Trace Element Bioaccumulation in Stone Curlew (Burhinus oedicnemus, Linnaeus, 1758): A Case Study from Sicily (Italy)
Abstract
1. Introduction
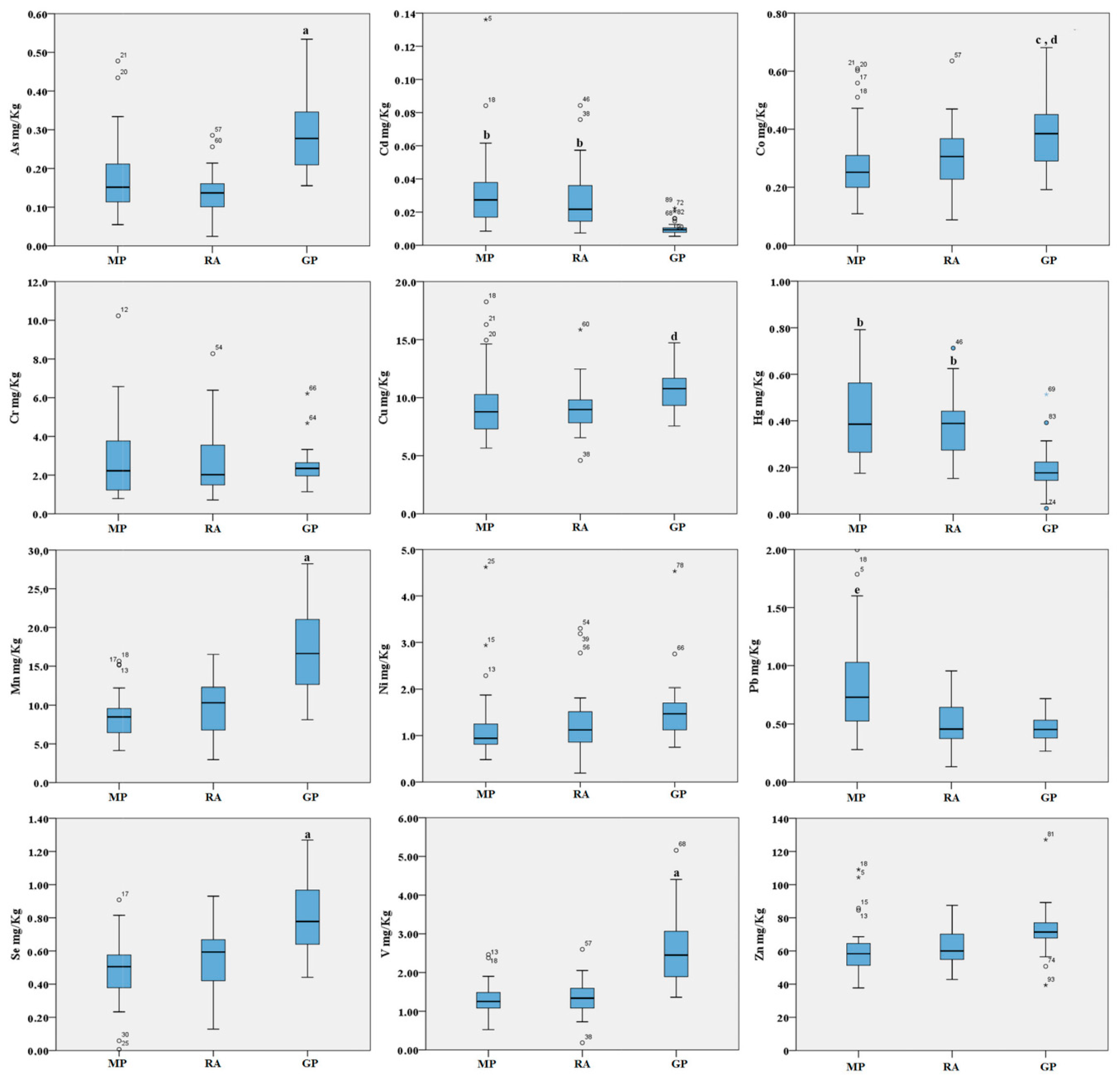
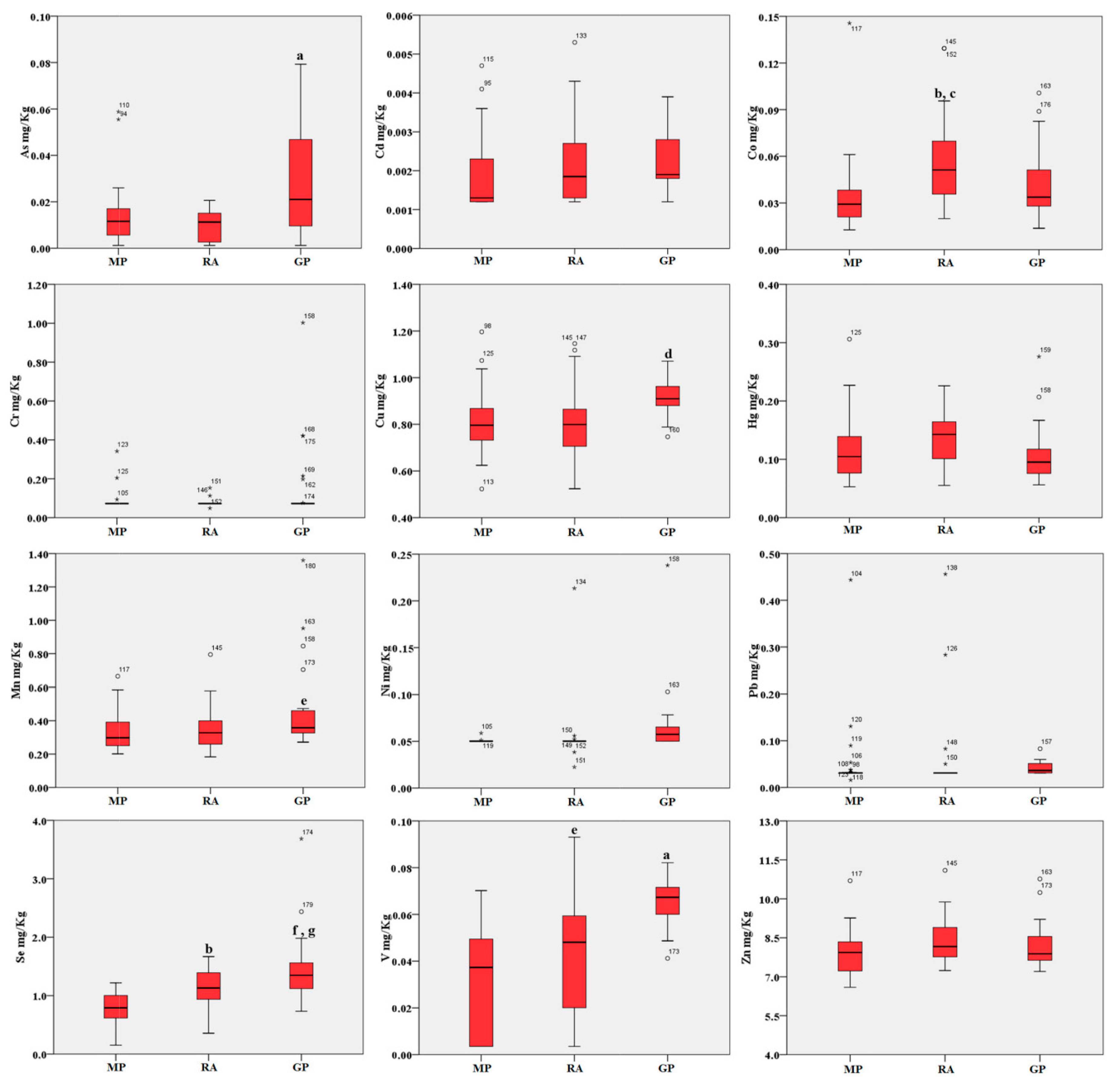
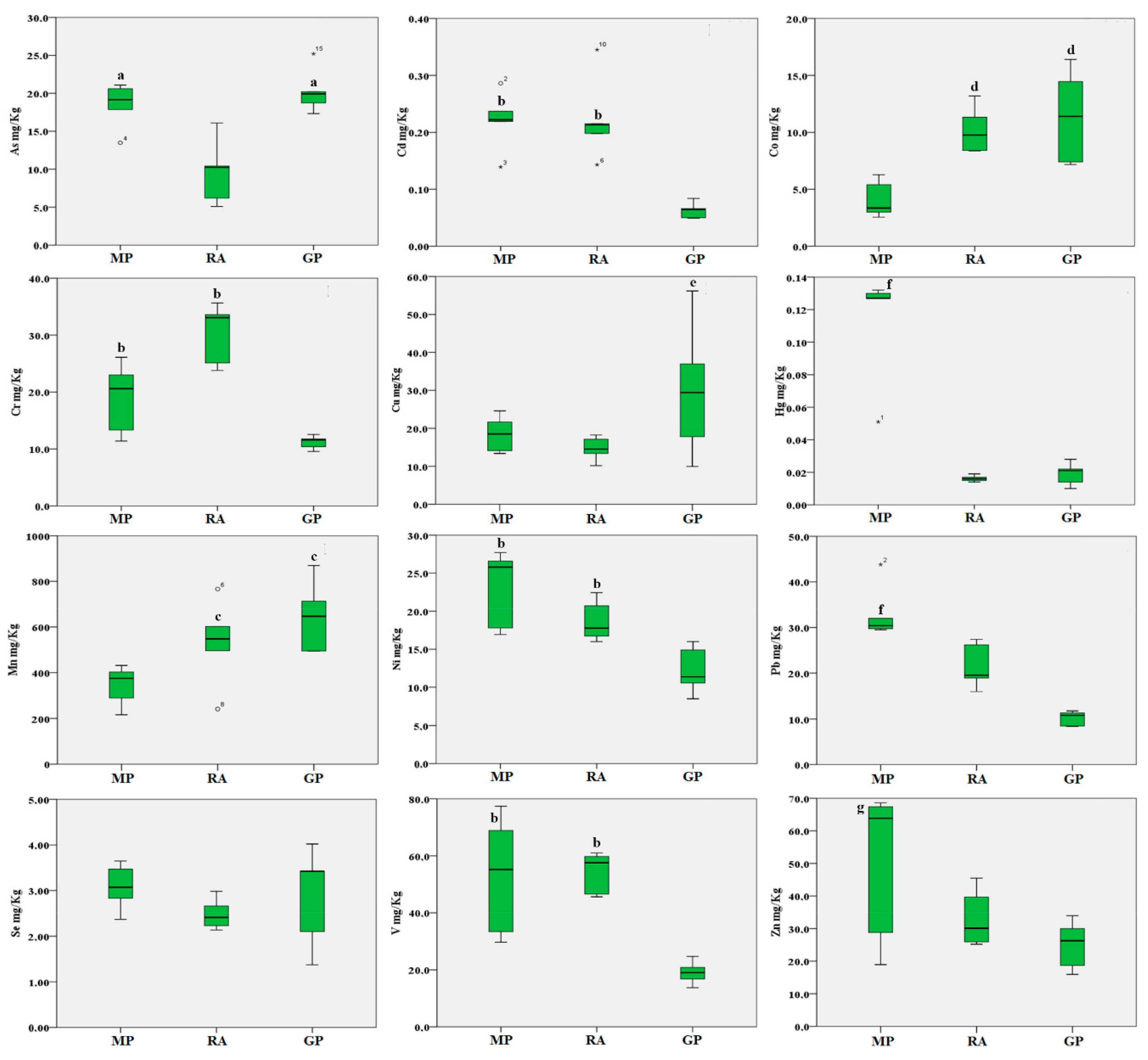
2. Results
3. Discussion
4. Materials and Methods
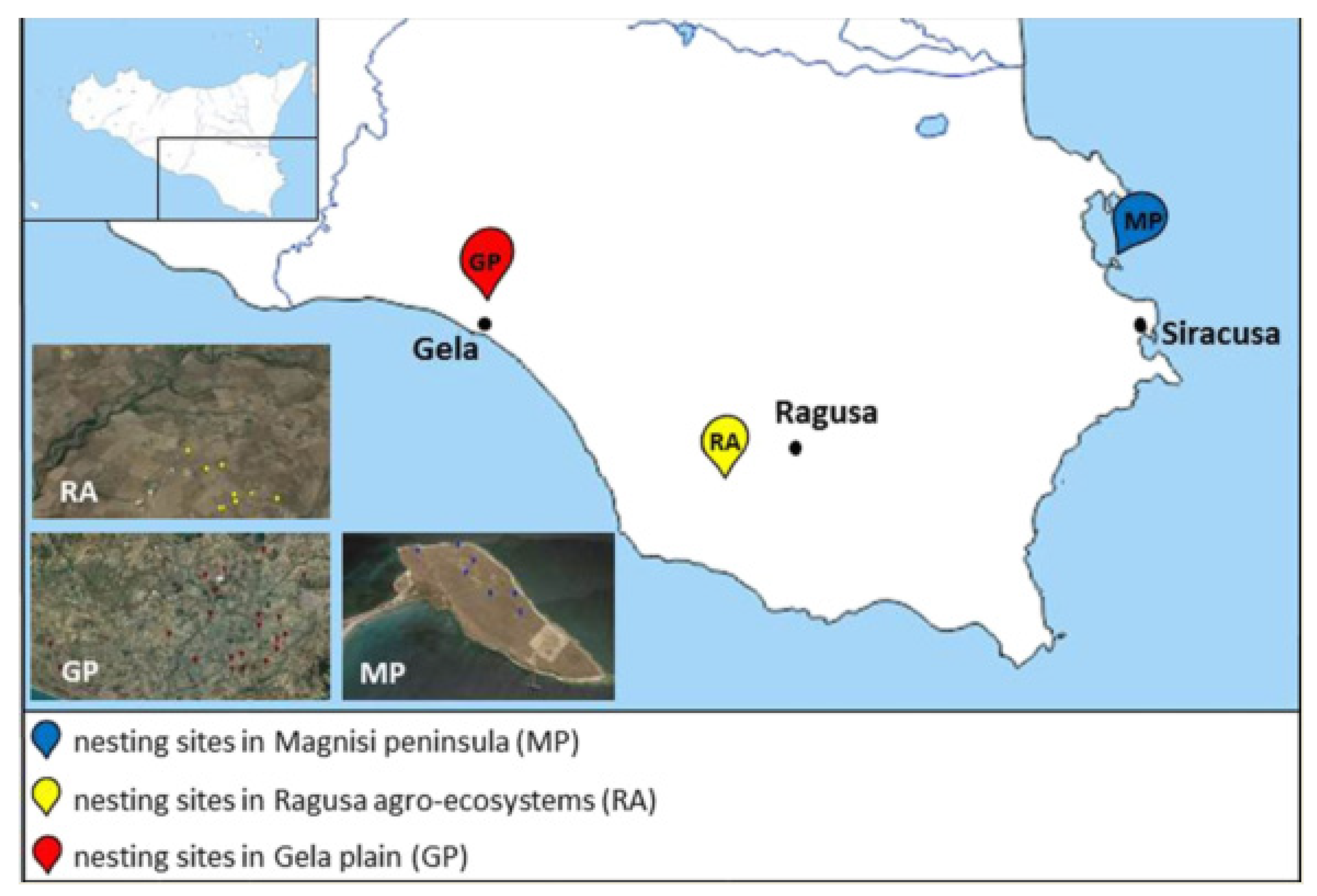
4.1. Sampling Areas
- The Magnisi peninsula (MP), located in the south-eastern coast of Sicily between the Augusta and Siracusa gulfs, a few kilometers away from the residential area of Priolo Gargallo (SR), has a rectangular shape with long sides, parallel to the coast, and about 2 km long with the short sides about 1 km. For more informations about geomorphological aspects refer to literature [90]. The peninsula has a flat surface that reaches the maximum height of 19 m above sea level. It shows a bare ground with sporadic buildings and wild fields, involved in cow pasture since the beginning of the last century. The proximity to the Augusta–Priolo–Melilli petrochemical plant, as well as the