Meta-Analysis of Gene Expressions in Testicular Germ Cell Tumor Histologies
Abstract
1. Introduction
2. Results
2.1. Comparisons of Three Datasets
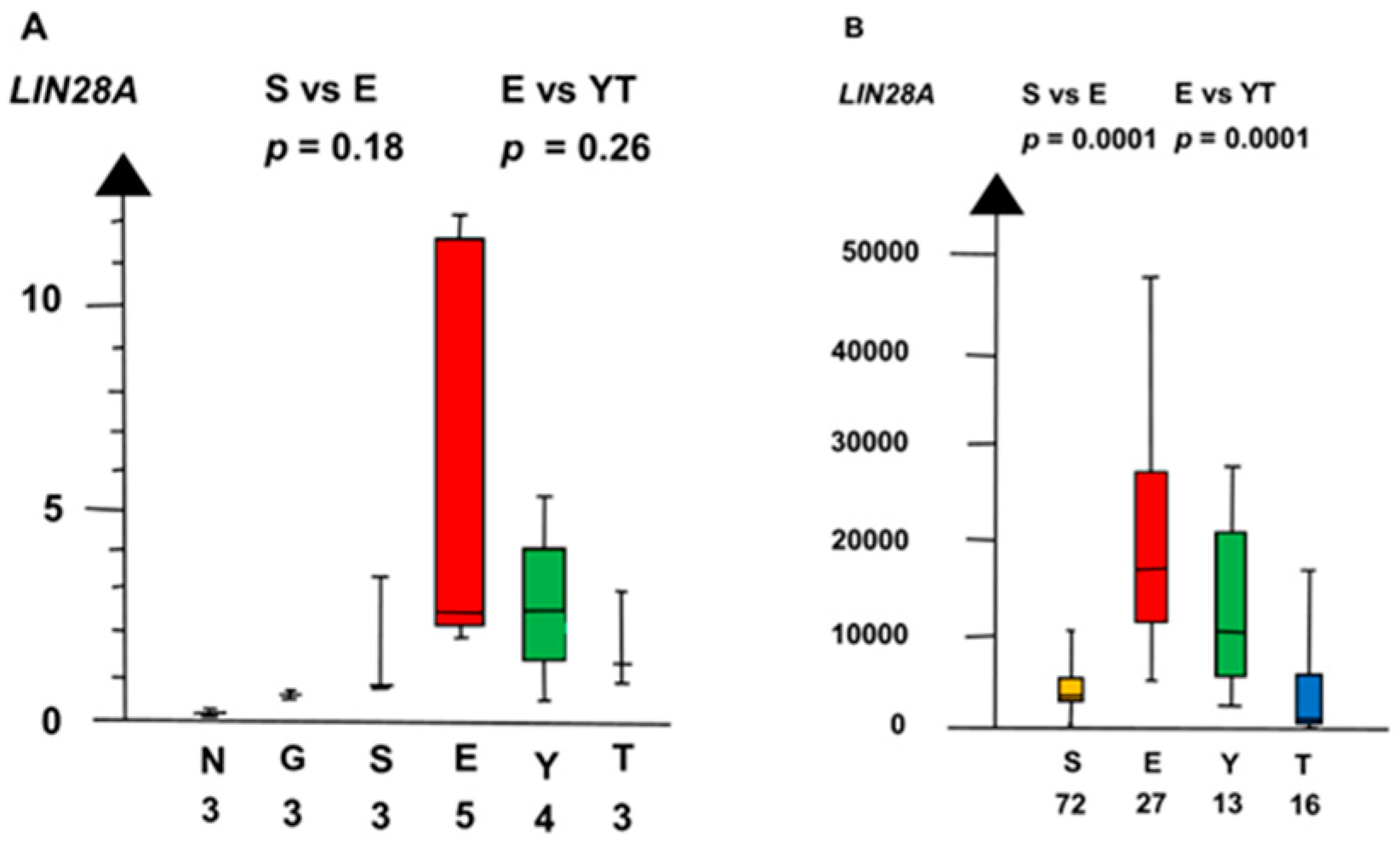
2.2. Significant Genes
2.3. Nonsignificant Genes
2.4. Role of Genes for the Development of Testicular Germ Cell Tumor Type II (TGCT)
3. Discussion
4. Materials and Methods
4.1. Data Sources
4.2. Candidate Genes
4.3. Definitions and Evaluations
4.4. Statistical Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hermanek, P.; International Union Against Cancer. The TNM Atlas: Illustrated Guide to the TNM/pTNM Classification of Malignant Tumours, 4th ed.; Springer: Berlin, NY, USA, 1987. [Google Scholar]
- Looijenga, L.H.J.; Kao, C.S.; Idrees, M.T. Predicting Gonadal Germ Cell Cancer in People with Disorders of Sex Development; Insights from Developmental Biology. Int. J. Mol. Sci. 2019, 20, 5017. [Google Scholar] [CrossRef] [PubMed]
- Wilms, M. The teratoid testicular tumors including so called cystoid and enchondroma, In German. Beitr. Der Pathol. Anat. 1896, 36, 266–337. [Google Scholar]
- Dixon, F.J.; Moore, R.A. Testicular tumors: A clinicopathological study. Cancer 1953, 6, 427–454. [Google Scholar] [CrossRef]
- Teilum, G. Endodermal sinus tumors of the ovary and testis. Comparative morphogenesis of the so-called mesoephroma ovarii (Schiller) and extraembryonic (yolk sac-allantoic) structures of the rat’s placenta. Cancer 1959, 12, 1092–1105. [Google Scholar] [CrossRef]
- De Jong, B.; Oosterhuis, J.W.; Castedo, S.M.; Vos, A.; te Meerman, G.J. Pathogenesis of adult testicular germ cell tumors: A cytogenetic model. Cancer Genet. Cytogenet. 1990, 48, 143–167. [Google Scholar] [CrossRef]
- Von Eyben, F.E. Chromosomes, Genes, and Development of Testicular Germ Cell Tumors. Cancer Genet. Cytogenet. 2004, 151, 93–138. [Google Scholar] [CrossRef]
- Alagaratnam, S.; Lind, G.E.; Kraggerud, S.M.; Lothe, R.A.; Skotheim, R.I. The testicular germ cell tumour transcriptome. Int. J. Androl. 2011, 34, e133–e150, discussion e50–e51. [Google Scholar] [CrossRef]
