Anti-Proliferative Effects of Standardized Cornus officinalis on Benign Prostatic Epithelial Cells via the PCNA/E2F1-Dependent Cell Cycle Pathway
Abstract
1. Introduction
2. Results
2.1. Chemical Profiling Analysis of COFE
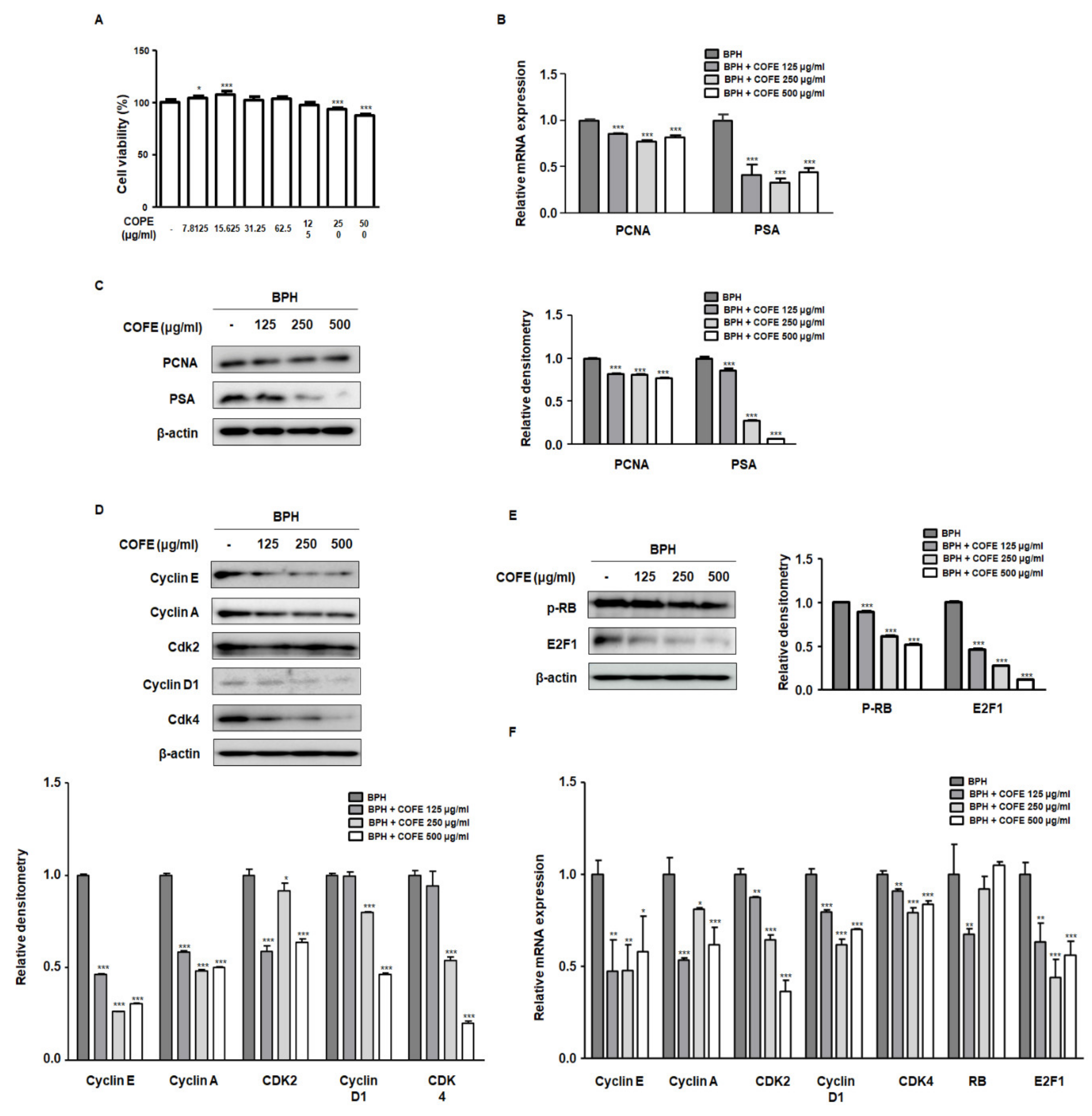
2.2. COFE Inhibited Markers of Cell Proliferation in BPH-1 Cells
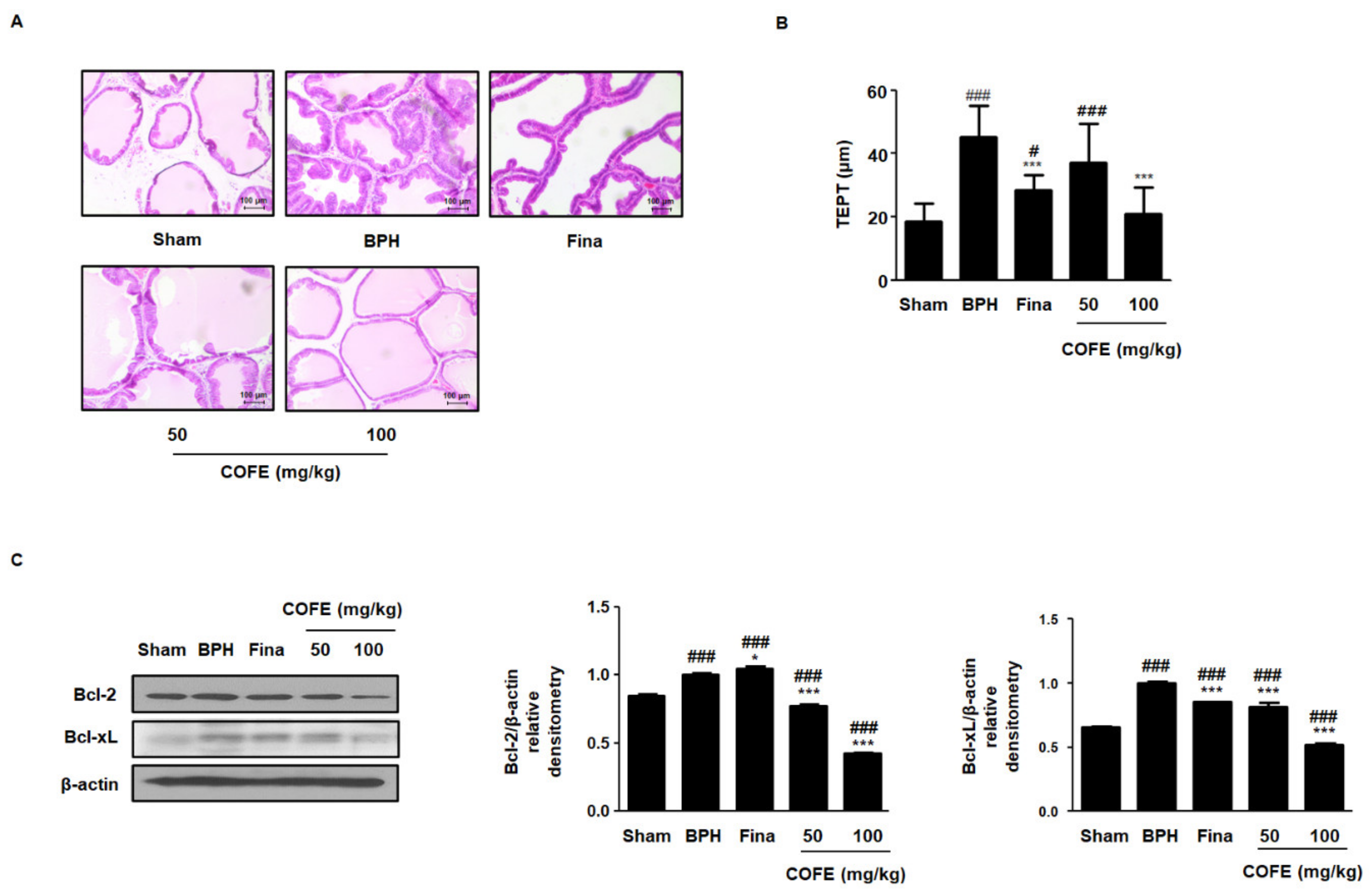
2.3. COFE Suppressed Androgen-Dependent Prostate Enlargement in Testosterone-Treated BPH Rats
2.4. COFE Improved Histological Changes via Regulation of Anti-Apoptosis Proteins in Testosterone-Treated BPH Rats
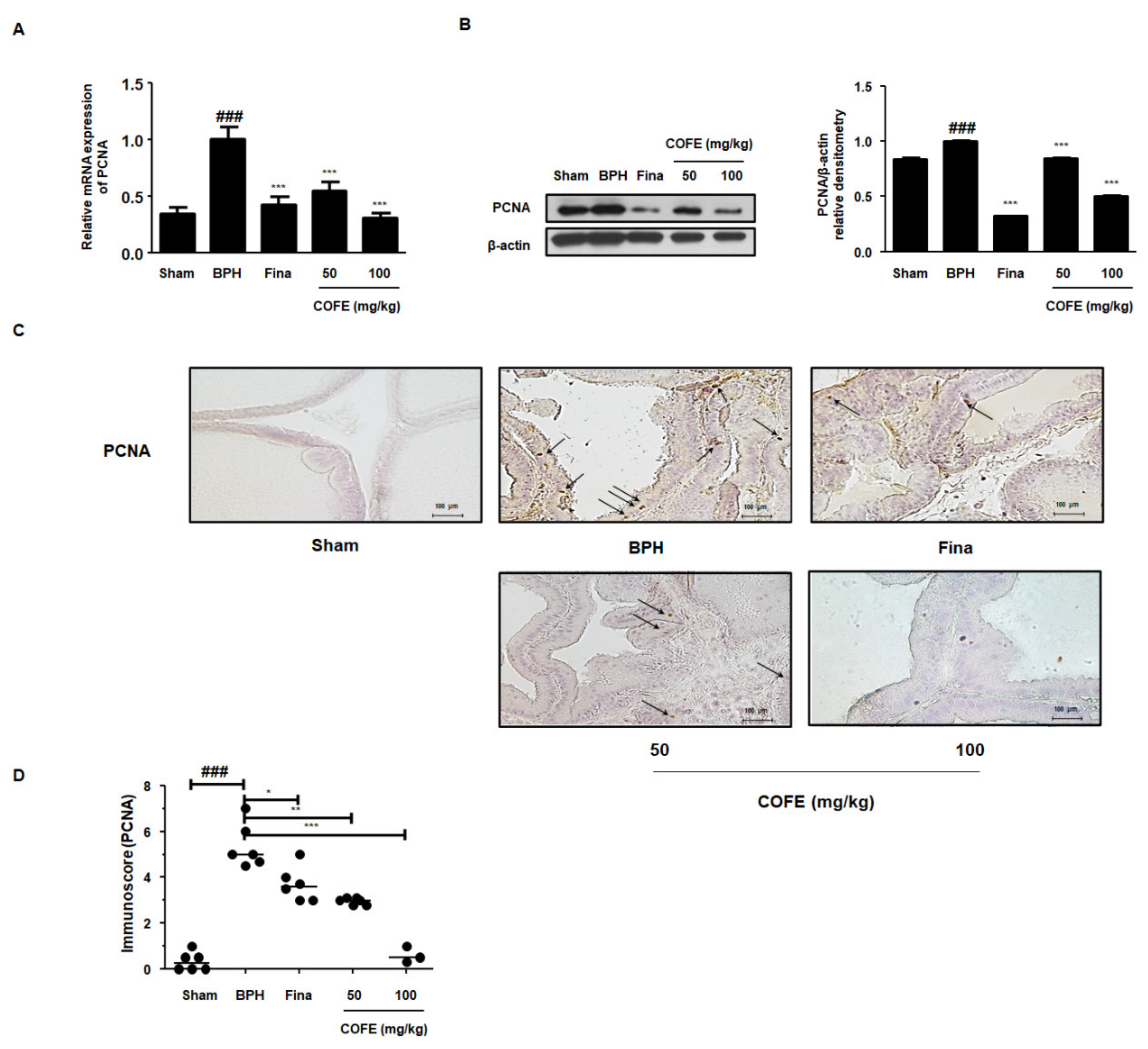
2.5. COFE Suppressed Expression of PCNA in Testosterone-Treated BPH Rats
2.6. COFE Regulated the Cell Cycle Markers in Testosterone-Treated BPH Rats
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of the COFE
4.3. Chromatography Conditions
4.4. Calibration Curves, Limits of Detection, and Limits of Quantification
