The Telocytes: Ten Years after Their Introduction in the Scientific Literature. An Update on Their Morphology, Distribution, and Potential Roles in the Gut
Abstract
1. Introduction
2. Telocytes
2.1. The Telocytes in the Gut Form Networks
The Telocyte Network Is the Scaffold of the Gut Wall
2.2. The Telocytes in the Gut Interact with Other Cells
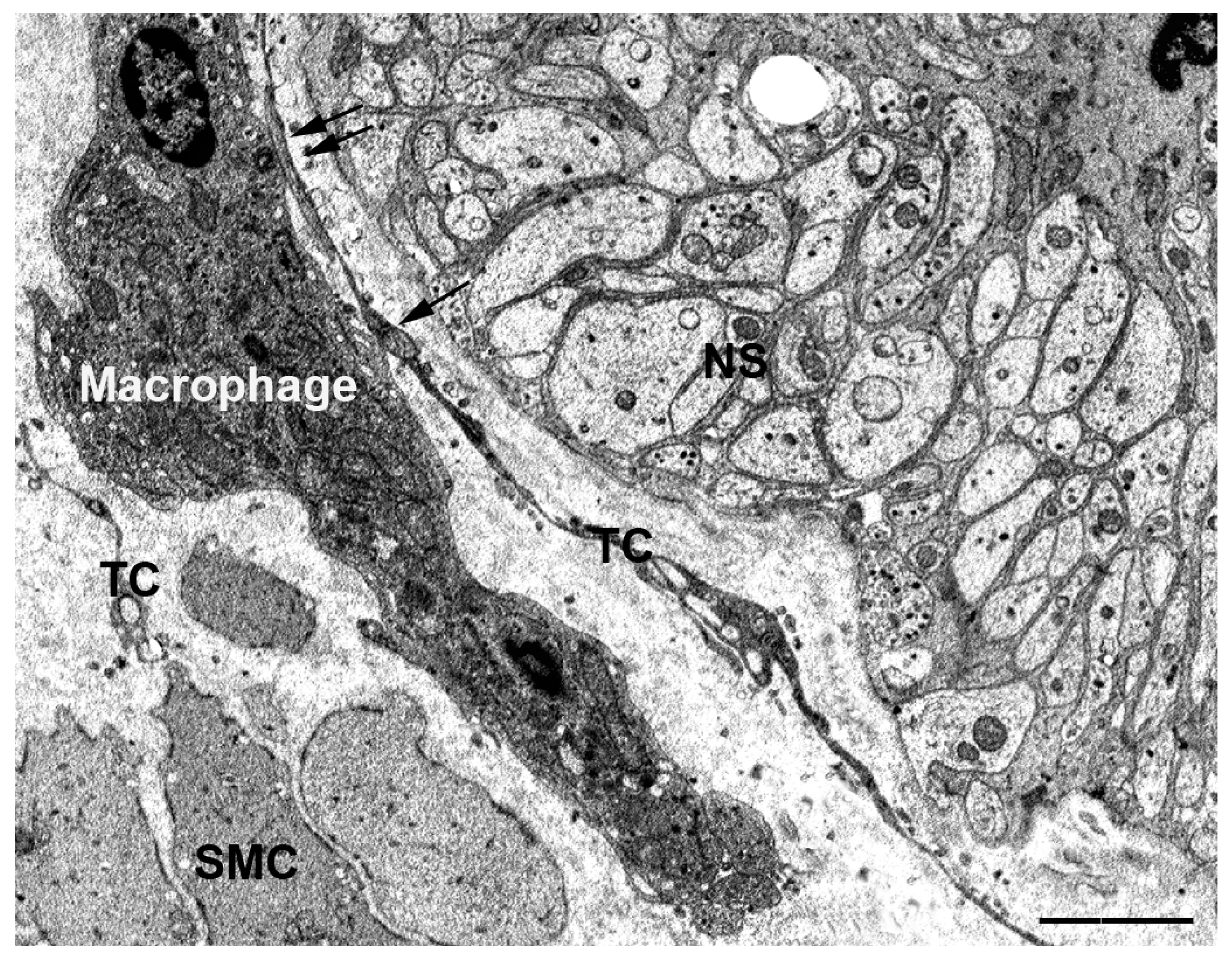
2.2.1. The TCs Interact with Isolated Connective Cells
2.2.2. The TCs Interact with Cells Organized in Networks
- (i)
- The TCs might be adult stromal mesenchymal cells able to differentiate in ICCs [16,26]. Notably, while ICCs undergo apoptosis with time, their number does not change significantly in aging [33]. However, images of ICCs mitosis were never seen. Thus, the existence of a pool of stem cells (the TCs?) committed to become ICCs when needed is reasonable. The expression of PDGFRα by the TCs reinforces such a hypothesis since the literature data indicate that PDGF/PDGFR signaling plays critical roles in mammalian organogenesis and morphogenesis [34,35].