- Nettersheim, D.; Westernstroer, B.; Haas, N.; Leinhaas, A.; Brustle, O.; Schlatt, S.; Schorle, H. Establishmlent of a versatile seminoma model indicates cellular plasticity of germ cell tumor cells. Genes Chromosomes Cancer 2012, 51, 717–726. [Google Scholar] [CrossRef]
- Nettersheim, D.; Jostes, S.; Schneider, S.; Schorle, H. Elucidating human male germ cell development by studying germ cell cancer. Reproduction 2016, 152, R101–R113. [Google Scholar] [CrossRef]
- von Eyben, F.E.; Jensen, M.B.; Hoyer, S. Frequency and Markers of Precursor Lesions and Implications for the Pathogenesis of Testicular Germ Cell Tumors. Clin. Genitourin. Cancer. 2018, 16, e211–e221. [Google Scholar] [CrossRef]
- Shen, H.; Shih, J.; Hollern, D.P.; Wang, L.; Bowlby, R.; Tickoo, S.K.; Thorsson, V.; Mungall, A.J.; Newton, Y.; Hedge, A.M.; et al. Integrated Molecular Characterization of Testicular Germ Cell Tumors. Cell Rep. 2018, 23, 3392–3406. [Google Scholar] [CrossRef] [PubMed]
- Gashaw, I.; Grummer, R.; Klein-Hitpass, L.; Dushaj, O.; Bergmann, M.; Brehm, R.; Grobholz, R.; Kliesch, S.; Neuvians, T.P.; Schmid, K.V.; et al. Gene signatures of testicular seminoma with emphasis on expression of ets variant gene 4. Cell. Mol. Life Sci. 2005, 62, 2359–2368. [Google Scholar] [CrossRef]
- Skotheim, R.I.; Lind, G.E.; Monni, O.; Nesland, J.M.; Abeler, V.M.; Fossa, S.D.; Duale, N.; Brunborg, G.; Kallioniemi, O.; Andrews, P.W.; et al. Differentiation of human embryonal carcinomas in vitro and in vivo reveals expression profiles relevant Duale, to normal development. Cancer Res. 2005, 65, 5588–5598. [Google Scholar] [CrossRef]
- Tobias, V. Meta-analysis of p values. Stata technical Bulletin. Stata Tech. Bull. 1999, 49, 15–17. [Google Scholar]
- Von Eyben, F.E.; Jacobsen, G.K.; Skotheim, R.I. Microinvasive germ cell tumor of the testis. Virchows Arch. 2005, 447, 610–625. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, N.K.; Abatti, L.E.; Mitchell, J.A. KLF4 protein stability regulated by interaction with pluripotency transcription factors overrides transcriptional control. Genes Dev. 2019, 33, 1069–1082. [Google Scholar] [CrossRef]
