Functional Heterogeneity of Protein Kinase A Activation in Multipotent Stromal Cells
Abstract
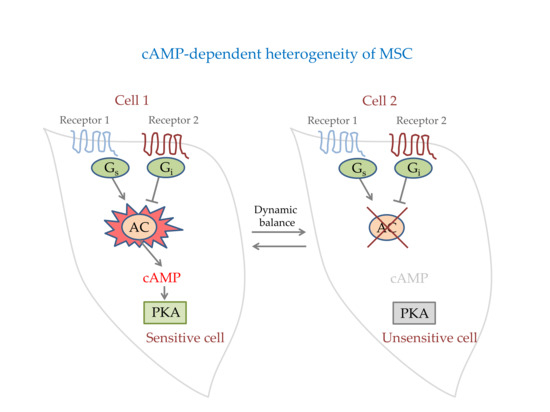
1. Introduction
2. Results
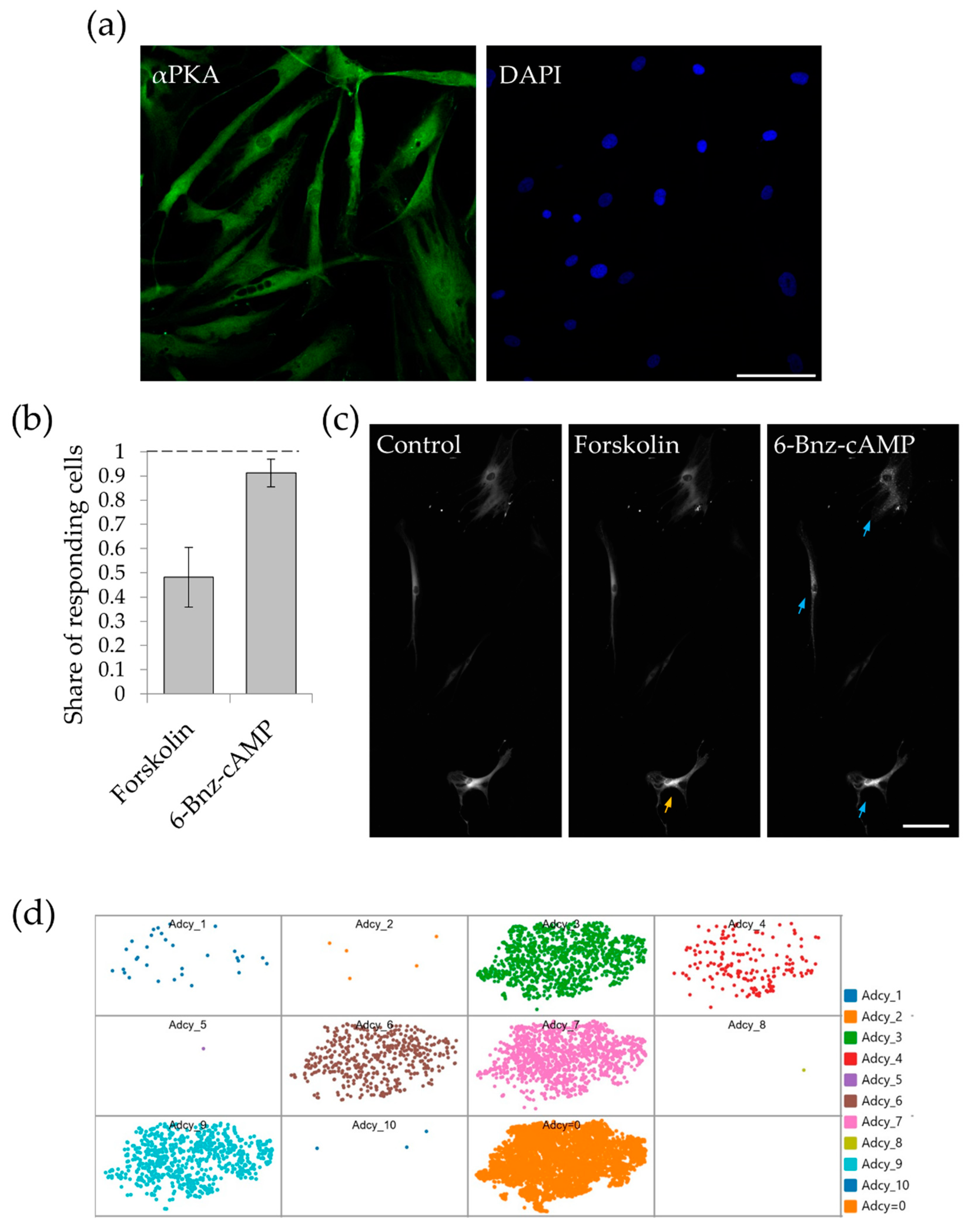
2.1. MSCs Subpopulations Demonstrate Distinct PKA Activation
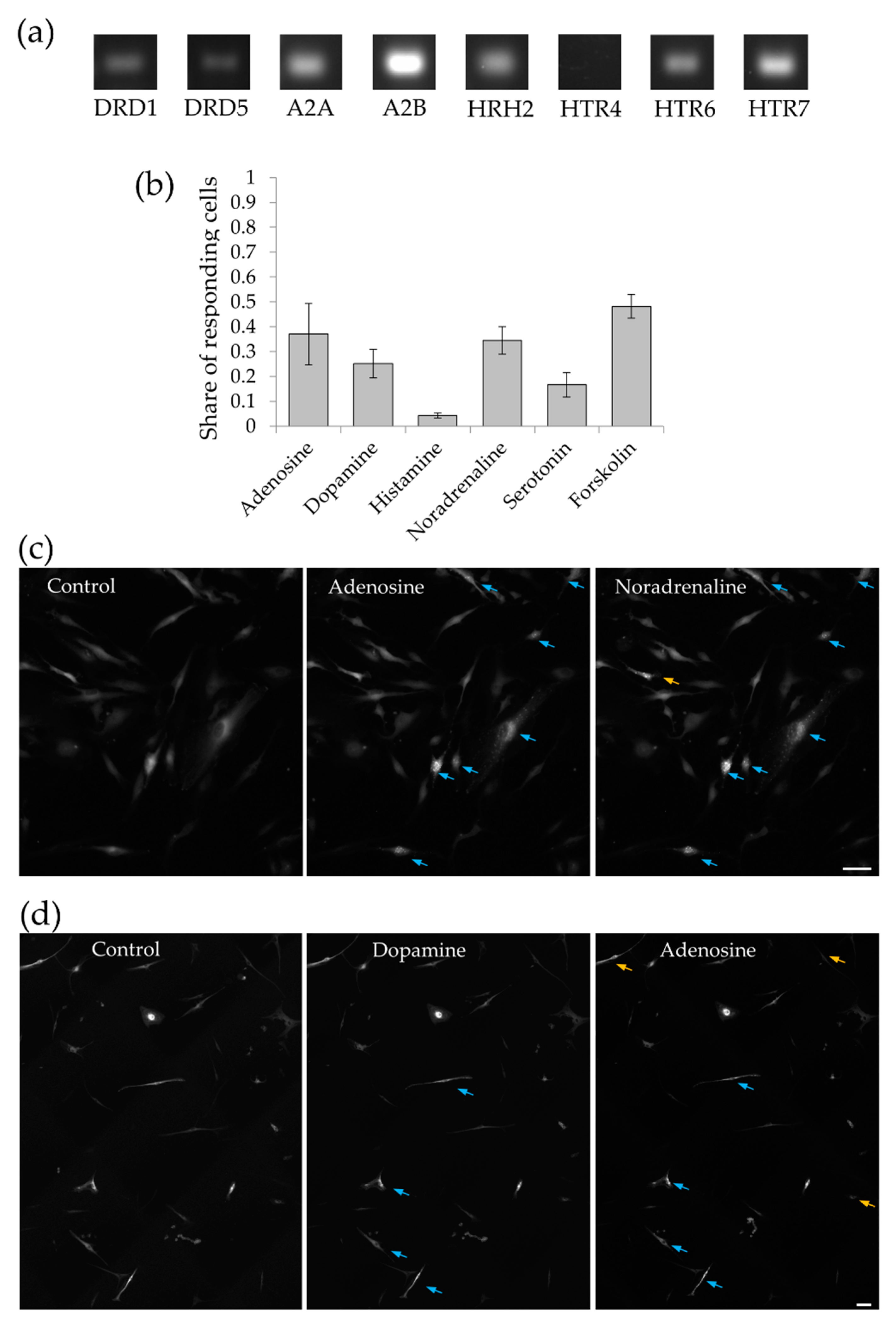
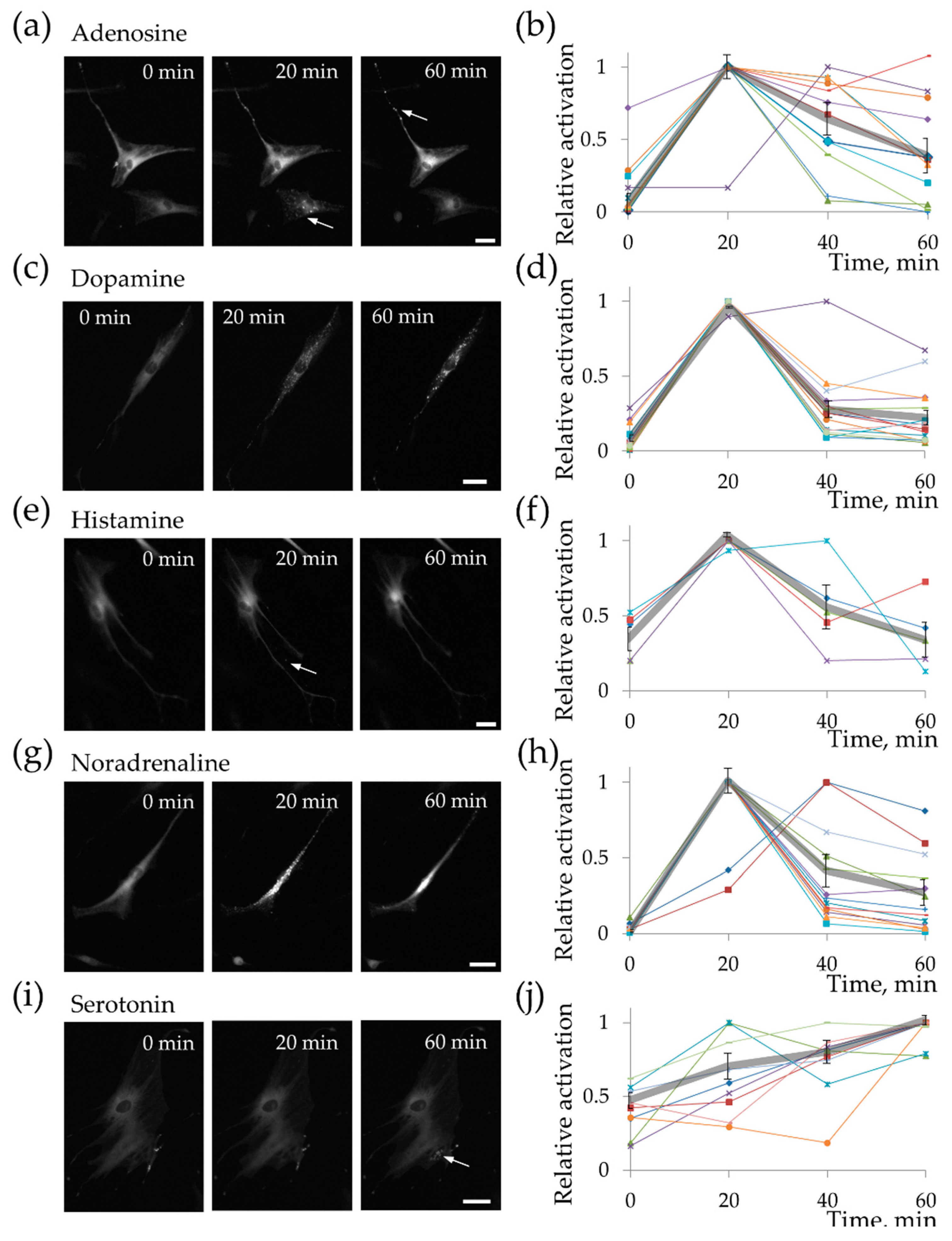
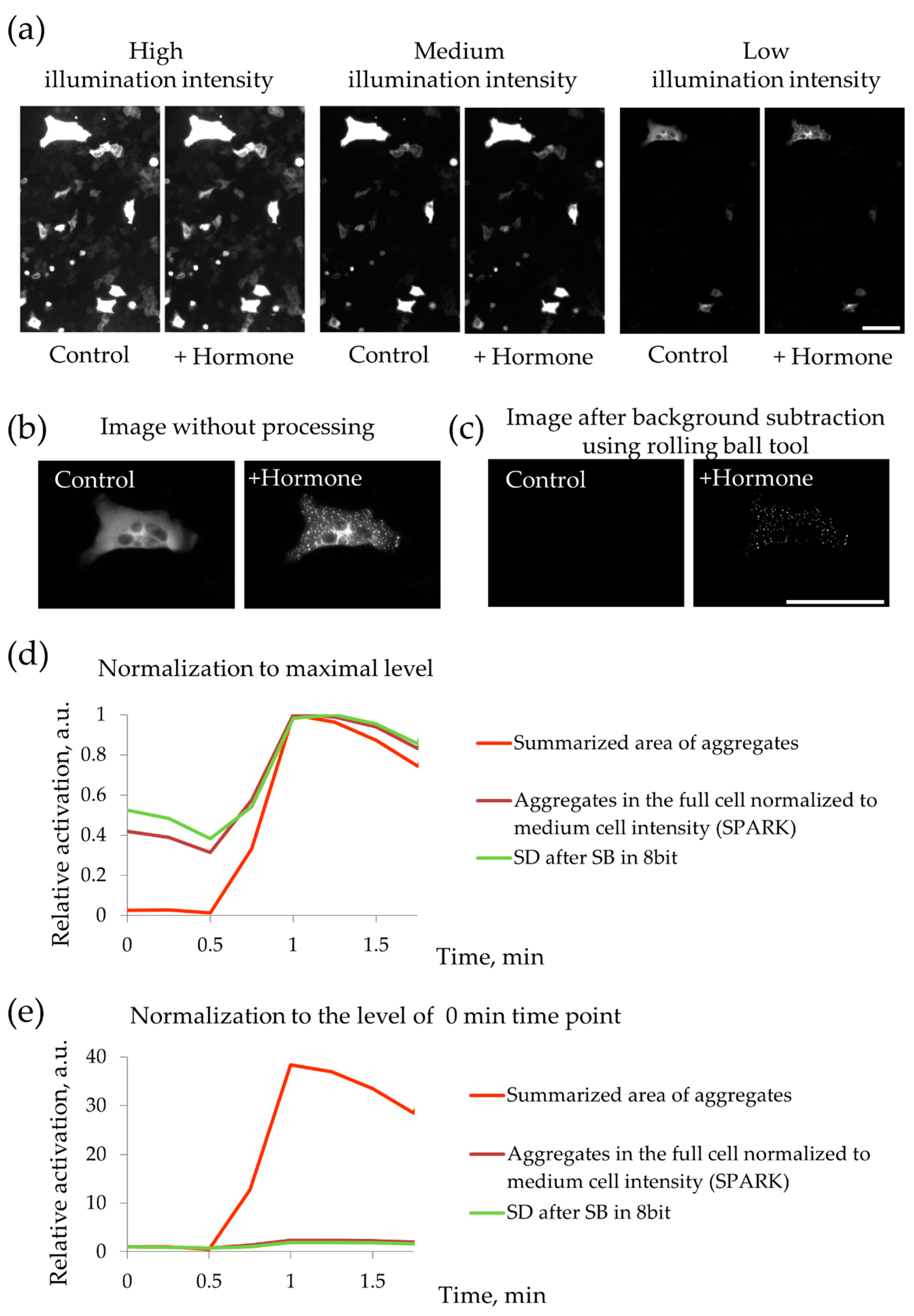
2.2. MSC Demonstrate Heterogeneous Response to the Hormonal Stimuli
2.3. MSC Functional Heterogeneity Is Highly Dynamic
3. Discussion
4. Materials and Methods
4.1. MSC Culture
4.2. RNA Isolation and PCR Detection of Receptors
4.3. Lentiviral Transduction of MSC
4.4. Registration of PKA Activation with PKA-Spark
4.5. Immunofluorescent Analysis of PKA Expression in MSCs
4.6. scRNAseq Data Processing
4.7. MSC Sorting and Clonal Analysis
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MSC | Multipotent stromal cells, Mesenchymal stem/stromal cells |
| PKA | Protein kinase A |
| AC | Adenylyl cyclase |
References
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, N.I.; Sysoeva, V.Y.; Rubina, K.A.; Parfenova, Y.V.; Tkachuk, V.A. Mesenchymal stem cells in tissue growth and repair. Acta Nat. 2011, 3, 30–37. [Google Scholar] [CrossRef]
- Schwalie, P.C.; Dong, H.; Zachara, M.; Russeil, J.; Alpern, D.; Akchiche, N.; Caprara, C.; Sun, W.; Schlaudraff, K.U.; Soldati, G.; et al. A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature 2018, 559, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Burl, R.B.; Ramseyer, V.D.; Rondini, E.A.; Pique-Regi, R.; Lee, Y.H.; Granneman, J.G. Deconstructing Adipogenesis Induced by beta3-Adrenergic Receptor Activation with Single-Cell Expression Profiling. Cell Metab. 2018, 28, 300–309.e304. [Google Scholar] [CrossRef] [PubMed]
- Hoogduijn, M.J.; Cheng, A.; Genever, P.G. Functional nicotinic and muscarinic receptors on mesenchymal stem cells. Stem Cells Dev. 2009, 18, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Gharibi, B.; Abraham, A.A.; Ham, J.; Evans, B.A. Adenosine receptor subtype expression and activation influence the differentiation of mesenchymal stem cells to osteoblasts and adipocytes. J. Bone Miner. Res. 2011, 26, 2112–2124. [Google Scholar] [CrossRef]
- Sysoeva, V.Y.; Ageeva, L.V.; Tyurin-Kuzmin, P.A.; Sharonov, G.V.; Dyikanov, D.T.; Kalinina, N.I.; Tkachuk, V.A. Local angiotensin II promotes adipogenic differentiation of human adipose tissue mesenchymal stem cells through type 2 angiotensin receptor. Stem Cell Res. 2017, 25, 115–122. [Google Scholar] [CrossRef]
- Kotova, P.D.; Bystrova, M.F.; Rogachevskaja, O.A.; Khokhlov, A.A.; Sysoeva, V.Y.; Tkachuk, V.A.; Kolesnikov, S.S. Coupling of P2Y receptors to Ca2+ mobilization in mesenchymal stromal cells from the human adipose tissue. Cell Calcium. 2018, 71, 1–14. [Google Scholar] [CrossRef]
