Sex Chromosomes Are Severely Disrupted in Gastric Cancer Cell Lines
Abstract
1. Introduction
2. Results
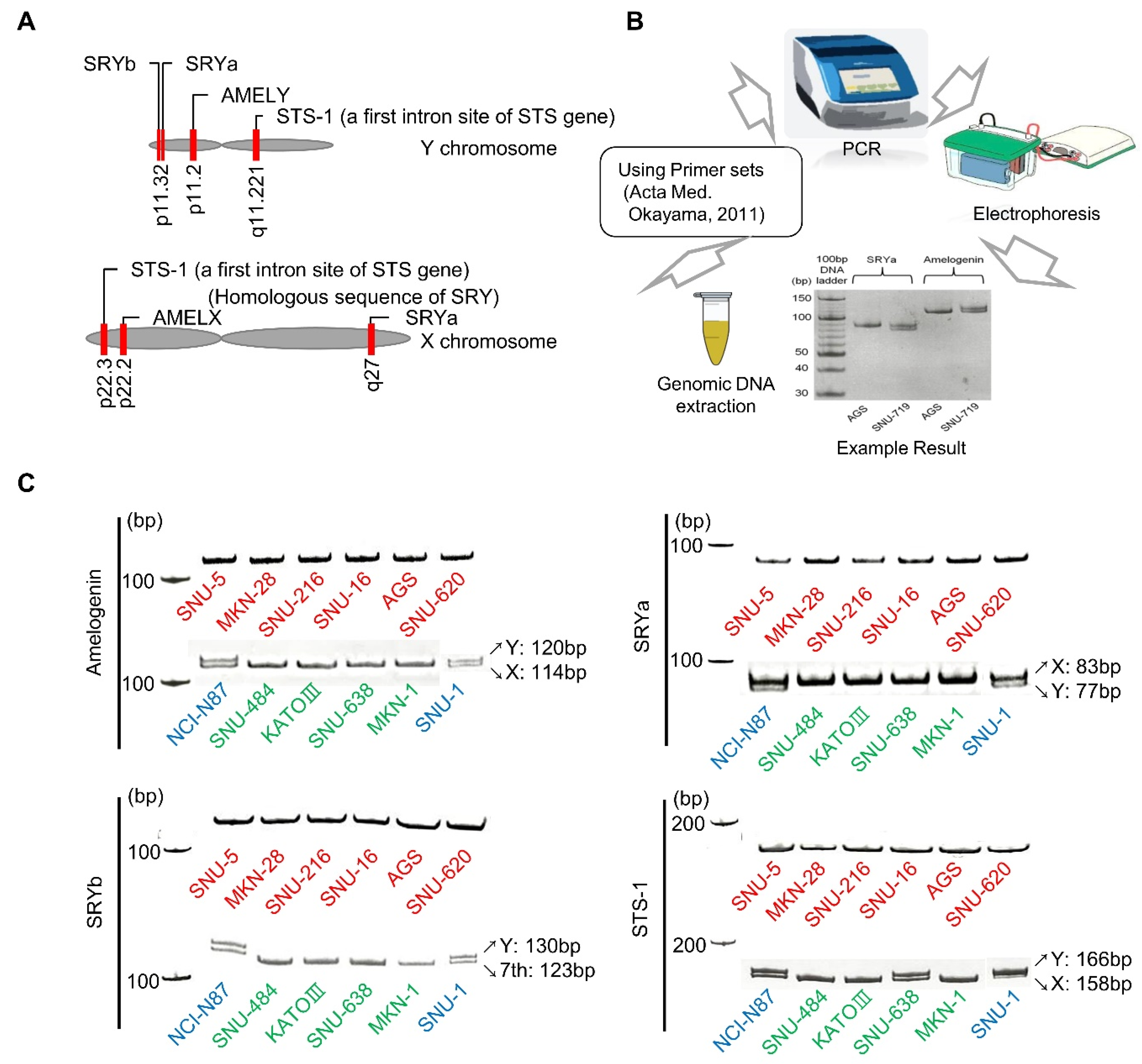
2.1. Polymerase Chain Reaction (PCR) of Sex Chromosomes
2.2. Short Tandem Repeat (STR) Profiling of the Cell Lines
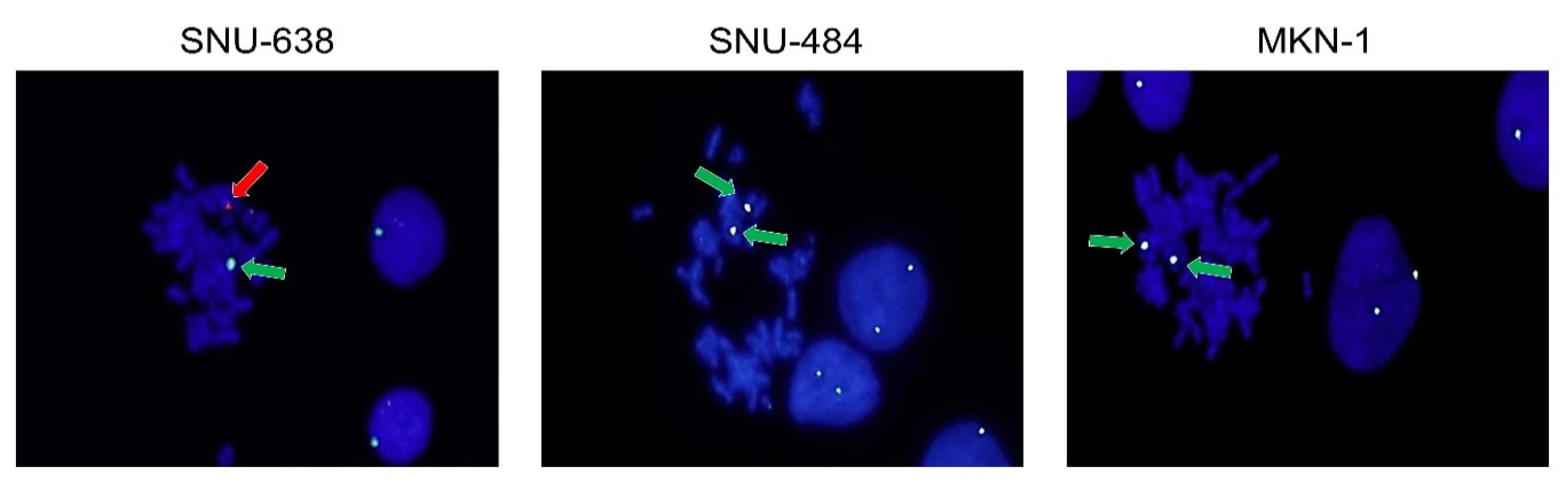
2.3. Fluorescence in Situ Hybridization (FISH) Analysis for Sex Chromosomes
2.4. Copy Number Variation (CNV) Analysis
2.5. Giemsa Banding
3. Discussion
4. Materials and Methods
4.1. Cells
4.2. Genomic DNA
4.3. PCR Detection of Sex Chromosomes
4.4. TR Profiling of the Cell Lines
4.5. FISH Analysis for Sex Chromosomes
4.6. CNV Analysis
4.7. Giemsa Banding
Author Contributions
Funding
Conflicts of Interest
References
- Becker, J.B.; Arnold, A.P.; Berkley, K.J.; Blaustein, J.D.; Eckel, L.A.; Hampson, E.; Herman, J.P.; Marts, S.; Sadee, W.; Steiner, M.; et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinology 2005, 146, 1650–1673. [Google Scholar] [CrossRef]
- Zucker, I.; Beery, A.K. Males still dominate animal studies. Nature 2010, 465, 690. [Google Scholar] [CrossRef] [PubMed]
- Reusch, J.E.B.; Kumar, T.R.; Regensteiner, J.G.; Zeitler, P.S. Identifying the Critical Gaps in Research on Sex Differences in Metabolism Across the Life Span. Endocrinology 2018, 159, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Nascimbeni, F.; Ballestri, S.; Fairweather, D.; Win, S.; Than, T.A.; Abdelmalek, M.F.; Suzuki, A. Sex Differences in Nonalcoholic Fatty Liver Disease: State of the Art and Identification of Research Gaps. Hepatol. (Baltim. Md.) 2019, 70, 1457–1469. [Google Scholar] [CrossRef] [PubMed]
- Clayton, J.A.; Collins, F.S. Policy: NIH to balance sex in cell and animal studies. Nature 2014, 509, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K. Sex as an important biological variable in biomedical research. Bmb Rep. 2018, 51, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Deasy, B.M.; Lu, A.; Tebbets, J.C.; Feduska, J.M.; Schugar, R.C.; Pollett, J.B.; Sun, B.; Urish, K.L.; Gharaibeh, B.M.; Cao, B.; et al. A role for cell sex in stem cell-mediated skeletal muscle regeneration: Female cells have higher muscle regeneration efficiency. J. Cell Biol. 2007, 177, 73–86. [Google Scholar] [CrossRef]