- (ii)
- ICCs are constantly and often richly innervated and participate in the control of the muscle wall activity as intermediaries of neuronal actions [15,36,37,38]. Although under light microscopy excitatory and inhibitory nerve fibers are often seen in the vicinity of the TCs [19,20,27,39], using TEM, we demonstrated in the GI muscle coat of mice and humans that the nerve endings never established cell-to-cell contacts with TCs [27,32]. Nevertheless, the TCs, through the contacts with the ICCs, might be involved in neurotransmission, possibly contributing to spread the slow waves or amplify the nervous stimulus generated in the ICC [16,19,20].
- (iii)
- TCs and ICC are involved in the SIP (Smooth muscle cells-Interstitial cells of Cajal-PDGFRα-positive cells) syncytium (see below).
2.2.3. The TCs Interact with the Smooth Muscle Cells
2.2.4. Are TCs Target of Neural Signals
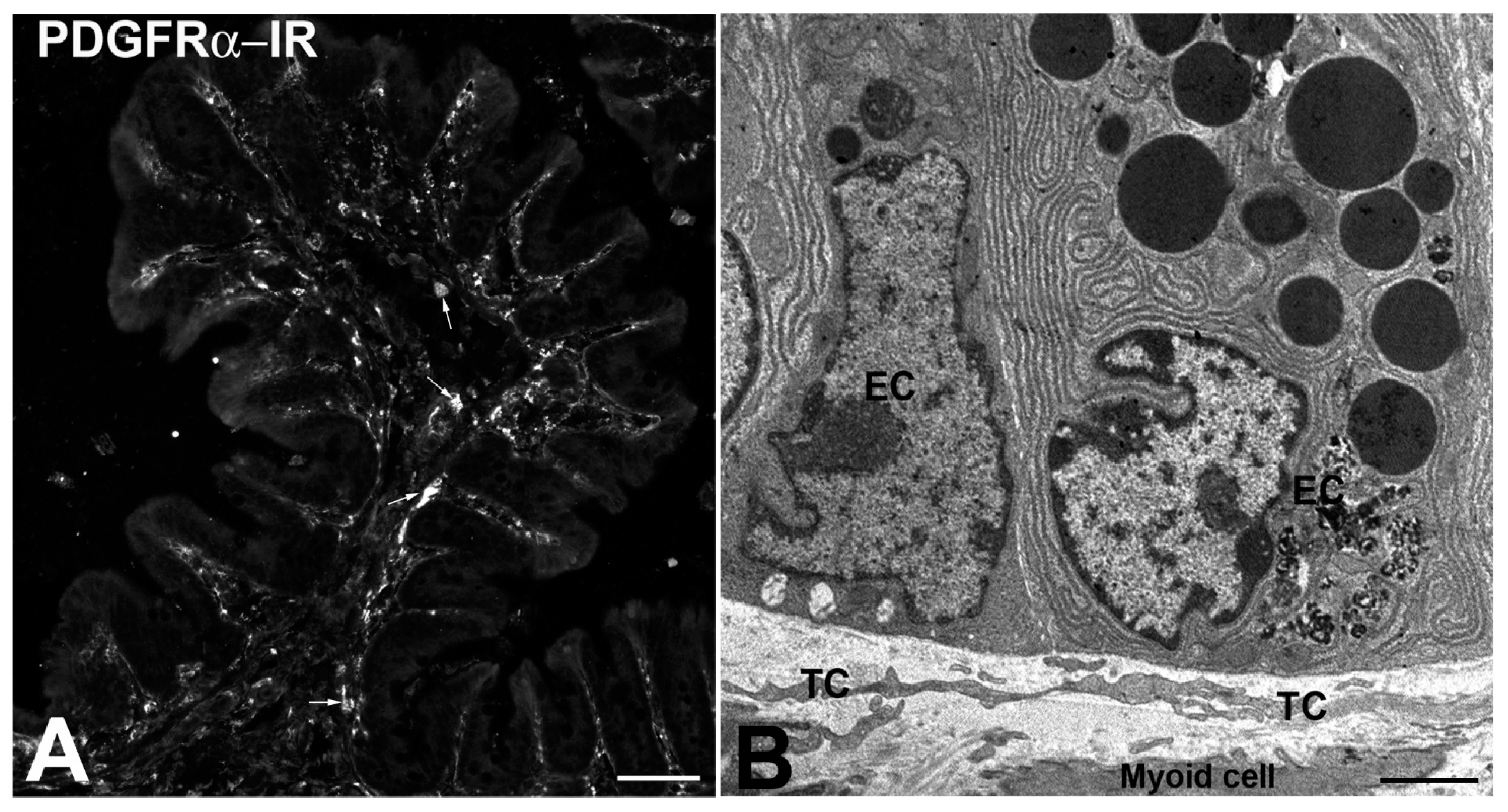
2.3. Telocyte Presence and Role in the Gut Mucosa
2.3.1. The TCs in the Gut Mucosa Transduce Sensory Signals
2.3.2. The TCs in the Gut Mucosa Are Nurse Cells for Stem Cell Niches
3. Conclusions
- TCs are the mechanical support during gut movements.
