Burns in the Elderly: Potential Role of Stem Cells
Abstract
:1. Introduction
2. Elderly Skin
2.1. Anatomical Composition of the Elderly Skin
2.2. Cell Biology of Elderly Skin
2.2.1. Oxidative Stress
2.2.2. Telomere Shortening
2.2.3. Cellular Senescence
2.2.4. Immunosenescence
3. Stem Cells
3.1. Stem Cell Classification
3.2. MSC Applications in Regenerative Medicine and Wound Healing
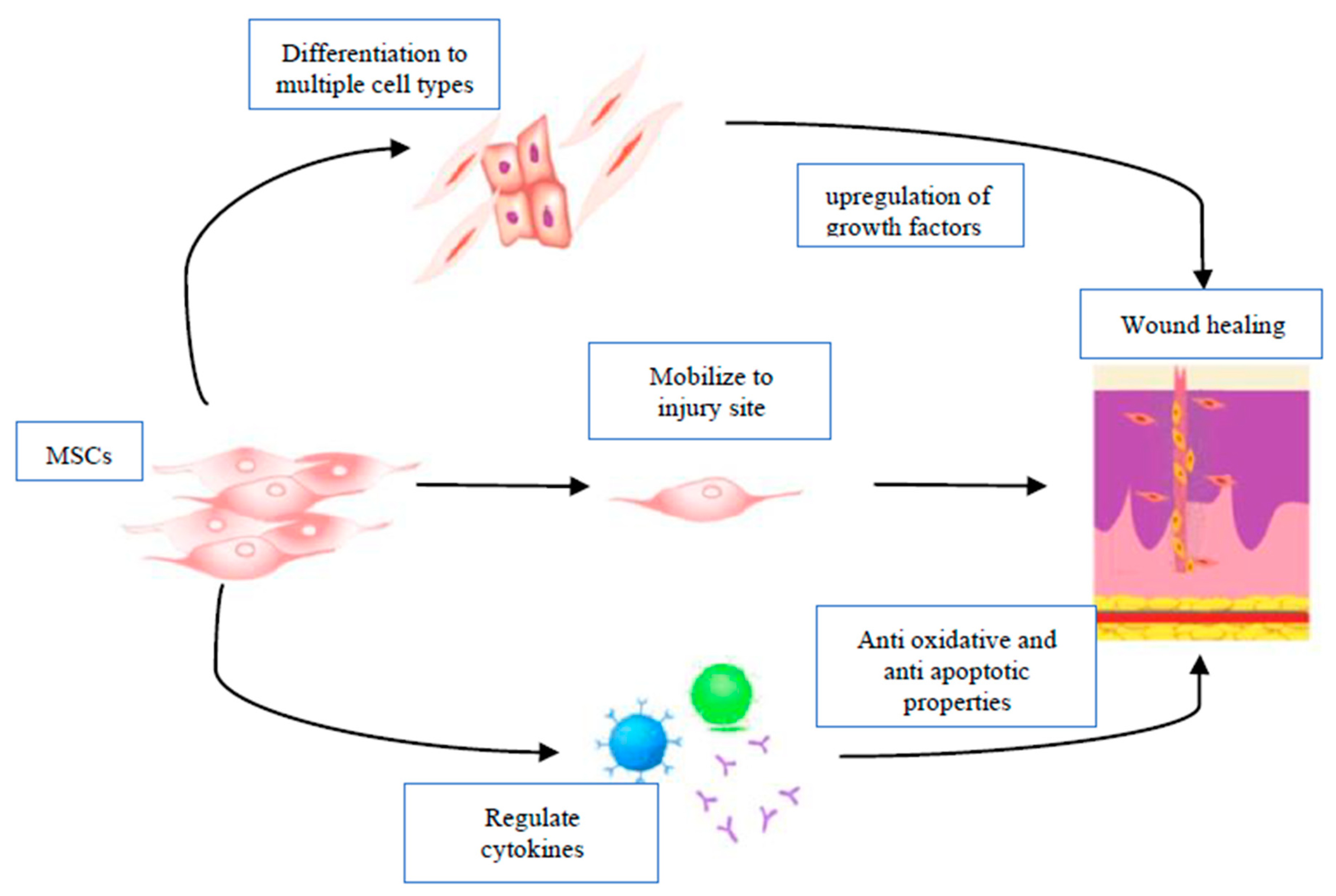
4. Stem Cells and Wound Repair
4.1. MSC Migration and Differentiation
4.2. MSCs and Collagen Deposition
4.3. Angiogenesis and Vascularization
4.4. Immunomodulatory Effects of Stem Cells
5. Prospective on Elderly Burns
5.1. Stem Cell Role in Repairing Elderly Burn Wounds
5.2. Skin Grafts and Donor Site Wound Repair in the Elderly Using Stem Cells
6. Conclusions
Funding
Conflicts of Interest
References
- Parachute. The Cost of Injury in Canada; Parachute: Toronto, ON, Canada, 2015; pp. 1–164. [Google Scholar]
- American Burn Association. National Burn Repository 2019 Update: Report of Data from 2009 to 2018; American Burn Association: Chicago, IL, USA, 2019. [Google Scholar]
- Ahn, C.S.; Maitz, P.K.M. The true cost of burn. Burns 2012, 38, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Eser, T.; Kavalci, C.; Aydogan, C.; Kayipmaz, A.E. Epidemiological and cost analysis of burn injuries admitted to the emergency department of a tertiary burn center. SpringerPlus 2016, 5, 1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endo, A.; Shiraishi, A.; Otomo, Y.; Fushimi, K.; Murata, K. Volume-outcome relationship on survival and cost benefits in severe burn injury: A retrospective analysis of a Japanese nationwide administrative database. J. Intensive Care 2019, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.K.; Kym, D.; Yim, H.; Yang, H.T.; Cho, Y.S.; Kim, J.H.; Hur, J.; Chun, W. Epidemiological trends and risk factors in major burns patients in South Korea: A 10-year experience. Burns 2015, 41, 181–187. [Google Scholar] [CrossRef]
- Scott-Conner, C.; Meydrech, E.; Wheeler, W.; Coil, J., Jr. Quantitation of Rate of Wound Closure and the Prediction of Death Following Major Burns. Burns Incl. Therm. Inj. 1988, 14, 373–378. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Pinto, R.; Costford, S.R.; Amini-Nik, S. Threshold age and burn size associated with poor outcomes in the elderly after burn injury. Burns 2016, 42, 276–281. [Google Scholar] [CrossRef] [Green Version]
- Maxson, S.; Lopez, E.A.; Yoo, D.; Danilkovitch-Miagkova, A.; LeRoux, M.A. Concise Review: Role of Mesenchymal Stem Cells in Wound Repair. Stem Cells Transl. Med. 2012, 1, 142–149. [Google Scholar] [CrossRef]
- Du, X. 3D Bio-Printing Review. In Proceedings of the The 5th Annual International Conference on Material Science and Environmental Engineering (MSEE2017); IOP Publishing: Xiamen, China, 2017; Volume 301, p. 012023. [Google Scholar]