- Penaloza, C.; Estevez, B.; Orlanski, S.; Sikorska, M.; Walker, R.; Smith, C.; Smith, B.; Lockshin, R.A.; Zakeri, Z. Sex of the cell dictates its response: Differential gene expression and sensitivity to cell death inducing stress in male and female cells. Faseb J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2009, 23, 1869–1879. [Google Scholar] [CrossRef]
- Klein, S.L. Immune cells have sex and so should journal articles. Endocrinology 2012, 153, 2544–2550. [Google Scholar] [CrossRef]
- Park, M.N.; Park, J.H.; Paik, H.Y.; Lee, S.K. Insufficient sex description of cells supplied by commercial vendors. Am. J. Physiol. Cell Physiol. 2015, 308, C578–C580. [Google Scholar] [CrossRef]
- Santos, F.R.; Pandya, A.; Tyler-Smith, C. Reliability of DNA-based sex tests. Nat. Genet. 1998, 18, 103. [Google Scholar] [CrossRef] [PubMed]
- Phua, A.C.; Abdullah, R.B.; Mohamed, Z. A PCR-based sex determination method for possible application in caprine gender selection by simultaneous amplification of the Sry and Aml-X genes. J. Reprod. Dev. 2003, 49, 307–311. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakahori, Y.; Hamano, K.; Iwaya, M.; Nakagome, Y. Sex identification by polymerase chain reaction using X-Y homologous primer. Am. J. Med Genet. 1991, 39, 472–473. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Yamamoto, Y.; Miyaishi, S. A new method for sex determination based on detection of SRY, STS and amelogenin gene regions with simultaneous amplification of their homologous sequences by a multiplex PCR. Acta Med. Okayama 2011, 65, 113–122. [Google Scholar] [CrossRef]
- Lattanzi, W.; Di Giacomo, M.C.; Lenato, G.M.; Chimienti, G.; Voglino, G.; Resta, N.; Pepe, G.; Guanti, G. A large interstitial deletion encompassing the amelogenin gene on the short arm of the Y chromosome. Hum. Genet. 2005, 116, 395–401. [Google Scholar] [CrossRef]
- Butler, E.R. Genetic Markers for Sex Identification in Forensic DNA Analysis. J Forensic Investig. 2014, 2, 10. [Google Scholar] [CrossRef][Green Version]
- Yadav, S.K.; Kumari, A.; Ali, S. Fate of the human Y chromosome linked genes and loci in prostate cancer cell lines DU145 and LNCaP. Bmc Genom. 2013, 14, 323. [Google Scholar] [CrossRef][Green Version]
