The Diverse Piscidin Repertoire of the European Sea Bass (Dicentrarchus labrax): Molecular Characterization and Antimicrobial Activities
Abstract
:1. Introduction
2. Results
2.1. Molecular Characterization of Sea Bass Piscidins
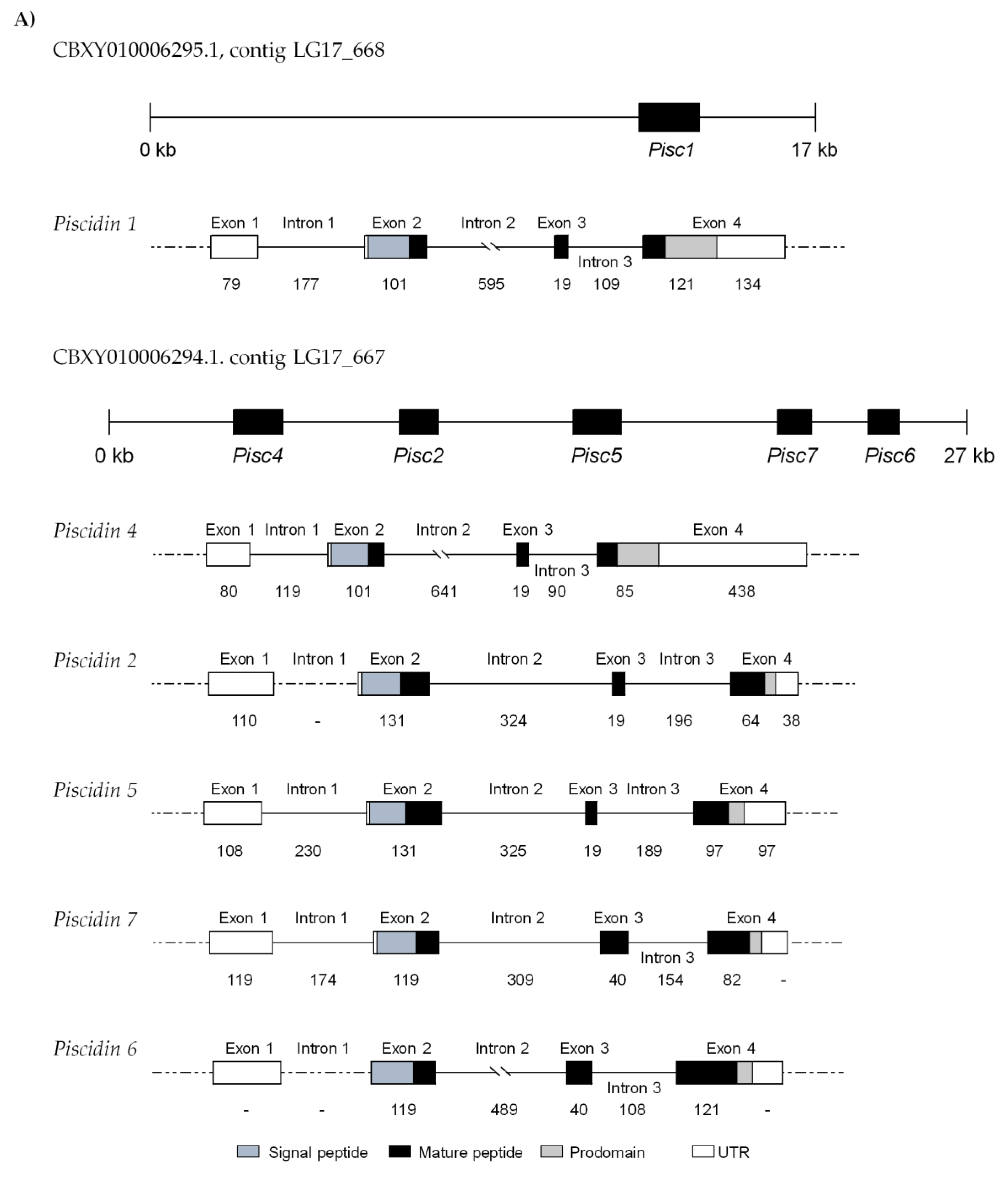
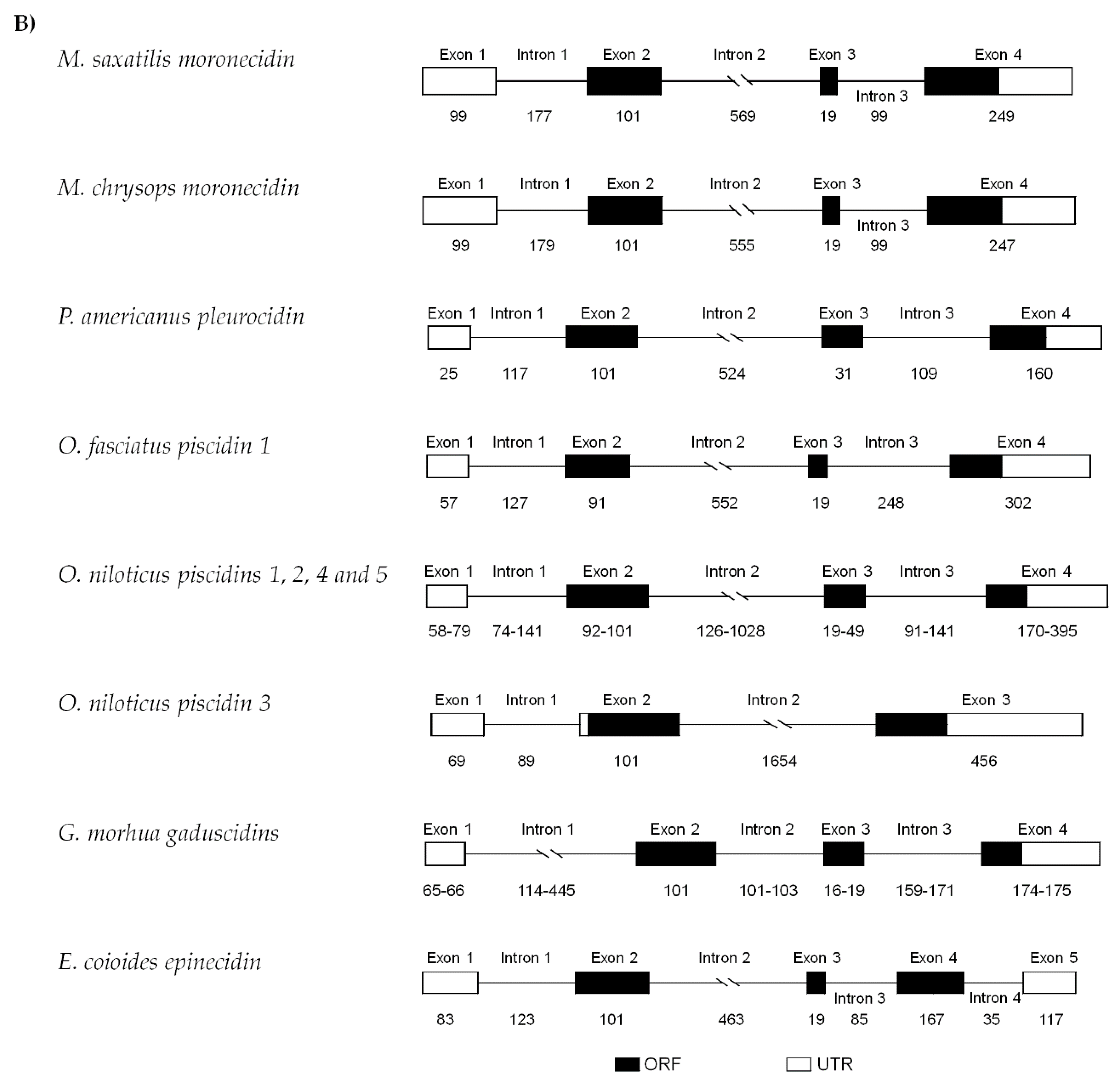
2.2. Genomic Organization
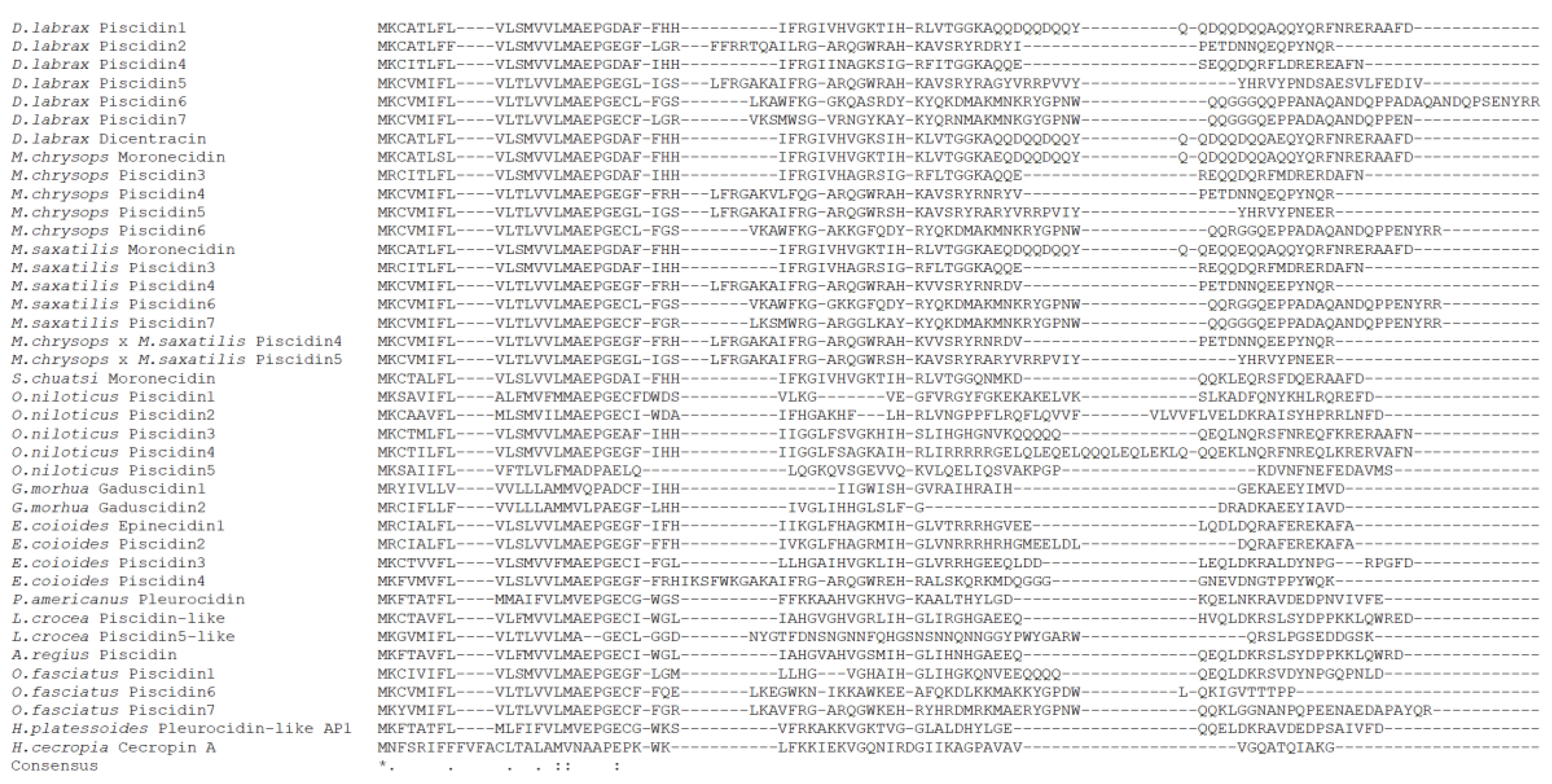
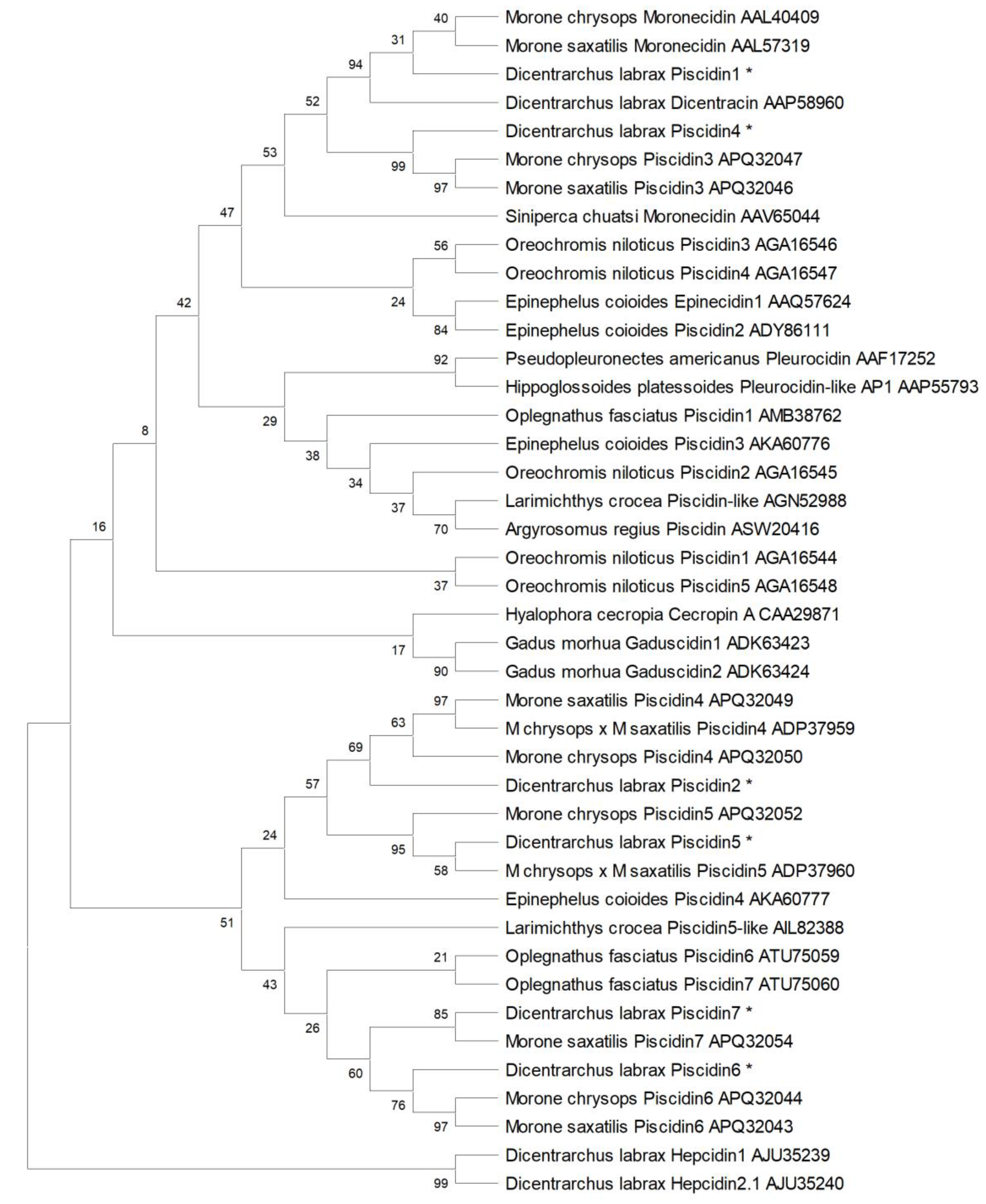
2.3. Sequence Comparison and Phylogenetic Analysis
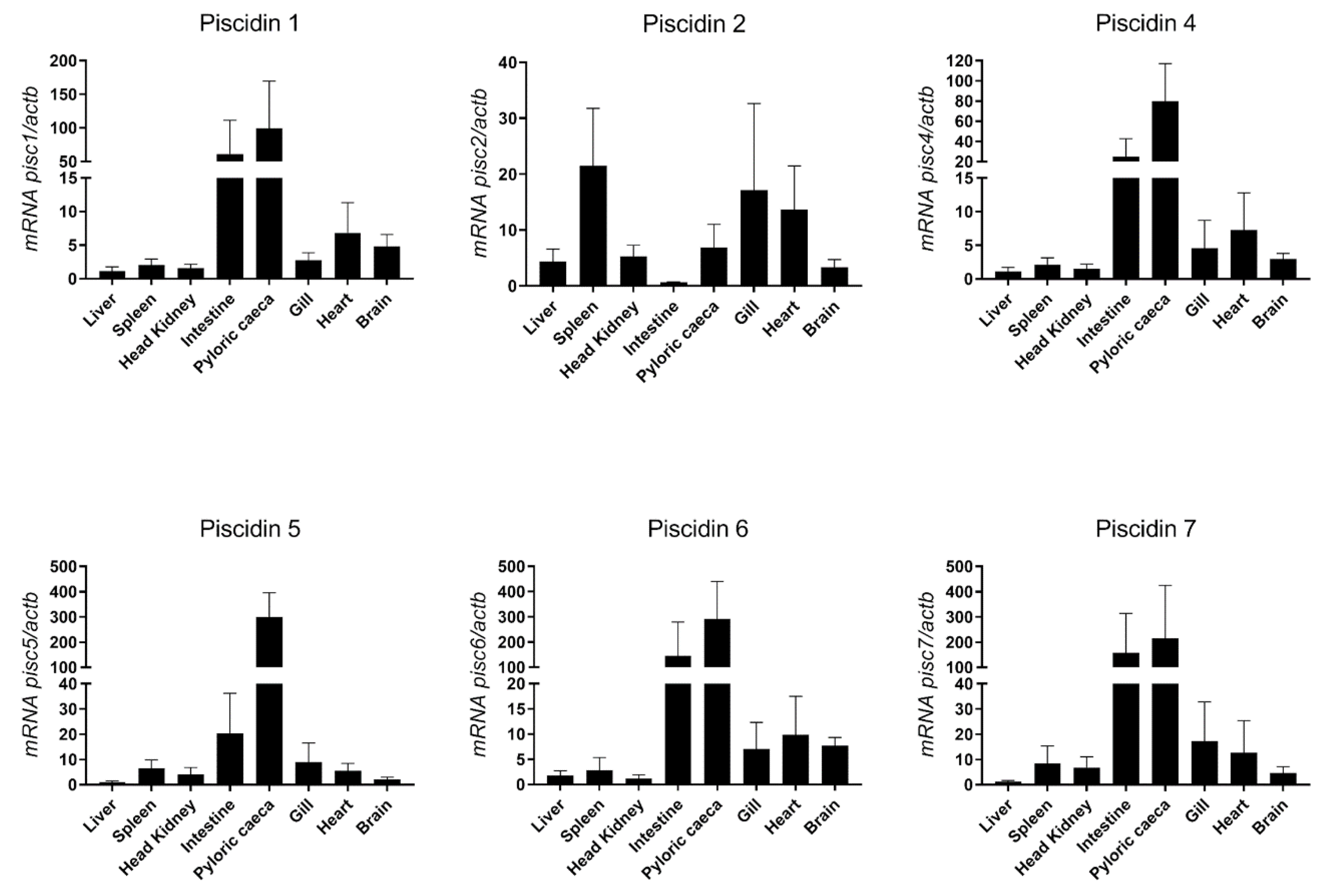
2.4. Basal Expression of Sea Bass Piscidins
2.5. Modeling of Sea Bass Piscidins
2.6. Antibacterial Activity of Sea Bass Piscidins
2.7. Antiparasitic Activity of Sea Bass Piscidins
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Isolation of Sea Bass Piscidins
4.3. Genomic Organization
4.4. Amplification of 5’ and 3’ Flanking Regions
4.5. Alignment and Phylogenetic Analysis
4.6. RNA Isolation and cDNA Synthesis
4.7. Basal Expression of Sea Bass Piscidins
4.8. Antibacterial Activity of Sea Bass Piscidin Peptides
4.9. Antiparasitic Activity of Sea Bass Piscidin Peptides
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Magnadottir, B. Immunological control of fish diseases. Mar. Biotechnol. 2010, 12, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Masso-Silva, J.A.; Diamond, G. Antimicrobial peptides from fish. Pharmaceuticals 2014, 7, 265–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katzenback, B.A. Antimicrobial Peptides as Mediators of Innate Immunity in Teleosts. Biology 2015, 4, 607–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaturvedi, P.; Bhat, R.A.H.; Pande, A. Antimicrobial peptides of fish: Innocuous alternatives to antibiotics. Rev. Aquac. 2020, 12, 85–106. [Google Scholar] [CrossRef]
- Cole, A.M.; Weis, P.; Diamond, G. Protein chemistry and structure: Isolation and Characterization of Pleurocidin, an Antimicrobial Peptide in the Skin Secretions of Winter Flounder. J. Biol. Chem. 1997, 272, 12008–12013. [Google Scholar] [CrossRef] [Green Version]
- Silphaduang, U.; Noga, E.J. Peptide antibiotics in mast cells of fish. Nature 2001, 414, 268–269. [Google Scholar] [CrossRef]
- Pan, C.Y.; Chen, J.Y.; Ni, I.H.; Wu, J.L.; Kuo, C.M. Organization and promoter analysis of the grouper (Epinephelus coioides) epinecidin-1 gene. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 150, 358–367. [Google Scholar] [CrossRef]