- TCs organize the ECM, compartmentalize, and restrain the other IC (i.e., macrophages or other immune cells) inside their meshes.
- TCs take several types of cell-to-cell contacts with almost all the other IC and, through the production of exosomes, might interact with all the cells present in the gut wall, neurons included.
- TCs express receptors and contain molecules involved in the neurotransmission and might participate in the control of the GI functions in terms of absorption and motility.
- TCs might be mesenchymal stem cells as precursors of the ICCs (and possible of other IC);
- TCs lining the gut epithelium are likely involved in controlling the proliferation and differentiation of cryptal stem cells behaving as nurse cells.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TEM | Transmission electron microscope |
| TCs | Telocytes |
| ICs | Interstitial cells |
| GI | Gastrointestinal |
| ICCs | Interstitial cells of Cajal |
| Myo | Myofibroblasts |
| Tps | Telopodes |
| IHC | Immunohistochemical/immunohistochemistry |
| NS | Nerve strands |
| ECM | Extra-cellular matrix |
| SMC | Smooth muscle cells |
| LP | Lamina Propria |
| IR | Immunoreactivity |
| PDGFRα | Platelet derived growth factor receptor-α |
| EC | Epithelial cells |
Appendix A
The Interstitial Cells of Cajal (ICCs)
Appendix B
Myofibroblasts
Appendix C
The Myoid Cells
References
- Faussone-Pellegrini, M.S.; Popescu, L.M. Telocytes. BioMol. Concepts 2011, 2, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Popescu, L.M.; Faussone-Pellegrini, M.S. TELOCYTES—A Case of Serendipity: The Winding Way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to TELOCYTES. J. Cell. Mol. Med. 2010, 4, 729–740. [Google Scholar] [CrossRef]
- Vanderwinden, J.M.; Rumessen, J.J.; De Laet, M.H.; Vanderhaeghen, J.J.; Schiffmann, S.N. CD34-cells in human intestine are fibroblasts adjacent to, but distinct from, interstitial cells of Cajal. Lab Invest. 1999, 79, 59–65. [Google Scholar]
- Johnston, L.; Woolsey, S.; Cunningham, R.M.; O’Kane, H.; Duggan, B.; Keane, P.; McCloskey, K.D. Morphological expression of KIT positive interstitial cells of Cajal in human bladder. J. Urol. 2010, 184, 370–377. [Google Scholar] [CrossRef]
- McCloskey, K.D. Bladder interstitial cells: An updated review of current knowledge. Acta. Physiol. 2013, 207, 7–15. [Google Scholar] [CrossRef]
- Vanderwinden, J.M.; Rumessen, J.J.; de Kerchove d’Exaerde, A.; Gillard, K.; Panthier, J.J.; de Laet, M.H.; Schiffmann, S.N. Kit-negative fibroblast-like cells expressing SK3, a Ca2+-activated K+ channel, in the gut musculature in health and disease. Cell Tissue Res. 2002, 310, 349–358. [Google Scholar] [CrossRef]
- Gherghiceanu, M.; Popescu, L.M. Interstitial Cajal-like cells (ICLC) in human resting mammary gland stroma. Transmission electron microscope (TEM) identification. J. Cell Mol. Med. 2005, 9, 893–910. [Google Scholar] [CrossRef]
- Hinescu, M.E.; Popescu, L.M.; Gherghiceanu, M.; Faussone-Pellegrini, M.S. Interstitial Cajal-like cells in rat mesentery: An ultrastructural and immunohistochemical approach. J. Cell Mol. Med. 2008, 12, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Cretoiu, S.M.; Popescu, L.M. Telocytes revisited. BioMol. Concepts 2014, 5, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, M.G.; Traini, C.; Guasti, D.; Del Popolo, G.; Faussone-Pellegrini, M.S. Telocytes subtypes in human urinary bladder. J. Cell Mol. Med. 2014, 18, 2000–2008. [Google Scholar] [CrossRef] [PubMed]
- Gevaert, T.; Vanstreels, E.; Daelemans, D.; Franken, J.; Van Der Aa, F.; Roskams, T.D.; Ridder, D. Identification of different phenotypes for the interstitial cells in the upper and deep lamina propria of the dome of the human bladder. J. Urol. 2014, 192, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, M.G.; Faussone-Pellegrini, M.S. The Telocyte Subtypes. Adv. Exp. Med. Biol. 2016, 913, 115–126. [Google Scholar] [PubMed]