- Chaerkady, R.; Kerr, C.L.; Kandasamy, K.; Marimuthu, A.; Gearhart, J.D.; Pandey, A. Comparative proteomics of human embryonic stem cells and embryonal carcinoma cells. Proteomics 2010, 10, 1359–1373. [Google Scholar] [CrossRef]
- De Jong, J.; Weeda, S.; Gillis, A.J.; Oosterhuis, J.W.; Looijenga, L.H. Differential methylation of the OCT3/4 upstream region in primary human testicular germ cell tumors. Oncol. Rep. 2007, 18, 127–132. [Google Scholar] [CrossRef]
- Nettersheim, D.; Jostes, S.; Sharma, R.; Schneider, S.; Hofmann, A.; Ferreira, H.J.; Hoffman, P.; Kristiansen, G.; Esteller, M.B.; Schorle, H. BMP Inhibition in Seminomas Initiates Acquisition of Pluripotency via NODAL Signaling Resulting in Reprogramming to an Embryonal Carcinoma. PLoS Genet. 2015, 11, e1005415. [Google Scholar] [CrossRef]
- Jostes, S.; Nettersheim, D.; Fellermeyer, M.; Schneider, S.; Hafezi, F.; Honecker, F.; Schumacher, V.; Geyer, M.; Kristiansen, G.; Schorle, H. The bromodomain inhibitor JQ1 triggers growth arrest and apoptosis in testicular germ cell tumours in vitro and in vivo. J. Cell. Mol. Med. 2017, 21, 1300–1314. [Google Scholar] [CrossRef]
- Almstrup, K.; Leffers, H.; Lothe, R.A.; Skakkebaek, N.E.; Sonne, S.B.; Nielsen, J.E.; Rajpert-De Meyts, E.; Skotheim, R.I. Improved gene expression signature of testicular carcinoma in situ. Int. J. Androl. 2007, 30, 292–302, discussion 3. He. [Google Scholar] [CrossRef] [PubMed]
- Buganim, Y.; Markoulaki, S.; van Wietmarschen, N.; Hoke, H.; Wu, T.; Ganz, K.; Akhtar-Zaidi, B.; He, Y.; Abraham, B.J.; Porubsky, D.; et al. The developmental potential of iPSCs is greatly influenced by reprogramming factor selection. Cell Stem Cell 2014, 15, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Rajpert-De Meyts, E.; Hanstein, R.; Jorgensen, N.; Graem, N.; Vogt, P.H.; Skakkebaek, N.E. Developmental expression of POU5F1 (OCT-3/4) in normal and dysgenetic human gonads. Hum. Reprod. 