- Browne, M.J.; Feng, C.Y.; Booth, V.; Rise, M.L. Characterization and expression studies of Gaduscidin-1 and Gaduscidin-2; paralogous antimicrobial peptide-like transcripts from Atlantic cod (Gadus morhua). Dev. Comp. Immunol. 2011, 35, 399–408. [Google Scholar] [CrossRef]
- Peng, K.C.; Lee, S.H.; Hour, A.L.; Pan, C.Y.; Lee, L.H.; Chen, J.Y. Five Different Piscidins from Nile Tilapia, Oreochromis niloticus: Analysis of Their Expressions and Biological Functions. PLoS ONE 2012, 7, e50263. [Google Scholar] [CrossRef]
- Niu, S.F.; Jin, Y.; Xu, X.; Qiao, Y.; Wu, Y.; Mao, Y.; Su, Y.Q.; Wang, J. Characterization of a novel piscidin-like antimicrobial peptide from Pseudosciaena crocea and its immune response to Cryptocaryon irritans. Fish Shellfish Immunol. 2013, 35, 513–524. [Google Scholar] [CrossRef]
- Cole, A.M.; Darouiche, R.O.; Legarda, D.; Connell, N.; Diamond, G. Characterization of a fish antimicrobial peptide: Gene expression, subcellular localization, and spectrum of activity. Antimicrob. Agents Chemother. 2000, 44, 2039–2045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patrzykat, A.; Gallant, J.; Seo, J.; Pytyck, J.; Douglas, S.E. Novel antimicrobial peptides derived from flatfish genes. Antimicrob. Agents Chemother. 2003, 47, 2464–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Z.X.; He, W.; Chen, W.J.; Yan, J.H.; Yang, J.N.; Chan, S.M.; He, J.G. Cloning, expression and antimicrobial activity of an antimicrobial peptide, epinecidin-1, from the orange-spotted grouper, Epinephelus coioides. Aquaculture 2006, 253, 204–211. [Google Scholar] [CrossRef]
- Sun, B.J.; Xie, H.X.; Song, Y.; Nie, P. Gene structure of an antimicrobial peptide from mandarin fish, Siniperca chuatsi (Basilewsky), suggests that moronecidins and pleurocidins belong in one family: The piscidins. J. Fish Dis. 2007, 30, 335–343. [Google Scholar] [CrossRef]
- Umasuthan, N.; Mothishri, M.S.; Thulasitha, W.S.; Nam, B.H.; Lee, J. Molecular, genomic, and expressional delineation of a piscidin from rock bream (Oplegnathus fasciatus) with evidence for the potent antimicrobial activities of Of-Pis1 peptide. Fish Shellfish Immunol. 2016, 48, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.-R.; Yang, X.-D.; Huang, X.-Z.; Gu, H.-X.; Wei, H.-Y.; He, Y.-J.; Deng, L. Three new piscidins from orange-spotted grouper (Epinephelus coioides): Phylogeny, expression and functional characterization. Fish Shellfish Immunol. 2017, 66, 240–253. [Google Scholar] [CrossRef]