- Infographic: Canada’s Seniors Population Outlook: Uncharted Territory. Available online: https://www.cihi.ca/en/infographic-canadas-seniors-population-outlook-uncharted-territory (accessed on 3 March 2020).
- Hu, M.S.; Borrelli, M.R.; Lorenz, H.P.; Longaker, M.T.; Wan, D.C. Mesenchymal Stromal Cells and Cutaneous Wound Healing: A Comprehensive Review of the Background, Role, and Therapeutic Potential. Stem Cells Int. 2018, 2018, 6901983. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, Y.; Yoshikawa, K. Cutaneous Wound Healing: An Update. J. Dermatol. 2001, 28, 521–534. [Google Scholar] [CrossRef]
- Isakson, M.; de Blacam, C.; Whelan, D.; McArdle, A.; Clover, A.J.P. Mesenchymal Stem Cells and Cutaneous Wound Healing: Current Evidence and Future Potential. Stem Cells Int. 2015, 2015, 831095. [Google Scholar] [CrossRef] [Green Version]
- ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT01443689, Allogenic Stem Cell Therapy in Patients with Acute Burn. 2006 September 30 [Update 2012 November 28]. Available online: https://clinicaltrials.gov/ct2/show/NCT01443689?term=stem+cells&cond=burn&draw=3&rank=1 (accessed on 3 March 2020).
- ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT04235296, Mesenchymal Stem Cell Conditioned Medium-derived Pleiotropic Factor in Treating Residual Burn Wound. 2020 January 21 [Update 2020 May 12]. Available online: https://clinicaltrials.gov/ct2/show/NCT04235296?term=stem+cells&cond=burn&draw=3&rank=2 (accessed on 3 March 2020).
- ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT02104713, Stem Cell Therapy to Improve Burn Wound Healing. 2014 April 4 [Update 2020 April 30]. Available online: https://clinicaltrials.gov/ct2/show/NCT02104713?term=stem+cells&cond=burn&draw=3&rank=3 (accessed on 3 March 2020).
- ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT03686449, Autologous Keratinocyte Suspension Versus Adipose-Derived Stem Cell-Keratinocyte Suspension for Post-Burn Raw Area. 2018 September 27 [Update 2020 May 12]. Available online: https://clinicaltrials.gov/ct2/show/NCT03686449?term=stem+cells&cond=burn&draw=3&rank=5 (accessed on 3 March 2020).
- ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT02394873, A Study to Evaluate the Safety of ALLO-ASC-DFU in the Subjects with Deep Second-Degree Burn Wound. 2015 March 20 [Update 2015 December 30]. Available online: https://clinicaltrials.gov/ct2/show/NCT02394873?term=stem+cells&cond=burn&draw=3&rank=10 (accessed on 3 March 2020).
- ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT03113747, Allogeneic ADSCs and Platelet-Poor Plasma Fibrin Hydrogel to Treat the Patients with Burn Wounds (ADSCs-BWs) (ADSCs-BWs). 2017 April 14 [2017 April 26]. Available online: https://clinicaltrials.gov/ct2/show/NCT03113747?term=stem+cells&cond=burn&draw=3&rank=13 (accessed on 3 March 2020).