2004, 19, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Gainetdinov, I.V.; Kondratieva, S.A.; Skvortsova, Y.V.; Zinovyeva, M.V.; Stukacheva, E.A.; Klimov, A.; Tryakin, A.A.; Azhikina, T.L. Distinguishing epigenetic features of preneoplastic testis tissues adjacent to seminomas and nonseminomas. Oncotarget 2016, 7, 22439–22447. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Korkola, J.E.; Houldsworth, J.; Chadalavada, R.S.; Olshen, A.B.; Dobrzynski, D.; Reuter, V.E.; Bosl, G.J.; Chaganti, R.S. Down-regulation of stem cell genes, including those in a 200-kb gene cluster at 12p13.31, is associated with in vivo differentiation of human male germ cell tumors. Cancer Res. 2006, 66, 820–827. [Google Scholar] [CrossRef]
- Nettersheim, D.; Biermann, K.; Gillis, A.J.; Steger, K.; Looijenga, L.H.; Schorle, H. NANOG promoter methylation and expression correlation during normal and malignant human germ cell development. Epigenetics 2011, 6, 114–122. [Google Scholar] [CrossRef]
- Almstrup, K.; Nielsen, J.E.; Mlynarska, O.; Jansen, M.T.; Jorgensen, A.; Skakkebaek, N.E.; Rajpert De Meyts, E. Carcinoma in situ testis displays permissive chromatin modifications similar to immature foetal germ cells. Br. J. Cancer 2010, 103, 1269–1276. [Google Scholar] [CrossRef]
- Kristensen, D.G.; Skakkebaek, N.E.; Rajpert-De Meyts, E.; Almstrup, K. Epigenetic features of testicular germ cell tumours in relation to epigenetic characteristics of foetal germ cells. Int. J. Dev. Biol. 2013, 57, 309–317. [Google Scholar] [CrossRef]
- Van Der Zwan, Y.G.; Stoop, H.; Rossello, F.; White, S.J.; Looijenga, L.H. Role of epigenetics in the etiology of germ cell cancer. Int. J. Dev. Biol. 2013, 57, 299–308. [Google Scholar] [CrossRef]
- Heurtier, V.; Owens, N.; Gonzalez, I.; Mueller, F.; Proux, C.; Mornico, D.; Clerc, P.; Dubois, A.; Navarro, P. The molecular logic of Nanog-induced self-renewal in mouse embryonic stem cells. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Eldar-Geva, T.; Gross-Tsur, V.; Hirsch, H.J.; Altarescu, G.; Segal, R.; Zeligson, S.; Golomb, E.; Epsztejn-Litman, S.; Eiges, R. Incomplete methylation of a germ cell tumor (Seminoma) in a Prader-Willi male. Mol. Genet. Genomic Med. 2018, 6, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Almstrup, K.; Hoei-Hansen, C.E.; Wirkner, U.; Blake, J.; Schwager, C.; Ansorge, W.; Nielsen, J.E.; Skakkebaek, N.E.; Rajpert De Meyts, E. Embryonic stem cell-like features of testicular carcinoma in situ revealed by genome-wide gene expression profiling. Cancer Res. 2004, 64, 4736–4743. [Google Scholar] [CrossRef]
- Salvatori, D.C.F.; Dorssers, L.C.J.; Gillis, A.J.M.; Perretta, G.; van Agthoven, T.; Gomes Fernandes, M.; Stoop, H.; Prins, J.B.; Oosterhuis, J.W.; Mummery, C.; et al. The MicroRNA-371 Family as Plasma Biomarkers for Monitoring Undifferentiated and Potentially Malignant Human Pluripotent Stem Cells in Teratoma Assays. Stem. Cell Rep. 2018, 11, 1493–1505. [Google Scholar] [CrossRef]
- Nettersheim, D.; Arndt, I.; Sharma, R.; Riesenberg, S.; Jostes, S.; Schneider, S.; Holzel, M.; Kristiansen, G.; Schorle, H. The cancer/testis antigen PRAME supports the pluripotency network and expresses somatic and germ cell differentiation programs in seminomas. Br. J. Cancer 2016, 115, 454–464. [Google Scholar] [CrossRef]
- Gillis, A.J.; Stoop, H.; Biermann, K.; van Gurp, R.J.; Swartzman, E.; Cribbes, S.; Ferlinz, A.; Shannon, M.; Oosterhuis, J.W.; Looijenga, L.H. Expression 8and interdependencies of pluripotency factors LIN28, OCT3/4, NANOG and SOX2 in human testicular germ cells and tumours of the testis. Int. J. Androl. 2011, 34, e160–e174. [Google Scholar] [CrossRef]
- Tomasetti, C.; Marchionni, L.; Nowak, M.A.; Parmigiani, G.; Vogelstein, B. Only three driver gene mutations are required for the development of lung and colorectal cancers. Proc. Natl. Acad. Sci. USA 2015, 112, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Von Eyben, F.E.; Jacobsen, G.K.; Pedersen, H.; Jacobsen, M.; Clausen, P.P.; Zibrandtsen, P.C.; Gullberg, B. Multivariate analysis of risk factors in patients with metastatic testicular germ cell tumors treated with vinblastine and bleomycin. Invasion Metastasis 1982, 2, 125–135. [Google Scholar]