- Noga, E.J.; Silphaduang, U.; Park, N.G.; Seo, J.K.; Stephenson, J.; Kozlowicz, S. Piscidin 4, a novel member of the piscidin family of antimicrobial peptides. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009, 152, 299–305. [Google Scholar] [CrossRef]
- Salger, S.A.; Reading, B.J.; Baltzegar, D.A.; Sullivan, C.V.; Noga, E.J. Molecular characterization of two isoforms of piscidin 4 from the hybrid striped bass (Morone chrysops × Morone saxatilis). Fish Shellfish Immunol. 2011, 30, 420–424. [Google Scholar] [CrossRef]
- Salger, S.A.; Cassady, K.R.; Reading, B.J.; Noga, E.J. A diverse family of host-defense peptides (piscidins) exhibit specialized anti-bacterial and anti-protozoal activities in fishes. PLoS ONE 2016, 11, e0159423. [Google Scholar] [CrossRef] [Green Version]
- Tennessen, J.A. Enhanced synonymous site divergence in positively selected vertebrate antimicrobial peptide genes. J. Mol. Evol. 2005, 61, 445–455. [Google Scholar] [CrossRef]
- Fernandes, J.M.O.; Ruangsri, J.; Kiron, V. Atlantic cod piscidin and its diversification through positive selection. PLoS ONE 2010, 5, e9501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buonocore, F.; Randelli, E.; Casani, D.; Picchietti, S.; Belardinelli, M.C.; de Pascale, D.; De Santi, C.; Scapigliati, G. A piscidin-like antimicrobial peptide from the icefish Chionodraco hamatus (Perciformes: Channichthyidae): Molecular characterization, localization and bactericidal activity. Fish Shellfish Immunol. 2012, 33, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.J.; Su, Y.Q.; Niu, S.F.; Liu, M.; Qiao, Y.; Wang, J. Discovery and molecular cloning of piscidin-5-like gene from the large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 2014, 41, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-P.; Chen, D.-W.; Pan, Y.-Q.; Deng, L. Two isoforms of piscidin from Malabar grouper, Epinephelus malabaricus: Expression and functional characterization. Fish Shellfish Immunol. 2016, 57, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.S.; Jeong, J.M.; Nam, B.H.; Kim, J.W.; Park, J.Y.; Park, C. Il Gene expressions and biological activities of two novel antimicrobial peptides (AMPs) isolated from leucocytes of the rock bream, Oplegnathus fasciatus. Aquaculture 2018, 495, 35–43. [Google Scholar] [CrossRef]