- Diaz-Flores, L.; Gutierrez, R.; Garcia, M.P.; Gonzalez, M.; Saez, F.J.; Aparicio, F.; Diaz-Flores, L., Jr.; Madrid, J.F. Human resident CD34+ stromal cells/telocytes have progenitor capacity and are a source of alphaSMA+ cells during repair. Histol. Histopathol. 2015, 30, 615–627. [Google Scholar]
- Traini, C.; Fausssone-Pellegrini, M.S.; Guasti, D.; Del Popolo, G.; Frizzi, J.; Sergio Serni, S.; Vannucchi, M.G. Adaptive changes of telocytes in the urinary bladder of patients affected by neurogenic detrusor overactivity. J. Cell Mol. Med. 2018, 22, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, M.G.; Traini, C. Interstitial cells of Cajal and telocytes in the gut: Twins, related or simply neighbor cells? BioMol. Concepts 2016, 7, 93–102. [Google Scholar] [CrossRef]
- Pieri, L.; Vannucchi, M.G.; Faussone-Pellegrini, M.S. Histochemical and ultrastructural characteristics of an interstitial cell type different from ICC and resident in the muscle coat of human gut. J. Cell Mol. Med. 2008, 12, 1944–1955. [Google Scholar] [CrossRef]
- Vannucchi, M.G.; Traini, C.; Manetti, M.; Ibba-Manneschi, L.; Faussone-Pellegrini, M.S. Telocytes express PDGFRα in the human gastrointestinal tract. J. Cell Mol. Med. 2013, 17, 1099–1108. [Google Scholar] [CrossRef]
- Traserra, S.; Villarte, S.; Traini, C.; Palacin, S.; Vergara, P.; Vannucchi, M.G.; Jimenez, M. The asymmetric innervation of the circular and longitudinal muscle of the mouse colon differently modulates myogenic slow phasic contractions. Neurogastroenterol. Motil. 2020, 32, e13778. [Google Scholar] [CrossRef]
- Kurahashi, M.; Nakano, Y.; Hennig, G.W.; Ward, S.M.; Sanders, K.M. Platelet-derived growth factor receptor α-positive cells in the tunica muscularis of human colon. J. Cell Mol. Med. 2012, 16, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Kurahashi, M.; Nakano, Y.; Peri, L.E.; Townsend, J.B.; Ward, S.M.; Sanders, K.M. A novel population of subepithelial platelet-derived growth factor receptor α-positive cells in the mouse and human colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G823–G834. [Google Scholar] [CrossRef] [PubMed]
- Cretoiu, D.; Roatesi, S.; Bica, I.; Plesca, C.; Stefan, A.; Bajenaru, O.; Condrat, C.E.; Cretoiu, S.M. Simulation and Modeling of Telocytes Behavior in Signaling and Intercellular Communication Processes. Int. J. Mol. Sci. 2020, 21, 2615. [Google Scholar] [CrossRef] [PubMed]
- Dìaz-Flores, L.; Gutierrez, R.; Garcia, M.P.; Sáez, F.J.; Díaz-Flores, L., Jr.; Valladares, F.; Madrid, J.F. CD34+ stromal cells/fibroblasts/fibrocytes/telocytes as a tissue reserve and a principal source of mesenchymal cells. Location, morphology, function and role in pathology. Histol. Histopathol. 2014, 29, 831–870. [Google Scholar] [PubMed]
- Milia, A.F.; Ruffo, M.; Manetti, M.; Rosa, I.; Conte, D.; Fazi, M.; Messerini, L.; Ibba-Manneschi, L. Telocytes in Crohn’s disease. J. Cell Mol. Med. 2013, 17, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Manetti, M.; Rosa, I.; Messerini, L.; Ibba-Manneschi, L. A loss of telocytes accompanies inflammation-driven fibrotic remodelling of the colonic wall in ulcerative colitis. J. Cell Mol. Med. 2015, 19, 62–73. [Google Scholar] [CrossRef]
- Cretoiu, S.M.; Cretoiu, D.; Suciu, L.; Popescu, L.M. Interstitial Cajal-like cells of human Fallopian tube express estrogen and progesterone receptors. J. Mol. Histol. 2009, 40, 387–394. [Google Scholar] [CrossRef]
- Vannucchi, M.G.; Bani, D.; Faussone-Pellegrini, M.S. Telocytes Contribute as Cell Progenitors and Differentiation Inductors in Tissue Regeneration. Current Stem Cell Res. Ther. 2016, 11, 383–389. [Google Scholar] [CrossRef]
- Faussone-Pellegrini, M.S.; Gherghiceanu, M. Telocyte’s contacts. Sem. Cell Dev. Biol. 2016, 55, 3–8. [Google Scholar] [CrossRef]