- Marcoli, M.; Candiani, S.; Tonachini, L.; Monticone, M.; Mastrogiacomo, M.; Ottonello, A.; Cervetto, C.; Paluzzi, P.; Maura, G.; Pestarino, M. In vitro modulation of gamma amino butyric acid (GABA) receptor expression by bone marrow stromal cells. Pharmacol. Res. 2008, 57, 374–382. [Google Scholar] [CrossRef]
- Kotova, P.D.; Sysoeva, V.Y.; Rogachevskaja, O.A.; Bystrova, M.F.; Kolesnikova, A.S.; Tyurin-Kuzmin, P.A.; Fadeeva, J.I.; Tkachuk, V.A.; Kolesnikov, S.S. Functional expression of adrenoreceptors in mesenchymal stromal cells derived from the human adipose tissue. Biochim. Biophys. Acta 2014, 1843, 1899–1908. [Google Scholar] [CrossRef]
- Nemeth, K.; Wilson, T.; Rada, B.; Parmelee, A.; Mayer, B.; Buzas, E.; Falus, A.; Key, S.; Masszi, T.; Karpati, S. Characterization and function of histamine receptors in human bone marrow stromal cells. Stem Cells 2012, 30, 222–231. [Google Scholar] [CrossRef]
- Tyurin-Kuzmin, P.A.; Dyikanov, D.T.; Fadeeva, J.I.; Sysoeva, V.Y.; Kalinina, N.I. Flow cytometry analysis of adrenoceptors expression in human adipose-derived mesenchymal stem/stromal cells. Sci. Data 2018, 5, 180196. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.-M.; Namkung, J.; Go, Y.; Shong, K.E.; Kim, K.; Kim, H.; Park, B.-Y.; Lee, H.W.; Jeon, Y.H.; Song, J. Regulation of systemic energy homeostasis by serotonin in adipose tissues. Nat. Commun. 2015, 6, 6794. [Google Scholar] [CrossRef] [PubMed]