- Zouboulis, C.C.; Makrantonaki, E. Clinical aspects and molecular diagnostics of skin aging. Clin. Dermatol. 2011, 29, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Characteristics of the Aging Skin. Adv. Wound Care New Rochelle 2013, 2, 5–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeschke, M.G.; Peck, M.D. Burn Care of the Elderly. J. Burn Care Res. 2017, 38, e625–e628. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, B.A.; Unger, J.G.; Rohrich, R.J. Reversal of Skin Aging with Topical Retinoids. Plast. Reconstr. Surg. 2014, 133, 481e–490e. [Google Scholar] [CrossRef]
- Gosain, A.; DiPietro, L.A. Aging and Wound Healing. World J. Surg. 2004, 28, 321–326. [Google Scholar] [CrossRef]
- Abu-Sittah, G.; Chahine, F.; Janom, H. Management of Burns in the Elderly. Ann. Burns Fire Disasters 2016, 29, 249–254. [Google Scholar]
- Kligman, A.; Balin, A. Aging of Human Skin; Raven Press: New York, NY, USA, 1989. [Google Scholar]
- Fore, J. A Review of Skin and the Effects of Aging on Skin Structure and Function. Ostomy Wound Manag. 2006, 52, 24–35. [Google Scholar]
- Elias, P.M. Stratum corneum acidification: How and why? Exp. Dermatol. 2015, 24, 179–180. [Google Scholar] [CrossRef] [Green Version]
- Jia, Q.; Nash, J. Pathology of Aging Skin. In Textbook of Aging Skin; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Karimipour, D.J.; Rittié, L.; Hammerberg, C.; Min, V.K.; Voorhees, J.J.; Orringer, J.S.; Sachs, D.L.; Hamilton, T.; Fisher, G.J. Molecular Analysis of Aggressive Microdermabrasion in Photoaged Skin. Arch. Dermatol. 2009, 145, 1114–1122. [Google Scholar] [CrossRef] [Green Version]
- Calleja-Agius, J.; Muscat-Baron, Y.; Brincat, M. Skin aging. Menopause Int. 2007, 13, 60–64. [Google Scholar] [CrossRef] [Green Version]
- Grey, J.E.; Harding, K.G.; Enoch, S. ABC of wound healing: Pressure ulcers. BMJ 2006, 332, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Barbul, A. Understanding the role of immune regulation in wound healing. Am. J. Surg. 2004, 187, 11S–16S. [Google Scholar] [CrossRef]
- Jiang, J.; Fisher, E.M.; Murasko, D.M. CD8 T cell responses to influenza virus infection in aged mice. Ageing Res. Rev. 2011, 10, 422–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso-Fernández, P.; De la Fuente, M. Role of the Immune System in Aging and Longevity. Curr. Aging Sci. 2011, 4, 78–100. [Google Scholar] [CrossRef]
- Huertas, A.; Schmelzer, C.; Hoehenwarter, W.; Heyroth, F.; Heinz, A. Molecular level insights into aging processes of skin elastin. Biochimie 2016. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef]
- Yaar, M.; Gilchrest, B. Skin Aging: Postulated Mechanisms and Consequent Changes in Structure and Function. Clin. Geriatr. Med. 2001, 17, 617–630. [Google Scholar] [CrossRef]
- Yaar, M.; Eller, M.S.; Gilchrest, B.A. Fifty Years of Skin Aging. J. Investig. Dermatol. Symp. Proc. 2002, 7, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, L.T. Effect of lifestyle, gender and age on collagen formation and degradation. Hernia 2006, 10, 456–461. [Google Scholar] [CrossRef]
- Rani, M.; Schwacha, M.G. Aging and the Pathogenic Response to Burn. Aging Dis. 2012, 3, 171–180. [Google Scholar]
- Parihar, A.; Parihar, M.S.; Milner, S.; Bhat, S. Oxidative stress and anti-oxidative mobilization in burn injury. Burns 2008, 34, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Gutteridge, J.; Halliwell, B. Iron Toxicity and Oxygen Radicals. Baillières Clin. Haematol. 1989, 2, 195–256. [Google Scholar] [CrossRef]
- Sies, H. Strategies of antioxidant defense. Eur. J. Biochem. 1993, 215, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Aust, S.; Thomas, C.; Morehouse, L.; Saito, M.; Bucher, J. Active oxygen and toxicity. Adv. Exp. Med. Biol. 1986, 197, 513–526. [Google Scholar] [CrossRef]
- Guemouri, L.; Artur, Y.; Herbeth, B.; Jeandel, C.; Cuury, G.; Siest, G. Biological Variability of Superoxide Dismutase, Glutathione Peroxidase, and Catalase in Blood. Clin. Chem. 1991, 37, 1932–1937. [Google Scholar] [CrossRef]
- Poljšak, B.; Dahmane, R.G.; Godić, A. Intrinsic skin aging: The role of oxidative stress. Acta Dermatovenerol. Alp. Pannonica Adriat. 2012, 21, 33–36. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.; Larsson, N. Mitochondrial DNA mutations in disease and aging. J. Cell Biol. 2011, 193, 809–818. [Google Scholar] [CrossRef] [Green Version]