- De Schepper, S.; Verheijden, S.; Aguilera-Lizarraga, J.; Viola, M.F.; Boesmans, W.; Stakenborg, N.; Voytyuk, I.; Schmidt, I.; Boeckx, B.; Dierckx de Casterlé, I.; et al. Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell 2019, 176, 676. [Google Scholar] [CrossRef]
- Pierce, J.H.; Di Marco, E.; Cox, G.W.; Lombardi, D.; Ruggiero, M.; Varesio, L.; Wang, L.M.; Choudhury, G.G.; Sakaguchi, A.Y.; Di Fiore, P.P. Macrophage-colony-stimulating factor (CSF-1) induces proliferation, chemotaxis, and reversible monocytic differentiation in myeloid progenitor cells transfected with the human c-fms/CSF-1 receptor cDNA. Proc. Natl. Acad. Sci. USA 1990, 87, 5613–5617. [Google Scholar] [CrossRef]
- Gabanyi, I.; Muller, P.A.; Feighery, L.; Oliveira, T.Y.; Costa-Pinto, F.A.; Mucida, D. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell 2016, 164, 378–391. [Google Scholar] [CrossRef]
- Muller, P.A.; Koscsó, B.; Rajani, G.M.; Stevanovic, K.; Berres, M.L.; Hashimoto, D.; Mortha, A.; Leboeuf, M.; Li, X.M.; Mucida, D.; et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 2014, 158, 1210. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Traini, C.; Mischopoulou, M.; Gibbons, S.J.; Ligresti, G.; Faussone-Pellegrini, M.S.; Sha, L.; Farrugia, G.; Vannucchi, M.G.; Cipriani, G. Muscularis macrophages establish cell-to-cell contacts with telocytes/PDGFRα-positive cells and smooth muscle cells in the human and mouse gastrointestinal tract. Neurogastroenterol. Motil. 2020. (under review). [Google Scholar]
- Gibbons, S.J.; De Giorgio, R.; Faussone Pellegrini, M.S.; Garrity-Park, M.M.; Miller, S.M.; Schmalz, P.F.; Young-Fadok, T.M.; Larson, D.W.; Dozois, E.J.; Camilleri, M.; et al. Apoptotic cell death of human interstitial cells of Cajal. Neurogastroenterol. Motil. 2009, 2, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Hoch, R.V.; Soriano, P. Roles of PDGF in animal development. Development 2003, 130, 4769–4784. [Google Scholar] [CrossRef]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008, 22, 276–312. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, M.G. Receptors in interstitial cells of Cajal: Identification and possible physiological roles. Microsc. Res. Tech. 1999, 47, 325–335. [Google Scholar] [CrossRef]
- Chaudhury, A. Furthering the debate on the role of interstitial cells of Cajal in enteric inhibitory neuromuscular neurotransmission. Am. J. Physiol. Cell Physiol. 2016, 311, C479–C481. [Google Scholar] [CrossRef]
- Cipriani, G.; Gibbons, S.J.; Saravanaperumal, S.A.; Malysz, J.; Sha, L.; Szurszewski, J.H.; Linden, D.R.; Evangelista, S.; Faussone-Pellegrini, M.S.; Vannucchi, M.G.; et al. Changes in nitrergic and tachykininergic pathways in rat proximal colon in response to chronic treatment with otilonium bromide. Neurogastroenterol. Motil. 2015, 27, 997–1009. [Google Scholar] [CrossRef]
- Sanders, K.M.; Ward, S.M.; Koh, S.D. Interstitial cells: Regulators of smooth muscle function. Physiol. Rev. 2014, 94, 859–907. [Google Scholar] [CrossRef]
- Blair, P.J.; Rhee, P.L.; Sanders, K.M.; Ward, S.M.J. The significance of interstitial cells in neurogastroenterology. Neurogastroenterol. Motil. 2014, 20, 294–317. [Google Scholar] [CrossRef]
- Peri, L.E.; Sanders, K.M.; Mutafova-Yambolieva, V.N. Differential expression of genes related to purinergic signaling in smooth muscle cells, PDGFRα-positive cells, and interstitial cells of Cajal in the murine colon. Neurogastroenterol. Motil. 2013, 25, e609–e620. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M.; Kito, Y.; Hwang, S.J.; Ward, S.M. Regulation of Gastrointestinal Smooth Muscle Function by Interstitial Cells. Physiology 2016, 31, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, J.W.; Corrias, A.; Buist, M.L. A Mechanistic Model of a PDGFRα(+) Cell. J. Theor. Biol. 2016, 408, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Huang, X.; Lu, H.L.; Liu, S.H.; Zang, J.Y.; Li, Y.J.; Chen, J.; Xu, W.X. Different distributions of interstitial cells of Cajal and platelet-derived growth factor receptor-α positive cells in colonic smooth muscle cell/interstitial cell of Cajal/platelet-derived growth factor receptor-α positive cell syncytium in mice. World J. Gastroenterol. 2018, 24, 4989–5004. [Google Scholar] [CrossRef]