- Ziebe, S.; Lundin, K.; Loft, A.; Bergh, C.; Nyboe Andersen, A.; Selleskog, U.; Nielsen, D.; Grøndahl, C.; Kim, H.; Arce, J.C. FISH analysis for chromosomes 13, 16, 18, 21, 22, X and Y in all blastomeres of IVF pre-embryos from 144 randomly selected donated human oocytes and impact on pre-embryo morphology. Hum. Reprod. 2003, 18, 2575–2581. [Google Scholar] [CrossRef]
- Sugita, S.; Hasegawa, T. Practical use and utility of fluorescence in situ hybridization in the pathological diagnosis of soft tissue and bone tumors. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2017, 22, 601–612. [Google Scholar] [CrossRef]
- Schreck, R.R.; Distèche, C.M. Chromosome banding techniques. Curr. Protoc. Hum. Genet. 2001, Chapter 4. Unit4.2. [Google Scholar] [CrossRef]
- Zarrei, M.; MacDonald, J.R.; Merico, D.; Scherer, S.W. A copy number variation map of the human genome. Nat. Rev.. Genet. 2015, 16, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Bucan, M. Copy Number Variation Detection via High-Density SNP Genotyping. Csh Protoc. 2008, 2008. pdb.top46. [Google Scholar] [CrossRef] [PubMed]
- Schröck, E.; du Manoir, S.; Veldman, T.; Schoell, B.; Wienberg, J.; Ferguson-Smith, M.A.; Ning, Y.; Ledbetter, D.H.; Bar-Am, I.; Soenksen, D.; et al. Multicolor spectral karyotyping of human chromosomes. Science 1996, 273, 494–497. [Google Scholar] [CrossRef]
- Jang, W.; Chae, H.; Kim, J.; Son, J.O.; Kim, S.C.; Koo, B.K.; Kim, M.; Kim, Y.; Park, I.Y.; Sung, I.K. Identification of small marker chromosomes using microarray comparative genomic hybridization and multicolor fluorescent in situ hybridization. Mol. Cytogenet. 2016, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Fadl-Elmula, I.; Kytölä, S.; Pan, Y.; Lui, W.O.; Derienzo, G.; Forsberg, L.; Mandahl, N.; Gorunova, L.; Bergerheim, U.S.; Heim, S.; et al. Characterization of chromosomal abnormalities in uroepithelial carcinomas by G-banding, spectral karyotyping and FISH analysis. Int. J. Cancer 2001, 92, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Ji, M.F.; Wang, X.R.; Luo, R.L.; Ren, X.; Liu, M.; Wang, Q.K. Detection of human chromosomal abnormalities using a new technique combining 4’,6-diamidino-2-phenyl-indole staining and image analysis. Clin. Genet. 