- Mantel, C.R.; O’Leary, H.A.; Chitteti, B.R.; Huang, X.; Cooper, S.; Hangoc, G.; Brustovetsky, N.; Srour, E.F.; Lee, M.R.; Messina-Graham, S.; et al. Enhancing hematopoietic stem cell transplantation efficacy by mitigating oxygen shock. Cell 2015, 161, 1553–1565. [Google Scholar] [CrossRef]
- Garcia-Prat, L.; Martinez-Vicente, M.; Perdiguero, E.; Ortet, L.; Rodriguez-Ubreva, J.; Rebollo, E.; Ruiz-Bonilla, V.; Gutarra, S.; Ballestar, E.; Serrano, A.; et al. Autophagy maintains stemness by preventing senescence. Nature 2016, 529, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Kosmadaki, M.G.; Gilchrest, B.A. The role of telomeres in skin aging/photoaging. Micron 2004, 35, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Wright, W.E.; Shay, J.W. Cellular senescence as a tumor-protection mechanism: The essential role of counting. Curr. Opin. Genet. Dev. 2001, 11, 98–103. [Google Scholar] [CrossRef]
- Richter, T.; von Zglinicki, T. A continuous correlation between oxidative stress and telomere shortening in fibroblasts. Exp. Gerontol. 2007, 42, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Serra, V.; von Zglinicki, T.; Lorenz, M.; Saretzki, G. Extracellular Superoxide Dismutase Is a Major Antioxidant in Human Fibroblasts and Slows Telomere Shortening. J. Biol. Chem. 2003, 278, 6824–6830. [Google Scholar] [CrossRef] [Green Version]
- Buckingham, E.M.; Klingelhutz, A.J. The role of telomeres in the ageing of human skin: Telomeres and skin ageing. Exp. Dermatol. 2011, 20, 297–302. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [Green Version]
- Panich, U.; Sittithumcharee, G.; Rathviboon, N.; Jirawatnotai, S. Ultraviolet Radiation-Induced Skin Aging: The Role of DNA Damage and Oxidative Stress in Epidermal Stem Cell Damage Mediated Skin Aging. Stem Cells Int. 2016, 2016, 7370642. [Google Scholar] [CrossRef] [Green Version]
- Ning, M.S.; Andl, T. Control by a hair’s breadth: The role of microRNAs in the skin. Cell. Mol. Life Sci. 2013, 70, 1149–1169. [Google Scholar] [CrossRef] [Green Version]
- Van Deursen, J. The role of senescent cells in aging. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [Green Version]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Toutfaire, M.; Bauwens, E.; Debacq-Chainiaux, F. The impact of cellular senescence in skin ageing: A notion of mosaic and therapeutic strategies. Biochem Pharm. 2017, 142, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Demaria, M.; Ohtani, N.; Youssef, S.; Rodier, F.; Toussaint, W.; Mitchell, J.; Laberge, R.M.; Vijg, J.; Van Steeg, H.; Dollé, M.; et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 2014, 31, 722–733. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.; Dreesen, O. Biomarkers of Cellular Senescence and Skin aging. Front. Genet. 2019, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Swift, M.E.; Burns, A.L.; Gray, K.L.; DiPietro, L.A. Age-Related Alterations in the Inflammatory Response to Dermal Injury. J. Investig. Dermatol. 2001, 117, 1027–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging: An Evolutionary Perspective on Immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Nomellini, V.; Gomez, C.R.; Gamelli, R.L.; Kovacs, E.J. Aging and Animal Models of Systemic Insult: Trauma, Burn, and Sepsis. Shock 2009, 31, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Stanojcic, M.; Chen, P.; Xiu, F.; Jeschke, M.G. Impaired Immune Response in Elderly Burn Patients: New Insights Into the Immune-senescence Phenotype. Ann. Surg. 2016, 264, 195–202. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, S4–S9. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Tredget, E.E.; Yu, Y.M. The metabolic effects of thermal injury. World J. Surg. 1992, 16, 68–79. [Google Scholar] [CrossRef]
- Till, G.; Hatherill, J.R.; Tourtellotte, W.; Lutz, M.; Ward, P.A. Lipid Peroxidation and Acute Lung Injury After Thermal Trauma to Skin. Evidence of a Role for Hydroxyl Radical. Am. J. Pathol. 1985, 119, 376–384. [Google Scholar] [PubMed]