- Price, S.T.; Beckham, T.H.; Cheng, J.C.; Lu, P.; Liu, X.; Norris, J.S. Sphingosine 1-phosphate receptor 2 regulates the migration, proliferation, and differentiation of mesenchymal stem cells. Int. J. Stem Cell Res. Ther. 2015, 2, 14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tyurin-Kuzmin, P.A.; Fadeeva, J.I.; Kanareikina, M.A.; Kalinina, N.I.; Sysoeva, V.Y.; Dyikanov, D.T.; Stambolsky, D.V.; Tkachuk, V.A. Activation of beta-adrenergic receptors is required for elevated alpha1A-adrenoreceptors expression and signaling in mesenchymal stromal cells. Sci. Rep. 2016, 6, 32835. [Google Scholar] [CrossRef]
- Li, H.; Fong, C.; Chen, Y.; Cai, G.; Yang, M. Beta-adrenergic signals regulate adipogenesis of mouse mesenchymal stem cells via cAMP/PKA pathway. Mol. Cell Endocrinol. 2010, 323, 201–207. [Google Scholar] [CrossRef]
- Mendez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; Macarthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, H.; Zhang, L.; Wu, R.; Chung, C.I.; Zhang, S.Q.; Torra, J.; Schepis, A.; Coughlin, S.R.; Kornberg, T.B.; et al. Visualizing Dynamics of Cell Signaling In Vivo with a Phase Separation-Based Kinase Reporter. Mol. Cell 2018, 69, 347. [Google Scholar] [CrossRef]
- Liu, X.; Xiang, Q.; Xu, F.; Huang, J.; Yu, N.; Zhang, Q.; Long, X.; Zhou, Z. Single-cell RNA-seq of cultured human adipose-derived mesenchymal stem cells. Sci. Data 2019, 6, 190031. [Google Scholar] [CrossRef]
- Tyurin-Kuzmin, P.A.; Kalinina, N.I.; Kulebyakin, K.Y.; Balatskiy, A.V.; Sysoeva, V.Y.; Tkachuk, V.A. Angiotensin receptor subtypes regulate adipose tissue renewal and remodelling. FEBS J. 2020, 287, 1076–1087. [Google Scholar] [CrossRef]