4.5. Cell Culture and Sample Treatment
4.6. Cell Viability Assays
4.7. Isolation of Total RNA and Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
4.8. Western Blot Analysis
4.9. Animals
4.10. Serum Concentrations for DHT Analysis
4.11. H&E Staining
4.12. Immunohistochemistry (IHC)
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BPH | benign prostatic hyperplasia |
| COFE | C. officinalis 30% ethanol extract |
| DHT | dihydrotestosterone |
| PCNA | proliferating cell nuclear antigen |
| TEPT | thickness of epithelium from prostate tissues |
References
- Elkahwaji, J.E. The role of inflammatory mediators in the development of prostatic hyperplasia and prostate cancer. Res. Rep. Urol. 2012, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chughtai, B.; Lee, R.; Te, A.; Kaplan, S. Role of inflammation in benign prostatic hyperplasia. Rev. Urol. 2011, 13, 147–150. [Google Scholar] [PubMed]
- Meikle, A.W.; Stephenson, R.A.; Lewis, C.M.; Middleton, R.G. Effects of age and sex hormones on transition and peripheral zone volumes of prostate and benign prostatic hyperplasia in twins. J. Clin. Endocrinol. Metab. 1997, 82, 571–575. [Google Scholar] [CrossRef]
- Miah, S.; Catto, J. BPH and prostate cancer risk. Indian J. Urol. IJU J. Urol. Soc. India 2014, 30, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Kelman, Z.; O’Donnell, M. Structural and functional similarities of prokaryotic and eukaryotic DNA polymerase sliding clamps. Nucleic Acids Res. 1995, 23, 3613–3620. [Google Scholar] [CrossRef] [PubMed]
- Nigg, E.A. Cyclin-dependent protein kinases: Key regulators of the eukaryotic cell cycle. Bioessays 1995, 17, 471–480. [Google Scholar] [CrossRef]
- Funk, J.O. Cell cycle checkpoint genes and cancer. e LS 2001. [Google Scholar] [CrossRef]