- Haycoskl, J.W.; Ralstox, R.; Morris, B.; Freedlander, E.; MasNeil’, S. Oxidative damage to protein and alterations to antioxidant levels in human cutaneous thermal injury. Burns 1997, 23, 533–540. [Google Scholar] [CrossRef]
- Horton, J.W. Free radicals and lipid peroxidation mediated injury in burn trauma: The role of antioxidant therapy. Toxicology 2003, 189, 75–88. [Google Scholar] [CrossRef]
- O’Toole, E.; Goel, M.; Woodley, D. Hydrogen Peroxide Inhibits Human Keratinocyte Migration. Search Results Web Result Site Links Dermatol. Surg. 1996, 22, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Lindblom, L.; Cassuto, J.; Yregård, L.; Mattsson, U.; Tarnow, P.; Sinclair, R. Importance of nitric oxide in the regulation of burn oedema, proteinuria and urine output. Burns 2000, 26, 13–17. [Google Scholar] [CrossRef]
- Schwacha, M.; Anantha Samy, T.; Catania, R.; Chaudry, I. Thermal injury alters macrophage responses to prostaglandin E2: Contribution to the enhancement of inducible nitric oxide synthase activity. J. Leukoc. Biol. 1998, 64, 740–746. [Google Scholar] [CrossRef]
- Huang, F.; Niedbala, W.; Wei1, X.-Q.; Xu, D.; Feng, G.; Robinson, J.; Lam, C.; Liew, F. Nitric oxide regulates Th1 cell development through the inhibition of IL-12 synthesis by macrophages. Eur. J. Immunol. 1998, 28, 4062–4070. [Google Scholar] [CrossRef]
- Kao, C.; Garner, W. Acute Burns. Plast. Reconstr. Surg. 2000, 105, 2482–2492. [Google Scholar] [CrossRef]
- Grimble, R.F. Inflammatory response in the elderly. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 21–29. [Google Scholar] [CrossRef]
- Lundgren, R.S.; Kramer, C.B.; Rivara, F.P.; Wang, J.; Heimbach, D.M.; Gibran, N.S.; Klein, M.B. Influence of Comorbidities and Age on Outcome Following Burn Injury in Older Adults. J. Burn Care Res. 2009, 30, 307–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, C.V.; Garcia-Lavandeira, M.; Garcia-Rendueles, M.E.R.; Diaz-Rodriguez, E.; Garcia-Rendueles, A.R.; Perez-Romero, S.; Vila, T.V.; Rodrigues, J.S.; Lear, P.V.; Bravo, S.B. Defining stem cell types: Understanding the therapeutic potential of ESCs, ASCs, and iPS cells. J. Mol. Endocrinol. 2012, 49, R89–R111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Romito, A.; Cobellis, G. Pluripotent Stem Cells: Current Understanding and Future Directions. Stem Cells Int. 2016, 2016, 9451492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzaei, H.; Sahebkar, A.; Sichani, L.S.; Moridikia, A.; Nazari, S.; Sadri Nahand, J.; salehi, H.; Stenvang, J.; Masoudifar, A.; Mirzaei, H.R.; et al. Therapeutic application of multipotent stem cells. J. Cell. Physiol. 2018, 233, 2815–2823. [Google Scholar] [CrossRef]
- Mahla, R.S. Stem Cells Applications in Regenerative Medicine and Disease Therapeutics. Int. J. Cell Biol. 2016, 2016, 6940283. [Google Scholar] [CrossRef] [Green Version]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells—Current trends and future prospective. Biosci. Rep. 2015, 35. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Amini-Nik, S.; Dolp, R.; Eylert, G.; Datu, A.-K.; Parousis, A.; Blakeley, C.; Jeschke, M.G. Stem cells derived from burned skin—The future of burn care. EBioMedicine 2018, 37, 509–520. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, K.; Jadhav, S.S. The application of mesenchymal stem cells to treat thermal and radiation burns. Adv. Drug Deliv. Rev. 2018, 123, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Butler, K.L.; Goverman, J.; Ma, H.; Fischman, A.; Yu, Y.-M.; Bilodeau, M.; Rad, A.M.; Bonab, A.A.; Tompkins, R.G.; Fagan, S.P. Stem Cells and Burns: Review and Therapeutic Implications. J. Burn Care Res. 2010, 31, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Maranda, E.; Rodriguez-Menocal, L.; Badiavas, E. Role of Mesenchymal Stem Cells in Dermal Repair in Burns and Diabetic Wounds. Curr. Stem Cell Res. Ther. 2017, 12, 61–70. [Google Scholar] [CrossRef]
- Oh, E.J.; Lee, H.W.; Kalimuthu, S.; Kim, T.J.; Kim, H.M.; Baek, S.H.; Zhu, L.; Oh, J.M.; Son, S.H.; Chung, H.Y.; et al. In vivo migration of mesenchymal stem cells to burn injury sites and their therapeutic effects in a living mouse model. J. Control. Release 2018, 279, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Abe, R.; Fujita, Y.; Ando, S.; Inokuma, D.; Shimizu, H. Mesenchymal Stem Cells Are Recruited into Wounded Skin and Contribute to Wound Repair by Transdifferentiation into Multiple Skin Cell Type. J. Immunol. 2008, 180, 2581–2587. [Google Scholar] [CrossRef] [PubMed]