- Langeberg, L.K.; Scott, J.D. A-kinase-anchoring proteins. J. Cell Sci. 2005, 118, 3217–3220. [Google Scholar] [CrossRef] [PubMed]
- Sadana, R.; Dessauer, C.W. Physiological roles for G protein-regulated adenylyl cyclase isoforms: Insights from knockout and overexpression studies. Neurosignals 2009, 17, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Hardiman, O.; Sweeney, D.J.; Keenan, A.K. Adenylyl cyclase activity in clonally derived human myoblast cultures: Evidence for myoblast heterogeneity. Neuromuscul. Disord. 1996, 6, 283–291. [Google Scholar] [CrossRef]
- Blake, W.J.; Kærn, M.; Cantor, C.R.; Collins, J.J. Noise in eukaryotic gene expression. Nature 2003, 422, 633–637. [Google Scholar] [CrossRef]
- Chang, H.H.; Hemberg, M.; Barahona, M.; Ingber, D.E.; Huang, S. Transcriptome-wide noise controls lineage choice in mammalian progenitor cells. Nature 2008, 453, 544–547. [Google Scholar] [CrossRef]
- Kaern, M.; Elston, T.C.; Blake, W.J.; Collins, J.J. Stochasticity in gene expression: From theories to phenotypes. Nat. Rev. Genet. 2005, 6, 451–464. [Google Scholar] [CrossRef]
- Krinner, A.; Zscharnack, M.; Bader, A.; Drasdo, D.; Galle, J. Impact of oxygen environment on mesenchymal stem cell expansion and chondrogenic differentiation. Cell Prolif. 2009, 42, 471–484. [Google Scholar] [CrossRef]
- Longo, P.A.; Kavran, J.M.; Kim, M.-S.; Leahy, D.J. Transient mammalian cell transfection with polyethylenimine (PEI). Methods Enzymol. 2013, 529, 227–240. [Google Scholar]