- Colorni, A.; Ullal, A.; Heinisch, G.; Noga, E.J. Activity of the antimicrobial polypeptide piscidin 2 against fish ectoparasites. J. Fish Dis. 2008, 31, 423–432. [Google Scholar] [CrossRef]
- Corrales, J.; Mulero, I.; Mulero, V.; Noga, E.J. Detection of antimicrobial peptides related to piscidin 4 in important aquacultured fish. Dev. Comp. Immunol. 2010, 34, 331–343. [Google Scholar] [CrossRef]
- Bae, J.S.; Jung, J.M.; An, C.M.; Kim, J.W.; Hwang, S.D.; Kwon, M.G.; Park, M.A.; Kim, M.C.; Park, C. Il Piscidin: Antimicrobial peptide of rock bream, Oplegnathus fasciatus. Fish Shellfish Immunol. 2016, 51, 136–142. [Google Scholar] [CrossRef]
- Hu, B.; Pan, Y.; Li, Z.; Yuan, W.; Deng, L. EmPis-1L, an Effective Antimicrobial Peptide against the Antibiotic-Resistant VBNC State Cells of Pathogenic Bacteria. Probiotics Antimicrob. Proteins 2019, 11, 667–675. [Google Scholar] [CrossRef]
- Huang, H.N.; Pan, C.Y.; Chen, J.Y. Grouper (Epinephelus coioides) antimicrobial peptide epinecidin-1 exhibits antiviral activity against foot-and-mouth disease virus in vitro. Peptides 2018, 106, 91–95. [Google Scholar] [CrossRef]
- Milne, D.J.; Fernández-Montero, Á.; Gundappa, M.K.; Wang, T.; Acosta, F.; Torrecillas, S.; Montero, D.; Zou, J.; Sweetman, J.; Secombes, C.J. An insight into piscidins: The discovery, modulation and bioactivity of greater amberjack, Seriola dumerili, piscidin. Mol. Immunol. 2019, 114, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zheng, L.B.; Mao, Y.; Wang, J.; Lin, L.S.; Su, Y.Q.; Li, Y. The antibacterial activity and mechanism analysis of piscidin 5 like from Larimichthys crocea. Dev. Comp. Immunol. 2019, 92, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Salerno, G.; Parrinello, N.; Roch, P.; Cammarata, M. cDNA sequence and tissue expression of an antimicrobial peptide, dicentracin; a new component of the moronecidin family isolated from head kidney leukocytes of sea bass, Dicentrarchus labrax. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 146, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Silphaduang, U.; Colorni, A.; Noga, E.J. Evidence for widespread distribution of piscidin antimicrobial peptides in teleost fish. Dis. Aquat. Organ. 