- Ringe, J.; Strassburg, S.; Neumann, K.; Endres, M.; Notter, M.; Burmester, G.-R.; Kaps, C.; Sittinger, M. Towards in situ tissue repair: Human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL. J. Cell. Biochem. 2007, 101, 135–146. [Google Scholar] [CrossRef]
- Hocking, A.M.; Gibran, N.S. Mesenchymal stem cells: Paracrine signaling and differentiation during cutaneous wound repair. Exp. Cell Res. 2010, 316, 2213–2219. [Google Scholar] [CrossRef] [Green Version]
- Arno, A.I.; Amini-Nik, S.; Blit, P.H.; Al-Shehab, M.; Belo, C.; Herer, E.; Tien, C.; Jeschke, M.G. Human Wharton’s jelly mesenchymal stem cells promote skin wound healing through paracrine signaling. Stem Cell Res. Ther. 2014, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.N.; Willis, E.; Chan, V.T.; Muffley, L.A.; Isik, F.F.; Gibran, N.S.; Hocking, A.M. Mesenchymal stem cells induce dermal fibroblast responses to injury. Exp. Cell Res. 2010, 316, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Bliley, J.M.; Argenta, A.; Satish, L.; McLaughlin, M.M.; Dees, A.; Tompkins-Rhoades, C.; Marra, K.G.; Rubin, J.P. Administration of adipose-derived stem cells enhances vascularity, induces collagen deposition, and dermal adipogenesis in burn wounds. Burns 2016, 42, 1212–1222. [Google Scholar] [CrossRef]
- Baraniak, P.R.; McDevitt, T.C. Stem cell paracrine actions and tissue regeneration. Regen. Med. 2010, 5, 121–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franck, C.L.; Senegaglia, A.C.; Leite, L.M.B.; de Moura, S.A.B.; Francisco, N.F.; Ribas Filho, J.M. Influence of Adipose Tissue-Derived Stem Cells on the Burn Wound Healing Process. Stem Cells Int. 2019, 2019, 2340725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fathke, C.; Wilson, L.; Hutter, J.; Kapoor, V.; Smith, A.; Hocking, A.; Isik, F. Contribution of Bone Marrow-Derived Cells to Skin: Collagen Deposition and Wound Repair. Stem Cells 2004, 22, 812–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Huang, S.; Enhe, J.; Ma, K.; Yang, S.; Sun, T.; Fu, X. Bone marrow-derived mesenchymal stem cell attenuates skin fibrosis development in mice: A cell-based therapy to alleviating skin fibrosis. Int. Wound J. 2014, 11, 701–710. [Google Scholar] [CrossRef]
- Watt, S.M.; Gullo, F.; van der Garde, M.; Markeson, D.; Camicia, R.; Khoo, C.P.; Zwaginga, J.J. The angiogenic properties of mesenchymal stem/stromal cells and their therapeutic potential. Br. Med. Bull. 2013, 108, 25–53. [Google Scholar] [CrossRef]
- Kong, P.; Xie, X.; Li, F.; Liu, Y.; Lu, Y. Placenta mesenchymal stem cell accelerates wound healing by enhancing angiogenesis in diabetic Goto-Kakizaki (G.K.) rats. Biochem. Biophys. Res. Commun. 2013, 438, 410–419. [Google Scholar] [CrossRef]
- Liu, L.; Yu, Y.; Hou, Y.; Chai, J.; Duan, H.; Chu, W.; Zhang, H.; Hu, Q.; Du, J. Human Umbilical Cord Mesenchymal Stem Cells Transplantation Promotes Cutaneous Wound Healing of Severe Burned Rats. PLoS ONE 2014, 9, e88348. [Google Scholar] [CrossRef] [Green Version]
- Ipaktchi, K.; Mattar, A.; Niederbichler, A.D.; Hoesel, L.M.; Vollmannshauser, S.; Hemmila, M.R.; Su, G.L.; Remick, D.G.; Wang, S.C.; Arbabi, S. Attenuating Burn Wound Inflammatory Signaling Reduces Systemic Inflammation and Acute Lung Injury. J. Immunol. 2006, 177, 8065–8071. [Google Scholar] [CrossRef] [Green Version]
- Gauglitz, G.G.; Song, J.; Herndon, D.N.; Finnerty, C.C.; Boehning, D.; Barral, J.M.; Jeschke, M.G. Characterization of the inflammatory response during acute and post-acute phases after severe burn. Shock 2008, 30, 503–507. [Google Scholar] [CrossRef] [Green Version]
- Schwacha, M.G.; Thobe, B.M.; Daniel, T.; Hubbard, W.J. Impact of Thermal Injury on Wound Infiltration and the Dermal Inflammatory Response. J. Surg. Res. 2010, 158, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Kim, A.; Lang, T.; Xue, M.; Wijewardana, A.; Jackson, C.; Vandervord, J. The Role of Th-17 Cells and γδ T-Cells in Modulating the Systemic Inflammatory Response to Severe Burn Injury. Int. J. Mol. Sci. 2017, 18, 758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; La, X.; Fan, L.; Li, P.; Yu, Y.; Huang, Y.; Ding, J.; Xing, Y. Immunosuppressive effects of mesenchymal stem cell transplantation in rat burn models. Int. J. Clin. Exp. Pathol. 2015, 8, 5129–5136. [Google Scholar] [PubMed]