- Grover, M.; Bernard, C.E.; Pasricha, P.J.; Parkman, H.P.; Abell, T.L.; Nguyen, L.A.; Snape, W.; Shen, K.R.; Sarr, M.; Swain, J.; et al. Platelet derived growth factor receptor α (PDGFRα)-expressing “fibroblast-like cells” in diabetic and idiopathic gastroparesis of humans. Neurogastroenterol. Motil. 2012, 24, 844–852. [Google Scholar] [CrossRef]
- Coyle, D.; O’Donnell, A.M.; Puri, P. Altered distribution of small-conductance calcium-activated potassium channel SK3 in Hirschsprung’s disease. J. Pediatr. Surg. 2015, 50, 1659–1664. [Google Scholar] [CrossRef]
- Song, N.N.; Lu, H.L.; Lu, C.; Tong, L.; Huang, S.Q.; Huang, X.; Chen, J.; Kim, Y.C.; Xu, W.X. Diabetes-induced colonic slow transit mediated by the up-regulation of PDGFRα+ cells/SK3 in streptozotocin-induced diabetic mice. Neurogastroenterol. Motil. 2018. [Google Scholar] [CrossRef]
- Furuya, K.; Sokabe, M.; Furuya, S. Characteristics of subepithelial fibroblasts as a mechano-sensor in the intestine: Cell-shape dependent ATP release and P2Y1 signaling. J. Cell Sci. 2005, 118, 3289–3304. [Google Scholar] [CrossRef]
- Faussone-Pellegrini, M.S.; Bani, D. Relationships between telocytes and cardiomyocytes during pre- and post-natal life. J. Cell Mol. Med. 2010, 14, 1061–1063. [Google Scholar] [CrossRef]
- Bani, D.; Formigli, L.; Gherghiceanu, M.; Faussone-Pellegrini, M.S. Telocytes as supporting cells for myocardial tissue organization in developing and adult heart. J. Cell Mol. Med. 2010, 14, 2531–2538. [Google Scholar] [CrossRef]
- Albulescu, R.; Tanase, C.; Codrici, E.; Popescu, D.I.; Cretoiu, S.M.; Popescu, L.M. The secretome of myocardial telocytes modulates the activity of cardiac stem cells. J. Cell Mol. Med. 2015, 19, 1783–1794. [Google Scholar] [CrossRef]
- Kostin, S. Cardiac telocytes in normal and diseased hearts. Semin. Cell Dev. Biol. 2016, 55, 22–30. [Google Scholar] [CrossRef]
- Buller, N.V.; Rosekrans, S.L.; Westerlund, J.; van den Brink, G.R. Hedgehog signaling and maintenance of homeostasis in the intestinal epithelium. Physiology 2012, 27, 148–155. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Greicius, G.; Kabiri, Z.; Sigmundsson, K.; Liang, C.; Bunte, R.; Singh, M.K.; Virshup, D.M. PDGFRα+ pericryptal stromal cells are the critical source of Wnts and RSPO3 for murine intestinal stem cells in vivo. Proc. Natl. Acad. Sci. USA 2018, 115, E3173–E3181. [Google Scholar] [CrossRef] [PubMed]
- Shoshkes-Carmel, M.; Wang, Y.J.; Wangensteen, K.J.; Tóth, B.; Kondo, A.; Massassa, E.E.; Itzkovitz, S.; Kaestner, K.H. Subepithelial telocytes are an important source of Wnts that supports intestinal crypts. Nature 2018, 557, 242–246. [Google Scholar] [CrossRef]
- Kinchen, J.; Chen, H.H.; Parikh, K.; Gervais, F.; Koohy, H.; Simmons, A. Structural Remodeling of the Human Colonic Mesenchyme in Inflammatory Bowel Disease. Cell 2018, 175, 372–386. [Google Scholar] [CrossRef]
- Kondo, A.; Kaestner, K.H. Emerging diverse roles of telocytes. Development 2019, 146, 175018. [Google Scholar] [CrossRef]
- Kaestner, K.H. The Intestinal Stem Cell Niche: A Central Role for Foxl1-Expressing Subepithelial Telocytes. Cell Mol. Gastroenterol. Hepatol. 2019, 8, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Mifflin, R.C.; Pinchuk, I.V.; Saada, J.I.; Powell, D.W. Intestinal myofibroblasts: Targets for stem cell therapy. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G684–G696. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, M.G.; Traini, C.; (University of Florence, Florence, Italy). Personal Communication, 2020.