- Phillips, S.M.; Barton, C.M.; Lee, S.J.; Morton, D.G.; Wallace, D.M.; Lemoine, N.R.; Neoptolemos, J.P. Loss of the retinoblastoma susceptibility gene (rb1) is a frequent and early event in prostatic tumorigenesis. Br. J. Cancer 1994, 70, 1252–1257. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.; Dong, L.; Gao, Q.; Yin, L.; Quan, H.; Chen, R.; Fu, X.; Lin, D. Ethnopharmacology, phytochemistry, and pharmacology of cornus officinalis sieb. Et zucc. J. Ethnopharmacol. 2018, 213, 280–301. [Google Scholar] [CrossRef]
- Lee, W.C.; Wu, C.C.; Chuang, Y.C.; Tain, Y.L.; Chiang, P.H. Ba-wei-die-huang-wan (hachimi-jio-gan) can ameliorate cyclophosphamide-induced ongoing bladder overactivity and acidic adenosine triphosphate solution-induced hyperactivity on rats prestimulated bladder. J. Ethnopharmacol. 2016, 184, 1–9. [Google Scholar] [CrossRef]
- Dharmananda, S. Herbal therapy for benign prostratic hypertrophy. Int. J. Inst. Trad. Med. Prev. Health Care 2002. Available online: http://www.itmonline.org/journal/arts/bph.htm (accessed on 15 December 2020).