2006, 69, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Pfitzinger, H.; Ludes, B.; Mangin, P. Sex determination of forensic samples: Co-amplification and simultaneous detection of a Y-specific and an X-specific DNA sequence. Int. J. Leg. Med. 1993, 105, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Castedo, S.; Correia, C.; Gomes, P.; Seruca, R.; Soares, P.; Carneiro, F.; Sobrinho-Simões, M. Loss of Y chromosome in gastric carcinoma. Fact or artifact? Cancer Genet. Cytogenet. 1992, 61, 39–41. [Google Scholar] [CrossRef]
- van Dekken, H.; Pizzolo, J.G.; Kelsen, D.P.; Melamed, M.R. Targeted cytogenetic analysis of gastric tumors by in situ hybridization with a set of chromosome-specific DNA probes. Cancer 1990, 66, 491–497. [Google Scholar] [CrossRef]
- Ochi, H.; Douglass, H.O., Jr.; Sandberg, A.A. Cytogenetic studies in primary gastric cancer. Cancer Genet. Cytogenet. 1986, 22, 295–307. [Google Scholar] [CrossRef]
- Chun, Y.H.; Kil, J.I.; Suh, Y.S.; Kim, S.H.; Kim, H.; Park, S.H. Characterization of chromosomal aberrations in human gastric carcinoma cell lines using chromosome painting. Cancer Genet. Cytogenet. 2000, 119, 18–25. [Google Scholar] [CrossRef]
- Masuzawa, Y.; Hayata, I.; Ichikawa, T.; Ichikawa, Y.; Toida, T. Cytogenetic Study on a Subclone of KATO-III with HSR, a Cell Line derived from Human Gastric Carcinoma. Proc. Jpn. Acad. Ser. B 1988, 64, 57–60. [Google Scholar] [CrossRef][Green Version]
- Spatz, A.; Borg, C.; Feunteun, J. X-chromosome genetics and human cancer. Nat. Rev. Cancer 2004, 4, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Visakorpi, T.; Hyytinen, E.; Kallioniemi, A.; Isola, J.; Kallioniemi, O.P. Sensitive detection of chromosome copy number aberrations in prostate cancer by fluorescence in situ hybridization. Am. J. Pathol. 1994, 145, 624–630. [Google Scholar] [PubMed]
- Di Oto, E.; Monti, V.; Cucchi, M.C.; Masetti, R.; Varga, Z.; Foschini, M.P. X chromosome gain in male breast cancer. Hum. Pathol. 2015, 46, 1908–1912. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Peng, X.; Chen, Y.; Zhang, Y.; Ma, Q.; Liang, L.; Carter, A.C.; Lu, X.; Wu, C.I. Free-living human cells reconfigure their chromosomes in the evolution back to uni-cellularity. eLife 2017, 6. [Google Scholar] [CrossRef]