- Karlsson, L.; Lindahl, P.; Heath, J.K.; Betsholtz, C. Abnormal gastrointestinal development in PDGF-A and PDGFR-(alpha) deficient mice implicates a novel mesenchymal structure with putative instructive properties in villus morphogenesis. Development 2000, 127, 457–466. [Google Scholar]
- Kurahashi, M.; Niwa, Y.; Cheng, J.; Ohsaki, Y.; Fujita, A.; Goto, H.; Fujimoto, T.; Torihashi, S. Platelet- derived growth factor signals play critical roles in differentiation of longitudinal smooth muscle cells in mouse embryonic gut. Neurogastroenterol. Motil. 2008, 20, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Cajal, S.R. Histologie du Systeme Nerveux de L’Homme et des Vertebres. Volume 2; Maloine: Paris, France, 1911. [Google Scholar]
- Faussone-Pellegrini, M.S.; Cortesini, C.; Romagnoli, P. Ultrastructure of the tunica muscularis of the cardial portion of the human esophagus and stomach, with special reference to the so-called Cajal’s interstitial cells. Arch. Ital. Anat. Embriol. 1977, 82, 157–177. [Google Scholar]
- Thuneberg, L. Interstitial cells of Cajal: Intestinal pacemaker cells? Adv. Anat. Embryol. Cell Biol. 1982, 71, 1–130. [Google Scholar] [PubMed]
- Lecoin, L.; Gabella, G.; Le Douarin, N. Origin of the c-kit positive interstitial cells in the avian bowel. Development 1996, 122, 725–733. [Google Scholar] [PubMed]
- Komuro, T.; Tokui, K.; Zhou, D.S. Identification of the interstitial cells of Cajal. Histol. Histopathol. 1996, 11, 769–786. [Google Scholar]
- Sanders, K.M.; Ordög, T.; Koh, S.D.; Torihashi, S.; Ward, S.M. Development and plasticity of interstitial cells of Cajal. Neurogastroenterol. Motil. 1999, 11, 311–338. [Google Scholar] [CrossRef]
- Al-Sajee, D.; Huizinga, J.D. Interstitial Cells of Cajal: Pathology, Injury and Repair. Sultan Qaboos Univ. Med. J. 2012, 12, 411–421. [Google Scholar] [CrossRef]
- Faussone-Pellegrini, M.S.; Thuneberg, L. Guide to the identification of interstitial cells of Cajal. Microsc. Res. Tech. 1999, 47, 248–266. [Google Scholar] [CrossRef]
- Sanders, K.M.; Koh, S.D.; Ward, S.M. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu Rev. Physiol. 2006, 68, 307–343. [Google Scholar] [CrossRef]
- Ward, S.M.; Sanders, K.M.; Hirst, G.D. Role of interstitial cells of Cajal in neural control of gastrointestinal smooth muscles. Neurogastroenterol. Motil. 2004, 16, 112–117. [Google Scholar] [CrossRef]
- Vannucchi, M.G.; Evangelista, S. Neurokinin receptors in the gastrointestinal muscle wall: Cell distribution and possible roles. BioMol. Concepts 2013, 4, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Traini, C.; Faussone-Pellegrini, M.S.; Evangelista, S.; Mazzaferro, K.; Cipriani, G.; Santicioli, P.; Vannucchi, M.G. Inner and outer portions of colonic circular muscle: Ultrastructural and immunohistochemical changes in rat chronically treated with otilonium bromide. PLoS ONE 2014, 9, e103237. [Google Scholar] [CrossRef] [PubMed]
- Powley, T.L.; Phillips, R.J. Vagal intramuscular array afferents form complexes with interstitial cells of Cajal in gastrointestinal smooth muscle: Analogues of muscle spindle organs? Neuroscience 2011, 186, 188–200. [Google Scholar] [CrossRef]
- Faussone-Pellegrini, M.S.; Vannucchi, M.G.; Alaggio, R.; Strojna, A.; Midrio, P.J. Morphology of the interstitial cells of Cajal of the human ileum from foetal to neonatal life. J. Cell Mol. Med. 2007, 11, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Radenkovic, G.; Radenkovic, D.; Velickov, A.J. Development of interstitial cells of Cajal in the human digestive tract as the result of reciprocal induction of mesenchymal and neural crest cells. J. Cell Mol. Med. 2018, 22, 778–785. [Google Scholar] [CrossRef]