- Jeng, H.; Wu, C.M.; Su, S.J.; Chang, W.C. A substance isolated from cornus officinalis enhances the motility of human sperm. Am. J. Chin. Med. 1997, 25, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Feng, Z.L.; Chen, H.B.; Wang, F.S.; Lu, J.H. Corni fructus: A review of chemical constituents and pharmacological activities. Chin. Med. 2018, 13, 34. [Google Scholar] [CrossRef]
- Hwangbo, H.; Kwon, D.H.; Choi, E.O.; Kim, M.Y.; Ahn, K.I.; Ji, S.Y.; Kim, J.S.; Kim, K.I.; Park, N.J.; Kim, B.H.; et al. Corni fructus attenuates testosterone-induced benign prostatic hyperplasia by suppressing 5alpha-reductase and androgen receptor expression in rats. Nutr. Res. Pract. 2018, 12, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Van den Heuvel, S.; Dyson, N.J. Conserved functions of the prb and e2f families. Nat. Rev. Mol. Cell Biol. 2008, 9, 713–724. [Google Scholar] [CrossRef]
- Chen, W.; Zouboulis, C.C.; Fritsch, M.; Blume-Peytavi, U.; Kodelja, V.; Goerdt, S.; Luu-The, V.; Orfanos, C.E. Evidence of heterogeneity and quantitative differences of the type 1 5alpha-reductase expression in cultured human skin cells--evidence of its presence in melanocytes. J. Investig. Dermatol. 1998, 110, 84–89. [Google Scholar] [CrossRef]
- Hipfner, D.R.; Cohen, S.M. Connecting proliferation and apoptosis in development and disease. Nat. Rev. Mol. Cell Biol. 2004, 5, 805–815. [Google Scholar] [CrossRef]
- Taftachi, R.; Ayhan, A.; Ekici, S.; Ergen, A.; Ozen, H. Proliferating-cell nuclear antigen (pcna) as an independent prognostic marker in patients after prostatectomy: A comparison of pcna and ki-67. BJU Int. 2005, 95, 650–654. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, H.; Beach, D. D type cyclins associate with multiple protein kinases and the DNA replication and repair factor pcna. Cell 1992, 71, 505–514. [Google Scholar] [CrossRef]
- DeGregori, J.; Kowalik, T.; Nevins, J.R. Cellular targets for activation by the e2f1 transcription factor include DNA synthesis- and g1/s-regulatory genes. Mol. Cell. Biol. 1995, 15, 4215–4224. [Google Scholar] [CrossRef]
- Thompson, I.M.; Goodman, P.J.; Tangen, C.M.; Lucia, M.S.; Miller, G.J.; Ford, L.G.; Lieber, M.M.; Cespedes, R.D.; Atkins, J.N.; Lippman, S.M.; et al. The influence of finasteride on the development of prostate cancer. N. Engl. J. Med. 2003, 349, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.H.; Lin, W.L.; Lui, S.L.; Cai, X.Y.; Wong, V.T.; Ziea, E.; Zhang, Z.J. Efficacy and safety of chinese herbal medicine for benign prostatic hyperplasia: Systematic review of randomized controlled trials. Asian J. Androl. 2013, 15, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.H.; Lee, S.H.; Roehrborn, C.G.; Siami, P.F.; Major-Walker, K.; Wilson, T.H.; Montorsi, F.; Comb, A.T.S.G. Comparison of the response to treatment between asian and caucasian men with benign prostatic hyperplasia: Long-term results from the combination of dutasteride and tamsulosin study. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2012, 19, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Roehrborn, C. Pathology of benign prostatic hyperplasia. Int. J. Impot. Res. 2008, 20, S11–S18. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.R.; Kim, H.J.; Kim, E.Y.; Chung, T.W.; Ha, K.T.; An, H.J. 6′-sialyllactose ameliorates in vivo and in vitro benign prostatic hyperplasia by regulating the e2f1/prb-ar pathway. Nutrients 2019, 11, 2203. [Google Scholar] [CrossRef]