- Pastor, D.M.; Poritz, L.S.; Olson, T.L.; Kline, C.L.; Harris, L.R.; Koltun, W.A.; Chinchilli, V.M.; Irby, R.B. Primary cell lines: False representation or model system? a comparison of four human colorectal tumors and their coordinately established cell lines. Int. J. Clin. Exp. Med. 2010, 3, 69–83. [Google Scholar]
- Kaur, G.; Dufour, J.M. Cell lines: Valuable tools or useless artifacts. Spermatogenesis 2012, 2, 1–5. [Google Scholar] [CrossRef]


| Locus | Cell Lines | |||
|---|---|---|---|---|
| KATOⅢ | SNU-484 | SNU-638 | MKN-1 | |
| TPOX | 11 | 8 | 8, 11 | 8 |
| D3S1358 | 15, 16 | 18 | 15 | 15, 17 |
| FGA | 23, 24 | 19, 23 | 17, 27 | 20, 23 |
| D5S818 | 10, 11 | 10 | 10, 13, 14 | 11 |
| CSF1PO | 7, 11 | 9 | 10, 12 | 9, 12 |
| D7S820 | 8, 12 | 12 | 9, 10 | 10 |
| TH01 | 7, 9 | 7 | 7 | 9 |
| vWA | 14, 16 | 18 | 14, 19 | 16 |
| D13S317 | 8, 12 | 11, 12 | 10 | 10, 12 |
| amelogenin | X | X | X | X |
| D16S539 | 10, 12 | 13 | 11, 12, 13 | 11, 12 |
| D21S11 | 30, 31 | 30 | 30.2, 31 | 29 |
| D18S51 | 12 | 13, 15 | 14, 16 | 13, 16 |
| D8S1179 | 13, 14 | 12, 14 | 11, 13 | 14 |
| D2S1338 | 18, 20 | 23 | 18, 22 | 22 |
| D19S433 | 13, 16 | 13 | 11, 14 | 13, 14 |
| Penta D | 13, 14 | 12 | 9 | 10 |
| Penta E | 13, 18, 19 | 22 | 14, 18 | 21 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, S.; Min, K.; Kim, M.; Lee, S.K. Sex Chromosomes Are Severely Disrupted in Gastric Cancer Cell Lines. Int. J. Mol. Sci. 2020, 21, 4598. https://doi.org/10.3390/ijms21134598
Oh S, Min K, Kim M, Lee SK. Sex Chromosomes Are Severely Disrupted in Gastric Cancer Cell Lines. International Journal of Molecular Sciences. 2020; 21(13):4598. https://doi.org/10.3390/ijms21134598
Chicago/Turabian StyleOh, Sooeun, Kyoungmi Min, Myungshin Kim, and Suk Kyeong Lee. 2020. "Sex Chromosomes Are Severely Disrupted in Gastric Cancer Cell Lines" International Journal of Molecular Sciences 21, no. 13: 4598. https://doi.org/10.3390/ijms21134598
APA StyleOh, S., Min, K., Kim, M., & Lee, S. K. (2020). Sex Chromosomes Are Severely Disrupted in Gastric Cancer Cell Lines. International Journal of Molecular Sciences, 21(13), 4598. https://doi.org/10.3390/ijms21134598