presence of a pyrite ashes dump, pose a threat to the environmental health of the area. Despite these issues, the site shows naturalistic peculiarities, for instance the presence of endemic plants, such as Limonium syracusanum Brullo, as well as a bird biodiversity ranging from migratory to non-migratory species [91]. In particular, the population density recorded for the Stone Curlew is far greater compared to the other southern regions of Italy [91,92]: it depends on the presence of bovine excrements, which turn into a substantial source of invertebrates on whom the birds feed, and therefore cause a decrease in intraspecific competition [93].
- The Ragusa agro-ecosystem (RA) is characterized by a rural landscape, where arable lands delimited by dry stone walls alternate with the association of olive and carob trees (Oleo sylvestris–Ceratonion siliquae Br.- Bl. ex Guinochet and Drouineau). The woodlands overlooking the canyons represent an excellent refuge for several vertebrate taxa, as well as an important nesting site for numerous species of birds.
- The Gela plain (GP), with an extension of 447.8 km2, is the second largest alluvial plain in Sicily and southern Italy in terms of size. It features an extensive agricultural mosaic, in which the Stone Curlew nests in the cultivated crops areas (56%, mainly cereals), artichokes land (22%), abandoned pastures and garrigues (17%) and olive tree groves (3%). The anthropic pressure on the area is focused in particular on the coast while the fields, subject to intensive farming and full of irrigation canals, are sprayed with pesticides, fertilizers and herbicides. We chose the Gela Plain due to the presence of a petrochemical plant, abandoned since 2014, with the aim of identifying possible relationships between pollution and the use of the area. Moreover, the rural area and its surroundings are crossed by several provincial and rural roads. On the other hand, there are also semi-natural environments, such as olive groves, wetlands, pastures, garrigues and small woods. The alternation of these habitats increases the fauna and flora communities.