- Cheon, S.Y.; Jin, B.R.; Kim, H.J.; An, H.J. Oleanolic acid ameliorates benign prostatic hyperplasia by regulating pcna-dependent cell cycle progression in vivo and in vitro. J. Nat. Prod. 2020, 83, 1183–1189. [Google Scholar] [CrossRef]
- Shin, I.-S.; Lee, M.-Y.; Jung, D.-Y.; Seo, C.-S.; Ha, H.-K.; Shin, H.-K. Ursolic acid reduces prostate size and dihydrotestosterone level in a rat model of benign prostatic hyperplasia. Food Chem. Toxicol. 2012, 50, 884–888. [Google Scholar] [CrossRef]
- McConnell, J.D. Prostatic growth: New insights into hormonal regulation. Br. J. Urol. 1995, 76 (Suppl. 1), 5–10. [Google Scholar]
- Mundy, A.R.; Fitzpatrick, J.; Neal, D.E.; George, N.J. The Scientific Basis of Urology; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Altintas, D.M.; Shukla, M.S.; Goutte-Gattat, D.; Angelov, D.; Rouault, J.P.; Dimitrov, S.; Samarut, J. Direct cooperation between androgen receptor and e2f1 reveals a common regulation mechanism for androgen-responsive genes in prostate cells. Mol. Endocrinol. 2012, 26, 1531–1541. [Google Scholar] [CrossRef]
- Kyprianou, N.; Tu, H.; Jacobs, S.C. Apoptotic versus proliferative activities in human benign prostatic hyperplasia. Hum. Pathol. 1996, 27, 668–675. [Google Scholar] [CrossRef]
- Lee, E.C.; Tenniswood, M. Programmed cell death and survival pathways in prostate cancer cells. Arch. Androl. 2004, 50, 27–32. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, X.; Chen, M.W.; Ng, A.; Ng, P.Y.; Lee, C.; Rubin, M.; Olsson, C.A.; Buttyan, R. Abnormal prostate development in c3(1)-bcl-2 transgenic mice. Prostate 1997, 32, 16–26. [Google Scholar] [CrossRef]
- Reshi, L.; Wang, H.V.; Hui, C.F.; Su, Y.C.; Hong, J.R. Anti-apoptotic genes bcl-2 and bcl-xl overexpression can block iridovirus serine/threonine kinase-induced bax/mitochondria-mediated cell death in gf-1 cells. Fish Shellfish. Immunol. 2017, 61, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Strzalka, W.; Ziemienowicz, A. Proliferating cell nuclear antigen (pcna): A key factor in DNA replication and cell cycle regulation. Ann. Bot. 2011, 107, 1127–1140. [Google Scholar] [CrossRef] [PubMed]
- Koundrioukoff, S.; Jonsson, Z.O.; Hasan, S.; de Jong, R.N.; van der Vliet, P.C.; Hottiger, M.O.; Hubscher, U. A direct interaction between proliferating cell nuclear antigen (pcna) and cdk2 targets pcna-interacting proteins for phosphorylation. J. Biol. Chem. 2000, 275, 22882–22887. [Google Scholar] [CrossRef]
- Hinds, P.W.; Weinberg, R.A. Tumor suppressor genes. Curr. Opin. Genet. Dev. 1994, 4, 135–141. [Google Scholar] [CrossRef]