2006, 72, 241–252. [Google Scholar] [CrossRef]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Bioinformatics 1992, 8, 275–282. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Terova, G.; Cattaneo, A.G.; Preziosa, E.; Bernardini, G.; Saroglia, M. Impact of acute stress on antimicrobial polypeptides mRNA copy number in several tissues of marine sea bass (Dicentrarchus labrax). BMC Immunol. 2011, 12, 69. [Google Scholar] [CrossRef] [Green Version]
- Meloni, M.; Candusso, S.; Galeotti, M.; Volpatti, D. Preliminary study on expression of antimicrobial peptides in European sea bass (Dicentrarchus labrax) following in vivo infection with Vibrio anguillarum. A time course experiment. Fish Shellfish Immunol. 2015, 43, 82–90. [Google Scholar] [CrossRef]
- Dezfuli, B.S.; Pironi, F.; Giari, L.; Noga, E.J. Immunocytochemical localization of piscidin in mast cells of infected seabass gill. Fish Shellfish Immunol. 2010, 28, 476–482. [Google Scholar] [CrossRef]
- Lauth, X.; Shike, H.; Burns, J.C.; Westerman, M.E.; Ostland, V.E.; Carlberg, J.M.; Van Olst, J.C.; Nizet, V.; Taylor, S.W.; Shimizu, C.; et al. Discovery and characterization of two isoforms of moronecidin, a novel antimicrobial peptide from hybrid striped bass. J. Biol. Chem. 2002, 277, 5030–5039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruangsri, J.; Salger, S.A.; Caipang, C.M.A.; Kiron, V.; Fernandes, J.M.O. Differential expression and biological activity of two piscidin paralogues and a novel splice variant in Atlantic cod (Gadus morhua L.). Fish Shellfish Immunol. 2012, 32, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.E.; Patrzykat, A.; Pytyck, J.; Gallant, J.W. Identification, structure and differential expression of novel pleurocidins clustered on the genome of the winter flounder, Pseudopleuronectes americanus (Walbaum). Eur. J. Biochem. 2003, 270, 3720–3730. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Van De Peer, Y. From 2R to 3R: Evidence for a fish-specific genome duplication (FSGD). BioEssays 2005, 27, 937–945. [Google Scholar] [CrossRef] [Green Version]
- Padhi, A.; Verghese, B. Evidence for positive Darwinian selection on the hepcidin gene of Perciform and Pleuronectiform fishes. Mol. Divers. 2007, 11, 119–130. [Google Scholar] [CrossRef]
- Ravi, V.; Venkatesh, B. Rapidly evolving fish genomes and teleost diversity. Curr. Opin. Genet. Dev. 2008, 18, 544–550. [Google Scholar] [CrossRef]