4.2. Sampling
4.3. Trace Element Extraction and Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jerrett, M. The death toll from air-pollution sources. Nature 2015, 525, 330–331. [Google Scholar] [CrossRef] [PubMed]
- Wasana, H.M.S.; Perera, G.D.R.K.; Gunawardena, P.D.S.; Fernando, P.S.; Bandara, J. WHO water quality standards Vs Synergic effect(s) of fluoride, heavy metals and hardness in drinking water on kidney tissues. Sci. Rep. 2017, 7, 42516. [Google Scholar] [CrossRef] [PubMed]
- Weissmannová, H.D.; Pavlovský, J. Indices of soil contamination by heavy metals – methodology of calculation for pollution assessment (minireview). Environ. Monit. Assess. 2017, 189, 616. [Google Scholar] [CrossRef] [PubMed]
- Copat, C.; Arena, G.; Fiore, M.; Ledda, C.; Fallico, R.; Sciacca, S.; Ferrante, M. Heavy metals concentrations in fish and shellfish from eastern Mediterranean Sea: Consumption advisories. Food Chem. Toxicol. 2013, 53, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.; Spena, M.T.; Hernout, B.V.; Grasso, A.; Messina, A.; Grasso, R.; Agnelli, P.; Brundo, M.V.; Copat, C. Trace elements bioaccumulation in liver and fur of Myotis myotis from two caves of the eastern side of Sicily (Italy): A comparison between a control and a polluted area. Environ. Pollut. 2018, 240, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Hernout, B.V.; Arnold, K.E.; McClean, C.J.; Walls, M.; Baxter, M.; Boxall, A.B.A. A national level assessment of metal contamination in bats. Environ. Pollut. 2016, 214, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, V.; Longo, G.; Brundo, M.V.; Sinatra, F.; Copat, C.; Conti, G.O.; Ferrante, M. Bioaccumulation of cadmium and lead and its effects on hepatopancreas morphology in three terrestrial isopod crustacean species. Ecotoxicol. Environ. Saf. 2014, 110, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Copat, C.; Vinceti, M.; D’Agati, M.G.; Arena, G.; Mauceri, V.; Grasso, A.; Fallico, R.; Sciacca, S.; Ferrante, M. Mercury and selenium intake by seafood from the Ionian Sea: A risk evaluation. Ecotoxicol. Environ. Saf. 2014, 100, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.F. Arsenic toxicity and potential mechanisms of action. Toxicol. Lett. 2002, 133, 1–16. [Google Scholar] [CrossRef]
- Bernhoft, R.A. Mercury Toxicity and Treatment: A Review of the Literature. J. Environ. Public Health 2011, 2012, 1–10. [Google Scholar] [CrossRef]
- Maret, W.; Sigel, A.; Sigel, H.; Sigel, R.K.O. The Bioinorganic Chemistry of Lead in the Context of Its Toxicity. Lead Its Effects Environ. Health 2017, 17, 1–20. [Google Scholar] [CrossRef]
- Mansour, S.; Abdel-Hamid, A.A.; Ibrahim, A.W.; Mahmoud, N.H.; Moselhy, W.A. Toxicity of Some Pesticides, Heavy Metals and Their Mixtures to Vibrio fischeri Bacteria and Daphnia magna: Comparative Study. J. Boil. Life Sci. 2015, 6, 221. [Google Scholar] [CrossRef]
- Ferrante, M.; Signorelli, S.S.; Ferlito, S.L.; Grasso, A.; DiMartino, A.; Copat, C. Groundwater-based water wells characterization from Guinea Bissau (Western Africa): A risk evaluation for the local population. Sci. Total. Environ. 2018, 619, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Islam, E.U.; Yang, X.; He, Z.-L.; Mahmood, Q. Assessing potential dietary toxicity of heavy metals in selected vegetables and food crops. J. Zhejiang Univ. Sci. B 2006, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.S.; Roh, T.H.; Kim, M.K.; Kwon, Y.C.; Choi, S.M.; Kwack, S.J.; Kim, K.B.; Yoon, S.; Kim, H.S.; Lee, B.-M. Non-cancer, cancer, and dermal sensitization risk assessment of heavy metals in cosmetics. J. Toxicol. Environ. Heal. Part A 2018, 81, 432–452. [Google Scholar] [CrossRef]
- Hou, S.; Zheng, N.; Tang, L.; Ji, X.; Li, Y.; Hua, X. Pollution characteristics, sources, and health risk assessment of human exposure to Cu, Zn, Cd and Pb pollution in urban street dust across China between 2009 and 2018. Environ. Int. 2019, 128, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.; Pappalardo, A.M.; Ferrito, V.; Pulvirenti, V.; Fruciano, C.; Grasso, A.; Sciacca, S.; Tigano, C.; Copat, C. Bioaccumulation of metals and biomarkers of environmental stress in Parablennius sanguinolentus (Pallas, 1814) sampled along the Italian coast. Mar. Pollut. Bull. 2017, 122, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Van Der Bank, F.; Van Wyk, E.; Verdoorn, G.; Hofmann, D. Selected mineral and heavy metal concentrations in blood and tissues of vultures in different regions of South Africa. South Afr. J. Anim. Sci. 2001, 31, 57–64. [Google Scholar] [CrossRef]
- Evers, D.C.; Savoy, L.J.; DeSorbo, C.R.; Yates, D.E.; Hanson, W.; Taylor, K.M.; Siegel, L.S.; Cooley, J.H.; Bank, M.S.; Major, A.; et al. Adverse effects from environmental mercury loads on breeding common loons. Ecotoxicology 2007, 17, 69–81. [Google Scholar] [CrossRef]