- Tan, Z.; Wortman, M.; Dillehay, K.L.; Seibel, W.L.; Evelyn, C.R.; Smith, S.J.; Malkas, L.H.; Zheng, Y.; Lu, S.; Dong, Z. Small-molecule targeting of proliferating cell nuclear antigen chromatin association inhibits tumor cell growth. Mol. Pharmacol. 2012, 81, 811–819. [Google Scholar] [CrossRef]


| Index | Sham | BPH | Fina | COFE 50 | COFE 100 | |
|---|---|---|---|---|---|---|
| Group | ||||||
| Body weight (g) | 344.06 ± 26.34 | 311.17 ± 22.84 | 300.10 ± 18.88 | 314.74 ± 24.10 | 302.14 ± 21.94 | |
| Prostate weight (g) | 0.58 ± 0.12 | 1.41 ± 0.25 ### | 0.97 ± 0.15 ###, *** | 1.07 ± 0.11 ###,*** | 1.02 ± 0.12 ###,*** | |
| Dihydrotestosterone (ng/mL) | 1.45 ± 1.22 | 10.25 ± 1.52 ### | 6.51 ± 1.22 ###,** | 6.14 ± 1.01 ###, *** | 6.57 ± 0.88 ###,** | |
| Relative mRNA expression of 5α-reductase 2 | 1.06 ± 0.16 | 3.88 ± 0.80 ### | 0.76 ± 0.47 *** | 1.37 ± 0.64 ** | 1.15 ± 0.79 ** | |
| Gene Name | Forward Primers (5′-3′) | Reverse Primers (5′-3′) |
|---|---|---|
| human PCNA | TTAAACGGTTGCAGGCGTAG | AGGAAAGTCTAGCTGGTTTCGG |
| human PSA | ATAGGATTGCCCAGGCAGAA | CTAAGGGTAAAAGCAGGGAGAGAGT |
| human Cyclin E | GACGGGGAGCTCAAAACTGA | TACAACGGAGCCCAGAACAC |
| human Cyclin A | CGGTACTGAAGTCCGGGAAC | GTTCACAGCCAAATGCAGGG |
| human CDK2 | TTCTATGCCTGATTACAAGCC | CTGGCTTGGTCACATCCT |
| human Cyclin D1 | ACGGCCGAGAAGCTGTGCATC | CCTCCGCCTCTGGCATTTTGGAG |
| human CDK4 | ATGGCTACCTCTCGATATGAGC | CATTGGGGACTCTCACACTCT |
| human RB | ATGGTTCACCTCGAACACCC | TTTCGACACAACCCTGTCCC |
| human E2F1 | AAGAACCGCTGTTGTCCCG | TCGAGGCCGAAGTGGTAGTC |
| human β-actin | GGCCAGGTCATCACCATTGG | CTTTGCGGATGTCCACGTCA |
| rats 5α-reductase 2 | GGCAGCTACCAACTGTGACC | CTCCCGACGACACACTCTCT |
| rats PCNA | CTGCTGGGACATCAGTTCGG | GATCGCAGCGGTATGTGTCG |
| rats GAPDH | TGATTCTACCCACGGCAAGT | AGCATCACCCCATTTGATGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, B.-R.; Cheon, S.-Y.; Kim, H.-J.; Kim, M.-S.; Lee, K.-H.; An, H.-J. Anti-Proliferative Effects of Standardized Cornus officinalis on Benign Prostatic Epithelial Cells via the PCNA/E2F1-Dependent Cell Cycle Pathway. Int. J. Mol. Sci. 2020, 21, 9567. https://doi.org/10.3390/ijms21249567
Jin B-R, Cheon S-Y, Kim H-J, Kim M-S, Lee K-H, An H-J. Anti-Proliferative Effects of Standardized Cornus officinalis on Benign Prostatic Epithelial Cells via the PCNA/E2F1-Dependent Cell Cycle Pathway. International Journal of Molecular Sciences. 2020; 21(24):9567. https://doi.org/10.3390/ijms21249567
Chicago/Turabian StyleJin, Bo-Ram, Se-Yun Cheon, Hyo-Jung Kim, Myoung-Seok Kim, Kwang-Ho Lee, and Hyo-Jin An. 2020. "Anti-Proliferative Effects of Standardized Cornus officinalis on Benign Prostatic Epithelial Cells via the PCNA/E2F1-Dependent Cell Cycle Pathway" International Journal of Molecular Sciences 21, no. 24: 9567. https://doi.org/10.3390/ijms21249567
APA StyleJin, B.-R., Cheon, S.-Y., Kim, H.-J., Kim, M.-S., Lee, K.-H., & An, H.-J. (2020). Anti-Proliferative Effects of Standardized Cornus officinalis on Benign Prostatic Epithelial Cells via the PCNA/E2F1-Dependent Cell Cycle Pathway. International Journal of Molecular Sciences, 21(24), 9567. https://doi.org/10.3390/ijms21249567