- Sung, W.S.; Lee, J.; Lee, D.G. Fungicidal effect and the mode of action of piscidin 2 derived from hybrid striped bass. Biochem. Biophys. Res. Commun. 2008, 371, 551–555. [Google Scholar] [CrossRef]
- Olivieri, C.; Buonocore, F.; Picchietti, S.; Taddei, A.R.; Bernini, C.; Scapigliati, G.; Dicke, A.A.; Vostrikov, V.V.; Veglia, G.; Porcelli, F. Structure and membrane interactions of chionodracine, a piscidin-like antimicrobial peptide from the icefish Chionodraco hamatus. Biochim. Biophys. Acta Biomembr. 2015, 1848, 1285–1293. [Google Scholar] [CrossRef] [Green Version]
- Mihailescu, M.; Sorci, M.; Seckute, J.; Silin, V.I.; Hammer, J.; Perrin, B.S.; Hernandez, J.I.; Smajic, N.; Shrestha, A.; Bogardus, K.A.; et al. Structure and Function in Antimicrobial Piscidins: Histidine Position, Directionality of Membrane Insertion, and pH-Dependent Permeabilization. J. Am. Chem. Soc. 2019, 141, 9837–9853. [Google Scholar] [CrossRef]
- Chekmenev, E.Y.; Vollmar, B.S.; Forseth, K.T.; Manion, M.K.N.; Jones, S.M.; Wagner, T.J.; Endicott, R.L.M.; Kyriss, B.P.; Homem, L.M.; Pate, M.; et al. Investigating molecular recognition and biological function at interfaces using piscidins, antimicrobial peptides from fish. Biochim. Biophys. Acta Biomembr. 2006, 1758, 1359–1372. [Google Scholar] [CrossRef] [Green Version]
- Chekmenev, E.Y.; Jones, S.M.; Nikolayeva, Y.N.; Vollmar, B.S.; Wagner, T.J.; Gor’kov, P.L.; Brey, W.W.; Manion, M.N.; Daugherty, K.C.; Cotten, M. High-field NMR studies of molecular recognition and structure-function relationships in antimicrobial piscidins at the water-lipid bilayer interface. J. Am. Chem. Soc. 2006, 128, 5308–5309. [Google Scholar] [CrossRef] [PubMed]
- Campagna, S.; Saint, N.; Molle, G.; Aumelas, A. Structure and mechanism of action of the antimicrobial peptide piscidin. Biochemistry 2007, 46, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Park, N.G.; Silphaduang, U.; Moon, H.S.; Seo, J.K.; Corrales, J.; Noga, E.J. Structure-Activity Relationships of Piscidin 4, a Piscine Antimicrobial Peptide. Biochemistry 2011, 50, 3288–3299. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.S.; Shim, S.H.E.; Hwang, S.D.O.; Park, M.A.; Jee, B.Y.; An, C.M.; Kim, Y.O.; Kim, J.W.; Park, C. Il Expression analysis and biological activity of moronecidin from rock bream, Oplegnathus fasciatus. Fish Shellfish Immunol. 