- Lucia, M.; André, J.-M.; Gontier, K.; Diot, N.; Veiga, J.; Davail, S. Trace Element Concentrations (Mercury, Cadmium, Copper, Zinc, Lead, Aluminium, Nickel, Arsenic, and Selenium) in Some Aquatic Birds of the Southwest Atlantic Coast of France. Arch. Environ. Contam. Toxicol. 2009, 58, 844–853. [Google Scholar] [CrossRef]
- Markowski, M.; Kaliński, A.; Skwarska, J.; Wawrzyniak, J.; Bańbura, M.; Markowski, J.; Zieliński, P.; Bańbura, J. Avian Feathers as Bioindicators of the Exposure to Heavy Metal Contamination of Food. Bull. Environ. Contam. Toxicol. 2013, 91, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Espín, S.; Martínez-López, E.; Jiménez, P.; María-Mojica, P.; García-Fernández, A.-J. Effects of heavy metals on biomarkers for oxidative stress in Griffon vulture (Gyps fulvus). Environ. Res. 2014, 129, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Oh, J.M. Concentration of trace elements in feathers of waterfowl, Korea. Environ. Monit. Assess. 2014, 186, 8517–8525. [Google Scholar] [CrossRef] [PubMed]
- Ullah, K.; Hashmi, M.Z.; Malik, R.N. Heavy-Metal Levels in Feathers of Cattle Egret and Their Surrounding Environment: A Case of the Punjab Province, Pakistan. Arch. Environ. Contam. Toxicol. 2013, 66, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Fasola, M.; Muhammad, A.; Malik, S.A.; Bostan, N.; Bokhari, H.; Kamran, M.A.; Shafqat, M.N.; Alamdar, A.; Khan, M.; et al. Avian feathers as a non-destructive bio-monitoring tool of trace metals signatures: A case study from severely contaminated areas. Chemosphere 2015, 119, 553–561. [Google Scholar] [CrossRef]
- Beck, M.L.; Hopkins, W.A.; Jackson, B.P.; Hawley, D.M. The effects of a remediated fly ash spill and weather conditions on reproductive success and offspring development in tree swallows. Environ. Monit. Assess. 2015, 187. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, M.; Colaço, B.; Brandão, R.; Azorín, B.; Nicolás, O.; Colaço, J.; Pires, M.J.; Agustí, S.; Casas-Díaz, E.; Lavín, S.; et al. Assessment of the exposure to heavy metals in Griffon vultures (Gyps fulvus) from the Iberian Peninsula. Ecotoxicol. Environ. Saf. 2015, 113, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Manjula, M.; Mohanraj, R.; Devi, M.P. Biomonitoring of heavy metals in feathers of eleven common bird species in urban and rural environments of Tiruchirappalli, India. Environ. Monit. Assess. 2015, 187. [Google Scholar] [CrossRef] [PubMed]
- Sinka-Karimi, M.H.; Pourkhabbaz, A.R.; Hassanpour, M.; Levengood, J.M. Study on Metal Concentrations in Tissues of Mallard and Pochard from Two Major Wintering Sites in Southeastern Caspian Sea, Iran. Bull. Environ. Contam. Toxicol. 2015, 95, 292–297. [Google Scholar] [CrossRef]
- Ahmadpour, M.; Lan-Hai, L.; Ahmadpour, M.; Hoseini, S.H.; Mashrofeh, A.; Binkowski, L.J. Mercury concentration in the feathers of birds from various trophic levels in Fereydunkenar International wetland (Iran). Environ. Monit. Assess. 2016, 188, 666. [Google Scholar] [CrossRef]
- Dolan, K.J.; Ciesielski, T.M.; Lierhagen, S.; Eulaers, I.; Nygård, T.; Johnsen, T.V.; Gómez-Ramírez, P.; García-Fernández, A.-J.; Bustnes, J.O.; Ortiz-Santaliestra, M.E.; et al. Trace element concentrations in feathers and blood of Northern goshawk (Accipiter gentilis) nestlings from Norway and Spain. Ecotoxicol. Environ. Saf. 2017, 144, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Schmude, E.; Ertl, H.M.H.; Taylor, R.J.; Mora, M.A. Using Feathers to Evaluate Adverse Effects of Metals on Northern Bobwhites (Colinus virginianus) in Texas. Arch. Environ. Contam. Toxicol. 2018, 75, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, N.A.; Jaspers, V.L.B.; Chaudhry, M.J.I.; Ali, S.; Malik, R.N. Influence of taxa, trophic level, and location on bioaccumulation of toxic metals in bird’s feathers: A preliminary biomonitoring study using multiple bird species from Pakistan. Chemosphere 2015, 120, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Espín, S.; Martínez-López, E.; León-Ortega, M.; Calvo, J.F.; García-Fernández, A.-J. Factors that influence mercury concentrations in nestling Eagle Owls (Bubo bubo). Sci. Total. Environ. 2014, 470, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.; Gochfeld, M. Metal levels in feathers of 12 species of seabirds from Midway Atoll in the northern Pacific Ocean. Sci. Total. Environ. 2000, 257, 37–52. [Google Scholar] [CrossRef]
- Eisler, R. A Review of Arsenic Hazards to Plants and Animals with Emphasis on Fishery and Wildlife Resources; U.S. National Biological Survey, Patuxent Wildlife Research Center: Laurel, MD, USA, 1988.