| Target | Forward Primer | Reverse Primer | T Annealing | Product Length |
|---|---|---|---|---|
| HRH2 | CGT GTC CTT GGC TAT CAC TGA | GGC TGG TGT AGA TAT TGC AGA AG | 55 °C | 119 bp |
| HRH2 | CAG TTC GGG TCG CCA TCT C | CTG GTC TCG TTC CTG CTG TTC | 55.2 °C | 100 bp |
| DRD1 | AGG GAC TTC TCT GTT CGT ATC C | AGG GAC TTC TCT GTT CGT ATC C | 54.9 °C | 103 bp |
| DRD1 | TCT GTG CTG CCG TTA TCA GG | TTG TGG GTT TTG CCT TGT GC | 54.2 °C | 378 bp |
| DRD5 | CCG TGT CAG ACC TTT TCG TG | TGC GCT GAG TCA TCT TGC G | 54.7 °C | 216 bp |
| DRD5 | GGG CAG TTC GCT CTA TAC CAG | GGT CCA GAT GAT GAG TAG GGT C | 56 °C | 126 bp |
| A2B | CTG TCA CAT GCC AAT TCA GTT G | GCC TGA CCA TTC CCA CTC TTG | 55 °C | 134 bp |
| A2A | CGC TCC GGT ACA ATG GCT T | TTG TTC CAA CCT AGC ATG GGA | 54.5 °C | 109 bp |
| A2A | CTG GCT GCC CCT ACA CAT C | TCA CAA CCG AAT TGG TGT GGG | 54.3 °C | 116 bp |
| HTR4 | CTC ACG TTT CTC TCG ACG GTT | AGC AGA TCC GCA AAA GCA AGA | 54.6 °C | 137 bp |
| HTR4 | GAT CTG CTG GTT TCG GTG CT | CAG AGG GGT CAT CTT GTT CCT A | 54.6 °C | 213 bp |
| HTR6 | GCA ACA CGT CCA ACT TCT TCC | TGC AGC ACA TCA CGT CGA A | 54.1 °C | 159 bp |
| HTR7 | CGA AGA TGA TTC TCT CCG TCT G | GCG GTA GAG TAA ATC GTA TAG CC | 54.9 °C | 136 bp |