2014, 40, 345–353. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Huang, H.N.; Rajanbabu, V.; Pan, C.Y.; Chan, Y.L.; Wu, C.J.; Chen, J.Y. Use of the antimicrobial peptide Epinecidin-1 to protect against MRSA infection in mice with skin injuries. Biomaterials 2013, 34, 10319–10327. [Google Scholar] [CrossRef]
- Acosta, J.; Montero, V.; Carpio, Y.; Velázquez, J.; Garay, H.E.; Reyes, O.; Cabrales, A.; Masforrol, Y.; Morales, A.; Estrada, M.P. Cloning and functional characterization of three novel antimicrobial peptides from tilapia (Oreochromis niloticus). Aquaculture 2013, 372, 9–18. [Google Scholar] [CrossRef]
- Nussbaum, K.; Honek, J.; Cadmus, C.M.C.v.C.; Efferth, T. Trypanosomatid Parasites Causing Neglected Diseases. Curr. Med. Chem. 2010, 17, 1594–1617. [Google Scholar] [CrossRef]
- Gautier, R.; Douguet, D.; Antonny, B.; Drin, G. HELIQUEST: A web server to screen sequences with specific α-helical properties. Bioinformatics 2008, 24, 2101–2102. [Google Scholar] [CrossRef]



| Piscidin1 | Piscidin2 | Piscidin4 | Piscidin5 | Piscidin6 | Piscidin7 | |
|---|---|---|---|---|---|---|
| Accession number | MT066191 | MT066192 | MT066193 | MT066194 | MT066195 | MT066196 |
| ORF (bp) 1 | 240 | 213 | 204 | 246 | 279 | 240 |
| 5’ UTR (bp) 2 | 80 | 111 | 81 | 109 | - | - |
| 3’ UTR (bp) 2 | 134 | 38 | 438 | 97 | - | 120 |
| Prepropeptide (aa) 3 | 79 | 70 | 67 | 81 | 92 | 79 |
| Signal peptide (aa) | 22 | 22 | 22 | 22 | 22 | 22 |
| Mature peptide (aa) | 22 | 44 | 22 | 46 | 65 | 55 |
| Prodomain (aa) | 35 | 4 | 23 | 13 | 5 | 2 |
| Molecular weight (Da) | 2571 | 5362 | 2454 | 5406 | 7182 | 6174 |
| Isoelectric point (pI) | 14.00 | 11.82 | 14.00 | 12.03 | 10.34 | 10.52 |
| Net charge (at pH 7) | 4.4 | 7.1 | 4.2 | 12.2 | 5.0 | 6.0 |
| Piscidin1 | Piscidin2 | Piscidin4 | Piscidin5 | Piscidin6 | Piscidin7 | |
|---|---|---|---|---|---|---|
| Piscidin1 | 37.8% | 67.3% | 30.6% | 25.5% | 33.7% | |
| Piscidin2 | 37.8% | 48.0% | 58.2% | 28.6% | 43.9% | |
| Piscidin4 | 67.3% | 48.0% | 36.7% | 27.6% | 34.7% | |
| Piscidin5 | 30.6% | 58.2% | 36.7% | 38.8% | 48.0% | |
| Piscidin6 | 25.5% | 28.6% | 27.6% | 38.8% | 64.3% | |
| Piscidin7 | 33.7% | 43.9% | 34.7% | 48.0% | 64.3% |
| Bacteria | MIC (µM) | |||||
|---|---|---|---|---|---|---|
| Gram-Negative | Piscidin1 | Piscidin2 | Piscidin4 | Piscidin5 | Piscidin6 | Piscidin7 |