- Eyden, B. The myofibroblast: Phenotypic characterization as a prerequisite to understanding its functions in translational medicine. J. Cell. Mol. Med. 2008, 12, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Eyden, B. The myofibroblast, electron microscopy and cancer research. Int. J. Cancer 2009, 125, 1743–1745. [Google Scholar] [CrossRef]
- Drake, M.J.; Hedlund, P.; Andersson, K.E.; Brading, A.F.; Hussain, I.; Fowler, C.; Landon, D.N. Morphology, phenotype and ultrastructure of fibroblastic cells from normal and neuropathic human detrusor: Absence of myofibroblast characteristics. J. Urol. 2003, 169, 1573–1576. [Google Scholar] [CrossRef]
- Wiseman, O.J.; Fowler, C.J.; Landon, D.N. The role of the human bladder lamina propria myofibroblast. BJU Int. 2003, 91, 89–93. [Google Scholar] [CrossRef]
- Roosen, A.; Datta, S.N.; Chowdhury, R.A.; Patel, P.M.; Kalsi, V.; Elneil, S.; Dasgupta, P.; Kessler, T.M.; Khan, S.; Panicker, J.; et al. Suburothelial myofibroblasts in the human overactive bladder and the effect of botulinum neurotoxin type A treatment. Eur. Urol. 2009, 55, 1440–1449. [Google Scholar] [CrossRef]
- Chapple, C. Chapter 2: Pathophysiology of neurogenic detrusor overactivity and the symptom complex of “overactive bladder”. Neurourol. Urodyn. 2014, 33, S6–S13. [Google Scholar] [CrossRef] [PubMed]
- Fry, C.H.; Vahabi, B. The Role of the Mucosa in Normal and Abnormal Bladder Function. Basic Clin. Pharmacol. Toxicol. 2016, 119, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, M.G.; Traini, C. The telocytes/myofibroblasts 3-D network forms a stretch receptor in the human bladder mucosa. Is this structure involved in the detrusor overactive diseases? Ann. Anat. 2018, 218, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Komuro, T. Re-evaluation of fibroblasts and fibroblastlike cells. Anat. Embryol. 1990, 182, 103–112. [Google Scholar] [CrossRef]
- Faussone-Pellegrini, M.S.; Vannucchi, M.G. Substance P and Neurokinin 1 receptor—Expressionis affected in the ileum of mice with mutation in the W locus. J. Cell Mol. Med. 2006, 10, 511–518. [Google Scholar] [CrossRef]
- Faussone-Pellegrini, M.S. Relationships between neurokinin receptor-expressing interstitial cells of Cajal and tachykininergic nerves in the gut. J. Cell Mol. Med. 2006, 10, 20–32. [Google Scholar] [CrossRef]
- Southwell, B.R.; Woodman, H.L.; Rojal, S.J.; Furness, J.B. Movement of villi induces endocytosis of NK1 receptors in myenteric neurons from guinea-pig ileum. Cell Tissue Res. 1998, 292, 37–45. [Google Scholar] [CrossRef]


© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vannucchi, M.G. The Telocytes: Ten Years after Their Introduction in the Scientific Literature. An Update on Their Morphology, Distribution, and Potential Roles in the Gut. Int. J. Mol. Sci. 2020, 21, 4478. https://doi.org/10.3390/ijms21124478
Vannucchi MG. The Telocytes: Ten Years after Their Introduction in the Scientific Literature. An Update on Their Morphology, Distribution, and Potential Roles in the Gut. International Journal of Molecular Sciences. 2020; 21(12):4478. https://doi.org/10.3390/ijms21124478
Chicago/Turabian StyleVannucchi, Maria Giuliana. 2020. "The Telocytes: Ten Years after Their Introduction in the Scientific Literature. An Update on Their Morphology, Distribution, and Potential Roles in the Gut" International Journal of Molecular Sciences 21, no. 12: 4478. https://doi.org/10.3390/ijms21124478
APA StyleVannucchi, M. G. (2020). The Telocytes: Ten Years after Their Introduction in the Scientific Literature. An Update on Their Morphology, Distribution, and Potential Roles in the Gut. International Journal of Molecular Sciences, 21(12), 4478. https://doi.org/10.3390/ijms21124478




