12), 4329. https://doi.org/10.3390/ijms21124329