- Nogales, F.F.; Prat, J.; Schuldt, M.; Cruz-Viruel, N.; Kaur, B.; D’Angelo, E.; Matias-Guiu, X.; Vidal, A.; McCluggage, W.G.; Oosterhuis, J.W. Germ cell tumour growth patterns originating from clear cell carcinomas of the ovary and endometrium: A comparative immunohistochemical study favouring their origin from somatic stem cells. Histopathology 2018, 72, 634–647. [Google Scholar] [CrossRef]
- Lobo, J.; Gillis, A.J.M.; Jeronimo, C.; Henrique, R.; Looijenga, L.H.J. Human Germ Cell Tumors are Developmental Cancers: Impact of Epigenetics on Pathobiology and Clinic. Int. J. Mol. Sci. 2019, 20, 258. [Google Scholar] [CrossRef]
- Wermann, H.; Stoop, H.; Gillis, A.J.; Honecker, F.; van Gurp, R.J.; Ammerpohl, O.; Richter, J.; Oosterhuis, J.W.; Bokemeyer, C.; Looijenga, L.H. Global DNA methylation in fetal human germ cells and germ cell tumours: Association with differentiation and cisplatin resistance. J. Pathol. 2010, 221, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Oosterhuis, J.W.; Looijenga, L.H.J. Human germ cell tumours from a developmental perspective. Nat. Rev. Cancer 2019, 19, 522–537. [Google Scholar] [CrossRef] [PubMed]
- Sikora, K.; Evan, G.; Stewart, J.; Watson, J.V. Detection of the c-myc oncogene product in testicular cancer. Br. J. Cancer 1985, 52, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Godmann, M.; Gashaw, I.; Eildermann, K.; Schweyer, S.; Bergmann, M.; Skotheim, R.I.; Behr, R. The pluripotency transcription factor Kruppel-like factor 4 is strongly expressed in intratubular germ cell neoplasia unclassified and seminoma. Mol. Hum. Reprod. 2009, 15, 479–488. [Google Scholar] [CrossRef][Green Version]
- Jones, T.D.; Ulbright, T.M.; Eble, J.N.; Cheng, L. OCT4: A sensitive and specific biomarker for intratubular germ cell neoplasia of the testis. Clin. Cancer Res. 2004, 10, 8544–8547. [Google Scholar] [CrossRef]
- Datta, M.W.; Macri, E.; Signoretti, S.; Renshaw, A.A.; Loda, M. Transition from in situ to invasive testicular germ cell neoplasia is associated with the loss of p21 and gain of mdm-2 expression. Mod. Pathol. 2001, 14, 437–442. [Google Scholar] [CrossRef]