| HTR7 | TGG TGA TCT CCG TGT GCT TC | TCC AAA GAT CCA CTT GCC CC | 54.8 °C | 149 bp |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyurin-Kuzmin, P.A.; Karagyaur, M.N.; Kulebyakin, K.Y.; Dyikanov, D.T.; Chechekhin, V.I.; Ivanova, A.M.; Skryabina, M.N.; Arbatskiy, M.S.; Sysoeva, V.Y.; Kalinina, N.I.; et al. Functional Heterogeneity of Protein Kinase A Activation in Multipotent Stromal Cells. Int. J. Mol. Sci. 2020, 21, 4442. https://doi.org/10.3390/ijms21124442
Tyurin-Kuzmin PA, Karagyaur MN, Kulebyakin KY, Dyikanov DT, Chechekhin VI, Ivanova AM, Skryabina MN, Arbatskiy MS, Sysoeva VY, Kalinina NI, et al. Functional Heterogeneity of Protein Kinase A Activation in Multipotent Stromal Cells. International Journal of Molecular Sciences. 2020; 21(12):4442. https://doi.org/10.3390/ijms21124442
Chicago/Turabian StyleTyurin-Kuzmin, Pyotr A., Maxim N. Karagyaur, Konstantin Yu. Kulebyakin, Daniyar T. Dyikanov, Vadim I. Chechekhin, Anastasiya M. Ivanova, Mariya N. Skryabina, Mikhail S. Arbatskiy, Veronika Yu. Sysoeva, Natalia I. Kalinina, and et al. 2020. "Functional Heterogeneity of Protein Kinase A Activation in Multipotent Stromal Cells" International Journal of Molecular Sciences 21, no. 12: 4442. https://doi.org/10.3390/ijms21124442
APA StyleTyurin-Kuzmin, P. A., Karagyaur, M. N., Kulebyakin, K. Y., Dyikanov, D. T., Chechekhin, V. I., Ivanova, A. M., Skryabina, M. N., Arbatskiy, M. S., Sysoeva, V. Y., Kalinina, N. I., & Tkachuk, V. A. (2020). Functional Heterogeneity of Protein Kinase A Activation in Multipotent Stromal Cells. International Journal of Molecular Sciences, 21(12), 4442. https://doi.org/10.3390/ijms21124442