- Burger, J. Heavy metals in avian eggshells: Another excretion method. J. Toxicol. Environ. Heal. Part A 1994, 41, 207–220. [Google Scholar] [CrossRef]
- Stock, M.; Herber, R.; Geron, H. Cadmium levels in oystercatcher Haematopus ostralegus from the German Wadden Sea. Mar. Ecol. Prog. Ser. 1989, 53, 227–234. [Google Scholar] [CrossRef]
- Eisler, R. Mercury Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review. Biol. Rep. 1987, 85. [Google Scholar]
- Burger, J.; Gochfeld, M. Growth and behavioral effects of early postnatal chromium and manganese exposure in herring gull (Larus argentatus) chicks. Pharmacol. Biochem. Behav. 1995, 50, 607–612. [Google Scholar] [CrossRef]
- Meador, J.P. Environmental Contaminants in Wildlife: Interpreting Tissue Concentrations; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Ohlendorf, H.; Heinz, G. Selenium in Birds. In Environmental Contaminants in Biota: Interpreting Tissue Concentrations, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 669–701. [Google Scholar]
- Burger, J.; Gochfeld, M. Age differences in metals in the blood of herring (Larus argentatus) and Franklin’s (Larus pipixcan) gulls. Arch. Environ. Contam. Toxicol. 1997, 33, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Daury, R.W.; Schwab, F.E.; Bateman, M.C. Blood lead concentrations of waterfowl from unhunted and heavily hunted marshes of nova scotia and prince edward island, Canada. J. Wildl. Dis. 1993, 29, 577–581. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stout, J.D.; Brinker, D.F.; Driscoll, C.P.; Davison, S.; Murphy, L.A. Serum Biochemistry Values, Plasma Mineral Levels, and Whole Blood Heavy Metal Measurements in Wild Northern Goshawks (Accipiter gentilis). J. Zoo Wildl. Med. 2010, 41, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, L.; He, J.; Hu, M.; Zeng, F.; Li, Y.; Tian, H.; Luo, X. The Adverse Effects of Se Toxicity on Inflammatory and Immune Responses in Chicken Spleens. Boil. Trace Element Res. 2018, 185, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Chawla, R.; Filippini, T.; Loomba, R.; Cilloni, S.; Dhillon, K.S.; Vinceti, M. Exposure to a high selenium environment in Punjab, India: Biomarkers and health conditions. Sci. Total. Environ. 2020, 719, 134541. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Filippini, T.; Wise, L.A. Environmental Selenium and Human Health: An Update. Curr. Environ. Heal. Rep. 2018, 5, 464–485. [Google Scholar] [CrossRef]
- Vinceti, M.; Filippini, T.; Malagoli, C.; Violi, F.; Mandrioli, J.; Consonni, D.; Rothman, K.J.; Wise, L.A. Amyotrophic lateral sclerosis incidence following exposure to inorganic selenium in drinking water: A long-term follow-up. Environ. Res. 2019, 179, 108742. [Google Scholar] [CrossRef]
- Lemos, L.S.; Moura, J.; Hauser-Davis, R.A.; De Campos, R.C.; Siciliano, S. Small cetaceans found stranded or accidentally captured in southeastern Brazil: Bioindicators of essential and non-essential trace elements in the environment. Ecotoxicol. Environ. Saf. 2013, 97, 166–175. [Google Scholar] [CrossRef]
- Mallory, C.D.; Gilchrist, H.G.; Robertson, G.J.; Provencher, J.F.; Braune, B.M.; Forbes, M.R.; Mallory, M. Hepatic trace element concentrations of breeding female common eiders across a latitudinal gradient in the eastern Canadian Arctic. Mar. Pollut. Bull. 2017, 124, 252–257. [Google Scholar] [CrossRef]
- Vikøren, T.; Kristoffersen, A.B.; Lierhagen, S.; Handeland, K. A comparative study of hepatic trace element levels in wild moose, roe deer, and reindeer from norway. J. Wildl. Dis. 2011, 47, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Darriet, P.; Bouchilloux, P.; Poupot, C.; Bugaret, Y.; Clerjeau, M.; Sauris, P.; Medina, B.; Dubourdieu, D. Effects of copper fungicide spraying on volatile thiols of the varietal aroma of Sauvignon blanc, Cabernet Sauvignon and Merlot wines. Vitis 2001, 40, 93–99. [Google Scholar]
- Defarge, N.; De Vendômois, J.S.; Séralini, G.-E. Toxicity of formulants and heavy metals in glyphosate-based herbicides and other pesticides. Toxicol. Rep. 2017, 5, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.; Napoli, S.; Grasso, A.; Zuccarello, P.; Cristaldi, A.; Copat, C. Systematic review of arsenic in fresh seafood from the Mediterranean Sea and European Atlantic coasts: A health risk assessment. Food Chem. Toxicol. 2019, 126, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Campos, V. Arsenic in groundwater affected by phosphate fertilizers at São Paulo, Brazil. Environ. Earth Sci. 2002, 42, 83–87. [Google Scholar] [CrossRef]
- Pirastu, R.; Ancona, C.; Iavarone, I.; Mitis, F.; Zona, A.; Comba, P. SENTIERI Project. Mortality study of residents in Italian polluted sites: Evaluation of the epidemiological evidence. Epidemiol. Prev. 2011, 34, 1–2. [Google Scholar]
- Cubadda, F. Inductively Coupled Plasma-Mass Spectrometry for the Determination of Elements and Elemental Species in Food: A Review. J. AOAC Int. 2004, 87, 173–204. [Google Scholar] [CrossRef] [PubMed]
- Barbour, E.K.; Shaib, H.A.; Yaghi, R.H.; Sabra, A.H. Regression of the Level of Different Heavy Metals to Size of Marine Organisms Harvested from the “Jiyeh” Oil Spill Zone of the Eastern Mediterranean Sea. Bull. Environ. Contam. Toxicol. 2009, 83, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.V.; Rainha, K.P.; De Castro, E.V.R.; Filgueiras, P.R.; Carneiro, M.T.W.D.; Brandão, G. Exploratory data analysis using API gravity and V and Ni contents to determine the origins of crude oil samples from petroleum fields in the Espírito Santo Basin (Brazil). Microchem. J. 2016, 124, 26–30. [Google Scholar] [CrossRef]