- Li, X.; Liu, L.; Yang, J.; Yu, Y.; Chai, J.; Wang, L.; Ma, L.; Yin, H. Exosome Derived from Human Umbilical Cord Mesenchymal Stem Cell Mediates MiR-181c Attenuating Burn-induced Excessive Inflammation. EBioMedicine 2016, 8, 72–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yagi, H.; Soto-Gutierrez, A.; Kitagawa, Y.; Tilles, A.W.; Tompkins, R.G.; Yarmush, M.L. Bone Marrow Mesenchymal Stromal Cells Attenuate Organ Injury Induced by LPS and Burn. Cell Transplant. 2010, 19, 823–830. [Google Scholar] [CrossRef] [Green Version]
- Kuo, Y.-R.; Chen, C.-C.; Goto, S.; Huang, Y.-T.; Wang, C.-T.; Tsai, C.; Chen, C.-L. Immunomodulatory Effects of Bone Marrow-Derived Mesenchymal Stem Cells in a Swine Hemi-Facial Allotransplantation Model. PLoS ONE 2012, 7, e35459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartholomew, A.; Sturgeon, C.; Siatskas, M.; Ferrer, K.; McIntosh, K.; Patil, S.; Hardy, W.; Devine, S.; Ucker, D.; Deans, R.; et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 2002, 30, 42–48. [Google Scholar] [CrossRef]
- Larocca, R.A.; Moraes-Vieira, P.M.; Bassi, Ê.J.; Semedo, P.; de Almeida, D.C.; da Silva, M.B.; Thornley, T.; Pacheco-Silva, A.; Câmara, N.O.S. Adipose Tissue-Derived Mesenchymal Stem Cells Increase Skin Allograft Survival and Inhibit Th-17 Immune Response. PLoS ONE 2013, 8, e76396. [Google Scholar] [CrossRef]
- Lewis, G.; Heimbach, D.; Gibran, N. Chapter 10—Evaluation of the burn wound: Management decisions. In Total Burn Care; Saunders Elsevier: Philadelphia, PA, USA, 2012; pp. 125–130. [Google Scholar]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Moon, K.M.; Park, Y.-H.; Lee, J.S.; Chae, Y.-B.; Kim, M.-M.; Kim, D.-S.; Kim, B.-W.; Nam, S.-W.; Lee, J.-H. The Effect of Secretory Factors of Adipose-Derived Stem Cells on Human Keratinocytes. Int. J. Mol. Sci. 2012, 13, 1239–1257. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.-S.; Park, B.-S.; Sung, J.-H.; Yang, J.-M.; Park, S.-B.; Kwak, S.-J.; Park, J.-S. Wound healing effect of adipose-derived stem cells: A critical role of secretory factors on human dermal fibroblasts. J. Dermatol. Sci. 2007, 48, 15–24. [Google Scholar] [CrossRef]
- Ono, I.; Yamashita, T.; Hida, T.; Jin, H.-Y.; Ito, Y.; Hamada, H.; Akasaka, Y.; Ishii, T.; Jimbow, K. Combined administration of basic fibroblast growth factor protein and the hepatocyte growth factor gene enhances the regeneration of dermis in acute incisional wounds: Wound Repair and Regeneration. Wound Repair Regen. 2004, 12, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, L.; Scott, P.G.; Tredget, E.E. Mesenchymal Stem Cells Enhance Wound Healing Through Differentiation and Angiogenesis. Stem Cells 2007, 25, 2648–2659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, L.; Cammer, M.; Lehman, J.; Nielsen, S.; Guerra, C.; Veland, I.; Stock, C.; Hoffmann, E.; Yoder, B.; Schwab, A.; et al. Directional Cell Migration and Chemotaxis in Wound Healing Response to PDGF-AA are Coordinated by the Primary Cilium in Fibroblasts. Cell. Physiol. Biochem. 2010, 25, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Amini-Nik, S.; Cambridge, E.; Yu, W.; Guo, A.; Whetstone, H.; Nadesan, P.; Poon, R.; Hinz, B.; Alman, B.A. β-Catenin–regulated myeloid cell adhesion and migration determine wound healing. J. Clin. Investig. 2014, 124, 2599–2610. [Google Scholar] [CrossRef] [Green Version]
- Jeschke, M.G.; Patsouris, D.; Stanojcic, M.; Abdullahi, A.; Rehou, S.; Pinto, R.; Chen, P.; Burnett, M.; Amini-Nik, S. Pathophysiologic Response to Burns in the Elderly. EBioMedicine 2015, 2, 1536–1548. [Google Scholar] [CrossRef] [Green Version]
- Procházka, V.; Gumulec, J.; Jaluvka, F.; Salounova, D.; Jonszta, T.; Czerny, D.; Krajca, J.; Urbanec, R.; Klement, P.; Martinek, J.; et al. Cell Therapy, a New Standard in Management of Chronic Critical Limb Ischemia and Foot Ulcer. Cell Transplant. 2010, 19, 1413–1424. [Google Scholar] [CrossRef] [Green Version]