- Bartkova, J.; Rajpert-de Meyts, E.; Skakkebaek, N.E.; Bartek, J. D-type cyclins in adult human testis and testicular cancer: Relation to cell type, proliferation, differentiation, and malignancy. J. Pathol. 1999, 187, 573–581. [Google Scholar] [CrossRef]
- Pierconti, F.; Milardi, D.; Martini, M.; Grande, G.; Cenci, T.; Gulino, G.; Larocca, L.M.; Rindi, G.; Pontecorvi, A.; De Marinis, L. Pituitary-tumour-transforming-gene 1 expression in testicular cancer. Andrologia 2015, 47, 427–432. [Google Scholar] [CrossRef]
- Schmidt, B.A.; Rose, A.; Steinhoff, C.; Strohmeyer, T.; Hartmann, M.; Ackermann, R. Up-regulation of cyclin-Dependent kinase 4/cyclin D2 expression but down-regulation of cyclin-dependent kinase 2/cyclin E in testicular germ cell tumors. Cancer Res. 2001, 61, 4214–4221. [Google Scholar]
- Grande, G.; Milardi, D.; Martini, M.; Cenci, T.; Gulino, G.; Mancini, F.; Bianchi, A.; Pontecorvi, A.; Pierconti, F. Protein Expression of PTTG-1, OCT-4, and KLF-4 in Seminoma: A Pilot Study. Front. Endocrinol. (Lausanne) 2019, 10, 619. [Google Scholar] [CrossRef]
- Bartkova, J.; Lukas, C.; Sorensen, C.S.; Rajpert-De Meyts, E.; Skakkebaek, N.E.; Lukas, J.; Bartek, J. Deregulation of the RB pathway in human testicular germ cell tumours. J. Pathol. 2003, 200, 149–156. [Google Scholar] [CrossRef]
- Bartkova, J.; Thullberg, M.; Rajpert-De Meyts, E.; Skakkebaek, N.E.; Bartek, J. Cell cycle regulators in testicular cancer: Loss of p18INK4C marks progression from carcinoma in situ to invasive germ cell tumours. Int. J. Cancer 2000, 85, 370–375. [Google Scholar] [CrossRef]
- Di Vizio, D.; Cito, L.; Boccia, A.; Chieffi, P.; Insabato, L.; Pettinato, G.; Motti, M.L.; Shepis, F.; D’Amico, W.; Fabiani, F.; et al. Loss of the tumor suppressor gene PTEN marks the transition from intratubular germ cell neoplasias (ITGCN) to invasive germ cell tumors. Oncogene 2005, 24, 1882–1894. [Google Scholar] [CrossRef] [PubMed]
- Bartkova, J.; Thullberg, M.; Rajpert-De Meyts, E.; Skakkebaek, N.E.; Bartek, J. Lack of p19INK4d in human testicular germ-cell tumours contrasts with high expression during normal spermatogenesis. Oncogene 2000, 19, 4146–4150. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Strohmeyer, T.; Reissmann, P.; Cordon-Cardo, C.; Hartmann, M.; Ackermann, R.; Slamon, D. Correlation between retinoblastoma gene expression and differentiation in human testicular tumors. Proc. Natl. Acad. Sci. USA 1991, 88, 6662–6666. [Google Scholar] [CrossRef] [PubMed]