- Salvo, A.; La Torre, G.L.; Mangano, V.; Casale, K.E.; Bartolomeo, G.; Santini, A.; Granata, T.; Dugo, G. Toxic inorganic pollutants in foods from agricultural producing areas of Southern Italy: Level and risk assessment. Ecotoxicol. Environ. Saf. 2018, 148, 114–124. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, A. Cadmium toxicity in fish: An overview. GERF Bull. Biosci. 2010, 1, 41–47. [Google Scholar]
- Olsson, P.E. Disorders associated with heavy metal pollution. Fish Dis. Disord. 1998, 2, 105–131. [Google Scholar]
- Sprovieri, M.; Oliveri, E.; Di Leonardo, R.; Romano, E.; Ausili, A.; Gabellini, M.; Barra, M.; Tranchida, G.; Bellanca, A.; Neri, R.; et al. The key role played by the Augusta basin (southern Italy) in the mercury contamination of the Mediterranean Sea. J. Environ. Monit. 2011, 13, 1753. [Google Scholar] [CrossRef] [PubMed]
- Zahir, F.; Rizwi, S.J.; Haq, S.K.; Khan, R. Low dose mercury toxicity and human health. Environ. Toxicol. Pharmacol. 2005, 20, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Eagles-Smith, C.A.; Silbergeld, E.K.; Basu, N.; Bustamante, P.; Díaz-Barriga, F.; Hopkins, W.A.; Kidd, K.A.; Nyland, J.F. Modulators of mercury risk to wildlife and humans in the context of rapid global change. Ambio 2018, 47, 170–197. [Google Scholar] [CrossRef] [PubMed]
- Spahn, S.A.; Sherry, T.W. Cadmium and lead exposure associated with reduced growth rates, poorer fledging success of little blue heron chicks (Egretta caerulea) in south Louisiana wetlands. Arch. Environ. Contam. Toxicol. 1999, 37, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Veerle, J.; Tom, D.; Rianne, P.; Lieven, B.; Ronny, B.; Marcel, E. The importance of exogenous contamination on heavy metal levels in bird feathers. A field experiment with free-living great tits, Parus major. J. Environ. Monit. 2004, 6, 356. [Google Scholar] [CrossRef]
- Beyer, W.N.; Meador, J.P. Environmental Contaminants in Biota: Interpreting Tissue Concentrations, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Burger, J. Metals in avian feathers; bioindicators of environmental pollution. Rev. Environ. Toxicol. 1993, 5, 203–311. [Google Scholar]
- Hughes, K.; Ewins, P.; Clark, K. A comparison of mercury levels in feathers and eggs of osprey (Pandion haliaetus) in the North American Great Lakes. Arch. Environ. Contam. Toxicol. 1997, 33, 441–452. [Google Scholar] [CrossRef]
- Braune, B.M.; Gaskin, D.E. A Mercury Budget for the Bonaparte’s Gull during Autumn Moult. Ornis Scand. 1987, 18, 244. [Google Scholar] [CrossRef]
- Altmeyer, M.; Dittmann, J.; Dmowski, K.; Wagner, G.; Muller, P. Distribution of elements in flight feathers of a white-tailed eagle. Sci. Total. Environ. 1991, 105, 157–164. [Google Scholar] [CrossRef]
- Cramp, S.; Simmons, K.E.L. Handbook of the birds of Europe the Middle east and North Africa; Oxford University Press: Oxford, UK, 1983. [Google Scholar]
- Ginn, H.B.; Melville, D.S. Moult in Birds. BTO guide 19 British Trust for Ornithology; British Trust for Ornithology: Tring, UK, 1983. [Google Scholar]
- Vashist, S.; Luong, J. Handbook of Immunoassay Technologies - Approaches, Performances, and Applications; Elsevier - Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Spena, M.T.; Pollonara, E.; Grasso, R.; Baldaccini, N.E. Tradizioni agro-pastorali e conservazione della biodiversità: Il caso dell’Occhione (Burhinus oedicnemus). In Proceedings of the Atti del LXXI Congresso Nazionale del’Unione Zoologica Italiana, Palermo, Italy, 20–23 September 2010; pp. 87–91. [Google Scholar]
- Ausili, A.; Gabellini, M.; Cammarata, G.; Fattorini, D.; Benedetti, M.; Pisanelli, B.; Gorbi, S.; Regoli, F. Ecotoxicological and human health risk in a petrochemical district of southern Italy. Mar. Environ. Res. 2008, 66, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Di Leonardo, R.; Bellanca, A.; Neri, R.; Tranchida, G.; Mazzola, S. Distribution of REEs in box-core sediments offshore an industrial area in SE Sicily, Ionian Sea: Evidence of anomalous sedimentary inputs. Chemosphere 2009, 77, 778–784. [Google Scholar] [CrossRef] [PubMed]
- De Domenico, E.; Mauceri, A.R.; Giordano, D.; Maisano, M.; Gioffrè, G.; Natalotto, A.; D’Agata, A.; Ferrante, M.; Brundo, M.V.; Fasulo, S. Effects of “in vivo” exposure to toxic sediments on juveniles of sea bass (Dicentrarchus labrax). Aquat. Toxicol. 2011, 105, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, B.; Copat, C.; Pulvirenti, V.; Ferrito, V.; Ferrante, M.; Renis, M.; Sciacca, S.; Tigano, C. Biochemical and bioaccumulation approaches for investigating marine pollution using Mediterranean rainbow wrasse, Coris julis (Linneaus 1798). Ecotoxicol. Environ. Saf. 2012, 86, 168–175. [Google Scholar] [CrossRef]
- Romano, E.; Bergamin, L.; Ausili, A.; Magno, M.C.; Gabellini, M. Evolution of the anthropogenic impact in the Augusta Harbor (Eastern Sicily, Italy) in the last decades: Benthic foraminifera as indicators of environmental status. Environ. Sci. Pollut. Res. 2015, 23, 10514–10528. [Google Scholar] [CrossRef] [PubMed]
- Conti, E.; Dattilo, S.; Costa, G.; Puglisi, C. Bioaccumulation of trace elements in the sandhopper Talitrus saltator (Montagu) from the Ionian sandy coasts of Sicily. Ecotoxicol. Environ. Saf. 2016, 129, 57–65. [Google Scholar] [CrossRef]