- Otero-Vinas, M.; Falanga, V. Mesenchymal stem cells in chronic wounds: The spectrum from basic to advanced therapy. Adv. Wound Care 2016, 5, 149–163. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Jeong, S.Y.; Ha, J.; Kim, M.; Jin, H.J.; Kwon, S.-J.; Chang, J.W.; Choi, S.J.; Oh, W.; Yang, Y.S.; et al. Low immunogenicity of allogeneic human umbilical cord blood-derived mesenchymal stem cells in vitro and in vivo. Biochem. Biophys. Res. Commun. 2014, 446, 983–989. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Rehou, S.; McCann, M.R.; Shahrokhi, S. Allogeneic mesenchymal stem cells for treatment of severe burn injury. Stem Cell Res. Ther. 2019, 10, 337. [Google Scholar] [CrossRef]
- Lee, J.; Dibildox, M.; Jimenez, C.; Gallagher, J.; Sayeed, S.; Sheridan, R.; Herndon, D. Chapter 13—Operative wound management. In Total Burn Care; Saunders Elsevier: Philadelphia, PA, USA, 2012; pp. 157–172. [Google Scholar]
- McGwin, G.; Cross, J.; Ford, J.; Rue, L. Long-term trends in mortality according to age among adult burn patients. J. Burn Care Rehabil. 2003, 24, 21–25. [Google Scholar] [CrossRef]
- Aickara, D.; Candendo, A.; Guzman, W.; Rodriguez-Menocal, L. Use of mesenchymal stem cells to improve healing of second degree burn wound and improve split thickness skin graft outcome. J. Am. Acad. Derm. 2019, 81, AB144. [Google Scholar]
- Keck, M.; Lumenta, D.B.; Andel, H.; Kamolz, L.P.; Frey, M. Burn treatment in the elderly. Burns 2009, 35, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhi, S.; Arno, A.; Jeschke, M.G. The use of dermal substitutes in burn surgery: Acute phase: Artificial dermis for acute burn phase. Wound Repair Regen. 2014, 22, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Chua, A.W.C.; Khoo, Y.C.; Tan, B.K.; Tan, K.C.; Foo, C.L.; Chong, S.J. Skin tissue engineering advances in severe burns: Review and therapeutic applications. Burns Trauma 2016, 4, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motamed, S.; Taghiabadi, E.; Molaei, H.; Sodeifi, N.; Hassanpour, S.E.; Shafieyan, S.; Azargashb, E.; Farajzadeh-Vajari, F.; Aghdami, N.; Bajouri, A. Cell-based skin substitutes accelerate regeneration of extensive burn wounds in rats. Am. J. Surg. 2017, 214, 762–769. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Mitsuno, H.; Nonaka, I.; Sen, Y.; Kawanishi, K.; Inada, Y.; Takakura, Y.; Okuchi, K.; Nonomura, A. Wound Therapy by Marrow Mesenchymal Cell Transplantation. Plast. Reconstr. Surg. 2008, 121, 860–877. [Google Scholar] [CrossRef]
- Sánchez-Muñoz, I.; Granados, R.; Holguín Holgado, P.; García-Vela, J.A.; Casares, C.; Casares, M. The Use of Adipose Mesenchymal Stem Cells and Human Umbilical Vascular Endothelial Cells on a Fibrin Matrix for Endothelialized Skin Substitute. Tissue Eng. Part A 2015, 21, 214–223. [Google Scholar] [CrossRef]
- Leonardi, D.; Oberdoerfer, D.; Fernandes, M.C.; Meurer, R.T.; Pereira-Filho, G.A.; Cruz, P.; Vargas, M.; Chem, R.C.; Camassola, M.; Nardi, N.B. Mesenchymal stem cells combined with an artificial dermal substitute improve repair in full-thickness skin wounds. Burns 2012, 38, 1143–1150. [Google Scholar] [CrossRef]
- Nakagawa, H.; Akita, S.; Fukui, M.; Fujii, T.; Akino, K. Human mesenchymal stem cells successfully improve skin-substitute wound healing. Br. J. Dermatol. 2005, 153, 29–36. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elloso, M.; Kambli, A.; Aijaz, A.; van de Kamp, A.; Jeschke, M.G. Burns in the Elderly: Potential Role of Stem Cells. Int. J. Mol. Sci. 2020, 21, 4604. https://doi.org/10.3390/ijms21134604
Elloso M, Kambli A, Aijaz A, van de Kamp A, Jeschke MG. Burns in the Elderly: Potential Role of Stem Cells. International Journal of Molecular Sciences. 2020; 21(13):4604. https://doi.org/10.3390/ijms21134604
Chicago/Turabian StyleElloso, Margarita, Ankita Kambli, Ayesha Aijaz, Alex van de Kamp, and Mark G. Jeschke. 2020. "Burns in the Elderly: Potential Role of Stem Cells" International Journal of Molecular Sciences 21, no. 13: 4604. https://doi.org/10.3390/ijms21134604
APA StyleElloso, M., Kambli, A., Aijaz, A., van de Kamp, A., & Jeschke, M. G. (2020). Burns in the Elderly: Potential Role of Stem Cells. International Journal of Molecular Sciences, 21(13), 4604. https://doi.org/10.3390/ijms21134604