| P. damselae subsp. piscicida | 7.4 ± 1.6 | 9.1 ± 1.7 | 29.1 ± 12.0 | 3.3 ± 0.1 | N.A. | N.A. |
| P. damselae subsp. damselae | 15.0 ± 0.2 | 105.8 ± 22.1 | 81.3 ± 9.5 | 14.8 ± 2.0 | N.A. | N.A. |
| V. anguillarum | 16.3 ± 0.6 | 146.1 ± 21.4 | N.A. | 10.1 ± 2.7 | N.A. | N.A. |
| V. alginolyticus | 34.8 ± 0.3 | 66.2 ± 2.0 | N.A. | 36.9 ± 2.3 | N.A. | N.A. |
| A. salmonicida subsp. salmonicida | 64.2 ± 2.1 | 67.7 ± 0.2 | N.A. | 47.9 ± 6.1 | N.A. | N.A. |
| A. hydrophila subsp. hydrophila | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. |
| Y. ruckeri | 48.4 ± 3.8 | 137.6 ± 3.1 | N.A. | 25.4 ± 1.8 | N.A. | N.A. |
| E. tarda | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. |
| Gram-positive | ||||||
| L. garviae | 33.9 ± 3.8 | N.A. | 48.8 ± 40.1 | 42.3 ± 16.4 | N.A. | N.A. |
| S. parauberis | 2.4 ± 0.8 | 6.9 ± 2.8 | 1.8 ± 1.8 | 1.3 ± 1.2 | 87.7 ± 123.0 | N.A. |
| IC50 (µM) | ||||||
|---|---|---|---|---|---|---|
| Piscidin1 | Piscidin2 | Piscidin4 | Piscidin5 | Piscidin6 | Piscidin7 | |
| T. brucei brucei | 3.88 ± 0.11 | 1.74 ± 0.13 | 3.24 ± 0.16 | 4.42 ± 0.12 | 56.50 ± 1.61 | 7.30 ± 0.32 |
| L. infantum | 5.20 ± 0.03 | 5.52 ± 0.26 | 8.34 ± 0.81 | 7.24 ± 0.61 | N.A. | 66.77 ± 0.08 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barroso, C.; Carvalho, P.; Carvalho, C.; Santarém, N.; Gonçalves, J.F.M.; Rodrigues, P.N.S.; Neves, J.V. The Diverse Piscidin Repertoire of the European Sea Bass (Dicentrarchus labrax): Molecular Characterization and Antimicrobial Activities. Int. J. Mol. Sci. 2020, 21, 4613. https://doi.org/10.3390/ijms21134613
Barroso C, Carvalho P, Carvalho C, Santarém N, Gonçalves JFM, Rodrigues PNS, Neves JV. The Diverse Piscidin Repertoire of the European Sea Bass (Dicentrarchus labrax): Molecular Characterization and Antimicrobial Activities. International Journal of Molecular Sciences. 2020; 21(13):4613. https://doi.org/10.3390/ijms21134613
Chicago/Turabian StyleBarroso, Carolina, Pedro Carvalho, Carla Carvalho, Nuno Santarém, José F. M. Gonçalves, Pedro N. S. Rodrigues, and João V. Neves. 2020. "The Diverse Piscidin Repertoire of the European Sea Bass (Dicentrarchus labrax): Molecular Characterization and Antimicrobial Activities" International Journal of Molecular Sciences 21, no. 13: 4613. https://doi.org/10.3390/ijms21134613