- Conti, E.; Dattilo, S.; Costa, G.; Puglisi, C. The ground beetle Parallelomorphus laevigatus is a potential indicator of trace metal contamination on the eastern coast of Sicily. Ecotoxicol. Environ. Saf. 2017, 135, 183–190. [Google Scholar] [CrossRef]
- Longo, G.; Trovato, M.; Mazzei, V.; Ferrante, M.; Conti, G.O. Ligia italica (Isopoda, Oniscidea) as Bioindicator of Mercury Pollution of Marine Rocky Coasts. PLoS ONE 2013, 8, e58548. [Google Scholar] [CrossRef][Green Version]
- Hernberg, S. Lead poisoning in a historical perspective. Am. J. Ind. Med. 2000, 38, 244–254. [Google Scholar] [CrossRef]
- Mitra, P.; Sharma, S.; Purohit, P.; Sharma, P. Clinical and molecular aspects of lead toxicity: An update. Crit. Rev. Clin. Lab. Sci. 2017, 54, 506–528. [Google Scholar] [CrossRef] [PubMed]
- Burrows, D. Chromium: Metabolism and Toxicity; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Goede, A.; De Bruin, M. The use of bird feather parts as a monitor for metal pollution. Environ. Pollut. Ser. B Chem. Phys. 1984, 8, 281–298. [Google Scholar] [CrossRef]
- Lentini, F.; Carbone, S.; Catalano, S.; Grasso, M. Principali lineamenti strutturali della Sicilia nord-orientale. Stud. Geol. Camerti 1995, 319–329. [Google Scholar]
- Brichetti, P.; Fracasso, G. Ornitologia Italiana: Tetraonidae-Scolopacidae: Identificazione, Distribuzione, Consistenza e Movimenti degli Ucceli Italiani; Oasi Alberto Perdisa: Bologna, Italy, 2004; Volume 2. [Google Scholar]
- Maria, T.S.; Grasso, R.; Pollonara, E.; Giunchi, D.; Natale, E.B. Indagini preliminari sulla biologia riproduttiva e sugli aspetti ecologici dell’Occhione (Burhinus oedicnemus) nella penisola di Thapsos (Siracusa). Biogeogr. J. Integr. Biogeogr. 2011, 30, 651–658. [Google Scholar] [CrossRef]
- Spena, M.T.; Grasso, R. Gli Occhioni della penisola di Thapsos (Sicilia): Dalla gestione agropastorale allo sfruttamento industriale. In Proceedings of the L’occhione (Burhinus oedicnemus): Biologia e Conservazione di una Specie di Interesse Comunitario—Indicazioni per la Gestione del Territorio e delle Aree Protette, Giarola, Italy, 20 September 2008; Volume 7, pp. 16–17. [Google Scholar]
- Dauwe, T.; Bervoets, L.; Pinxten, R.; Blust, R.; Eens, M. Variation of heavy metals within and among feathers of birds of prey: Effects of molt and external contamination. Environ. Pollut. 2003, 124, 429–436. [Google Scholar] [CrossRef]
- Kahle, S.; Becker, P.H. Bird blood as bioindicator for mercury in the environment. Chemosphere 1999, 39, 2451–2457. [Google Scholar] [CrossRef]

| Trace Elements | Magnisi Peninsula | Ragusa Agro-Ecosystem | Gela Plain | Thresholds |
|---|---|---|---|---|
| As | 0.180 | 0.139 | 0.285 | 2 mg/kg w.w. [36] |
| Cd | 0.032 | 0.029 | 0.010 | 0.1–2 mg/kg d.w. [37]; 2 mg/kg d.w. [35,38] |
| Co | 0.288 | 0.303 | 0.393 | |
| Cr | 2.860 | 2.742 | 2.452 | 2.8 mg/kg d.w. [35] |
| Cu | 9.420 | 9.007 | 10.76 | |
| Hg | 0.416 | 0.376 | 0.193 | 40 mg/kg w.w. [19]; 5 mg/kg w.w. [39]; 5 mg/kg d.w. [35] |
| Mn | 8.564 | 10.06 | 17.24 | Teratogenic effects (such as micromelia, twisted limbs, haemorrhage, and neck defects), behaviour impairments, altered growth rates and reduction of haemoglobin formation [40] |
| Ni | 1.199 | 1.266 | 1.554 | Interferences with plumage intensity [41] |
| Pb | 0.848 | 0.497 | 0.458 | 4.0 mg/kg d.w. [35] |
| Se | 0.483 | 0.566 | 0.802 | 5 mg/kg d.w. [42] |
| V | 1.299 | 1.352 | 2.606 | |
| Zn | 60.85 | 62.09 | 72.56 |
| Trace Elements | Magnisi Peninsula | Ragusa Agro-Ecosystem | Gela Plain | Thresholds | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B mg/kg d.w. | B mg/kg w.w. | B ng/mL | B mg/kg d.w. | B mg/kg w.w. | B ng/mL | B mg/kg d.w. | B mg/kg w.w. | B ng/mL | ||
| As | 0.014 | 0.003 | 2.982 | 0.01 | 0.002 | 2.13 | 0.028 | 0.006 | 5.964 | 20 ng/mL [43] |
| Cd | 0.002 | 0.0004 | 0.426 | 0.002 | 0.0004 | 0.426 | 0.002 | 0.0004 | 0.426 | 0.5 ng/mL [22] |
| Hg | 0.117 | 0.026 | 24.92 | 0.136 | 0.03 | 28.97 | 0.106 | 0.024 | 22.58 | 1–3 mg/kg w.w [19]; 30 ng/mL [22] |
| Pb | 0.050 | 0.011 | 10.65 | 0.058 | 0.013 | 12.35 | 0.042 | 0.009 | 8.950 | 10 mg/kg * [44]; 150 ng/mL [22] |
| Se | 0.804 | 0.179 | 171.3 | 1.135 | 0.253 | 241.8 | 1.454 | 0.324 | 309.7 | 1 mg/kg w.w. [42]; 0.130–0.200 mg/kg w.w. [45] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Copat, C.; Ferrante, M.; Hernout, B.V.; Giunta, F.; Grasso, A.; Messina, A.; Grasso, R.; Spena, M.T. Trace Element Bioaccumulation in Stone Curlew (Burhinus oedicnemus, Linnaeus, 1758): A Case Study from Sicily (Italy). Int. J. Mol. Sci. 2020, 21, 4597. https://doi.org/10.3390/ijms21134597
Copat C, Ferrante M, Hernout BV, Giunta F, Grasso A, Messina A, Grasso R, Spena MT. Trace Element Bioaccumulation in Stone Curlew (Burhinus oedicnemus, Linnaeus, 1758): A Case Study from Sicily (Italy). International Journal of Molecular Sciences. 2020; 21(13):4597. https://doi.org/10.3390/ijms21134597
Chicago/Turabian StyleCopat, Chiara, Margherita Ferrante, Béatrice V. Hernout, Flavia Giunta, Alfina Grasso, Andrea Messina, Rosario Grasso, and Maria Teresa Spena. 2020. "Trace Element Bioaccumulation in Stone Curlew (Burhinus oedicnemus, Linnaeus, 1758): A Case Study from Sicily (Italy)" International Journal of Molecular Sciences 21, no. 13: 4597. https://doi.org/10.3390/ijms21134597
APA StyleCopat, C., Ferrante, M., Hernout, B. V., Giunta, F., Grasso, A., Messina, A., Grasso, R., & Spena, M. T. (2020). Trace Element Bioaccumulation in Stone Curlew (Burhinus oedicnemus, Linnaeus, 1758): A Case Study from Sicily (Italy). International Journal of Molecular Sciences, 21(13), 4597. https://doi.org/10.3390/ijms21134597