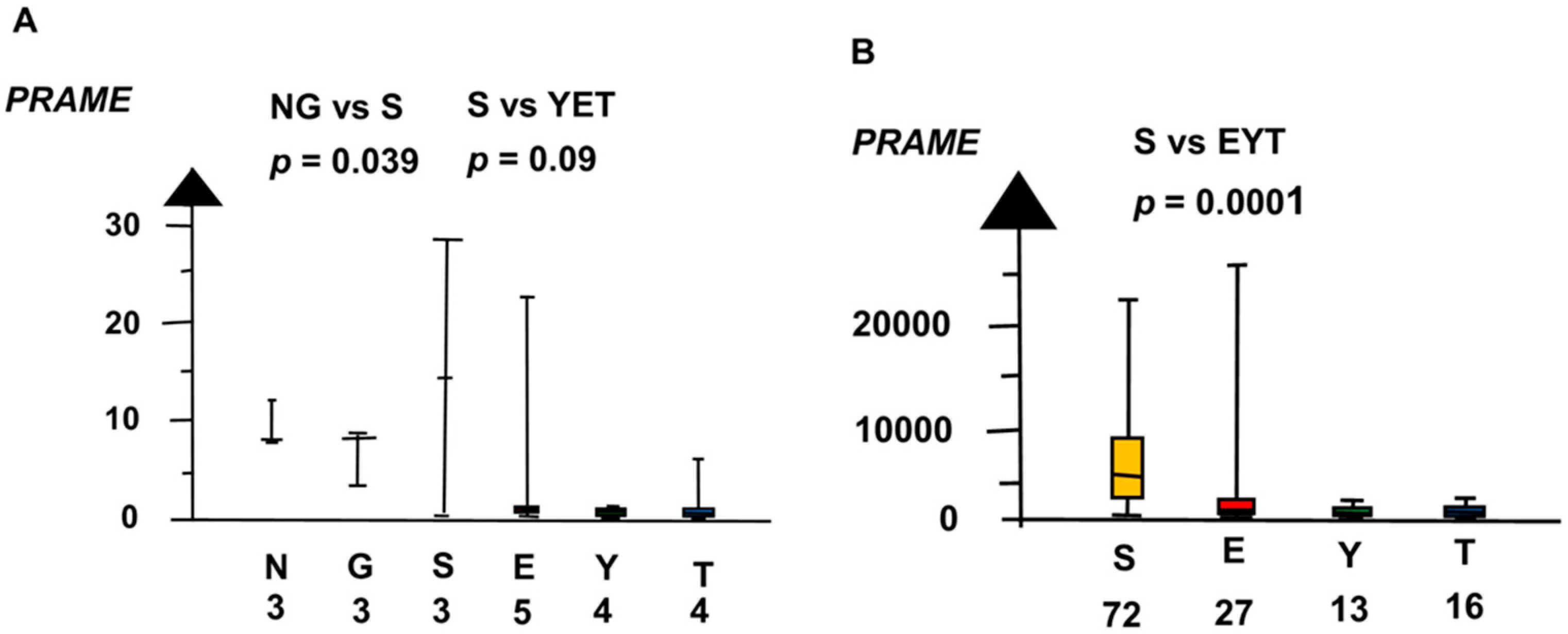
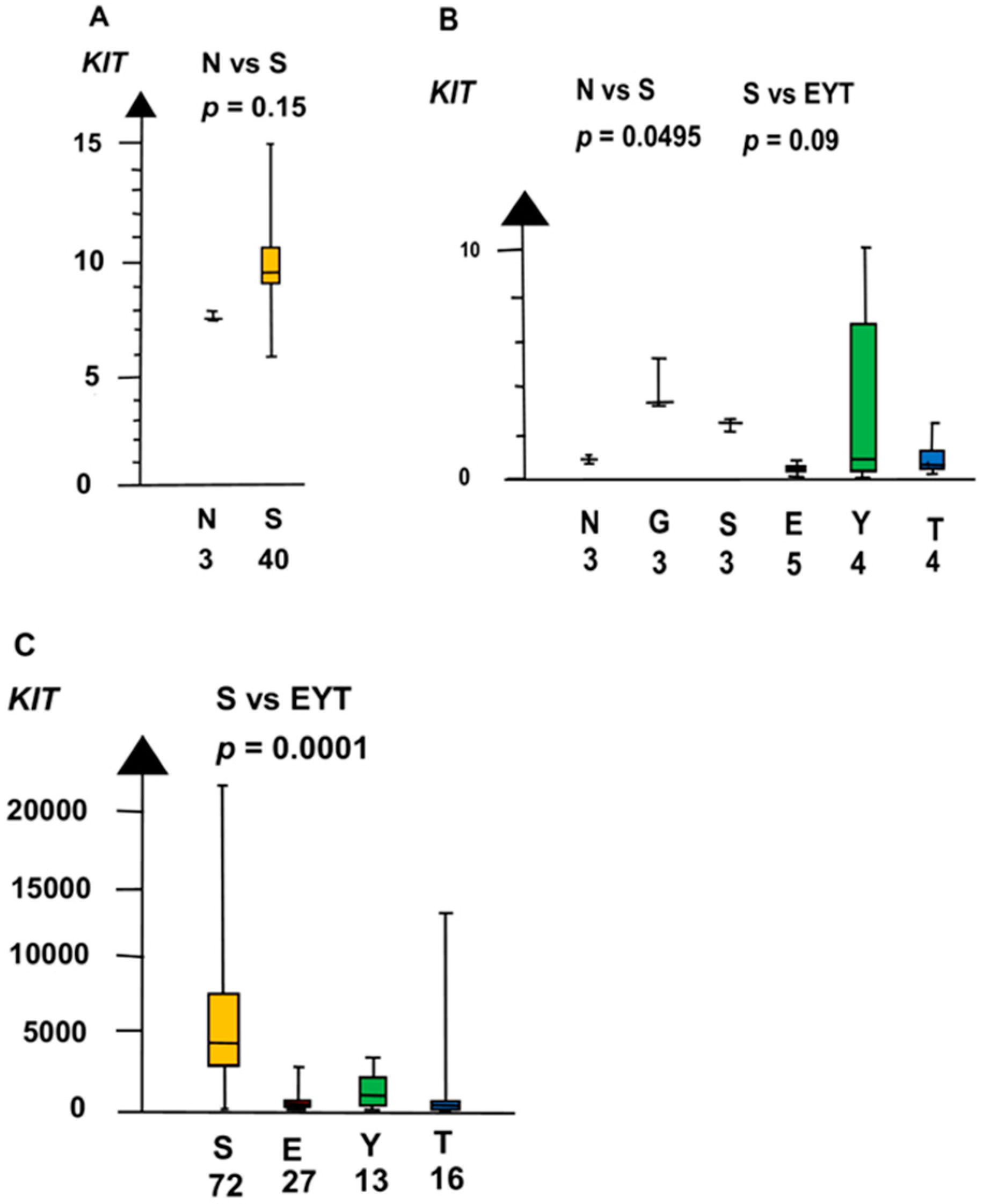


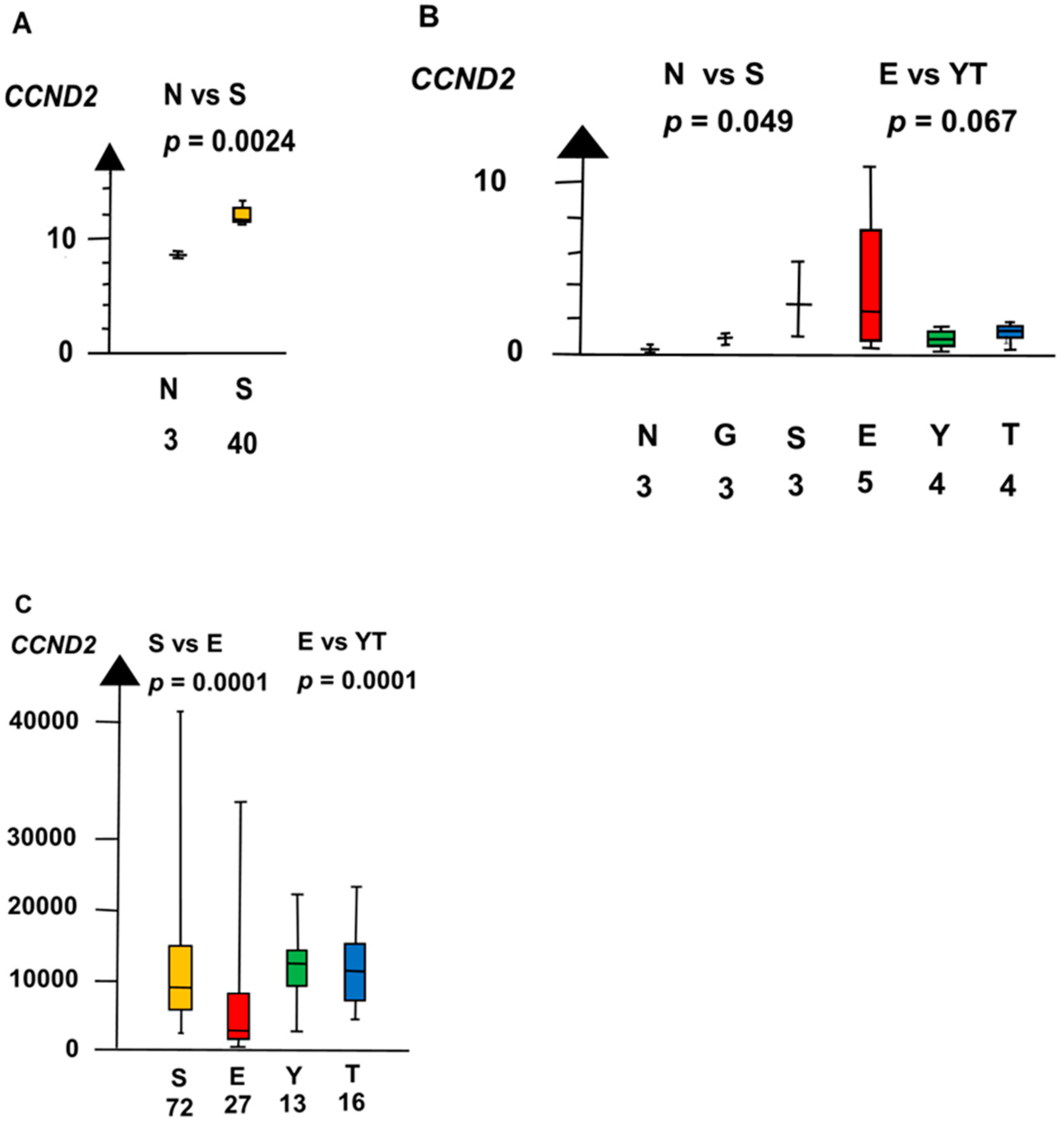
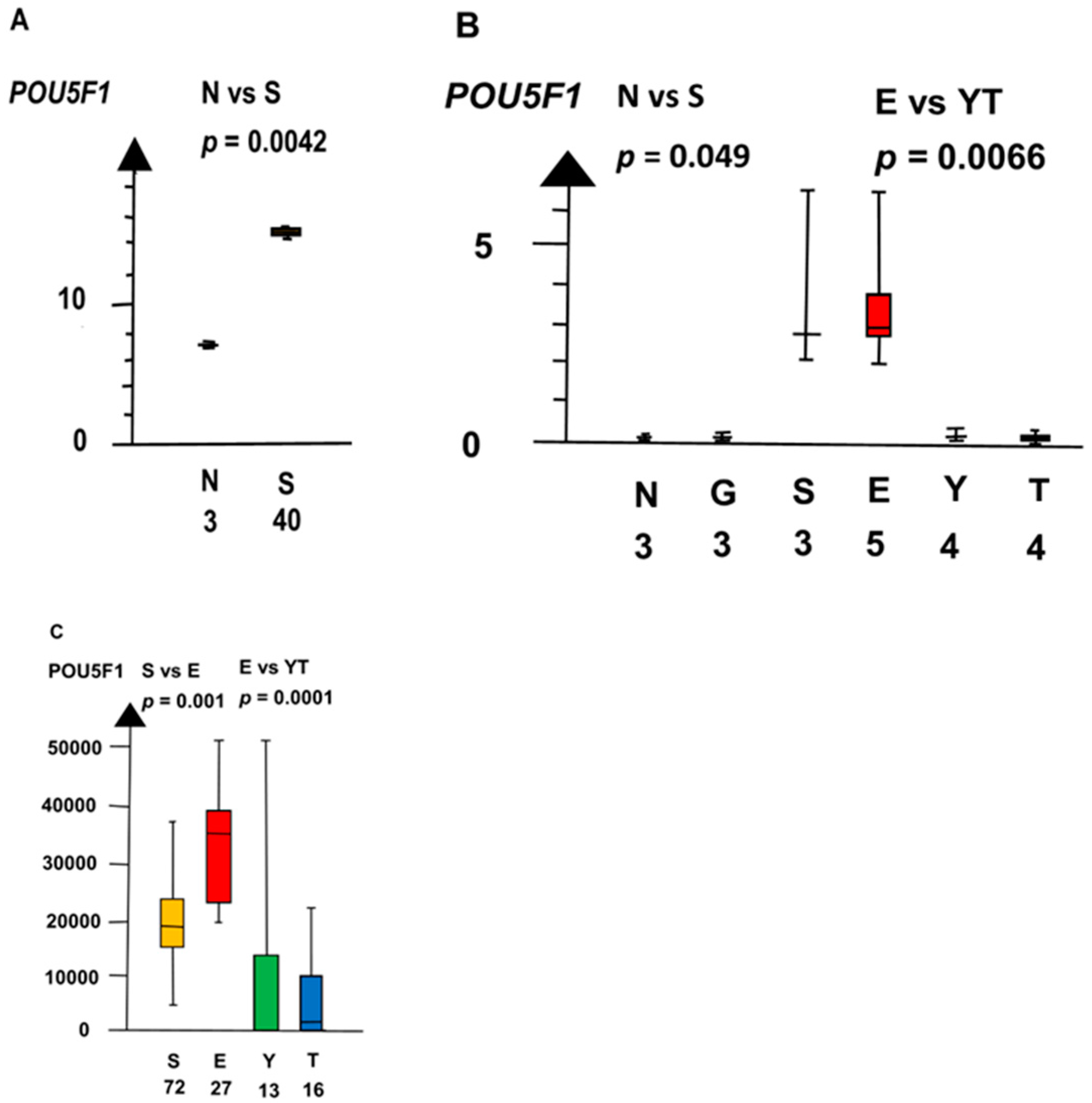
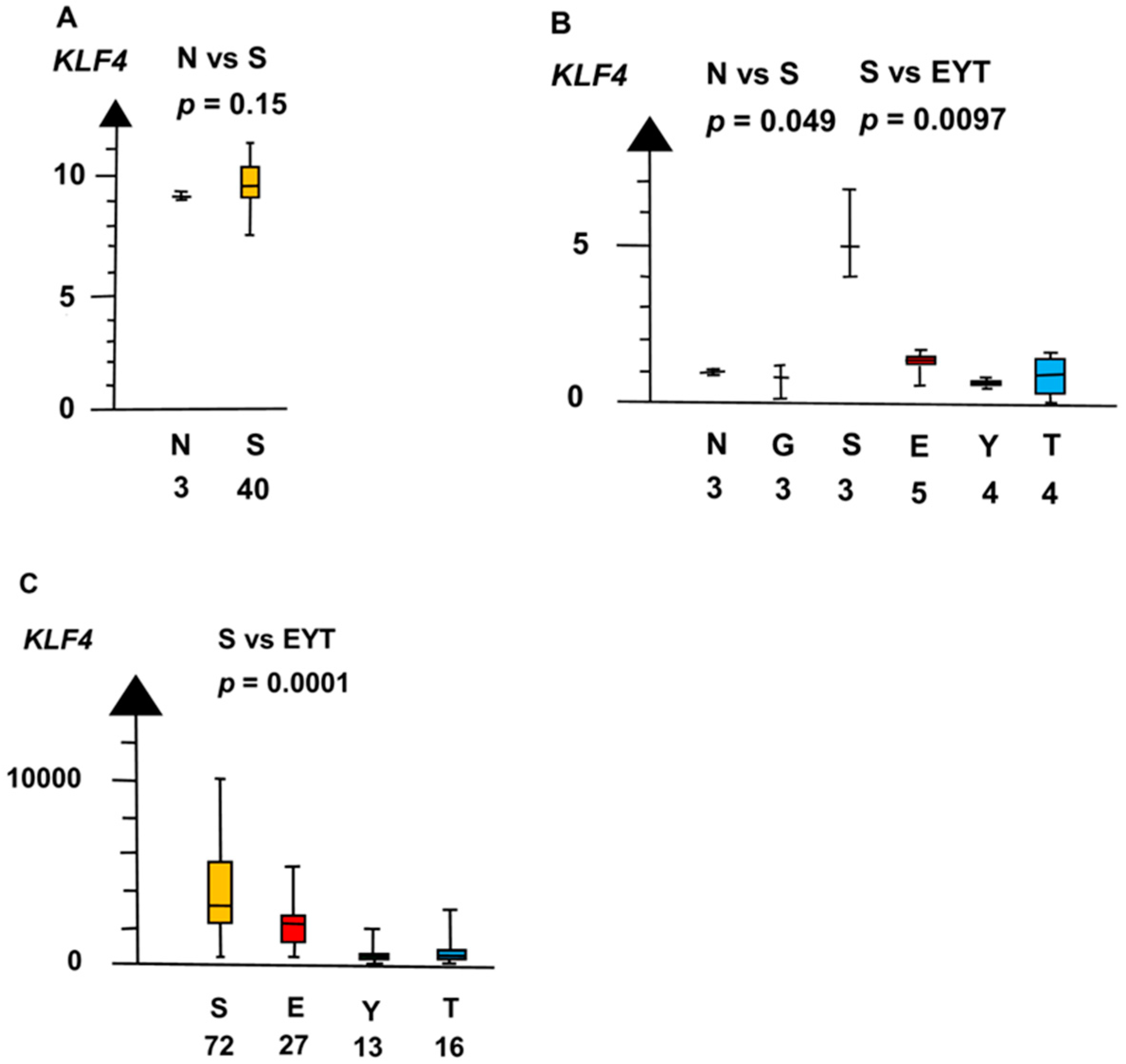
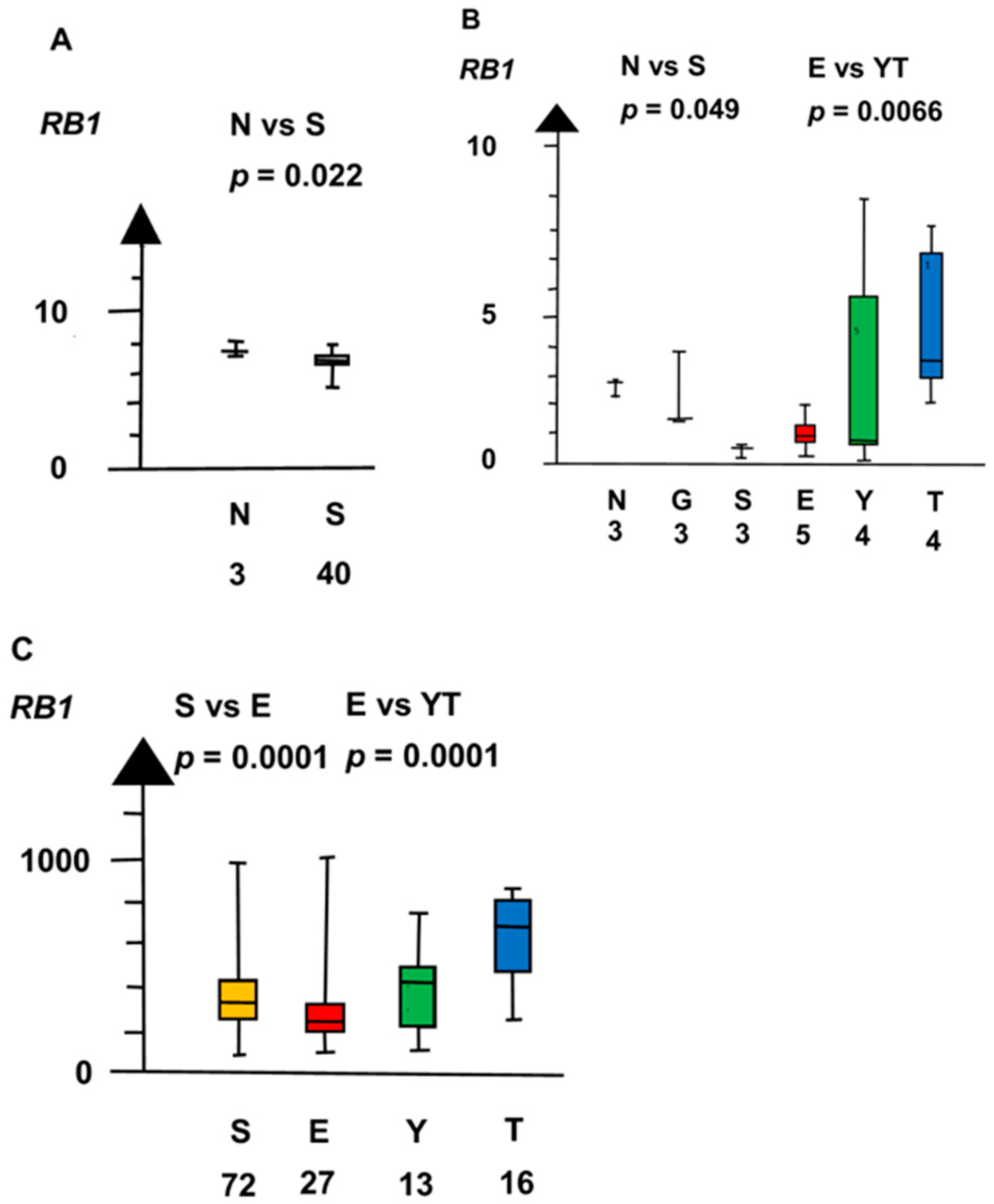
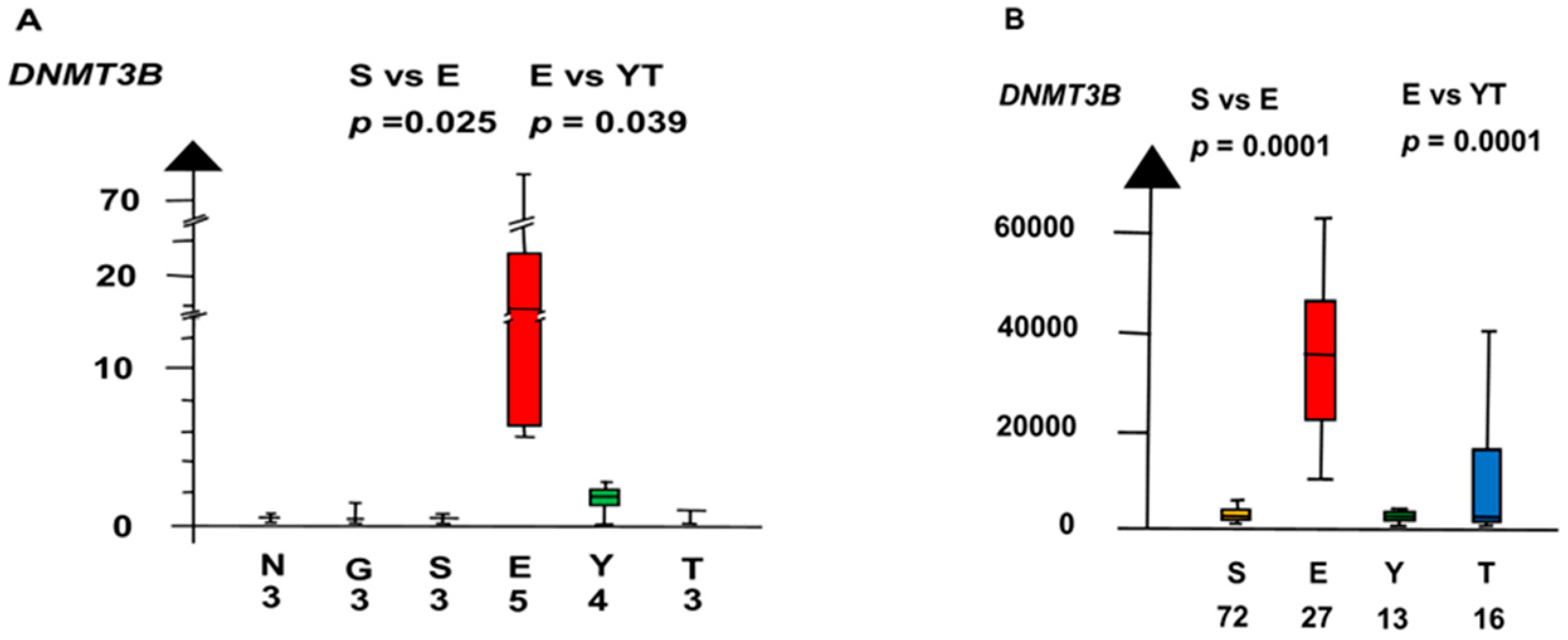


| Histologies | Genes | No Patients | Combined p Values |
|---|---|---|---|
| SvEYT | PRAME | 6 × 10−5 | |
| KLF4 | 1.4 × 10−5 | ||
| EvYT | CCND2 | 160 | 8.7 ×10−5 |
| POU5F1 | 160 | 1 × 10−5 | |
| RB1 | 160 | 1 × 10−5 | |
| DNMT3B | 160 | 5.2 × 10−5 |
| Histologies | Genes | No. of Patients | Combined p Values |
|---|---|---|---|
| NvS | KLF4 | 60 | 0.042 |
| KIT | 60 | 0.0022 | |
| RB1 | 60 | 0.008 | |
| POUF1 | 60 | 0.0019 | |
| CCND2 | 60 | 0.0011 | |
| SvE | SOX17 | 160 | 3.4 × 10−5 |
| KIT | 160 | 3.4 ×10−5 | |
| POU5F1 | 160 | 0.0019 | |
| LIN28A | 160 | 2.1 × 10−4 | |
| DNMT3B | 160 | 3.5 × 10−5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Eyben, F.E.; Parraga-Alava, J. Meta-Analysis of Gene Expressions in Testicular Germ Cell Tumor Histologies. Int. J. Mol. Sci. 2020, 21, 4487. https://doi.org/10.3390/ijms21124487
von Eyben FE, Parraga-Alava J. Meta-Analysis of Gene Expressions in Testicular Germ Cell Tumor Histologies. International Journal of Molecular Sciences. 2020; 21(12):4487. https://doi.org/10.3390/ijms21124487
Chicago/Turabian Stylevon Eyben, Finn Edler, and Jorge Parraga-Alava. 2020. "Meta-Analysis of Gene Expressions in Testicular Germ Cell Tumor Histologies" International Journal of Molecular Sciences 21, no. 12: 4487. https://doi.org/10.3390/ijms21124487
APA Stylevon Eyben, F. E., & Parraga-Alava, J. (2020). Meta-Analysis of Gene Expressions in Testicular Germ Cell Tumor Histologies. International Journal of Molecular Sciences, 21(12), 4487. https://doi.org/10.3390/ijms21124487